Abstract
Background
Androgen deprivation therapy (ADT) for prostate cancer with luteinizing hormone-releasing hormone (LHRH) agonists can be improved.
Objective
To assess safety, the frequency and severity of hot flushes (HFs), bone health, and antitumor effects of high-dose estetrol (HDE4) when combined with ADT.
Design, setting and participants
A phase II, double-blind, randomized, placebo-controlled study was conducted in advanced prostate cancer patients requiring ADT (the PCombi study).
Intervention
Patients receiving LHRH agonist treatment were randomized 2:1 to 40 mg HDE4 (n = 41) or placebo (n = 21) cotreatment for 24 wk.
Outcome measurements and statistical analysis
Coprimary endpoints were frequency/severity of HFs and levels of total and free testosterone (T). Secondary endpoints included assessments of bone metabolism (osteocalcin and type I collagen telopeptide [CTX1]), prostate-specific antigen (PSA), and follicle-stimulating hormone (FSH). Efficacy analysis was based on the selected per-protocol (PP) population.
Results and limitations
Of 62 patients included in the study, 57 were suitable for a PP analysis (37 HDE4; 20 placebo). No E4-related serious cardiovascular adverse events occurred at 24 wk. Weekly HFs were reported by 13.5% of patients with HDE4 and 60.0% with placebo (p < 0.001). Daily HFs occurred in 5.9% versus 55%. Bone turnover parameters decreased significantly with HDE4 (p < 0.0001). Total and free T decreased earlier (p < 0.05), and free T was suppressed further (p < 0.05). PSA suppression was more profound and earlier (p < 0.005). FSH levels were suppressed by 98% versus 57% (p < 0.0001). Estrogenic side effects were nipple sensitivity (34%) and gynecomastia (17%).
Conclusions
HDE4 cotreatment of ADT patients with advanced prostate cancer was well tolerated, and no treatment-related cardiovascular adverse events were observed at 24 wk. HFs and bone turnover were substantially reduced. Suppression of free T, PSA, and FSH was more rapid and profound, suggesting enhanced disease control by HDE4 cotreatment. Larger and longer-lasting studies are needed to confirm the results of the study reported here.
Patient summary
Cotreatment of androgen deprivation therapy with high-dose estetrol in advanced prostate cancer patients results in fewer occurrences of hot flushes, bone protection, and other antitumor benefits. Nipple sensitivity and gynecomastia may occur as side effects.
Keywords: Androgen deprivation therapy, Bone, Cardiovascular safety, Antitumor efficacy, Estetrol, Hot flushes, Luteinizing hormone-releasing hormone agonists, PCombi, Prostate cancer
Take Home Message
Androgen-deprivation therapy (ADT) for prostate cancer with luteinizing hormone-releasing hormone (LHRH) agonists can be improved. Cotreatment of ADT with high-dose estetrol in advanced prostate cancer patients results in fewer occurrences of hot flushes, bone protection, and other antitumor benefits.
1. Introduction
In 1941, Huggins and Hodges [1] were the first to demonstrate that metastatic prostate cancer is positively affected by castration. Since then, estrogens were used effectively for 30 yr to reduce testosterone (T) levels to inhibit the growth of androgen-sensitive adenocarcinoma of the prostate [2], [3]. However, estrogens had serious cardiovascular (CV) side effects [3]. The discovery of gonadotrophin-releasing hormone (GnRH) by the group of Schally [4], controlling the release of the gonadotrophins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by the pituitary, made the development of GnRH analogs possible. Replacement of estrogens by GnRH analogs may decrease the CV risk of androgen deprivation therapy (ADT), but causes serious estrogen deficiency, since the reduction of T also suppresses its metabolite estradiol (E2). Hot flushes (HFs) occur frequently, interfering with quality of life (QoL), and increased bone turnover predisposes to bone loss and fractures. Relevant other effects of estrogens are the following: (1) an increase of the hepatic protein sex hormone–binding globulin (SHBG), thereby decreasing free T [5], and (2) suppression of FSH, an independent prostate cancer growth inhibitor that is also associated with a lower risk of CV side effects during ADT [6].
Estetrol (E4) is a natural human estrogen, produced by the fetal liver during pregnancy only. Its physiological function is unknown, and it has no active or toxic metabolites. Oral administration of E4 has a low impact on coagulation and hemostatic liver factors, alone or at doses up to 15 mg in combination with the progestin drospirenone for contraception [7], [8], [9], [10], [11], [12], [13]. E4 has a different mechanism of action from those of other estrogens, since it does not stimulate the membrane estrogen receptor alpha (ERα), and in the presence of E2, E4 antagonizes the stimulatory membrane effects of E2 [14], [15].
In view of this different mode of action and its limited liver and hemostatic effects, oral E4 was considered to offer a new opportunity for estrogen treatment of advanced prostate cancer. An E4 dose-finding study resulted in the selection of a dose of 40 mg E4 for further development [5]. The phase II PCombi study was performed in patients with advanced prostate cancer who started ADT treatment with luteinizing hormone-releasing hormone (LHRH) agonists. In this study, safety, estrogen replacement effects on HFs and bone, and potential antitumor effects on total and free T, prostate-specific antigen (PSA) and FSH of high-dose estetrol (HDE4) were investigated and are reported here.
2. Patients and methods
2.1. Patients
The present study was performed in patients with regionally advanced infiltrating, nonmetastatic, or metastatic castration-sensitive prostate cancer that required ADT. Patients aged ≥18 yr were eligible if they qualified for ADT by LHRH agonist treatment (Supplementary material). In addition, patients had to have a body mass index (BMI) of 18.0–35.0 kg/m2, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1 [16], and life expectancy of at least 2 yr. The concomitant use of antiandrogens to prevent symptoms of a T flare was allowed during the first 14 d of the study. Patients with a history of venous thromboembolism or defect in the blood coagulation system could not participate. Patients were treated with standard doses and regimen of leuprolide (88%) or goserelin (12%) chosen by the investigator.
