Abstract
Context
The optimal management of oligometastatic prostate cancer (PCa) is still debated.
Objective
The purpose of the present systematic review and meta-analysis is to collect the available evidence to date to better define the role of stereotactic body radiotherapy (SBRT) in selected patients with oligorecurrent PCa.
Evidence acquisition
Study methodology complied with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA). All prospective studies including PCa patients with nodal and/or bone oligometastases (one to five lesions) were considered eligible. Heterogeneity between study-specific estimates was tested using chi-square statistics and measured with the I2 index. A pooled estimate was obtained by fitting both fixed-effect and DerSimonian and Laird random-effect model.
Evidence synthesis
Overall, six works (two randomized and the remainder observational) published between 2013 and 2020 were considered eligible. Globally, data from 445 patients were incorporated, of whom 396 were treated with SBRT (329 in observational studies and the remaining 67 in randomized ones). Regarding local progression-free survival (PFS), five studies reported values close to 100%, while one reported a value of 80% in the observation arm. The benefit in terms of biochemical PFS brought by SBRT was evident in all considered studies. Such a difference in cumulative probabilities between the intervention arm and the comparator arm is maintained even 24 mo after the baseline. All studies but one considered toxicity among the endpoints of interest. Most events were classified as either G1 or G2, and the only G ≥ 3 adverse event was reported in one trial.
Conclusions
SBRT is highly cost effective, safe, and with an almost inexistent toxicity risk that makes it the perfect candidate for the optimal management of PCa oligometastatic patients. However, more solid data and a higher level of evidence are needed to affirm its role in the management of these patients.
Patient summary
In this work, we reviewed available evidence on the use of stereotactic body radiotherapy in treating oligometastatic prostate cancer patients. We found good evidence that radiotherapy brings important benefits in overall treatment efficacy without major side effects.
Keywords: Meta-analysis, Metastasis-directed therapy, Oligometastases, Prostate cancer, Stereotactic body radiotherapy, Systematic review
Take Home Message
In this work, we reviewed available evidence of the use of stereotactic body radiotherapy in treating oligometastatic prostate cancer patients. We found good evidence that radiotherapy brings important benefits in overall treatment efficacy without major side effects.
1. Introduction
In the recent years, the therapeutic scenario for advanced prostate cancer (PCa) has changed profoundly [1]. The metastatic burden has emerged as the pivotal factor in the clinical decision-making process [2]. Systemic therapies with androgen deprivation therapy (ADT) and/or chemotherapy represented the standard of care for the management of metastatic PCa [3], [4], until from a growing body of evidence emerged the existence of an oligometastatic state [5].
Advances in imaging, such as the increasing use of next-generation imaging techniques, including gallium-68–labeled prostate-specific membrane antigen positron emission tomography/computed tomography (PSMA-PET/CT), choline-based positron emission tomography (PET), and whole-body magnetic resonance imaging (MRI), have highly increased the sensitivity in the detection of PCa with respect to conventional imaging modalities [6], [7]. By detecting early recurrence, PSMA-PET/CT has increased the relative proportion of metastatic patients diagnosed with low metastatic burden [8]. As no biomarkers are currently available for the identification of oligometastatic disease, so far imaging findings represent the most relevant method for this diagnosis.
In recent years, it has become progressively clearer that oligometastatic patients could benefit from local approaches, also referred to as metastasis-directed therapies (MDTs) [9]. Among MDTs, stereotactic body radiotherapy (SBRT) represents an appealing and cost-effective treatment option, thanks to its limited duration, low or no invasiveness, excellent local control rates, and acceptable toxicity profile [10], [11], [12].
The rationale of SBRT in this clinical setting is not only to eradicate malignant secondary lesions, but also to prevent further metastatic development and delay subsequent treatment escalation. One of the main advantages of prolonged treatment-free survival is the opportunity to defer the progression toward a castration-resistant state [3], [13].
Several studies, including both single-arm and randomized trials, are investigating the use of SBRT alone or in combination with ADT in oligorecurrent PCa patients [14], [15]. Already published data have shown a good local control with a low toxicity profile [3], [10], [11], [13], [16], [17].
Despite the lack of definitive evidence supporting the efficacy of SBRT in all detectable lesions in this subset of patients, three out of four respondents in the 2019 Advanced Prostate Cancer Consensus Conference (APCCC 2019) agreed on using systemic therapies and local approaches for all lesions in most patients with oligorecurrent metachronous PCa [18].
The purpose of the present study is to explore available evidence on the role of SBRT in terms of progression-free survival (PFS) and treatment escalation deferral in selected patients with oligorecurrent PCa. To this aim, we performed a systematic review and meta-analysis to synthesize the existing prospective evidence for PCa patients with a low metastatic burden. Moreover, to the best of our knowledge, we could provide a quantitative estimation of the maintenance of SBRT benefit over time—up to 24 mo—for this group of long-surviving patients.
2. Evidence acquisition
The study methodology complied with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [19]. The inclusion criteria were derived using the population, intervention, control, outcome, and study design (PICOS) approach (Supplementary Table 1). All prospective studies, either randomized or nonrandomized, including PCa patients with nodal and/or bone oligometastases (one to five lesions), were considered eligible. Systematic reviews and meta-analyses were screened for cross-reference. Only papers with full text written in English language were included. The endpoints of interest were identified and discussed by all authors, and encompassed the outcomes of benefit, that is, local PFS (l-PFS), biochemical PFS (b-PFS), distant metastases PFS (d-PFS), and treatment-free survival, and the outcomes of harm, that is, radiotherapy (RT)-related toxicity. Specifically, l-PFS refers to the survival free from local progression, b-PFS refers to the survival free from prostate-specific antigen (PSA) increase, d-PFS refers to the survival free from new distant metastases, and treatment-free survival refers to the survival free from any kind (systemic/local) of new treatment, all expressed in months.
