Abstract
Context
Long-term urinary and sexual outcomes after repair of anorectal malformations (ARMs) are currently affected by concomitant malformations of the urinary tract and genitalia, sacral anomalies, and the surgical approach. However, the overall prevalence of urinary and sexual dysfunction remains unclear.
Objective
To evaluate the prevalence of urinary and sexual dysfunction in patients aged >10 yr after repair of ARM in infancy.
Evidence acquisition
A systematic literature review was performed using the Medline, Embase, and Cochrane databases. Selected studies were reviewed according to the Consolidated Standards of Reporting Trials (CONSORT) and Standards for the Reporting of Diagnostic Accuracy Studies (STARD) criteria. We included studies reporting the prevalence of the following outcomes: urinary incontinence (UI), lower urinary tract symptoms (LUTS), neurogenic bladder dysfunction (NBD), sexual dysfunction (SD), erectile dysfunction (ED), ejaculatory dysfunction, and birth rate. We initially identified 588 studies, of which 17 were included for evidence synthesis.
Evidence synthesis
A probabilistic meta-analysis on each subgroup revealed the following combined prevalence estimates: UI 16% (95% confidence interval [CI] 7–27%), LUTS/NBD 36% (95% CI 13–62%), SD among women 50% (95% CI 34–66%), ED 12% (95% CI 7–18%), ejaculatory dysfunction 16% (95% CI 9–25%), and birth rate 20% (95% CI 7–38%). Subgroup analysis showed a higher prevalence of ED and ejaculatory dysfunction among patients with high ARM severity when compared to low ARM severity.
Conclusions
Among patients undergoing ARM repair, we found a high prevalence of long-term impairment of UI, ED, and SD. We stress the need for larger multicentre trials with more comparable populations to optimise treatment and follow-up regimens.
Patient summary
We reviewed long-term outcomes for patients with anorectal malformations who underwent surgery and found that both urinary incontinence and sexual dysfunctions are common for both males and females.
Keywords: Anorectal malformation, Urinary outcome, Sexual outcome, Paediatric surgery
Take Home Message
The prevalence of urinary incontinence, erectile dysfunction (ED), ejaculatory dysfunction (EJD), and sexual dysfunction is high after repair of anorectal malformation (ARM). The prevalence of ED and EJD was higher for patients with high-severity ARM than for patients with low-severity ARM. In general, the studies included in the review were small and heterogeneous.
1. Introduction
Anorectal malformations (ARMs) are inborn defects affecting from one in 2500 to one in 5000 newborns [1], [2]. Associated inborn anomalies are present in up to 70% of newborns with ARM, and they increase with the ARM severity. Anomalies of the urinary tract and genitalia are the most common, reported for 40–50% of ARM patients [3], [4], [5]. ARMs were previously classified as high, intermediate, or low according to the termination of the rectal pouch in relation to the levator muscle complex (Wingspread classification) [6]. In 2005 the Krickenbeck classification was introduced with a more descriptive approach and a focus on the fistula course [7]. The surgical approach was revolutionised four decades ago with the introduction of posterior sagittal anorectoplasty (PSARP) as an alternative to the former pull-through technique [8]. PSARP allows surgeons to view the junction of the rectum and the genitourinary tract and to use direct vision to repair it to avoid damaging the nerves responsible for urinary control and sexual function [9]. The laparoscopic approach was subsequently introduced, which in principle is somewhat similar to the old pull-through technique [10]. Long-term bowel functional outcomes after surgical repair of ARMs have been comprehensively assessed and reported [11], [12]. Urinary and sexual outcomes have received less attention, but it has been shown that they are affected by concomitant malformations of the urinary tract and genitalia, sacral anomalies, and the surgical approach [13]. In recent years, however, more studies have investigated long-term urinary and sexual outcomes among patients with ARMs. A multidisciplinary approach is needed to optimise treatment and improve prognosis. Against this background, now is a good time to investigate long-term urinary and sexual functional outcomes, including fertility, after surgical repair of ARMs via a contemporary systematic review and meta-analysis.
2. Evidence acquisition
We performed a systematic literature review in accordance with the European Association of Urology (EAU) methodology on the key steps in conducting a systematic review and the Preferred Reporting Item for Systematic Reviews and Meta-analyses (PRISMA) statement recommendations [14], [15]. The review was registered on the PROSPERO international register of systematic reviews (www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020153499).
2.1. Search strategy
A literature search was conducted on June 22, 2020 using the Medline and Embase databases and the Cochrane Library (Supplementary Tables 1 and 2). We limited the search to studies that dealt with human subjects. No other search limits were applied.
2.2. Inclusion criteria
We chose to include only journal articles that presented one of the following long-term outcomes: urinary incontinence (UI), lower urinary tract symptoms (LUTS), neurogenic bladder dysfunction (NBD), sexual dysfunction (SD), erectile dysfunction (ED), ejaculatory dysfunction, or any outcome measure concerning fertility. Birth rate, defined as the percentage of patients able to produce their own offspring, was the only outcome parameter to describe fertility that was reported. We choose to merge LUTS and NBD for outcome estimates owing to overlap of symptoms. Data were extracted for subgroups in some studies in order to avoid including patients with cloaca or Hirschsprung’s disease. Data on the type of UI and urinary tract infection (UTI) were also retrieved. None of the studies reporting on UI, LUTS, or NBD reported the prevalence of chronic kidney disease, dialysis, or kidney transplant.