The study was approved by the Independent Ethics Committee of the Foundation “Evaluation of Ethics in Biomedical Research,” Assen, the Netherlands, and conducted in accordance with the provisions of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Council for Harmonisation. All patients provided written informed consent.
2.2. Study design and treatments
PCombi was a phase 2, double-blind, randomized, placebo-controlled study, conducted at four sites in the Netherlands from March 2018 to May 2020 (ClinicalTrials.gov identifier: NCT03361969. EudraCT 2017-003708-34). Patients were randomized in a 2:1 ratio to once daily oral treatment with 40 mg E4 (HDE4) or placebo, in addition to ADT using an LHRH agonist. Blinded study treatment was initiated on the day of the first LHRH agonist injection and was continued for 24 wk.
2.3. Outcomes
The PCombi study had two primary efficacy endpoints: (1) the frequency and intensity of HFs, and (2) the levels of total T and free T. Occurrences of HFs were recorded in a diary (number and intensity) and in a nonvalidated Q-Man questionnaire (Supplementary material) registering hormonal side effects at every visit. The diary was completed during three 7-d periods in weeks 5, 13, and 23 of treatment. Secondary efficacy endpoints were the effect of HDE4 on PSA, LH, FSH, E2, SHBG, osteocalcin, type I collagen telopeptide (CTX1), and dehydroepiandrosterone-sulfate (DHEA-S). Special emphasis was paid to CV adverse events (AEs). Safety was further assessed through reporting of other AEs, clinical laboratory tests, vital signs, and physical examinations.
2.4. Statistical analysis
No formal sample size calculation was performed for this explorative phase II study. The number of patients to be recruited was based on a reduction of at least 50% of the frequency of HFs, known to occur in 55–60% of patients treated with ADT [17], [18], [19]. The per-protocol (PP) population was considered the primary population for the efficacy analysis. For all efficacy variables, the 24-wk visit was the primary endpoint. The proportion of patients experiencing at least one HF during the 7 d before the last visit was compared using Fisher’s exact test. Differences between treatment groups over time for total T, free T, and PSA were analyzed using a repeated-measure mixed model on log-transformed values. Endocrine and biochemical parameters at the 24-wk visit were compared using Kruskal-Wallis testing (Supplementary material).
The safety evaluation was based on all patients who received at least one dose of study medication.
3. Results
Five patients withdrew informed consent during the screening. In total, 63 patients were randomized and 62 received study medication (41 HDE4 and 21 placebo). Five patients discontinued treatment early. The PP population consisted of 57 patients: 37 on HDE4 and 20 on placebo (see Supplementary Fig. 1 for the CONSORT diagram). Baseline patient characteristics did not differ between the treatment groups, except for BMI and ECOG performance status (Table 1). Concomitant hormonal medication was not allowed, except for bicalutamide during the first 2 wk of treatment, used by 43% and 50% of patients on HDE4 and placebo, respectively.
Table 1.
Patient demographics and baseline characteristics (per-protocol population)
| Parameter | 40 mg estetrol | Placebo | Total | p valuea |
|---|---|---|---|---|
| (N = 37) | (N = 20) | (N = 57) | ||
| Age (yr), median (range) | 74 (59–85) | 75 (49–84) | 74 (49–85) | 0.650 |
| Weight (kg) | 82.9 (12.2) | 90.0 (14.8) | 85.4 (13.5) | 0.057 |
| BMI (kg/m2) | 26.1 (3.4) | 28.2 (3.7) | 26.8 (3.6) | 0.045 |
| Duration since PC diagnosis | ||||
| 0–3 mo | 21 (56.8) | 12 (60.0) | 33 (57.9) | 0.458 |
| 3–6 mo | 3 (8.1) | 3 (15.0) | 6 (10.5) | |
| 6 mo–1 yr | 2 (5.4) | 1 (5.0) | 3 (5.3) | |
| >1 yr | 11 (29.7) | 4 (20.0) | 15 (26.3) | |
| Distant metastasis | ||||
| M0 | 25 (67.6) | 14 (70.0) | 39 (68.4) | 0.382 |
| M1 | 7 (18.9) | 2 (10.0) | 9 (15.8) | |
| M1a | 2 (5.4) | – | 2 (3.5) | |
| M1b | 3 (8.1) | 3 (15.0) | 6 (10.5) | |
| M1c | – | 1 (5.0) | 1 (1.8) | |
| Gleason score, n (%) | ||||
| 6 | 2 (5.4) | 1 (5.0) | 3 (5.3) | 0.996 |
| 7 | 15 (40.5) | 8 (40.0) | 23 (40.4) | |
| ≥8 | 20 (54.1) | 11 (55.0) | 31 (54.4) | |
| ECOG performance status, n (%) | ||||
| 0 | 33 (89.2) | 12 (60.0) | 45 (78.9) | 0.010 |
| 1 | 4 (10.8) | 8 (40.0) | 12 (21.1) | |
| Previous prostatectomy, n (%) | 9 (24.3) | 3 (15.0) | 12 (21.1) | 0.410 |
| Radiotherapy for primary tumor, n (%) | 7 (18.9) | 2 (10.0) | 9 (15.8) | 0.378 |
| Previous hormone therapy, n (%) | – | 1 (5.0) | 1 (1.8) | 0.170 |
| Use of bicalutamide during baseline efficacy lab test, n (%) | 16 (43.2) | 10 (50.0) | 26 (45.6) | 0.625 |
BMI = body mass index; ECOG = Eastern Cooperative Oncology Group; LHRH = luteinizing hormone-releasing hormone; PC = prostate cancer.
Results are expressed in mean (standard deviation) or otherwise specified.
All patients were male patients with prostate cancer qualifying for treatment with an LHRH agonist with no history of radiotherapy to bone or no history of chemotherapy.
For categorical variables, chi-square exact test p value was reported; for continuous variables, t test p value was reported (p value is printed in bold if statistically significant).