2.1. Search strategy
Electronic databases (namely, National Center for Biotechnology Information PubMed, Elsevier EMBASE and Elsevier Scopus, and ClinicalTrials.gov) were screened up to September 2020 without date restrictions by an author experienced in bibliographic search (S.V.); no additional filters were applied in the first screening phase. The full search strategy is provided in the Supplementary material.
Findings from the above-reported search were screened independently and selected based on titles and abstract by two authors (S.V. and M.A.). Disagreements were resolved following consultation with a third author (G. Marvaso). The whole selection process is detailed in Supplementary Fig. 1.
2.2. Statistical analysis
Extracted data included study design, the number of included patients, median follow-up duration, and association measures, namely, hazard ratio (HR) with 95% confidence interval (CI). More details are available in Supplementary Table 2.
Data were processed and portrayed in the corresponding forest plots by an author (A.B.). Heterogeneity between study-specific estimates was tested using chi-square statistics [20] and measured with the I2 index [21]. Pooled estimate was obtained by fitting both the fixed-effect and the DerSimonian and Laird random-effect model [22]. All tests were considered statistically significant for p < 0.05. All analyses were performed by using R studio (version 1.3.959) with the package “Meta.”
3. Evidence synthesis
Overall, six studies published between 2013 and 2020 were included [3], [10], [11], [13], [16], [17]. Half of the studies were conducted in Australia [10], [11], [13], two in Europe [3], [17], and one in the USA [16].
Considering study design, four were observational studies [10], [11], [13], [17], while the remaining were randomized controlled trials (RCTs) [3], [16], as detailed in Table 1.
Table 1.
Characteristics of the included studies
| Study | Study Design | Sample size | Follow-up | Intervention | Control | Outcome measure |
|---|---|---|---|---|---|---|
| Kneebone et al (2018) [10] | Single-arm, prospective observational study | 57 patients | 16.0 (range: 5.0–31.0) mo | Median b-PFS: 11.0 mo (95% CI, 8.1–13.9) | NA | NA |
| PMID: 31158100 | SBRT | l-PFS: 100% | ||||
| Bowden et al (2020) [13] | Single-arm, prospective observational study, interim results | 199 patients, 176 patients available at last FUP | 35.1 (range: 6.5–51.3) mo, including patients lost to FUP | b-PFS: 41/176 (23.3%) at last FUP | NA | NA |
| PMID: 31199504 | SBRT | |||||
| Muacevic et al (2013) [17] | Single-arm, prospective observational study | 40 patients | 10.2 (range: 3.0–48.0) mo | l-PFS: | NA | NA |
| PMID: 21481619 | SRS | At 6 mo: 95.5% (95% CI, 83.0–98.8) | ||||
| At 12 mo: 95.5% (95% CI, 83.0–98.8) | ||||||
| At 24 mo: 95.5% (95% CI, 83.0–98.8) | ||||||
| Siva et al (2018) [11] | Single-arm, prospective observational study | 33 patients | 24.0 mo | l-PFS: | NA | NA |
| PMID: 30227924 | SABR | At 12 mo: 97.0% (95% CI, 91.0–100.0) | ||||
| At 24 mo: 93.0% (95% CI, 84.0–100.0) | ||||||
| Ost et al (2018) [3] | Randomized (1:1), prospective | 31 patients in both the intervention and the control group (n = 62) | 36 (IQR: 27.6–45.6) mo | Median b-PFS: 10.0 (80% CI, 8.0–13.0) mo | Median b-PFS: 6.0 (80% CI, 4.0–7.0) mo | b-PFS: HR, 0.53; 80% CI, 0.37–077; p = 0.03 |
| PMID: 29240541 | MDT vs observation, interim results | l-PFS: 100% | l-PFS: 80.6% (no. of events = 6) | l-PFS: not provided | ||
| Phillips et al (2020) [16] | Randomized (2:1), prospective | 36 patients in the intervention group and 18 in the control group (n = 54) | 18.8 (range: 5.8–35.0) mo | Median b-PFS not reached | Median b-PFS: 6.4 mo | b-PFS: HR, 0.31; 95% CI, 0.13–0.75; p = 0.002 |
| PMID: 32215577 | SBRT vs observation, interim results | b-PFS at 6 mo: 4 events/36, 11%; 95% CI, 3.9–26.1 | b-PFS at 6 mo: 9 events/18, 50%; 95% CI, 29.1–70.9 | b-PFS at 6 mo: p = 0.005 | ||
| l-PFS at 6 mo: 98.9% (1 event) | l-PFS: not provided | l-PFS: not provided |
b-PFS = biochemical progression-free survival; CI = confidence interval; FUP = follow-up; HR = hazard ratio; l-PFS = local progression-free survival; IQR = interquartile range; MDT = metastasis-directed treatment; NA = not applicable; SBRT = stereotactic body radiotherapy; SRS = robotic stereotactic radiosurgery; SABR = stereotactic ablative body radiotherapy.