ARM severity was classified as low, intermediate, or high in accordance with the Wingspread classification (Table 1) to facilitate comparison of the studies included [14].
Table 1.
Translation of the Krickenbeck classification to the Wingspread classification
| Krickenbeck classification | Wingspread classification |
|||
|---|---|---|---|---|
| Low | Intermediate | High | ||
| Males | Rectoperineal fistula | X | ||
| Rectourethral bulbar fistula | X | |||
| Rectourethral prostatic fistula | X | |||
| Rectobladderneck fistula | X | |||
| Imperforate anus without fistula | X | |||
| Females | Rectoperineal fistula | X | ||
| Rectovestibular fistula | X | |||
| Imperforated anus without fistula | X | |||
| Rectal atresia | X | |||
2.3. Exclusion criteria
We did not include review articles, conference abstracts, or case reports on fewer than five patients since prevalence estimates obtained from very small populations are considered unreliable. Studies investigating cloacal malformations were excluded unless it was possible to retrieve data on patients without cloaca in the individual studies. Cloaca is the most severe and complex type of ARM, for which long-term sequelae from the urinary tract and genitalia are near-obligate. Studies on patients with syndromes comprising ARM were also excluded. Only studies with patients aged >10 yr were included.
2.4. Data extraction
Two authors (T.B.-.M., M.E.) independently screened manuscript titles and abstracts. The same authors carried out subsequent full-text assessment and retrieval of relevant data.
2.5. Quality assessment
Two independent reviewers (T.B.-M., M.E.) assessed the quality of studies included. The Newcastle-Ottawa scale (NOS) for nonrandomised studies in systematic reviews was used to determine quality [16]. Scores were generated using a standardised star allocation system for three items: selection, comparability, and outcome. The maximum score was 9. A score of <5 was considered low quality, a score of 5 or 6 as medium quality, and a score >6 as high quality.
2.6. Statistical analysis
We extracted prevalence estimates from the individual studies for UI, LUTS/NBD, female SD, male ED and ejaculatory dysfunction, and the birth rate for both sexes. Data are presented as prevalence estimates with the range or standard deviation, depending on the reporting study. We used the metaprop command in Stata v16 (StataCorp, College Station, TX, USA) to conduct a meta-analysis of proportions for each of the six outcomes (UI, LUTS/NBD, SD, ED, ejaculatory dysfunction, and birth rate). We used random-effects models with Freeman-Tukey double arcsine transformation of the proportions and the exact method to calculate 95% confidence intervals (CIs) for the pooled estimates. We applied the I2 statistic to test for heterogeneity. Funnel plots and Egger’s test were used to investigate the presence of publication bias and small-study effects for each of the six outcomes. Meta regression was not applicable owing to the small number of studies included for each outcome.
Subgroup analysis presenting proportions for each study was conducted by dividing studies according to high, intermediate, and low degree of severity of ARM [6]. Data availability only allowed the construction of forest plots for high and low ARM severity.
3. Evidence synthesis
3.1. Study selection
The literature search generated 643 articles that were subsequently imported to Covidence.org (Fig. 1). Fifty-five duplicates were removed, leaving 588 studies. The article screening process was then carried out in two phases by two authors (T.B.-M., M.E.) independently. First, study titles and abstracts were screened using our pre-established criteria, and 534 studies were excluded. Second, the remaining 54 articles were read in detail and the predetermined inclusion and exclusion criteria were applied. This process resulted in 17 studies eligible for qualitative synthesis [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33]. Reference lists for the 17 papers included were screened (snowballing) without identifying additional eligible studies.
Fig. 1.
PRISMA flow diagram of the study selection process.
3.2. Study characteristics
3.2.1. Demographics
Only three of the 17 studies included were published before 2000 (Table 2) [31], [32], [33]. The majority of the studies were European (14/17). The three remaining studies were from Japan (n = 2) and the USA (n = 1) [28], [29], [30]. Fifteen studies had a cross-sectional design, while two were prospective cohort studies. The number of patients in the studies ranged from 17 to 83.
Table 2.