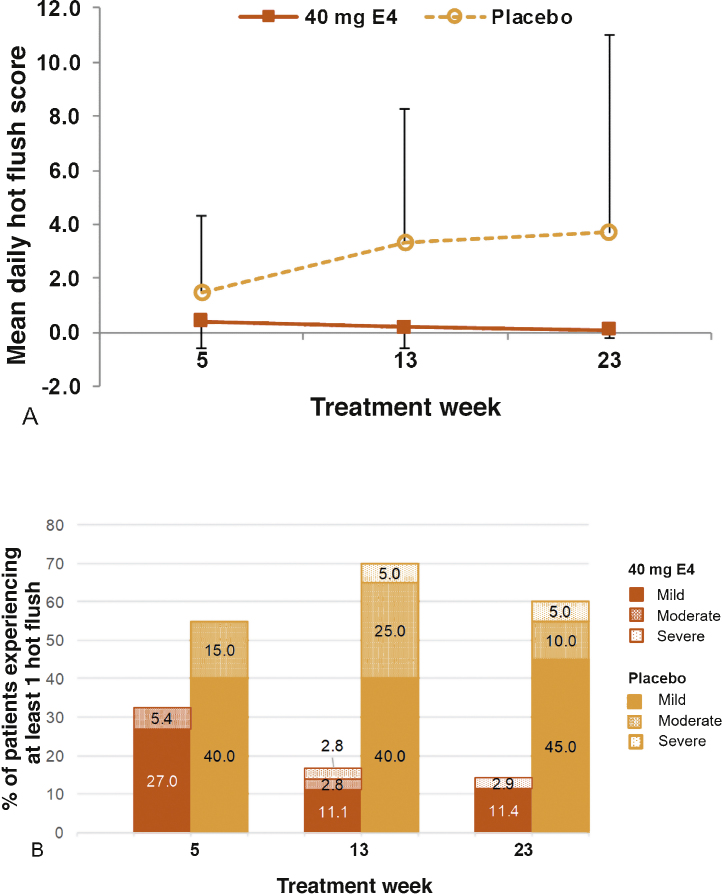
The mean daily frequency and intensity HF score were lower with HDE4 at all time points (Fig. 1A). During week 23, 14.3% of patients in the HDE4 group reported mild, moderate, or severe HFs in a diary compared with 60.0% in the placebo group (p < 0.001; Fig. 1B). At the week 24 visit, the Q-Man questionnaire (Supplementary material) revealed daily HFs in 5.9% of patients with HDE4 and in 55.0% with placebo. Temporary nipple sensitivity (34%) and gynecomastia (17%) occurred in the HDE4 group only.
Fig. 1.
(A) Mean daily hot flushes and (B) percentage of patients experiencing at least one hot flush measured during treatment weeks 5, 13, and 23 with 40 mg estetrol (E4) or placebo in patients with prostate cancer treated with an LHRH agonist (per-protocol population). Mean daily hot flush score is a calculated score based on the number and severity of hot flushes per day, averaged over the 1-wk period. Severity was recorded as mild (1), moderate (2), severe (3), and very severe (4). LHRH = luteinizing hormone-releasing hormone.
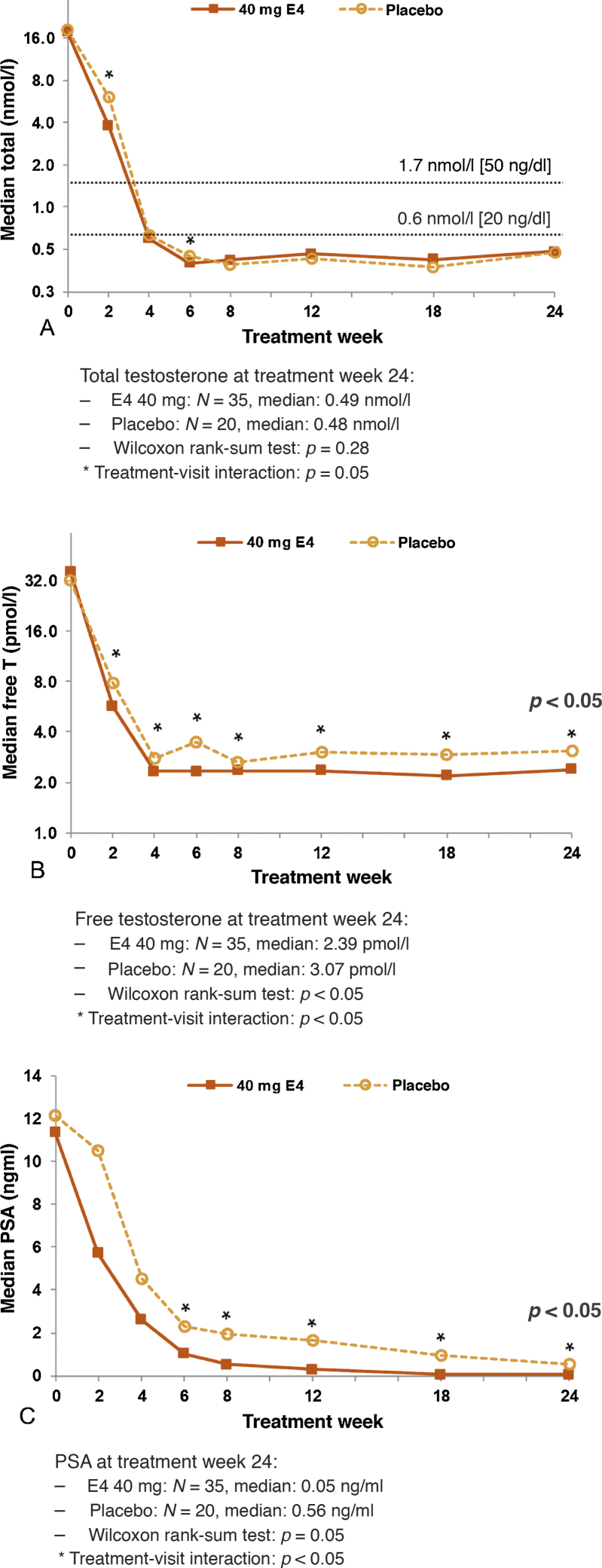
Patients using HDE4 showed earlier suppression of total T during the first 2–6 wk of treatment (treatment × visit interaction p < 0.05; Fig. 2A), which was also reflected in total T nadir levels being reached earlier with HDE4 (90 vs 101 d). Final total T and nadir levels were similar. Sustained suppression of total T below castrate levels of 1.7 nmol/l (50 ng/dl) from week 4 through week 24 was achieved in all patients in both treatment groups. Free T was lower in patients using HDE4 throughout the 24 wk (treatment × visit interaction p < 0.05; Fig. 2B and Table 2).
