Abstract
Context
Several newer device-based procedures have recently become available for treating men with lower urinary tract symptoms attributed to benign prostatic hyperplasia, but their effectiveness remains uncertain.
Objective
To assess the longer-term comparative effectiveness (defined as >12 mo of follow-up) of the newer treatment modalities prostatic urethral lift (PUL), transurethral prostate convective radiofrequency water vapor (Rezūm), Aquablation, and prostatic arterial embolization (PAE).
Evidence acquisition
Ovid Medline, the Cochrane Central Register of Controlled Trials (CENTRAL), and the Agency for Healthcare Research and Quality databases were searched through September 30, 2019; hand searches of references of relevant studies were also performed. Eligible studies were randomized controlled trials (RCTs) published in English language. We excluded observational studies.
Evidence synthesis
One RCT (n = 91) found that patients undergoing PUL may be less likely to respond (risk ratio [RR] 0.8; 95% confidence interval [CI] 0.7–1.0; low certainty of evidence [CoE]) and have a higher mean International Prostate Symptom Score (IPSS; mean difference 6.1; 95% CI 2.2–10.0; low CoE) than those undergoing transurethral resection of the prostate (TURP). Among patients undergoing PAE, one small RCT (n = 30) reported similar IPSS response rates (RR 0.9; 95% CI 0.7–1.1; low CoE) and one trial (n = 107) found similar mean IPSS (–0.7; 95% CI –1.3 to 2.7; moderate CoE) scores to those among patients undergoing TURP. A single study on Aquablation reported 12 mo of follow-up only, and a single 3-mo trial compared Rezūm with sham treatment.
Conclusions
The current best evidence underlying these newer therapies is limited to few trials (PUL and PAE), short-term follow-up of 12 mo (Aquablation and Rezūm), or sham comparison only (Rezūm).
Patient summary
Evidence for four of the newer surgical treatments for men with an enlarged prostate is limited to few small trials with short-term follow-up; only one trial compared a new treatment modality with sham surgery.
Keywords: Lower urinary tract symptoms, Benign prostatic hyperplasia, Systematic review, Comparative effectiveness, Randomized trials
Take Home Message
The current best evidence for newer treatment being used for benign prostatic obstruction is limited to few trials (prostatic urethral lift procedure and prostatic arterial embolization), short follow-up of ≤12 mo (Aquablation), or sham comparison only (for transurethral prostate convective radiofrequency water vapor).
1. Introduction
Surgical and interventional approaches play an important role in the treatment of lower urinary tract symptoms (LUTS) attributed to benign prostatic hyperplasia (BPH), especially for men who are refractory to lifestyle changes and behavioral management, as well as medical therapy. Recently, several new approaches have become available, prompting the American Urological Association (AUA) to commission an evidence report as the foundation of an update of their clinical practice guideline. As part of this AUA guideline process, we conducted a systematic review on the comparative effectiveness of these treatment modalities, with this report focusing on four newer modalities, namely, prostatic urethral lift (PUL; Urolift), transurethral prostate convective radiofrequency water vapor (Rezūm), Aquablation, and prostatic arterial embolization (PAE).
2. Evidence acquisition
We developed an a priori protocol with key input from the members of the AUA Guideline Panel for the surgical management LUTS attributed to BPH. This was registered with PROSPERO on July 17, 2017 (CRD42017072325). We included randomized controlled trials (RCTs) that tested the comparative effectiveness of newer minimally invasive surgical therapies, namely, PUL, Rezūm, Aquablation, and PAE, compared with transurethral resection of the prostate (TURP), sham procedures, or pharmacological therapy only in men aged ≥45 yr with LUTS attributed BPH, including bladder outlet obstruction, who were surgical candidates (Supplementary Table 1). Systematic reviews/meta-analyses of RCTs or controlled clinical trials were also reviewed to identify eligible trials. Studies were limited to English language only. The primary predefined outcomes of interest were changes reflecting clinically important differences in validated measures to assess LUTS (International Prostate Symptom Score [IPSS]/AUA Symptom Index: score ranges from 0 to 35 with higher scores indicating more severe symptoms), and prostate-related bother or quality of life (QoL; IPSS-QoL questionnaire; BPH/LUTS impact scale at >12 mo of follow-up; Supplementary Table 2). We also extracted the percentage of responders (those with minimally important change) to treatment based on changes in IPSS/AUA scores as defined by the study investigators (eg, reduction ≥30% or ≥8 points) and symptom recurrence within. If no longer-term data (>12 mo) were available for these outcomes, short-term outcomes were reported. In addition, we extracted data on the need for reoperation, and assessed common and serious adverse effects (AEs) associated with surgical therapies.