Globally, data from 445 patients were incorporated in the present analysis. The type of primary treatment they underwent is reported in Supplementary Table 3. Of these patients, 396 were treated with MDTs, which were SBRT in 390 cases (98.4%) and surgery in six cases (1.6%), with all the latter cases included in the STOMP trial [3]. Overall, of 396 patients, 329 were treated in the setting of observational studies [10], [11], [13], [17], and the remaining 67 were enrolled in an RCT [3], [16]. Conversely, a total of 49 patients from the ORIOLE and STOMP trials [3], [16] were randomized in the observation arm.
Several hypofractionation schedules were applied and ranged between 20 Gy in a single fraction [10], [11], [17] and 50 Gy in ten fractions [10], [13]. The outcomes of interest were heterogeneously distributed across the selected studies, as summarized in Supplementary Table 4. The most common, and therefore comparable, endpoints were b-PFS and l-PFS.
The imaging techniques used in considered studies included computed tomography (CT) [16], whole-body bone scan [13], [16], MRI [13], [16], sodium fluoride PET/CT [11], choline-PET/CT [13], [3], [17], and PSMA-PET/CT [10], [13].
Follow-up ranged from 10.2 to 35 mo for observational studies and from 18.8 to 36 mo for RCTs. The median b-PFS and l-PFS values were reported by a minority of studies [3], [10], as well as the corresponding HR for b-PFS [3], [16]; therefore, these were not suitable for the calculation of the summary estimates.
3.1. Local PFS
Two studies showed l-PFS equal to 100% [3], [10], while three studies [11], [16], [17] reported a value nearly to 100% at different time points. Only one study [3] displayed l-PFS at 80% for the observation arm.
3.2. Biochemical PFS
Overall, four articles were eligible for the quantitative analysis [3], [10], [13], [16].
3.2.1. RCTs and observational studies
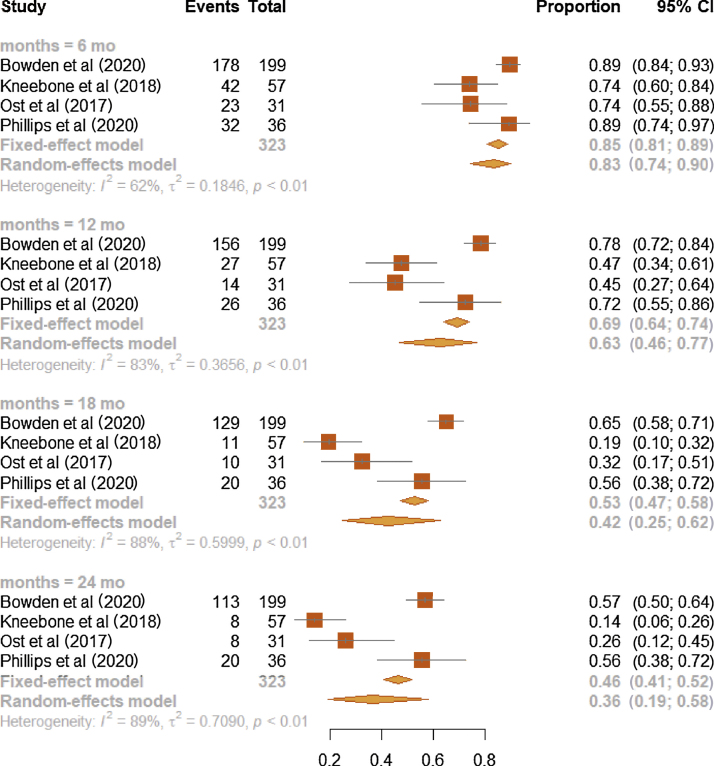
A progressive reduction of survival probabilities was noticed along follow-up in patients who underwent the intervention, with the corresponding b-PFS values being 0.83 (95% CI, 0.74–0.90; I2: 62%), 0.63 (95% CI, 0.46–0.77; I2: 83%), 0.42 (95% CI, 0.25–0.62; I2: 88%), and 0.36 (95% CI, 0.19–0.58; I2: 89%) at 6, 12, 18, and 24 mo since the beginning of SBRT, respectively (Fig. 1).
Fig. 1.
Forest plot on meta-analysis estimates of the b-PFS proportion in the intervention group (MDT or SBRT) for all four studies and stratified according to the time points of 6, 12, 18, 24 mo. Squares represent treatment estimate in the center, horizontal lines represent 95% CIs, and diamonds represent treatment estimate in the center and the right and left sides corresponding to lower and upper confidence limits with corresponding 95% CIs; p values are from testing for heterogeneity between study-specific estimates. b-PFS = biochemical progression-free survival; CI = confidence interval; MDT = metastasis-directed therapy; SBRT = stereotactic body radiotherapy.