Fig. 2.
Median levels of (A) total testosterone, (B) free testosterone, and (C) prostate-specific antigen (PSA) after 2, 4, 6, 8, 12, 18, and 24 wk of treatment with 40 mg estetrol (E4) or placebo in patients with prostate cancer treated with an LHRH agonist (per-protocol population). LHRH = luteinizing hormone-releasing hormone; T = testosterone.
Table 2.
Laboratory parameters at baseline and after 24 wk of treatment with 40 mg estetrol or placebo daily coadministration in patients with prostate cancer treated with an LHRH agonist (per-protocol population)
| Laboratory parameter | 40 mg Estetrol |
Placebo |
|||||
|---|---|---|---|---|---|---|---|
| (N = 37) |
(N = 20) |
||||||
| Baseline | End of treatment (week 24) | Percentage change from baseline | Baseline | End of treatment (week 24) | Percentage change from baseline | p valuea | |
| PSA (ng/ml) | 18.4 (22.10) | 0.6 (2.12) | –96.4 (5.3) | 33.5 (56.98) | 4.0 (9.54) | –83.1 (30.2) | 0.0033 |
| Testosterone, total (nmol/l) | 19.4 (8.54) | 0.5 (0.22) | –97.1 (1.6) | 19.1 (6.55) | 0.5 (0.21) | –97.4 (1.5) | 0.2819 |
| Testosterone, free (pmol/l) | 42.0 (17.36) | 2.4 (0.97) | –93.2 (4.0) | 37.7 (15.62) | 3.2 (1.57) | –89.7 (6.9) | 0.0389 |
| LH level (IU/l) | 10.2 (9.34) | 0.2 (0.17) | –97.6 (1.8) | 11.9 (11.49) | 0.17 (0.09) | –97.6 (2.8) | 0.6805 |
| FSH level (IU/l) | 11.3 (11.29) | 0.2 (0.09) | –97.8 (1.7) | 12.7 (9.48) | 5.5 (3.20) | –56.7 (44.8) | <0.0001 |
| Estradiol (pmol/l) | 98.5 (39.72) | 17.4 (12.40) | –81.6 (10.5) | 113.4 (47.71) | 17.1 (13.46) | –82.3 (14.9) | 0.7639 |
| SHBG (nmol/l) | 61.3 (26.80) | 166.6 (78.13) | +185.0 (111.4) | 69.1 (29.89) | 66.4 (28.25) | –2.5 (15.8) | <0.0001 |
| DHEA-S (µmol/l) | 3.5 (2.05) | 2.5 (1.35) | –26.8 (18.9) | 3.8 (1.97) | 2.8 (1.70) | –26.8 (18.8) | 0.5875 |
| Osteocalcin (ng/ml) | 18.2 (7.29) | 14.0 (6.63) | –22.0 (19.7) | 17.6 (5.19) | 25.0 (8.28) | +47.6 (47.2) | <0.0001 |
| CTX1 (ng/ml) | 309.5 (168.1) | 219.9 (117.9) | –24.8 (34.6) | 300.3 (124.7) | 678.6 (267.0) | +151.1 (109.1) | <0.0001 |
CTX1 = type I collagen telopeptide; DHEA-S = dehydroepiandrosterone-sulfate; FSH = follicle-stimulating hormone; PSA = prostate-specific antigen; LH = luteinizing hormone, LHRH = luteinizing hormone-releasing hormone; SHBG = sex hormone–binding globulin.
Results reported as mean (standard deviation).
Kruskall-Wallis test comparing week 24 laboratory levels of 40 mg estetrol with placebo (p values are printed in bold if statistically significant).