2.1. Information sources and literature search
We searched Ovid Medline (Supplemental Table 3) and the Cochrane Central Register of Controlled Trials (CENTRAL) with filters for study design to identify relevant studies published through September 30, 2019. We also searched for relevant systematic reviews for additional trials.
2.2. Study selection process, data extraction, and risk of bias in studies
Two independent investigators screened titles and abstracts to identify studies meeting the eligibility criteria. Data were extracted by one investigator, and reviewed and verified for accuracy by a second investigator. Risk of bias (RoB) of eligible studies was assessed using the Cochrane RoB tool and guidance from the Cochrane Handbook of Systematic reviews [1].
2.3. Synthesis of results
Data were analyzed in Review Manager Version 5.3 [2] to calculate risk ratios (RRs) and mean differences (MDs) and their corresponding 95% confidence intervals (CIs). Pooled analyses were conducted using a DerSimonian-Laird random-effect model if feasible and clinically appropriate [3]. We measured the magnitude of heterogeneity with the I2 statistic [4]. We interpreted efficacy and comparative effectiveness using established thresholds indicating clinical importance (Supplementary Table 2). We rated the certainty of evidence (CoE), our confidence in the estimates of effect, for the primary outcomes as high, moderate, low, or insufficient, based on the four domains of RoB, directness, consistency, and precision using GRADE [5].
3. Evidence synthesis
3.1. Results
3.1.1. Search results
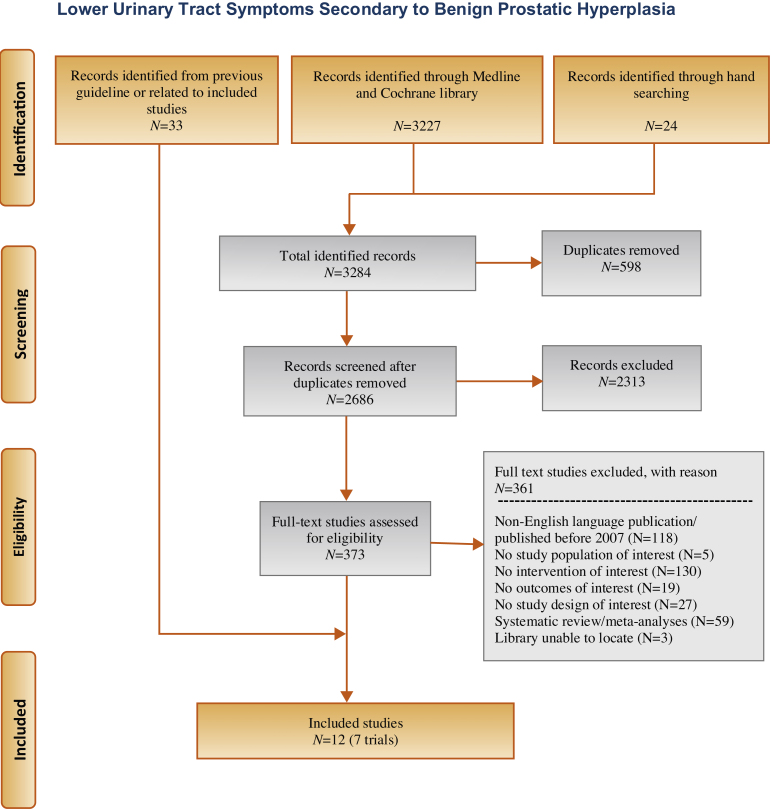
Our literature search identified 3284 references, of which 373 were selected for full-text review (Fig. 1). This process was mapped to 12 references reporting on seven unique RCTs: five reporting on comparison with TURP and two with sham. None were compared with medications.
Fig. 1.
Flowchart.