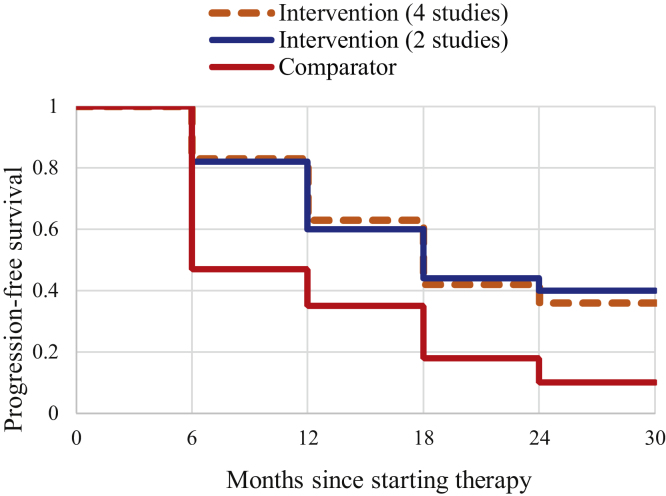
A subsequent analysis focused on assessing survival probabilities among the intervention and comparator arms, by pooling b-PFS proportions. The intervention arm was pictured by pooling patients from RCTs only, or both RCTs and observational studies. Compared with the intervention arm from the two RCTs [3], [16], b-PFS of the intervention group [3], [10], [13], [16] was slightly higher at 6 and 12 mo since starting the therapy, and slightly lower at 18 and 24 mo. This was confirmed by comparing the b-PFS of the intervention group, separately for four and two studies, with that of the observation arm and calculating rough disease-free ratios: (1) 1.77 versus 1.74 at 6 mo, (2) 1.80 versus 1.71 at 12 mo, (3) 2.33 versus 2.44 at 18 mo, and (4) 3.6 versus 4.0 at 24 mo (Fig. 2).
Fig. 2.
Kaplan-Meier survival curve for survivors to oligometastatic prostate cancer: intervention (four studies: observational studies and RCT; n = 323), intervention (two studies: RCT; n = 67), and comparison (two studies: RCT; n = 49). The dotted orange line represents all patients in the intervention arm (four studies). The blue line includes individuals in the intervention arm (two studies: RCT). The red line includes patients in the observation arm (two studies: RCT). RCT = randomized controlled trial.
Overall, the trend in cumulative probabilities of patients belonging to the intervention arm is very similar, considering RCTs alone or including all studies. Moreover, the clear difference in cumulative probabilities between the intervention and comparator arms is maintained even 24 mo after the baseline.
3.2.2. RCT pooling
When assessing the association between MDT or SBRT and the b-PFS reported by the two RCTs [3], [16], the summary HR showed a significant increase in the b-PFS associated with the intervention (HR = 0.45; 95% CI, 0.28–0.73) with very limited between-study heterogeneity (I2: 2%; Fig. 3).
Fig. 3.
Forest plots of study-specific and summary hazard ratios for the association between MDT or SBRT and b-PFS. Squares represent study-specific relative hazard estimates, horizontal lines represent 95% CIs, and diamonds represent summary hazard ratio estimates with corresponding 95% CIs; p values are from testing for heterogeneity between study-specific estimates. b-PFS = biochemical progression-free survival; CI = confidence interval; df = degree of freedom; IV = inverse variance; MDT = metastasis-directed therapy; SBRT = stereotactic body radiotherapy; SE = standard error.
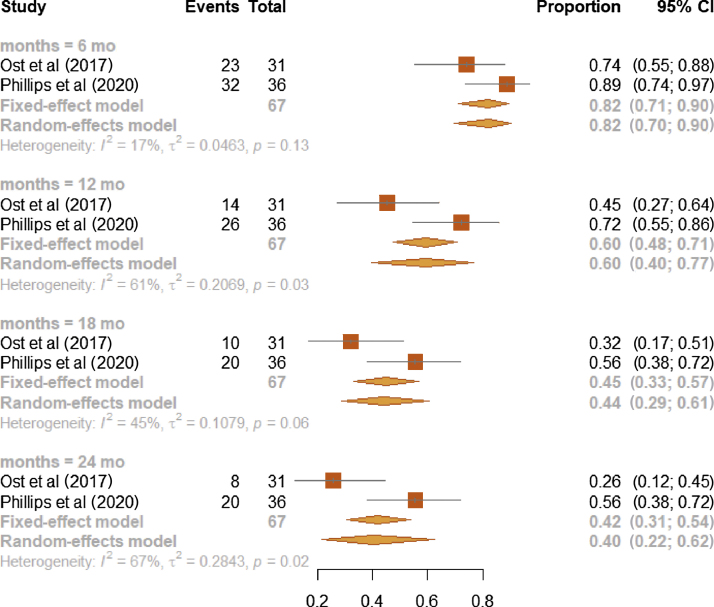
The b-PFS proportions of the MDT or SBRT arm studied in the two RCTs [3], [16] were (1) 0.82 at 6 mo (95% CI, 0.70–0.90; I2: 17%), (2) 0.60 at 12 mo (95% CI, 0.40–0.77; I2: 61%), (3) 0.44 at 18 mo (95% CI, 0.29–0.61; I2: 45%), and (4) 0.40 at 24 mo (95% CI, 0.22–0.62; I2: 67%; Fig. 4). At each time point, estimates from observational studies showed higher heterogeneity (Fig. 1) than those from RCTs (Fig. 4).
Fig. 4.
Forest plot on meta-analysis estimates of the b-PFS proportion in the intervention arm (MDT or SBRT) for RCT studies and stratified according to the time points of 6, 12, 18, and 24 mo. Squares represent treatment estimate in the center, horizontal lines represent 95% CIs, and diamonds represent treatment estimates in the center and the right and left sides corresponding to lower and upper confidence limits with corresponding 95% CIs; p values are from testing for heterogeneity between study-specific estimates. b-PFS = biochemical progression-free survival; CI = confidence interval; MDT = metastasis-directed therapy; RCT = randomized controlled trial; SBRT = stereotactic body radiotherapy.