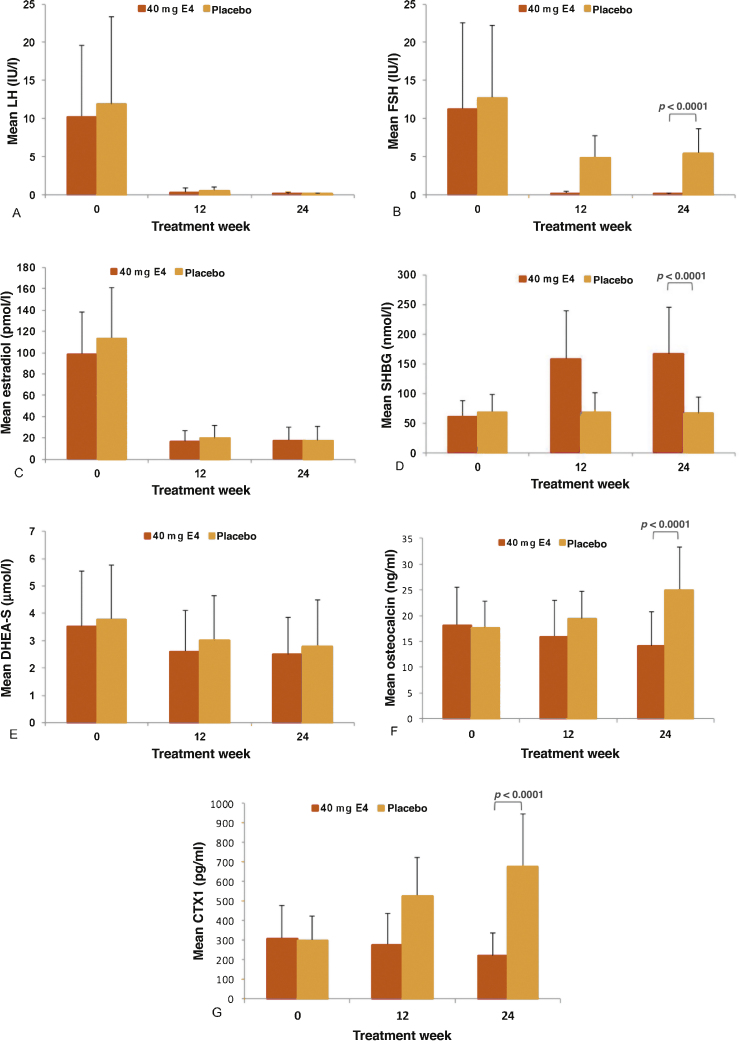
No differences between the two treatment groups were observed for LH levels (Fig. 3A and Table 2). Mean FSH levels decreased by 98% with HDE4 at 24 wk and by 57% with placebo (p < 0.0001; Fig. 3B and Table 2). The levels of E2 were suppressed equally in both groups by 82% at 24 wk of treatment (Fig. 3C and Table 2). At week 24, SHBG levels were increased by 185% in the HDE4 group, but no change occurred with placebo (p < 0.0001; Fig. 3D and Table 2). DHEA-S decreased by 27% in both treatment groups (Fig. 3E and Table 2). Bone metabolism markers increased in the placebo group by 48% for osteocalcin and by 151% for CTX1 at 24 wk. In the HDE4 group, osteocalcin decreased by 22% and CTX1 by 25% at 24 wk compared with baseline (p < 0.0001; Fig. 3F and G, and Table 2). PSA levels were significantly lower with HDE4 at all seven time points (p < 0.005; Fig. 2C and Table 2). PSA decreased earlier with a median PSA level of 5.7 ng/ml with HDE4 at 2-wk treatment compared with 10.5 ng/ml with placebo. After 24 wk, the median PSA with HDE4 was 0.05 ng/ml, compared with 0.56 ng/ml with placebo (p < 0.005). At 24 wk, the PSA level was <0.5 ng/ml in 31/37 HDE4 users (84%) versus in 9/20 placebo users (45%).
Fig. 3.
Mean levels of (A) LH, (B) FSH, (C) E2, (D) SHBG, (E) DHEA-S, and markers of bone turnover—(F) osteocalcin and (G) CTX1—after 12 and 24 wk of treatment with 40 mg estetrol (E4) or placebo in patients with prostate cancer treated with an LHRH agonist (per-protocol population). CTX1 = type I collagen telopeptide; DHEA-S = dehydroepiandrosterone sulfate; E2 = estradiol; E4 = estetrol; FSH = follicle-stimulating hormone; LH = luteinizing hormone; SHBG = sex hormone–binding globulin.
No deaths occurred during the study. There were seven CV AEs, all considered not related to study treatment by the investigators and the independent safety officer (Table 3): five of 41 (12.2%) patients on HDE4 and two of 21 (9.5%) patients on placebo. Four CV AEs were reported as serious: two (5%) with HDE4 (arrhythmia and atrioventricular block) and two (10%) with placebo (coronary artery disease and anemia with cardiac failure). Three AEs in the HDE4 group led to discontinuation of treatment (hypersensitivity reaction, depression, and peripheral edema), all considered not related to E4. No general safety problems or clinically relevant changes of lipids and hemostasis parameters were observed (Supplementary Table 1).
Table 3.
Overview of adverse events (all-patients-treated group)
| 40 mg Estetrol (N = 41) | Placebo (N = 21) | |
|---|---|---|
| No. of patients (%) | No. of patients (%) | |
| Any adverse events | 38 (92.7) | 18 (85.7) |
| Cardiovascular adverse eventsa | 5 (12.2) | 2 (9.5) |
| Serious adverse events | ||
| Arrhythmia | 1 (2.4) | – |
| Artery disease | – | 1 (4.8) |
| Hypersensitivity | 1 (2.4) | – |
| AV block 2nd degree | 1 (2.4) | – |
| Anemia with cardiac failure | – | 1 (4.8) |
| Toxicity to various agents (morphine) | 1 (2.4) | – |
| Adverse events with severe intensity | 3 (7.3) | 3 (14.3) |
| Drug-related adverse events | 4 ( 9.8) | 3 (14.3) |
| Adverse events leading to treatment discontinuation | 3 (7.3) | – |
| AE depression | ||
| AE peripheral edema (worsening of pre-existing condition) | ||
| SAE hypersensitivity | ||
| Adverse events that occurred in ≥5% of patients: | ||
| Hot flushes | 12 (29.3) | 11 (52.4) |
| Nipple pain | 14 (34.1) | – |
| Gynecomastia | 7 (17.1) | – |
| Fatigue | 11 (26.8) | 7 (33.3) |
| Edema, peripheral | 5 (12.2) | 1 (4.8) |
| Arthralgia | 3 (7.3) | 3 (14.3) |
| Back pain | 3 (7.3) | 1 (4.8) |
| Musculoskeletal stiffness | – | 2 (9.5) |
| Dysuria | 3 (7.3) | 1 (4.8) |
| Pollakiuria | 3 (7.3) | 2 (9.5) |
| Hematuria | 1 (2.4) | 2 (9.5) |
| Diarrhea | 3 (7.3) | 2 (9.5) |
| Dyspnea | 2 (4.9) | 2 (9.5) |
AV = atrioventricular; E4 = estetrol; SAE = serious adverse events.