Summary of the studies included in the review
| Study | Study design | Gender | Outcomes | NOS |
AAs (%) |
ARM severity |
ARM surgery | Questionnaire | Age (yr) | Sample (n) | Control group | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | C | O | T | UG | SP | High | Int. | Low | |||||||||
| Trovalusci 2020, Italy [16] | CSS, MC | Male | ED, EJD | 2 | 0 | 1 | 3 | NR | NR | Yes | Yes | Yes | PSARP, LAARP | IIEF | 26 (18–41) | 25 | No |
| Bjørsum-Meyer 2020, Denmark [17] | CSS, SC | Both | SD, ED, BR | 1 | 0 | 2 | 3 | 34.6 | 50.0 | Yes | Yes | Yes | PSARP 10 Perineal 9 Dilatation 4 APPT 2 Cutback 1 | FSFI, IIEF | 24 (18–32) | 26 | No |
| Eleuteri 2019, Italy [18] | CSS, SC | Male | ED, EJD | 1 | 0 | 1 | 2 | NR | NR | NR | NR | NR | NR | AIMAR | 28.8 ± 10.6 | 12 | No |
| Witvliet 2018, The Netherlands [19] | CSS, MC | Both | SD, ED | 3 | 0 | 2 | 5 | 21.6 | NR | Yes | No | Yes | PSARP, anoplasty, ASARP, Rehbein, PSARVUP, others | FSFI, IIEF | >18 | 37 | No |
| Kyrklund 2016, Finland [20] | CSS, SC | Both | LUTS | 2 | 0 | 1 | 3 | NR | 33.3 | No | Yes | Yes | PSARP/ ASARP | DPSS | >12 | 39 | No |
| Kyrklund 2016, Finland [21] | CSS, SC | Male | ED, EJD | 2 | 0 | 1 | 3 | 12.2 | 19.5 | Yes | Yes | Yes | PSARP 20 ASARP 10 Cutback/dilatation 11 | Erection Hardness Score | 21 (16–29) | 41 | Yes |
| Hondel 2015, The Netherlands [22] | CSS, MC | Both | SD, ED | 3 | 0 | 2 | 5 | 29 | NR | Yes | Yes | Yes | APPT, PSARP, perineal, others | FSFI, IIEF | >18 | 63 | No |
| Borg 2013, Sweden [23] | PS, SC | Both | LUTS | 3 | 0 | 3 | 6 | NR | NR | Yes | Yes | Yes | PSARP | Designed | 10 | 24 | No |
| Schmidt 2012, Germany [24] | CSS, MC | Both | EJD, BR | 1 | 0 | 1 | 2 | NR | NR | Yes | Yes | Yes | NR | NS | 19–35 | 55 | No |
| Schmidt 2012, Germany [25] | CSS, MC | Male | NBD | 1 | 0 | 1 | 2 | NR | NR | Yes | Yes | Yes | NR | Interview | 18–56 | 32 | No |
| Grano 2011, Italy [26] | PS, SC | Both | UI | 1 | 0 | 1 | 2 | NR | 16.7 | Yes | Yes | Yes | NR | HAQL | 17–49 | 36 | No |
| Davies 2010, USA [27] | CSS, SC | Both | UI, SD, ED, EJD, BR | 3 | 0 | 0 | 3 | NR | 43.8 | Yes | Yes | Yes | NR | ICS-male | 25.6 (18–46) | 74 | No |
| Iwai 2007, Japan [28] | CSS, SC | Both | ED, EJD, BR | 1 | 0 | 1 | 2 | 10.3 | 3.6 | Yes | Yes | Yes | APPT/ perineoplasty | NR | 26.3 (20–40) | 29 | No |
| Konuma 2006, Japan [29] | CSS, SC | Male | ED, EJD, NBD, BR | 1 | 0 | 1 | 2 | 17.7 | 35.3 | Yes | Yes | No | APPT, SP, ASP | Interview | 24 (20–29) | 17 | No |
| Rintala 1999, Finland [30] | CSS, SC | Male | UI | 1 | 0 | 2 | 3 | NR | 44.4 | Yes | No | No | ASP, PSARP | NR | 10–22 | 36 | No |
| Hassink 1993, The Netherlands [31] | CSS, SC | Both | UI | 1 | 1 | 0 | 2 | 45.0 | 48.0 | Yes | No | No | APPT, ASP, perineal | NR | 26.0 (18.1–56.9) | 58 | No |
| Rintala 1992, Finland [32] | CSS, SC | Both | UI, BR | 2 | 0 | 1 | 3 | NR | NR | No | No | Yes | Dilatations, cutback, pull-through | Unspecified questionnaire | 35.2 ± 4.1 | 83 | Yes |
CSS = cross-sectional study; PS = prospective study; MC = multicentre; SC = single-centre; ED = erectile dysfunction; EJD = ejaculatory dysfunction; SD = sexual dysfunction; BR = birth rate; LUTS = lower urinary tract symptoms; NBD = neurogenic bladder dysfunction; UI = urinary incontinence; NOS = Newcastle-Ottawa Scale (S = selection; C = comparability; O = outcome; T = total score); AAs = associated anomalies (UG = urogenital; SP = spinal); NR = not reported; ARM = anorectal malformation; PSARP = posterior sagittal anorectoplasty; LAARP = laparoscopic assisted anorectal pull-through; APPT = abdominoperineal pull-through; ASARP = anterior sagittal anorectoplasty; PSARVUP = posterior sagittal anorectal vaginal urethraplasty; SP = sacroperineal dissection; ASP = abdominosacroperineal dissection; IIEF = International Index of Erectile Function; FSFI = Female Sexual Function Index; AIMAR = Italian Association for Anorectal Malformations; DPSS = Danish Prostatic Symptom Score; HAQL = Hirschsprung Disease/Anorectal Malformation Quality of Life Questionnaire; ICS = International Continence Society.