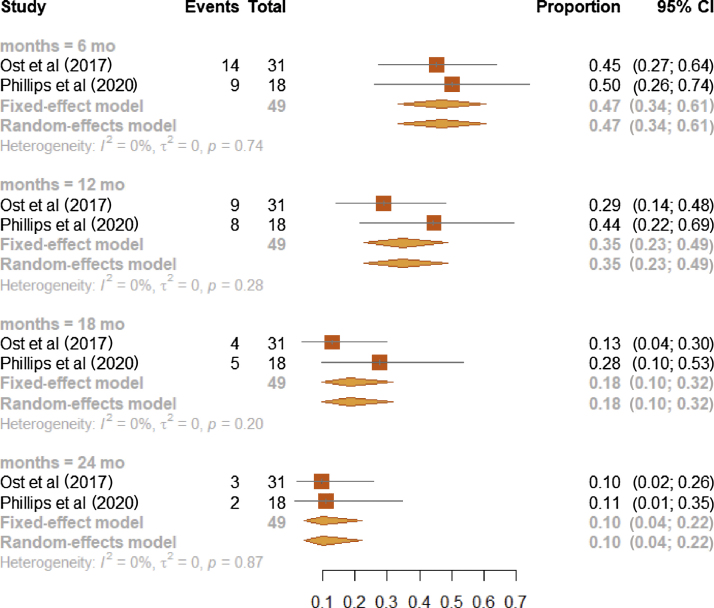
Survival probabilities of the comparison arm evaluated in the two RCT studies [3], [16] were (1) 0.47 at 6 mo (95% CI, 0.34–0.61; I2: 0%), (2) 0.35 at 12 mo (95% CI, 0.23–0.49; I2: 0%), (3) 0.18 at 18 mo (95% CI, 0.10–0.32; I2: 0%), and (4) 0.10 at 24 mo (95% CI, 0.04–0.22, I2: 0%; Fig. 5).
Fig. 5.
Forest plot on meta-analysis estimates of the b-PFS proportion in the observation arm (MDT or SBRT) for RCT studies and stratified according to the time points of 6, 12, 18, and 24 mo. Squares represent treatment estimate in the center, horizontal lines represent 95% CIs, and diamonds represent treatment estimate in the center and the right and left sides corresponding to lower and upper confidence limits with corresponding 95% CIs; p values are from testing for heterogeneity between study-specific estimates. b-PFS = biochemical progression-free survival; CI = confidence interval; MDT = metastasis-directed therapy; RCT = randomized controlled trial; SBRT = stereotactic body radiotherapy.
3.3. Toxicity outcomes
Of the six studies included in the analysis, five considered toxicity among the endpoints of interest. The only grade ≥3 adverse event was reported in the POPSTAR trial [11], in which the authors describe the occurrence of a vertebral fracture in one patient (1/33), treated with a single fraction of 20 Gy. The majority of events were classified as either G1 or G2.
Considering acute G2 toxicities, Kneebone et al [10] described urinary incontinence, which was observed also in one patient included in the ORIOLE trial. Moreover, Phillips et al [16] recorded one event of G2 esophagitis and one of dizziness.
Late toxicities were mainly graded as G1, with 5/57 events in the study by Kneebone et al [10] and 15/36 in the ORIOLE trial [18]. Two G2 late events were observed in the latter trial [16], both of genitourinary nature (urinary incontinence and bladder infection).
Siva et al [11] reported on the following G2 events within 24 mo since stereotactic ablative body RT completion, without discriminating between acute and late toxicities: back pain (2/33), fracture (2/33), diarrhea (1/33), myositis (1/33), and neuralgia (1/33); five additional unspecified G2 toxicities were also mentioned.
4. Conclusions
To our knowledge, this is the first systematic review of only prospective single-arm and randomized studies investigating the efficacy of SBRT. We found that PCa patients with a low metastatic burden may benefit from SBRT in terms of both local and biochemical control. In addition, we observed that SBRT consistently maintains its efficacy over time, which represents a novel and clinically meaningful finding for the management of PCa patients with a low metastatic burden.
In this clinical setting, these data support the biological rationale beyond MDTs. As the dissemination of subcellular clones from the metastatic sites to the rest of the body is prevented, patients’ oncological outcomes can be improved, and treatment-free survival prolonged, with a positive impact on quality of life (QoL). The benefits of MDTs can be higher than those of ADT, particularly in patients wishing to delay systemic treatments for QoL or comorbidity concerns. Data also reveal that MDTs are equivalent to systemic therapies from an economic perspective, as they have comparable cost effectiveness [12].
Overall, the oligometastatic state represents an intermediate step before the onset of widespread metastatic disease, with different chances of curability [9]. While recent phase III randomized trials [23] have clearly demonstrated an advantage in overall survival from irradiating the primary tumor for patients with synchronous metastatic disease and a low metastatic burden, the scenario in the metachronous setting is still under debate and suffers the lack of evidence-based indications [24].
Certainly, data supporting MDT, in particular SBRT, could not be ignored even if provided mostly by retrospective studies. A recent systematic review by Viani et al [25], including 23 observational studies with a total of 1441 lesions treated with SBRT, showed excellent rates of local control (0.976; 95% CI, 0.96–0.98) and ADT-free survival of 20.1 mo, with low rates of acute and late moderate-to-severe toxicity events (1.3% and 1.2%, respectively).
Comparable findings were reported by Yan et al [26], who identified ten, mainly observational, studies for a total of 653 patients and 1111 metachronous lesions treated with SBRT. Other than confirming both the safety and the efficacy of SBRT in this subset of patients, the authors focused on short- and long-term effects on ADT. In particular, they emphasized that a prolonged ADT-free interval may not only preserve patients’ QoL, but also limit the so-called “financial toxicity” for the healthcare systems, both in terms of ADT administration and ADT relatable side effects (ie, coronary artery disease, osteoporotic fractures, and metabolic syndrome).