Four out the seven cardiovascular (CV) adverse events (AEs) were reported as serious (arrhythmia [E4], AV block [E4], artery disease [placebo], anemia with cardiac failure [placebo]); the remaining three reported as AEs (atrial fibrillation [E4], angina pectoris [E4], supraventricular tachycardia [E4]). All CV adverse events were considered not related to study treatment by the investigators before the study code was broken.
4. Discussion
Reintroduction of the use of estrogens for the treatment of advanced prostate cancer, certainly when applied at high doses, raises CV safety concerns. However, not all estrogens are the same (Supplementary Fig. 2).
The present study was performed in patients with regionally advanced nonmetastatic or metastatic castration-sensitive prostate cancer that required ADT. Of note, this is an aging population with often multiple concomitant diseases and an increased risk of CV AEs anyhow [20]. In our relatively small study, no severe drug-related CV side effects occurred in 41 patients cotreated with a high oral dose of 40 mg estetrol (HDE4) for 24 wk. Mild estrogenic side effects (nipple sensitivity and gynecomastia) occurred occasionally without causing study discontinuation. The 40 mg dose of E4 is considered to be high because in preclinical and clinical studies the potency of E4 is known to be ten times lower than that of E2 (so 40 mg E4 equals 4 mg E2), whereas in women the standard dose of postmenopausal E2 treatment is 1–2 mg [21]. Transdermal estrogens have also been studied in advanced prostate cancer as a replacement of LHRH agonists in a head-to-head study design (thus not as a cotreatment) and have appeared to have comparable efficacy with the LHRH agonists [22], [23]. The effect on HFs seems to be less than that of HDE4 in the present study.
Increasing evidence supports the view that FSH levels are also related to CV risk in males with advanced prostate cancer by a direct effect of elevated FSH on arteriosclerotic plaques [6]. In a study comparing the LHRH agonist leuprolide with the oral GnRH antagonist relugolix, FSH suppression was significantly higher with relugolix (89%) than with leuprolide (64%) and considered to be responsible for the significantly better CV risk profile of relugolix [17]. We found that the combination of an LHRH agonist with HDE4 suppressed FSH almost completely (98%), whereas FSH levels were reduced only by 57% with LHRH agonists alone. This provides a mechanistic explanation for an anticipated better CV safety profile of HDE4 compared with the older estrogens used in the past [12]. In addition, the profound suppression of FSH may have an independent inhibitory effect on prostate cancer tumor growth [24], [25].
Both primary efficacy endpoints of the study were met with (1) a substantial and >50% reduction of HFs aimed at, and (2) significantly earlier suppression of total T and earlier and larger suppression of free T at all time points. The effects on T levels are due to E4 and not due to the LHRH agonists, since the suppression of LH was equal in both groups (98%).
Although the size and treatment duration of this phase II study do not allow final conclusions on the safety and the antitumor effects of HDE4, the data on important clinical parameters of estrogen deficiency were robust. First of all, the hypoestrogenicity caused by ADT was similar with 82% decreases of E2 in both treatment groups. Second, by cotreatment with a high dose of E4, two major problems caused by this LHRH agonist–induced estrogen deficiency were prevented: (1) HFs were substantially reduced and (2) the study generated significant data on a strong bone-sparing estrogenic effect of HDE4, with a decrease of bone turnover with HDE4 compared with an increase with LHRH agonists only. This suggests prevention of bone loss and the potential to decrease the risk of fractures.
HFs interfering with QoL are known to occur in 55–60% of ADT users, with both LHRH agonists and GnRH antagonists [17], [18], [19]. The number of patients suffering from HFs according to the diary was substantially reduced with HDE4, that is, to 14.3% versus 60.0% per week with placebo, and to 5.9% versus 55% per day. In addition, compared with placebo, the frequency of HFs per day per patient was reduced, and the intensity was less severe with HDE4. Side effects of HDE4 were temporary nipple tenderness and gynecomastia, but both events were no reason for study discontinuations.
The importance of suppressing biologically active free T is increasingly recognized as essential for effective prostate cancer treatment [26], [27]. The expected further lowering of free T, due to the 185% increase of SHBG [5], was confirmed by significantly lower free T levels observed at all time points. In addition, the suppression of total T and free T was earlier and greater with HDE4 after 2 wk of treatment. In men, 95% of T is produced by the testicular Leydig cells in response to LH. The remaining 5% is of adrenal origin as the metabolite of dehydroepiandrosterone (DHEA) and its sulfate DHEA-S [28], [29]. We found the same 27% decrease of DHEA-S in both groups, so HDE4 does not appear to affect adrenal androgens.
With ADT, there is a relationship between the efficacy of treatment and the suppression of PSA, with levels below 0.1, 0.2, or 0.5 ng/ml across studies being related to better prognosis and longer overall survival [30], [31], [32]. In the present study, suppression of PSA was effective in both groups, but PSA levels decreased earlier and suppression was significantly more profound with HDE4, whereas significantly more patients on HDE4 than on placebo were suppressed below the limit of 0.5 ng/ml (84% vs 45%).
4.1. Limitations
The current findings are robust in terms of the prevention and treatment of HFs, restoration of bone biochemistry, and suppression of FSH. However, due to the limited number of patients and the relatively short treatment period of 24 wk, a further 52-wk phase III study is needed to confirm the safety of HDE4, and to report QoL effects by validated questionnaires and measure bone mass. Longer-lasting studies with more patients will be necessary to investigate bone fractures and the clinical antitumor effect of HDE4, and future phase III studies with sufficient follow-up should allow concomitant treatment with, for example, abiraterone and/or docetaxel.
5. Conclusions
In patients with advanced prostate cancer treated with ADT, cotreatment with high-dose estetrol (HDE4) was well tolerated. Nipple tenderness and gynecomastia may occur as estrogenic side effects. No treatment-related CV AEs were observed at 24 wk. HFs and bone turnover were substantially reduced. Suppression of free T, PSA, and FSH was more rapid and profound, suggesting enhanced disease control by HDE4 cotreatment. Larger and longer-lasting studies are needed to confirm the results of the study reported here.