3.1.2. Prostatic urethral lift
We found one trial that compared PUL with sham [6] with comparative data limited to 3 mo of follow-up and one trial that compared PUL with TURP with a follow-up duration of up to 24 mo [7].
3.1.2.1. PUL versus sham
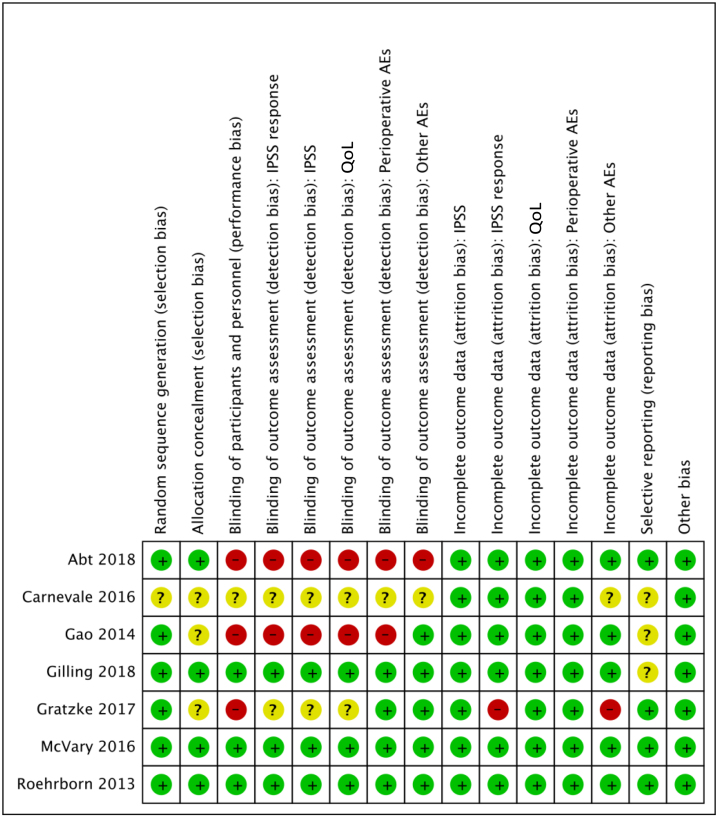
The LIFT study randomized 206 men, with an IPSS of ≥13, a maximum flow rate of 12 ml/s, and a prostate size of 30–80 cc, to PUL versus sham (Table 1) [6]. The sham procedure consisted of rigid cystoscopy with simulated sounds of a surgical intervention. The study was described as “double blinded,” and we rated the RoB as low (Fig. 2). The primary study endpoint was the comparison of IPSS at 3 mo. At this time point, patients were unblinded, and participants in the sham group were offered PUL and followed prospectively for up to 5 yr. This trial provided no information for the predefined primary outcomes of treatment response, IPSS and IPSS-QoL change, need for reoperation, or serious procedure-related complications (Table 2). Based on these findings, we are uncertain about the comparative risk of PUL versus sham (RR 1.4, 95% CI 0–34.5; very low-quality evidence). Reported long-term data [8] of the participants who underwent PUL were limited by the lack of a control group and considerable attrition; 5-yr data were limited to 87 of the 140 men (62.1%) for whom the retreatment rate was 13.6%.
Table 1.
Baseline characteristics of the eligible minimally invasive surgical therapies
| Study [reference]/location | Number randomized | Duration (mo) | Mean age | Mean IPSS | Mean prostate volume (ml) |
|---|---|---|---|---|---|
| Prostatic arterial embolization versus TURP | |||||
| Abt (2018) [10]/Switzerland | 103 | 3 | 66 | 18 | 52 |
| Carnevale (2016) [11]/Brazil | 30 | 12 | 65 | 26 | 60 |
| Gao (2014) [12]/China | 114 | 24 | 67 | 23 | 64 |
| Totals and means | 247 | 66 | 21 | 58 | |
| Prostatic urethral lift versus TURP | |||||
| Gratzke (2017) [7]/Europe | 91 | 24 | 64 | 22 | 39 |
| Prostatic urethral lift versus sham | |||||
| Roehrborn (2013) [6]/USA, Canada, Australia | 206 | 3 | 66 | 23 | 43 |
| Transurethral prostate convective radiofrequency water vapor (Rezūm system) versus sham | |||||
| McVary (2016) [15]/USA | 197 | 3 | 63 | 22 | 45 |
| Aquablation versus TURP | |||||
| Gilling (2019) [14]/International | 184 | 12 | 66 | 23 | 53 |
IPSS = International Prostate Symptom Score; TURP = transurethral prostate resection.