The only phase II studies included in our analysis [3], [16] have reported promising outcomes in terms of disease control in fairly comparable populations (namely, non–castration-resistant patients enrolled with one to three metastatic lesions). These findings were confirmed also considering a longer follow-up. In fact, during the last American Society of Clinical Oncology (ASCO) genitourinary meeting [27], 5-yr ADT-free survival was confirmed to be higher in the MDT arm than in the surveillance arm (34% vs 8%; HR = 0.57) in the STOMP trial [3]. Even though the outcomes of benefit were demonstrated clearly, it should be pointed out that in both trials, the control arm consisted of surveillance only, which could potentially affect the clinical implications of these results.
Nevertheless, while the results from comparative phase II and III trials are awaited [14], [15], this body of evidence, together with the one provided by observational trials [10], [11], [13], [17], currently represents the strongest evidence supporting the use of SBRT as a safe and effective MDT for metachronous PCa patients.
Other than focusing solely on disease control rates, which we know to be quite good, our analysis also considered the timeline of disease progression that quantifies how this control of disease is maintained over time.
We obtained clear results that the maximum efficacy in terms of b-PFS and survival probabilities is obtained within the first 6 mo following treatment completion. Notably, such oncological advantage is still maintained at 24 mo for a significant proportion of patients. As a consequence, it is straightforward to understand how the selection of proper patients is crucial. Specifically, identification of patients at a higher risk of rapid progression could better inform practitioners and lead to the prescription of more aggressive treatments.
As mentioned above, given the lack of specific biomarkers, the only method to identify the oligometastatic state of PCa with high precision relies on technological advances in diagnostic procedures. In recent years, PSMA-PET/CT has been emerging as one of the most sensitive imaging techniques to detect metastasis even in the presence of PSA levels inferior to 0.5 ng/ml [28]. The ORIOLE trial [16] has demonstrated that 16 out of 36 patients (44.4%) had baseline PET-avid lesions, which were not detected by conventional imaging (CT, MRI, or bone scan) and therefore not included in the RT treatment field. Importantly, as many as six out of these 16 patients (37.5%) experienced progression at 6 mo, while the same occurred only in one out of the 19 patients (5.2%) who had all the lesions treated, demonstrating that accurate imaging is of paramount importance in this setting of patients as it might highly impact the efficacy of MDTs. Finally, we need to classify patients based on other clinical characteristics such as the timing from the treatment of the primary tumor and the appearance of the metastases. This first disease-free interval could be a sign of illness aggressiveness and help identify patients at higher risk.
Besides imaging, the best approach to irradiate nodal oligorecurrent PCa patients is also discussed [29], [30]. Indeed, salvage RT can be delivered either to cover bilateral pelvic lymph node region (elective nodal radiotherapy [ENRT]) or to treat nodal relapse focally with SBRT. Specifically, ENRT allows for a better nodal coverage and improves PFS, while SBRT enables improvement of local control, deferment of systemic therapies, and thus improvement of QoL. According to limited data available, ENRT seems to reduce the number of local recurrences but at the cost of a potential increase in toxicity [31]. Overall, both strategies represent valuable MDT options in terms of efficacy and safety, and therefore the optimal RT regimen in the setting of nodal oligorecurrent PCa patients still remains a matter of debate [32]. Expected advances in diagnostic capability of imaging techniques could allow an improved detection of disease recurrences and thus a better selection of patients eligible for either technique [29].
Despite slight differences in the calculation of time from first diagnosis to primary treatment, a median time of 3.8 (interquartile range: 2.3–5.4) yr for the treatment of metachronous disease could be calculated for the whole population included in the analysis. Unfortunately, it was not possible to correlate this timing with the progression of disease. Moreover, the definition of the oligometastatic state was not consistent across publications, with the majority of studies (4/6) [10], [11], [16], [3] considering any patient with fewer than three lesions, staged as N1 and M1a or M1b, as oligometastatic. In the works of Bowden et al [13] and Siva et al [11], a minor proportion of the enrolled population was defined as castration resistant (14/199 and 6/33, respectively).
While Muacevic et al [17] considered only patients with oligometastatic bone involvement, the other works included in our analysis enrolled patients with both nodal and bone disease [13], [10], [16], [11], [3]. Interestingly, the location of metastases (node only vs bone only) was not correlated with the effect of MDT in three studies [3], [10], [13]. Nonetheless, Bowden et al [13] identified a trend toward an increased risk of disease progression, and subsequent treatment escalation, in patients presenting with both node and bony lesions, as compared with those having bone metastases only (HR = 2.12; 95% CI: 1.12–4.02; p = 0.022). Moreover, Siva et al [11] described a different incidence of treatment failure according to metastatic location, with patients with bone metastases showing a lower rate of disease progression than those with pelvic nodal disease (12/20 events, 60% vs 8/11 events, 73%). However, the authors could provide only a qualitative assessment of this phenomenon, probably due to the limited sample size of the analyzed population, and caution is advised in the generalization of these results.