Treatment concept was presented at various congresses (e.g. EAU, COGI, EMAS), but any results have not been presented.
Author contributions: Drs. Frans M.J. Debruyne, Herjan J.T. Coelingh Bennink, and Yvette Zimmerman had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Coelingh Bennink, Debruyne, Zimmerman, Reisman
Acquisition of data: Debruyne, van Moorselaar, Crawford, Shore, Saad, Schally, Coelingh Bennink, Zimmerman, Krijgh, Roos, Somford, Roeleveld, de Haan, van Melick, Reisman, van Osta.
Analysis and interpretation of data: Coelingh Bennink, Debruyne, van Moorselaar, Zimmerman.
Drafting of the manuscript: Coelingh Bennink, Debruyne, Zimmerman.
Critical revision of the manuscript for important intellectual content: Debruyne, van Moorselaar, Crawford, Shore, Saad, Schally, Coelingh Bennink, Zimmerman, Krijgh, Roos, Somford, Roeleveld, de Haan, van Melick, Reisman, van Osta.
Statistical analysis: van Osta.
Obtaining funding: None.
Administrative, technical, or material support: Zimmerman, Krijgh, Debruyne.
Supervision: Coelingh Bennink, Debruyne.
Other: Scientific input: Crawford, Shore, Saad, Schally.
Financial disclosures: Herjan J.T. Coelingh Bennink certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Dr. Debruyne is a paid consultant for Pantarhei Oncology BV. Dr. van Moorselaar received grants and fees from Astellas, Ipsen, Astra Zeneca, Bayer, and Janssen. Dr. Crawford was consultant for Astellas, Bayer, Dendreon, Ferring, Janssen, Pfizer, Sanofi, Tolmar, and MDxHealth. Dr. Shore is conducting research/consulting at Amgen, Astellas, AstraZeneca, Bayer, BMS, Dendreon, Ferring, Janssen, Merck, Myovant, Nymox, Pfizer, Sanofi‐Genzyme, and Tolmar. Dr. Saad has served as a consultant for, and received funding from, Amgen, Astellas, AstraZeneca, Bayer, BMS, Janssen, and Sanofi. Dr. Somford is a member of advisory boards of Astellas and Janssen, and research grant from Astellas. Dr. Coelingh Bennink is the president and a shareholder of Pantarhei Oncology, an affiliate of Pantarhei Bioscience BV. Dr. Zimmerman is the chief executive officer and a shareholder of Pantarhei Oncology. Dr. Krijgh is the chief medical officer of Pantarhei Oncology. The other authors declare no conflict of interest.
Funding/Support and role of the sponsor: All funding for the study was provided by the sponsor, Pantarhei Oncology. Pantarhei Oncology, in collaboration with academic authors, had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication. The sponsor did not have the right to veto publication. The decision regarding to which journal the paper was to be submitted was collaboratively made between the lead authors and the sponsor.
Data sharing: See the Supplementary material.
Acknowledgments: We thank the study staff of the clinical sites and the patients participating in the study. We are also grateful to Professor Marc Garnick (Harvard Medical School, Boston, MA, USA) for his valuable suggestions, and Professor Jan Rosing (Maastricht University, The Netherlands) for his advice on the hemostasis parameters. Carole Verhoeven (formerly at Pantarhei) and Monique Jansen are acknowledged for their participation in the setup and organization of the study, as well as Ilse Christ (Ilse Pharma, Breda, The Netherlands) who performed the monitoring. Amanda Prowse and Jan Egberts (at Terminal 4 Communications, Hilversum, The Netherlands) are acknowledged for their contribution to the preparation of the manuscript.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.euros.2021.04.005.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Huggins C., Hodges C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 2.Presti J.C., Jr Estrogen therapy for prostate carcinoma. JAMA. 1996;275:1153. doi: 10.1001/jama.275.15.1153. [DOI] [PubMed] [Google Scholar]
- 3.Turo R., Smolski M., Esler R. Diethylstilboestrol for the treatment of prostate cancer: past, present and future. Scand J Urol. 2014;48:4–14. doi: 10.3109/21681805.2013.861508. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo H., Baba Y., Nair R.M., Arimura A., Schally A.V. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43:1334–1339. doi: 10.1016/s0006-291x(71)80019-0. [DOI] [PubMed] [Google Scholar]
- 5.Coelingh Bennink H.J.T., Zimmerman Y., Verhoeven C. A dose-escalating study with the fetal estrogen estetrol in healthy men. J Clin Endocrinol Metab. 2018;103:3239–3249. doi: 10.1210/jc.2018-00147. [DOI] [PubMed] [Google Scholar]
- 6.Crawford E.D., Schally A.V. The role of FSH and LH in prostate cancer and cardiometabolic comorbidities. Can J Urol. 2020;27:10167–10173. [PubMed] [Google Scholar]
- 7.Hagen A.A., Barr M., Diczfalusy E. Metabolism of 17-beta-oestradiol-4-14-C in early infancy. Acta Endocrinol (Copenh) 1965;49:207–220. [PubMed] [Google Scholar]
- 8.Coelingh Bennink H.J., Holinka C.F., Diczfalusy E. Estetrol review: profile and potential clinical applications. Climacteric. 2008;11(Suppl 1):47–58. doi: 10.1080/13697130802073425. [DOI] [PubMed] [Google Scholar]
- 9.Coelingh Bennink H.J., Heegaard A.M., Visser M., Holinka C.F., Christiansen C. Oral bioavailability and bone-sparing effects of estetrol in an osteoporosis model. Climacteric. 2008;11(Suppl 1):2–14. doi: 10.1080/13697130701798692. [DOI] [PubMed] [Google Scholar]