Fig. 2.
Risk of bias of included studies. AEs = adverse events; IPSS = International Prostate Symptom Score; QoL = quality of life.
Table 2.
Evidence overview of prostatic urethral lift versus sham
| Outcome | Number of trials (evaluated) | Intervention | Control | Absolute risk difference (95% CI) | Relative risk (95% CI) | Quality of evidence |
|---|---|---|---|---|---|---|
| % (n/N) or mean change | % (n/N) or mean change | |||||
| Responders, based on the IPSS | NR | |||||
| IPSS, mean change from baseline: long-term follow-up (>12 mo) | No data long term; greater with PUL at 3 mo | |||||
| IPSS-QoL, mean change from baseline: long-term follow-up (>12 mo) | No data long term; greater with PUL at 3 mo | |||||
| Need for reoperation | 1 (206) | 0 (0/140) | 0 (0/66) | No difference between groups 0% (NA) | NA | |
| Transfusion | NR | |||||
| Urinary incontinence | 1 (206) | 4 (5/140) | 2 (1/66) | 2.1% (–2.2 to 6.3) | 2.4 (0.3–19.8) | Very lowa |
CI = confidence intervals; IPSS = International Prostate Symptom Score; NA = not applicable; NR = not reported; PUL = prostatic urethral lift; QoL = quality of life.
Downgraded based on the following:
Very wide confidence interval and few events.
3.1.2.2. PUL versus TURP
The BPH6 study randomized 91 men to either PUL or TURP [7]. It was a nonblinded, 24-mo trial that compared PUL with bipolar (66%) and monopolar TURP (Table 1) [7], [9]. RoB was moderate (Fig. 2). The proportion of responders to treatment, defined as IPSS reduction ≥30%, was lower in the PUL group than in the TURP group at month 12 (73% vs 91%; RR 0.8 [0.7–1.0]; Table 3 [9]). The MD in changes in IPSS from baseline through 24 mo was 6.1 points (95% CI 2.2–10.0), favoring the TURP group. Changes in IPSS-QoL were similar between groups throughout the trial. CoE was low for these outcomes.
Table 3.
Evidence overview of prostatic urethral lift versus TURP
| Outcome | Number of trials (evaluated) | Intervention | Control | Absolute risk difference (95% CI) | Relative risk (95% CI) | Quality of evidence |
|---|---|---|---|---|---|---|
| % (n/N) or mean change | % (n/N) or mean change | |||||
| Responders, based on IPSS reduction of ≥30% | 1 (73) | 73 (30/41) | 91 (29/32) | Greater with TURP | 0.8 (0.7–1.0) | Lowa, b |
| –17.5% (–34.4 to –0.5) | ||||||
| IPSS, mean change from baseline: long-term follow-up (>12 mo) | 1 (69) | –9.2 points | –15.3 points | Greater with TURP | Lowa, b | |
| MD 6.1 (2.2–10.0) | ||||||
| IPSS-QoL, mean change from baseline: long-term follow-up (>12 mo) | 1 (69) | –2.5 points | –3.3 points | MD 0.8 (–0.0 to 1.6) | Lowa, c | |
| Need for reoperation (2 yr) | 1 (79) | 14 (6/44) | 6 (2/35) | 8.0% (–4.8 to 20.6) | 2.4 (0.5–11.1) | Very lowa, d |
| Transfusion | NR | |||||
| Urinary incontinence e | 1 (79) | 2 (1/44) | 17 (6/35) | –15.0% (–28.1 to –1.6) | 0.1 (0.02–1.1) | Very low a, d |
CI = confidence intervals; IPSS = International Prostate Symptom Score; MD = mean difference; NR = not reported; QoL = quality of life; TURP = transurethral resection of the prostate.
Downgraded based on the following:
Risk of bias (moderate).
Imprecision.