3.2.2. Associated urogenital and spinal anomalies
Associated urogenital anomalies were reported in seven of the 17 studies, for which the prevalence range was 10.3–40.0% (Table 2) [18], [20], [22], [23], [29], [30], [32]. Concomitant anomalies of the sacrum and spine were reported in nine studies, for which the prevalence was 3.6–50% [18], [21], [22], [23], [27], [28], [29], [30], [31]. Only five of the 17 studies reported prevalence data for both urogenital and spinal anomalies.
3.2.3. Quality of the studies included
As presented in Table 2, three studies were considered to be of medium quality according to the NOS [20], [23], [24]. The remaining 14 studies were scored at ≤3 on the NOS and were thus categorised as low-quality studies.
3.3. Outcome measures
3.3.1. UI in both sexes
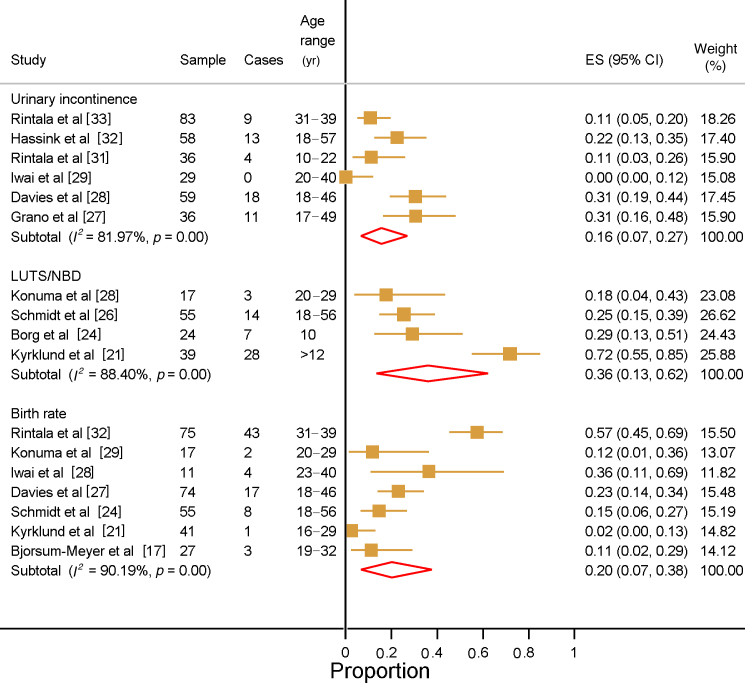
The prevalence of UI was reported in six studies involving a total of 316 patients (Fig. 2) [27], [28], [29], [31], [32], [33]. The number of patients in each study varied from 29 to 83. Four of the six studies only included adult patients [28], [29], [32], [33]. The combined prevalence of UI in these studies was 16% (95% CI 7–27%), ranging from 0% to 40.5%. One study only included males, for whom the UI prevalence was 11.1% [31]. Two studies reported UI prevalence of 11.1% and 22.4% among patients with high-severity ARM [31], [32]. One study reported UI for patients with low ARM severity (10.8%) [32]. One study compared patients to healthy controls who were matched by age and sex, but no differences in the prevalence of UI in subanalyses by gender and type of ARM were found [31]. A subgroup analysis of UI by level of ARM severity revealed an overall prevalence of 20% (95% CI 0–61%) among patients with low ARM severity (Supplementary Fig. 1) and 22% (95% CI 2–53%) among patient with intermediate or high ARM severity (Supplementary Fig. 2). Three studies specified the type of UI and one study also reported the number of patients with urinary tract reconstruction and use of catheterisation (Supplementary Table 3) [27], [28], [31].
Fig. 2.
Forest plots of the reported prevalence of urinary incontinence, lower urinary tract symptoms (LUTS)/neurogenic bladder dysfunction (NBD), and birth rate for patients with anorectal malformations.
ES = effect size; CI = confidence interval.
3.3.2. NBD/LUTS
The prevalence of NBD/LUTS was reported in four studies with a combined prevalence of 36% (95% CI 13–62%; Fig. 2), ranging from 18% to 72%. Two studies reported the prevalence of NBD among males, with a range of 17.6–31.3% for 49 patients in total [25], [30]. The study by Konuma et al [30] included only patients with intermediate/high ARM severity and reported prevalence of 17.6%. The prevalence of LUTS was reported in two studies as 29.2% and 71.8% [21], [24]. In the study by Kyrklund et al [21], which considered patients with low and intermediate ARM severity, LUTS prevalence was reported according to the presence or absence of associated spinal cord anomalies as 85% and 65%, respectively. The difference was not statistically significant. In a prospective study, Borg et al [24] found that the prevalence of non-NBD lower urinary tract dysfunction for patients aged 10 yr and 15 yr was 29% and 23%, respectively. Owing to the small number of studies presenting prevalence data for NBD and LUTS, we were unable to present a subgroup analysis by ARM severity. One study reported the type of UI and two studies presented data on UTI (Supplementary Table 3).