- 10.Mawet M., Maillard C., Klipping C., Zimmerman Y., Foidart J.M., Coelingh Bennink H.J. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Health Care. 2015;20:463–475. doi: 10.3109/13625187.2015.1068934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kluft C., Zimmerman Y., Mawet M. Reduced haemostatic effects with drospirenone-based oral contraceptives containing estetrol versus ethinyl estradiol. Contraception. 2017;95:140–147. doi: 10.1016/j.contraception.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Douxfils J., Klipping C., Duijkers I. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396–402. doi: 10.1016/j.contraception.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Coelingh Bennink H.J.T., Verhoeven C., Zimmerman Y., Visser M., Foidart J.M., Gemzell-Danielsson K. Pharmacodynamic effects of the fetal estrogen estetrol in postmenopausal women: results from a multiple-rising-dose study. Menopause. 2017;24:677–685. doi: 10.1097/GME.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 14.Abot A., Fontaine C., Buscato M. The uterine and vascular actions of estetrol delineate a distinctive profile of estrogen receptor alpha modulation, uncoupling nuclear and membrane activation. EMBO Mol Med. 2014;6:1328–1346. doi: 10.15252/emmm.201404112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnal J.F., Lenfant F., Metivier R. Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol Rev. 2017;97:1045–1087. doi: 10.1152/physrev.00024.2016. [DOI] [PubMed] [Google Scholar]
- 16.Azam F., Latif M.F., Farooq A. Performance status assessment by using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Case Rep Oncol. 2019;12:728–736. doi: 10.1159/000503095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shore N.D., Saad F., Cookson M.S. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. 2020;382:2187–2196. doi: 10.1056/NEJMoa2004325. [DOI] [PubMed] [Google Scholar]
- 18.Nishiyama T., Kanazawa S., Watanabe R., Terunuma M., Takahashi K. Influence of hot flashes on quality of life in patients with prostate cancer treated with androgen deprivation therapy. Int J Urol. 2004;11:735–741. doi: 10.1111/j.1442-2042.2004.00896.x. [DOI] [PubMed] [Google Scholar]
- 19.Dearnaley D.P., Saltzstein D.R., Sylvester J.E. The oral gonadotropin-releasing hormone receptor antagonist relugolix as neoadjuvant/adjuvant androgen deprivation therapy to external beam radiotherapy in patients with localised intermediate-risk prostate cancer: a randomised, open-label, parallel-group phase 2 trial. Eur Urol. 2020;78:184–192. doi: 10.1016/j.eururo.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Crawford E.D., Schally A.V., Pinthus J.H. The potential role of follicle-stimulating hormone in the cardiovascular, metabolic, skeletal, and cognitive effects associated with androgen deprivation therapy. Urol Oncol. 2017;35:183–191. doi: 10.1016/j.urolonc.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Maclennan A.H., Broadbent J.L., Lester S., Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2004;2004 doi: 10.1002/14651858.CD002978.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langley R.E., Cafferty F.H., Alhasso A.A. Cardiovascular outcomes in patients with locally advanced and metastatic prostate cancer treated with luteinising-hormone-releasing-hormone agonists or transdermal oestrogen: the randomised, phase 2 MRC PATCH trial (PR09) Lancet Oncol. 2013;14:306–316. doi: 10.1016/S1470-2045(13)70025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langley R.E., Gilbert D.C., Duong T. Transdermal oestradiol for androgen suppression in prostate cancer: long-term cardiovascular outcomes from the randomised Prostate Adenocarcinoma Transcutaneous Hormone (PATCH) trial programme. Lancet. 2021;397:581–591. doi: 10.1016/S0140-6736(21)00100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawford E.D., Tombal B., Keane T. FSH suppression and tumour control in patients with prostate cancer during androgen deprivation with a GnRH agonist or antagonist. Scand J Urol. 2018;52:349–357. doi: 10.1080/21681805.2018.1522372. [DOI] [PubMed] [Google Scholar]
- 25.Dizeyi N., Trzybulska D., Al-Jebari Y., Huhtaniemi I., Lundberg Giwercman Y. Cell-based evidence regarding the role of FSH in prostate cancer. Urol Oncol. 2019;37 doi: 10.1016/j.urolonc.2018.12.011. 290 e1–8. [DOI] [PubMed] [Google Scholar]
- 26.Rove K.O., Crawford E.D., Perachino M. Maximal testosterone suppression in prostate cancer—free vs total testosterone. Urology. 2014;83:1217–1222. doi: 10.1016/j.urology.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regis L., Planas J., Carles J. Free testosterone during androgen deprivation therapy predicts castration-resistant progression better than total testosterone. Prostate. 2017;77:114–120. doi: 10.1002/pros.23256. [DOI] [PubMed] [Google Scholar]
- 28.Labrie F. Blockade of testicular and adrenal androgens in prostate cancer treatment. Nat Rev Urol. 2011;8:73–85. doi: 10.1038/nrurol.2010.231. [DOI] [PubMed] [Google Scholar]
- 29.Nishiyama T., Hashimoto Y., Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004;10:7121–7126. doi: 10.1158/1078-0432.CCR-04-0913. [DOI] [PubMed] [Google Scholar]
- 30.McGuire S.E., Lee A.K., Cerne J.Z. PSA response to neoadjuvant androgen deprivation therapy is a strong independent predictor of survival in high-risk prostate cancer in the dose-escalated radiation therapy era. Int J Radiat Oncol Biol Phys. 2013;85:e39–46. doi: 10.1016/j.ijrobp.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harshman L.C., Chen Y.H., Liu G. Seven-month prostate-specific antigen is prognostic in metastatic hormone-sensitive prostate cancer treated with androgen deprivation with or without docetaxel. J Clin Oncol. 2018;36:376–382. doi: 10.1200/JCO.2017.75.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexander A., Crook J., Jones S. Is biochemical response more important than duration of neoadjuvant hormone therapy before radiotherapy for clinically localized prostate cancer? An analysis of the 3- versus 8-month randomized trial. Int J Radiat Oncol Biol Phys. 2010;76:23–30. doi: 10.1016/j.ijrobp.2009.01.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.