We are well aware of the limitations of our analysis. The most significant one derives from the design of available trials and is represented by the absence of a control group comparing SBRT with an active treatment such as ADT. While in most cases the primary endpoint was to defer ADT, we need to consider that hormonal therapy is still the standard of care in this setting of patients and that evidence is needed to support the role of MDT as an at least noninferior alternative. For this reason, our group decided to conduct a randomized phase II trial, named Radioablation With or Without Androgen DeprIvation Therapy in Metachronous Prostate Cancer OligometaStAsis (RADIOSA) [33] and registered at ClinicalTrials.gov as NCT03940235, which directly compares SBRT alone versus SBRT plus a short ADT course (6 mo). Owing to the importance of the role of systemic therapies in conjunction with MDTs, other similar trials have been registered recently, including the ADOPT trial, which aims at testing the hypothesis that the addition of ADT to MDT prolongs d-PFS compared with MDT alone [34]. A minor limitation is represented by the fact that, in the STOMP trial [3], a minority of patients (6/31) received surgery instead of SBRT to treat metastases.
Therapy for metastatic PCa patients continues to evolve, especially with the new drugs available that offer excellent results in terms of oncological outcomes. However, SBRT is highly cost effective, safe, and with an almost nonexistent toxicity risk that makes it the perfect candidate in this setting of patients. Despite this premise, more solid data and higher level of evidence are needed to affirm its role in the management of these patients.
Author contributions: Matteo Pepa had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Marvaso, Volpe.
Acquisition of data: Marvaso, Volpe, Pepa, Augugliaro, Corrao, Biffi.
Analysis and interpretation of data: Marvaso, Volpe, Pepa, Augugliaro, Biffi.
Drafting of the manuscript: Marvaso, Volpe, Pepa, Augugliaro, Biffi, Zaffaroni, Bergamaschi, La Fauci.
Critical revision of the manuscript for important intellectual content: Mistretta, Luzzago, Cattani, Musi, Petralia, Pravettoni, De Cobelli, Orecchia, Jereczek-Fossa.
Statistical analysis: Biffi.
Obtaining funding: Jereczek-Fossa.
Administrative, technical, or material support: Zaffaroni, Bergamaschi, La Fauci.
Supervision: Mistretta, Luzzago, Cattani, Musi, Petralia, Pravettoni, De Cobelli, Orecchia, Jereczek-Fossa.
Other: None.
Financial disclosures: Matteo Pepa certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This study was supported by a research grant from the Associazione Italiana per la Ricerca sul Cancro (AIRC) entitled “Radioablation ± hormonotherapy for prostate cancer oligorecurrences (RADIOSA trial): potential of imaging and biology” registered at ClinicalTrials.govNCT03940235, approved by the Ethics Committee of IRCCS Istituto Europeo di Oncologia and Centro Cardiologico Monzino (IEO-997). The institution of authors Giulia Marvaso, Gennaro Musi, Stefania Volpe, Matteo Pepa, Matteo Augugliaro, Giulia Corrao, Mattia Zaffaroni, Luca Bergamaschi, Francesco Maria La Fauci, Federica Cattani, Roberto Orecchia, and Barbara Alicja Jereczek-Fossa (IEO, European Institute of Oncology IRCCS, Milan) was also partially supported by the Italian Ministry of Health with Ricerca Corrente and 5 × 1000 funds. Mattia Zaffaroni was supported by a research grant from Accuray Inc., entitled “Data collection and analysis of Tomotherapy and CyberKnife breast clinical studies, breast physics studies and prostate study”. The sponsors did not play any role in the study design, collection, analysis, and interpretation of data; writing of the manuscript; or the decision to submit the manuscript for publication.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.euros.2021.02.008.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Heidegger I. Recent scientific developments in metastatic prostate cancer. Cancers. 2020;12:E3280. doi: 10.3390/cancers12113280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiota M., Terada N., Saito T. Differential prognostic factors in low- and high-burden de novo metastatic hormone-sensitive prostate cancer patients. Cancer Sci. 2020 doi: 10.1111/cas.14722. Epub ahead of print. Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ost P., Reynders D., Decaestecker K. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–453. doi: 10.1200/JCO.2017.75.4853. [DOI] [PubMed] [Google Scholar]
- 4.NCCN . 2020. Clinical practice guidelines in oncology: prostate cancer. [DOI] [PubMed] [Google Scholar]
- 5.Kyriakopoulos C.E., Chen Y.H., Carducci M.A. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol. 2018;36:1080–1087. doi: 10.1200/JCO.2017.75.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morigi J.J., Stricker P.D., van Leeuwen P.J. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–1190. doi: 10.2967/jnumed.115.160382. [DOI] [PubMed] [Google Scholar]
- 7.Trabulsi E.J., Rumble R.B., Jadvar H. Optimum imaging strategies for advanced prostate cancer: ASCO guideline. J Clin Oncol. 2020;38:1963–1996. doi: 10.1200/JCO.19.02757. [DOI] [PubMed] [Google Scholar]
- 8.Grubmuller B., Baltzer P., D’Andrea D. 68Ga-PSMA 11 ligand PET imaging in patients with biochemical recurrence after radical prostatectomy–diagnostic performance and impact on therapeutic decision-making. Eur J Nucl Med Mol Imaging. 2018;45:235–242. doi: 10.1007/s00259-017-3858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battaglia A., De Meerleer G., Tosco L. Novel insights into the management of oligometastatic prostate cancer: a comprehensive review. Eur Urol Oncol. 2019;2:174–188. doi: 10.1016/j.euo.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Kneebone A., Hruby G., Ainsworth H. Stereotactic body radiotherapy for oligometastatic prostate cancer detected via prostate-specific membrane antigen positron emission tomography. Eur Urol Oncol. 2018;1:531–537. doi: 10.1016/j.euo.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Siva S., Bressel M., Murphy D.G. Stereotactic ablative body radiotherapy (SABR) for oligometastatic prostate cancer: a prospective clinical trial. Eur Urol. 2018;74:455–462. doi: 10.1016/j.eururo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Parikh N.R., Chang E.M., Nickols N.G. Cost-effectiveness of metastasis-directed therapy in oligorecurrent hormone-sensitive prostate cancer. Int J Radiat Oncol Biol Phys. 2020;108:917–926. doi: 10.1016/j.ijrobp.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowden P., See A.W., Frydenberg M. Fractionated stereotactic body radiotherapy for up to five prostate cancer oligometastases: Interim outcomes of a prospective clinical trial. Int J Cancer. 2020;146:161–168. doi: 10.1002/ijc.32509. [DOI] [PubMed] [Google Scholar]
- 14.ClinicalTrials.gov. RADIOSA—Radioablation With or Without Androgen DeprIvation Therapy in Metachronous Prostate Cancer OligometaStAsis. https://clinicaltrials.gov/ct2/show/NCT03940235.