Very wide confidence.
Very wide confidence interval and few events.
Clavien-Dindo grade 1.
Serious perioperative bleeding requiring reintervention was reported for two TURP participants and none in the PUL group. The relative risks for reoperation due to symptom recurrence over 2 yr and for serious harms related to treatment at 12 mo were 2.4 (95% CI 0.5–11.1) and 0.6 (95% CI 0.2–2.2), respectively [7], [9]. No participants in the PUL group experienced adverse events related to sexual function. In comparison, erectile dysfunction and retrograde ejaculation occurred in, respectively, 9% and 20% of the participants in the TURP group.
3.1.3. Prostatic arterial embolization
We identified three RCTs (n = 247) that compared PAE with TURP [10], [11], [12]. Baseline characteristics of the included studies are provided in Table 1. One trial compared PAE with bipolar TURP [12] and two with monopolar TURP [10], [11]. One trial reported that 21% of the participants had an indwelling urethral catheter at baseline [10]. Two trials did not record preoperative urinary retention [11], [12]. RoB was moderate for both trials (Fig. 2).
One trial reported response to treatment, defined as achieving an IPSS of ≤8 points. The proportion of responders through 12 mo was similar between the PAE and TURP groups: 87% of the PAE participants compared with 100% in the TURP group (RR 0.9 [95% CI 0.7–1.1]; low CoE; Table 4) [11]. The one long-term (>12 mo) trial found that mean changes in IPSS and IPSS-QoL from baseline were similar between groups (MD 0.7 points [95% CI –1.3 to 2.7], moderate CoE; and MD 0.0 points [95% CI –0.3 to 0.3], moderate COE, respectively) [12].
Table 4.
Evidence overview of prostatic arterial embolization versus TURP
| Outcome | Number of trials (evaluated) | Intervention | Control | Results | Relative risk (95% CI) | Quality of evidence |
|---|---|---|---|---|---|---|
| % (n/N) or mean change | % (n/N) or mean change | Absolute risk difference (95% CI) | ||||
| Responders, based on IPSS reduction of ≥8 points | 1 (30) | 87 (13/15) | 100 (15/15) | Similar between groups | 0.9 (0.7 to 1.1) | Lowa, b |
| –13.3% (–33.1 to 6.4) | ||||||
| IPSS, mean change from baseline: long-term follow-up (>12 mo) | 1 (107) | –15.6 points | –16.3 points | Similar between groups | Moderatea | |
| MD –0.7 (–1.3 to 2.7) | ||||||
| IPSS-QoL, mean change from baseline: long-term follow-up (>12 mo) | 1 (107) | –3.2 points | –3.2 points | Similar between groups | Moderatea | |
| MD –0.0 (–0.0 to 0.0) | ||||||
| Need for reoperation | 2 (144) | 10 (7/72) | 3 (2/72) | 7% (–0.9% to 14.8) | 2.9 (0.7–11.9) | Very lowa, c |
| Transfusion | 2 (144) | 0 (0/72) | 3 (2/72) | –2.8% (–7.4 to 1.8) | 0.2 (0.01–4.1) | Very lowa, c |
| Urinary incontinence | 2 (129) | 0 (0/63) | 11 (7/66) | Greater with TURP | 0.1 (0.02–0.99) | Lowa, b |
| –10.6% (–18.5 to –2.8) |
CI = confidence intervals; IPSS = International Prostate Symptom Score; MD = mean difference; QoL = quality of life; TURP = transurethral resection of the prostate.
Downgraded based on the following:
Risk of bias (moderate).
Imprecision.
Very wide confidence interval and few events.
The need for a blood transfusion was reported for two TURP participants and none receiving PAE [11], [12]. The need for reoperation was reported for seven participants in the PAE groups compared with two in the TURP group [11], [12]. The CoE for both outcomes was very low. Two trials reported a total of seven incidences of urinary incontinence in the TURP group compared with none in the PAE group (low CoE) [11], [13]. One trial reported that all 15 TURP participants experienced retrograde ejaculation [11], and one trial reported that the incidence of ejaculatory dysfunction among participants in whom ejaculation was assessable was higher, but not significantly, in the TURP group than in the PAE group (84% vs 56%, p = 0.06) [13]. The largest trial reported a higher incidence of acute urinary retention requiring recatheterization in the PAE group (26%) than in the TURP group (6%, p = 0.004) [12]. The short-term trial reported three incidences of urinary retention in the TURP group and one in the PAE group [13]. Postoperative incidences of clot retention, strictures, or transurethral resection syndrome were infrequent [12]. No deaths were reported in any trial.