3.3.3. SD among females
SD was reported in four studies with a prevalence estimate of 50% (95% CI, 34–66%), as shown in Figure 3. The largest study included 26 patients with either a rectovestibular or a perineal fistula [18]. Three studies used the validated Female Sexual Function Index (FSFI) questionnaire to detect SD, defined as a score of <26 [18], [20], [23], [34]. In the study by Kyrklund et al [22], SD was reported as abnormal orgasm during sexual intercourse. Bjørsum-Meyer et al [18] assessed the prevalence of SD according to the presence of spinal anomalies, but found no statistically significant difference between patients with spinal anomalies and patients without. A subgroup analysis showed SD prevalence of 53% for patients with low ARM severity (Supplementary Fig. 1). The data did not allow for a similar subgroup analysis for intermediate or high ARM severity.
Fig. 3.
Forest plots of the reported prevalence of sexual dysfunction, erectile dysfunction, and ejaculatory dysfunction for patients with anorectal malformations.
ES = effect size; CI = confidence interval.
3.3.4. ED and ejaculatory dysfunction among males
Nine studies involving a total of 193 patients reported overall ED prevalence of 12% (95% CI, 7–18%). The largest study included 37 patients with ED prevalence of 16.2% [20]. In the study by Konuma et al [30], only patients with high/intermediate ARM severity were included and the ED prevalence was 11.8%. Kyrklund et al [22] reported ED prevalence of 5.6% among patients with low/intermediate ARM severity. Four studies used the International Index of Erectile Function (IIEF) questionnaire, and one of these used the short-form IIEF-5 instrument [17], [18], [20], [23], [35]. In three studies, ED was defined as an IIEF score of <26, and this applied for any level of dysfunction [17], [18], [20]. van den Hondel et al [23] only reported the prevalence of moderate to severe ED (27.3%). Three studies used questionnaires other than the IIEF, and two studies evaluated the presence of ED through interviews [19], [22], [28], [29], [30]. Higher ED prevalence was detected among patients with high ARM severity (18%) compared to patients with low ARM severity (7%; Supplementary Figs. 1 and 2).
Six studies reported the ejaculatory dysfunction status of 136 patients in total [19], [22], [25], [28], [29], [30]. The prevalence estimate was 16% (95% CI 9–25%), as shown in Figure 3. Two studies reported on high/intermediate and low/intermediate ARM severity, with ejaculatory dysfunction prevalence of 41.2% and 6.9%, respectively [22], [30]. In four studies, information on ejaculatory dysfunction was obtained through interviews [22], [25], [29], [30]. In the study by Konuma et al [30], which included seven patients with ejaculatory dysfunction, five reported incompetent ejaculation and two reported retrograde ejaculation. Similar to ED, the prevalence of ejaculatory dysfunction was higher for patients with high ARM severity (35%) than for patients with low ARM severity (6%), as shown in Supplementary Figures 1 and 2.
3.3.5. Birth rate for both sexes
Seven studies involving a total of 300 patients reported birth rates [18], [22], [25], [28], [29], [30], [33]. The number of patients in each study ranged from 17 to 75. As presented in Figure 2, the overall prevalence estimate was 20% (95% CI 7–38%). Konuma et al [30] reported a birth rate of 11.8% among patients with high/intermediate ARM severity. For patients with low/intermediate ARM severity, Kyrklund et al [22] reported a birth rate of 2.4%. The three studies with the highest birth rate (23.0–57.4%) also had the populations with highest mean/median age when compared to the remaining four studies [28], [29], [33]. Subgroup analysis according to ARM severity revealed a higher birth rate among patients with high ARM severity (32%) compared to patients with low ARM severity (20%; Supplementary Figs. 1 and 2).
3.4. Test of publication bias and small-study effect
We used Egger’s test for small-study effects and funnel plots to test for publication bias. Neither method returned concerning results, and we therefore see no obvious small-study effect or publication bias for the studies included (Supplementary Fig. 3).
4. Conclusions
4.1. Principal findings
We found high prevalence of long-term urinary and sexual dysfunction among adolescents and adults after ARM repair. The combined prevalence estimates were UI 16% (95% CI 7–27%), LUTS/NBD 36% (95% CI 13–62%), female SD 50% (95% CI 34–66%), ED 12% (95% CI 7–18%), ejaculatory dysfunction 16% (95% CI 9–25%), and birth rate 20% (95% CI, 7–38%). Subgroup analysis showed a higher prevalence of ED and ejaculatory dysfunction for patients with high ARM severity than for patients with low ARM severity, but the difference was not statistically significant. It has been reported that ED occurs in 2–9% of the general population of patients younger than 40 yr [36]. In a Danish study that included a sample of 2289 citizens, ED prevalence was 2.3% among males aged 20–29 yr [37]. The ED prevalence increased to 3.2% among subjects aged 30–39 yr. ED prevalence was positively associated with body mass index (BMI), alcohol consumption, and smoking status. None of the studies in the present review controlled for any of these factors, so because of these missing data we were unable to perform such an analysis. We found high combined prevalence of SD among females, with a wide range between studies. In three of the four studies, SD was diagnosed using the same questionnaire (FSFI) and defined as having a score of <26. Owing to the small study sizes and heterogeneous populations, we were unable to stratify the outcomes by type of ARM, type of repair, or associated anomalies, which would have been of great interest.