- 15.De Bruycker A., Spiessens A., Dirix P. PEACE V—Salvage Treatment of OligoRecurrent nodal prostate cancer Metastases (STORM): a study protocol for a randomized controlled phase II trial. BMC Cancer. 2020;20:406. doi: 10.1186/s12885-020-06911-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips R., Shi W.Y., Deek M. Outcomes of observation vs stereotactic ablative radiation for oligometastatic prostate cancer: the ORIOLE phase 2 randomized clinical trial. JAMA Oncol. 2020;6:650–659. doi: 10.1001/jamaoncol.2020.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muacevic A., Kufeld M., Rist C., Wowra B., Stief C., Staehler M. Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol Oncol. 2013;31:455–460. doi: 10.1016/j.urolonc.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Gillessen S., Attard G., Beer T.M. Management of patients with advanced prostate cancer: report of the Advanced Prostate Cancer Consensus Conference 2019. Eur Urol. 2020;77:508–547. doi: 10.1016/j.eururo.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 20.Cochran W.G. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 21.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Parker C.C., James N.D., Brawley C.D. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392:2353–2366. doi: 10.1016/S0140-6736(18)32486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burdett S., Boevé L.M., Ingleby F.C. Prostate radiotherapy for metastatic hormone-sensitive prostate cancer: a STOPCAP systematic review and meta-analysis. Eur Urol. 2019;76:115–124. doi: 10.1016/j.eururo.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viani G.A., Arruda C.V., Hamamura A.C., Faustino A.C., Freitas Bendo Danelichen A., Guimarães F.S. Stereotactic body radiotherapy for oligometastatic prostate cancer recurrence: a meta-analysis. Am J Clin Oncol. 2020;43:73–81. doi: 10.1097/COC.0000000000000635. [DOI] [PubMed] [Google Scholar]
- 26.Yan M., Moideen N., Bratti V.F., Moraes F.Y. Stereotactic body radiotherapy (SBRT) in metachronous oligometastatic prostate cancer: a systematic review and meta-analysis on the current prospective evidence. Br J Radiol. 2020;93 doi: 10.1259/bjr.20200496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nanda S. STOMP 5-year results confirm potential of metastasis-directed therapy in prostate cancer. https://oncology.medicinematters.com/asco-gu-2020/prostate-cancer/stomp-5-year-results-metastasis-directed-therapy/17706826.
- 28.Kishan AU, Nickols NG, Spratt DE. Prostate-specific membrane antigen positron emission tomography-guided radiotherapy. Eur Urol Focus. In press. 10.1016/j.euf.2020.09.020. [DOI] [PubMed]
- 29.Tran S., Jorcano S., Falco T., Lamanna G., Miralbell R., Zilli T. Oligorecurrent nodal prostate cancer: long-term results of an elective nodal irradiation approach. Am J Clin Oncol. 2018;41:960–962. doi: 10.1097/COC.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 30.Vaugier L., Palpacuer C., Rio E. Early toxicity of a phase 2 trial of combined salvage radiation therapy and hormone therapy in oligometastatic pelvic node relapses of prostate cancer (OLIGOPELVIS GETUG P07) Int J Radiat Oncol Biol Phys. 2019;103:1061–1067. doi: 10.1016/j.ijrobp.2018.12.020. [DOI] [PubMed] [Google Scholar]
- 31.De Bleser E., Jereczek-Fossa B.A., Pasquier D. Metastasis-directed therapy in treating nodal oligorecurrent prostate cancer: a multi-institutional analysis comparing the outcome and toxicity of stereotactic body radiotherapy and elective nodal radiotherapy. Eur Urol. 2019;76:732–739. doi: 10.1016/j.eururo.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Achard V., Bottero M., Rouzaud M. Radiotherapy treatment volumes for oligorecurrent nodal prostate cancer: a systematic review. Acta Oncol. 2020;59:1224–1234. doi: 10.1080/0284186X.2020.1775291. [DOI] [PubMed] [Google Scholar]
- 33.Marvaso G., Ciardo D., Corrao G. Radioablation +/- hormonotherapy for prostate cancer oligorecurrences (RADIOSA trial): potential of imaging and biology (AIRC IG-22159) BMC Cancer. 2019;19:903. doi: 10.1186/s12885-019-6117-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ClinicalTrials.gov. Androgen Deprivation Therapy for Oligo-recurrent Prostate Cancer in Addition to radioTherapy (ADOPT). https://clinicaltrials.gov/ct2/show/NCT04302454. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.