The incidence of UI was only slightly higher among patients with high ARM severity compared to those with low ARM severity. A Danish survey of the prevalence of UI in the general population revealed that 20.4% of Danish women aged 20–29 yr had UI [38] and UI was associated with high BMI and age. Stress-induced UI was the dominant type among younger women. None of the six studies reporting the prevalence of UI considered in the present review described either the UI type or severity. In order to stratify patients with ARM for an optimal treatment and follow-up regimen, future studies need to evaluate the prevalence of UI types.
We found a higher birth rate among patients with high ARM severity as compared to those with low ARM severity. This finding seems contradictory as the incidence of associated genital malformations increases with ARM severity [39], [40]. There are several potential explanations for this discrepancy. We did not include patients with cloacal malformations, of whom 30% have hydrocolpos in the newborn period, which can reduce fertility due to infection and scarring [41]. Furthermore, a double müllerian system has been reported for 40% of patients with cloaca, and this condition is associated with pregnancy loss and preterm birth [42]. The three studies in this review that reported the highest birth rate also had the highest mean or median age, and this, at least in part, explains the differences in birth rates reported. In general, the populations in this study were younger than the average age at which adults become parents in the community. Moreover, it is expected that the birth rate would be higher if it was measured only among patients attempting to become parents.
4.2. Strengths and weaknesses
This systematic review is the first study to specifically collect and present long-term urinary and sexual outcomes among ARM patients at least a decade after surgical correction. This study benefited from the large number of studies included and the absence of the small-study effect and publication bias.
To properly interpret the results of this systematic review, several weaknesses need to be addressed. The study populations were small, so it was not possible to stratify for baseline parameters including age, BMI, gender, and associated inborn anomalies. In community studies of UI, it has been shown that age, BMI, and gender are positively associated with higher incidence of UI. None of the studies included controlled for demographics or other relevant and potentially confounding effects. Another major limitation of the review is the quality of the studies included. Fourteen of the 17 studies were of poor quality as assessed using the NOS [16]. Owing to heterogeneity, we were not able to delineate outcomes by type of ARM, type of surgical repair, or associated anomalies. The methods for reporting outcomes were also largely heterogeneous because of the different questionnaires used in the studies and poor or absent definitions of reported outcomes. The small number of studies for each outcome did not allow for meta-regression, and the aforementioned heterogeneity did not allow for adjustment for associated anomalies, which are present in approximately two-thirds of patients with ARM.
4.3. Perspectives
It is of the utmost importance to identify subgroups of ARM patients who have a higher risk of long-term urinary and sexual dysfunction in order to initiate controls and timely treatment and to properly inform parents. We stress the need for large, multicentre, prospective studies with standardised treatments, patient care, and follow-up programmes in a multidisciplinary setting. Large studies allow stratification analyses by type of ARM, surgical approach, and associated anomalies, which can help to identify subgroups in need of special attention. Another important issue to address is the need for uniformity of reporting outcomes. Consensus guidelines regarding the choice of questionnaire and reporting systems are recommended to facilitate comparison of studies.
Author contributions: Thomas Bjoersum-Meyer had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bjoersum-Meyer, Qvist.
Acquisition of data: Bjoersum-Meyer, Ellebaek.
Analysis and interpretation of data: Bjoersum-Meyer, Kaalby.
Drafting of the manuscript: Bjoersum-Meyer, Qvist, Ellebaek.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Bjoersum-Meyer, Kaalby.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Qvist.
Other: None.
Financial disclosures: Thomas Bjoersum-Meyer certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Associate Editor: Silvia Proietti
References
- 1.Levitt M.A., Pena A. Anorectal malformations. Orphanet J Rare Dis. 2007;2:33. doi: 10.1186/1750-1172-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuschieri A., EUROCAT Working Group Anorectal anomalies associated with or as part of other anomalies. Am J Med Genet. 2002;110:122–130. doi: 10.1002/ajmg.10371. [DOI] [PubMed] [Google Scholar]
- 3.Stoll C., Alembik Y., Dott B., Roth M.P. Associated malformations in patients with anorectal anomalies. Eur J Med Genet. 2007;50:281–290. doi: 10.1016/j.ejmg.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Pena A. Anorectal malformations. Semin Pediatr Surg. 1995;4:35–47. [PubMed] [Google Scholar]
- 5.Hassink E.A., Rieu P.N., Hamel B.C., Severijnen R.S., van der Staak F.H., Festen C. Additional congenital defects in anorectal malformations. Eur J Pediatr. 1996;155:477–482. doi: 10.1007/BF01955185. [DOI] [PubMed] [Google Scholar]
- 6.Stephens F.D. Wingspread anomalies, rarities, and super rarities of the anorectum and cloaca. Birth Defects Orig Artic Ser. 1988;24:581–585. [PubMed] [Google Scholar]
- 7.Holschneider A., Hutson J., Pena A. Preliminary report on the International Conference for the Development of Standards for the Treatment of Anorectal Malformations. J Pediatr Surg. 2005;40:1521–1526. doi: 10.1016/j.jpedsurg.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Pena A., Devries P.A. Posterior sagittal anorectoplasty: important technical considerations and new applications. J Pediatr Surg. 1982;17:796–811. doi: 10.1016/s0022-3468(82)80448-x. [DOI] [PubMed] [Google Scholar]
- 9.Hong A.R., Acuna M.F., Pena A., Chaves L., Rodriguez G. Urologic injuries associated with repair of anorectal malformations in male patients. J Pediatr Surg. 2002;37:339–344. doi: 10.1053/jpsu.2002.30810. [DOI] [PubMed] [Google Scholar]
- 10.Georgeson K.E., Inge T.H., Albanese C.T. Laparoscopically assisted anorectal pull-through for high imperforate anus—a new technique. J Pediatr Surg. 2000;35:927–930. doi: 10.1053/jpsu.2000.6925. [DOI] [PubMed] [Google Scholar]
- 11.Bjørsum-Meyer T., Christensen P., Jakobsen M.S., Baatrup G., Qvist N. Correlation of anorectal manometry measures to severity of fecal incontinence in patients with anorectal malformations – a cross-sectional study. Sci Rep. 2020;10:6016. doi: 10.1038/s41598-020-62908-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyrklund K., Pakarinen M.P., Rintala R.J. Long-term bowel function, quality of life and sexual function in patients with anorectal malformations treated during the PSARP era. Semin Pediatr Surg. 2017;26:336–342. doi: 10.1053/j.sempedsurg.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Wood R.J., Levitt M.A. Anorectal malformations. Clin Colon Rectal Surg. 2018;31:61–70. doi: 10.1055/s-0037-1609020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knoll T., Omar M.I., Maclennan S. Key steps in conducting systematic reviews for underpinning clinical practice guidelines: methodology of the European Association of Urology. Eur Urol. 2018;73:290–300. doi: 10.1016/j.eururo.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital. www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 17.Trovalusci E., Rossato M., Gamba P., Midrio P. Testicular function and sexuality in adult patients with anorectal malformation. J Pediatr Surg. 2020;55:1839–1845. doi: 10.1016/j.jpedsurg.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Bjørsum-Meyer T., Lund L., Christensen P., Jakobsen M.S., Asmussen J., Qvist N. Impact of spinal defects on urinary and sexual outcome in adults with anorectal malformations—a cross-sectional study. Urology. 2020;139:207–213. doi: 10.1016/j.urology.2020.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Eleuteri S., Aminoff D., Lucidi F., Violani C., Grano C. Sexual well-being in adolescent and young adults born with arm: the perspective of the patients. Pediatr Surg Int. 2019;35:945–951. doi: 10.1007/s00383-019-04507-z. [DOI] [PubMed] [Google Scholar]
- 20.Witvliet M.J., van Gasteren S., van den Hondel D., Hartman E., van Heurn L., van der Steeg A. Predicting sexual problems in young adults with an anorectal malformation or Hirschsprung disease. J Pediatr Surg. 2018;53:1555–1559. doi: 10.1016/j.jpedsurg.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Kyrklund K., Pakarinen M.P., Taskinen S., Kivisaari R., Rintala R.J. Spinal cord anomalies in patients with anorectal malformations without severe sacral abnormalities or meningomyelocele: outcomes after expectant, conservative management. J Neurosurg Spine. 2016;25:782–789. doi: 10.3171/2016.4.SPINE1641. [DOI] [PubMed] [Google Scholar]
- 22.Kyrklund K., Taskinen S., Rintala R.J., Pakarinen M.P. Sexual function, fertility and quality of life after modern treatment of anorectal malformations. J Urol. 2016;196:1741–1746. doi: 10.1016/j.juro.2016.08.079. [DOI] [PubMed] [Google Scholar]
- 23.van den Hondel D., Sloots C.E., Bolt J.M., Wijnen R.M., de Blaauw I., IJsselstijn H. Psychosexual well-being after childhood surgery for anorectal malformation or Hirschsprung’s disease. J Sex Med. 2015;12:1616–1625. doi: 10.1111/jsm.12886. [DOI] [PubMed] [Google Scholar]
- 24.Borg H.C., Holmdahl G., Gustavsson K., Doroszkiewicz M., Sillen U. Longitudinal study of bowel function in children with anorectal malformations. J Pediatr Surg. 2013;48:597–606. doi: 10.1016/j.jpedsurg.2012.10.056. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt D., Winter S., Jenetzky E., Zwink N., Schmiedeke E., Maerzheuser S. Sexual function in adults with anorectal malformation: psychosocial adaptation. German Network for Congenital Uro-REctal Malformations (CURE-Net) Pediatr Surg Int. 2012;28:789–792. doi: 10.1007/s00383-012-3119-1. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt D., Jenetzky E., Zwink N., Schmiedeke E., Maerzheuser S. Postoperative complications in adults with anorectal malformation: a need for transition. German Network for Congenital Uro-REctal Malformations (CURE-Net) Pediatr Surg Int. 2012;28:793–795. doi: 10.1007/s00383-012-3120-8. [DOI] [PubMed] [Google Scholar]
- 27.Grano C., Aminoff D., Lucidi F., Violani C. Long-term disease-specific quality of life in adult anorectal malformation patients. J Pediatr Surg. 2011;46:691–698. doi: 10.1016/j.jpedsurg.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Davies M.C., Liao L.M., Wilcox D.T., Woodhouse C.R., Creighton S.M. Anorectal malformations: what happens in adulthood? BJU Int. 2010;106:398–404. doi: 10.1111/j.1464-410X.2009.09031.x. [DOI] [PubMed] [Google Scholar]
- 29.Iwai N., Deguchi E., Kimura O., Kubota Y., Ono S., Shimadera S. Social quality of life for adult patients with anorectal malformations. J Pediatr Surg. 2007;42:313–317. doi: 10.1016/j.jpedsurg.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Konuma K., Ikawa H., Kohno M., Okamoto S., Masuyama H., Fukumoto H. Sexual problems in male patients older than 20 years with anorectal malformations. J Pediatr Surg. 2006;41:306–309. doi: 10.1016/j.jpedsurg.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Rintala R.J., Lindahl H.G. Posterior sagittal anorectoplasty is superior to sacroperineal-sacroabdominoperineal pull-through: a long-term follow-up study in boys with high anorectal anomalies. J Pediatr Surg. 1999;34:334–337. doi: 10.1016/s0022-3468(99)90203-8. [DOI] [PubMed] [Google Scholar]
- 32.Hassink E.A., Rieu P.N., Severijnen R.S., van der Staak F.H., Festen C. Are adults content or continent after repair for high anal atresia? A long-term follow-up study in patients 18 years of age and older. Ann Surg. 1993;218:196–200. doi: 10.1097/00000658-199308000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rintala R., Mildh L., Lindahl H. Fecal continence and quality of life in adult patients with an operated low anorectal malformation. J Pediatr Surg. 1992;27:902–905. doi: 10.1016/0022-3468(92)90394-m. [DOI] [PubMed] [Google Scholar]
- 34.Wiegel M., Meston C., Rosen R. The Female Sexual Function Index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 35.Rosen R.C., Riley A., Wagner G., Osterloh I.H., Kirkpatrick J., Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 36.Prins J., Blanker M.H., Bohnen A.M., Thomas S., Bosch J.L. Prevalence of erectile dysfunction: a systematic review of population-based studies. Int J Impot Res. 2002;14:422–432. doi: 10.1038/sj.ijir.3900905. [DOI] [PubMed] [Google Scholar]
- 37.Schreiber Pedersen L., Lose G., Hoybye M.T., Elsner S., Waldmann A., Rudnicki M. Prevalence of urinary incontinence among women and analysis of potential risk factors in Germany and Denmark. Acta Obstet Gynecol Scand. 2017;96:939–948. doi: 10.1111/aogs.13149. [DOI] [PubMed] [Google Scholar]
- 38.Paulsen L.H., Sorensen Bakke L., Jarbol D.E., Balasubramaniam K., Hansen D.G. Associations between lifestyle, erectile dysfunction and healthcare seeking: a population-based study. Scand J Prim Health Care. 2020;38:176–183. doi: 10.1080/02813432.2020.1753347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metts J.C., 3rd, Kotkin L., Kasper S., Shyr Y., Adams M.C., Brock J.W., 3rd Genital malformations and coexistent urinary tract or spinal anomalies in patients with imperforate anus. J Urol. 1997;158:1298–1300. doi: 10.1097/00005392-199709000-00168. [DOI] [PubMed] [Google Scholar]
- 40.Nah S.A., Ong C.C., Lakshmi N.K., Yap T.L., Jacobsen A.S., Low Y. Anomalies associated with anorectal malformations according to the Krickenbeck anatomic classification. J Pediatr Surg. 2012;47:2273–2278. doi: 10.1016/j.jpedsurg.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 41.Levitt M.A., Pena A. Cloacal malformations: lessons learned from 490 cases. Semin Pediatr Surg. 2010;19:128–138. doi: 10.1053/j.sempedsurg.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 42.Rackow B.W., Arici A. Reproductive performance of women with müllerian anomalies. Curr Opin Obstet Gynecol. 2007;19:229–237. doi: 10.1097/GCO.0b013e32814b0649. [DOI] [PubMed] [Google Scholar]