3.1.4. Aquablation
The WATER study (n = 184) was a double-blinded (patient and follow-up team) trial that compared water jet–based prostate resection (Aquablation) with monopolar (55%) and bipolar TURP, and reported outcomes at 6 and 12 mo (Table 1) [14]. RoB was low (Fig. 2).
The proportion of responders to treatment at 12 mo, defined as an IPSS reduction of ≥50%, was similar between the Aquablation and TURP groups (93% and 87%, respectively; RR 1.1 [95% CI 0.96–1.2]; Table 5). Both groups had a mean change in the IPSS from baseline of 15.1 points (MD 0 points [95% CI –2.3 to 2.3]). Improvement from baseline in the IPSS-QoL was also similar between groups (–3.2 and –3.5 points for the Aquablation and TURP groups, respectively, MD 0.3 points [95% CI –0.2 to 0.8]).
Table 5.
Evidence overview of Aquablation versus TURP
| Outcome | Number of trials (evaluated) | Intervention | Control | Absolute risk difference (95% CI) | Relative risk (95% CI) | Quality of evidence |
|---|---|---|---|---|---|---|
| % (n/N) or Mean change | % (n/N) or mean change | |||||
| Responders, based on IPSS reduction of ≥50% | No data long term | |||||
| IPSS, mean change from baseline: long-term follow-up (>12 mo) | No data long term | |||||
| IPSS-QoL, mean change from baseline: long-term follow-up (>12 mo) | No data long term | |||||
| Need for reoperation | 1 (181) | 3 (3/116) | 2 (1/65) | 1.0% (–3.1 to 5.2) | 1.7 (0.2–15.8) | Very lowa |
| Transfusion | 1 (181) | 1 (1/116) | 0 (0/65) | 0.9% (–2.1 to 3.8) | 1.7 (0.07–41.0) | Very lowa |
| Urinary incontinence | NR, but no differences in combined urinary urgency, frequency, difficulty, or leakage | |||||
CI = confidence intervals; IPSS = International Prostate Symptom Score; NR = not reported; QoL = quality of life; TURP = transurethral resection of the prostate.
Downgraded based on the following:
Very wide confidence interval and few events.
The CoE for harms was very low. The need for a blood transfusion was reported for one Aquablation participant and none receiving TURP (RR 1.7 [95% CI 0.7–41.0]). Three participants in the Aquablation group and one in the TURP group required a reoperation due to LUTS within the 12-mo follow-up. At 3 mo, Aquablation resulted in fewer harms, classified as Clavien-Dindo grade ≥2, than TURP (26% vs 42%, RR 0.62 [95% CI 0.41–0.95]). Rates of retrograde ejaculation were also higher with TURP (23%) than with Aquablation (6%; RR 0.26 [95% CI 0.11–0.61]).
3.1.5. Transurethral prostate convective radiofrequency water vapor (Rezūm)
One double-blind (patient and questionnaire administrator) 3-mo trial (n = 197) compared the Rezūm system with sham [15]. RoB was low (Fig. 2). Following the double-blind phase, the 136 patients randomized to Rezūm thermal therapy only were followed up for 24 mo [16].
The proportion of responders to treatment at 3 mo, defined as an improvement in IPSS by ≥8 points, was much greater in the Rezūm therapy group than in the sham group (74% vs 31%, RR 2.4 [95% CI 1.6–3.5]; Table 6). The mean change from baseline in the IPSS was greater in the Rezūm therapy group than in the sham group and was greater than the minimally important difference of >3 points (MD –6.9 points [95% CI –9.1 to –4.8]). Improvement in the IPSS-QoL also favored the thermal therapy group and was greater than the minimally important difference of >1 point (MD –1.2 points [95% CI –1.7 to –0.7]).
Table 6.
Evidence overview of transurethral prostate convective radiofrequency water vapor (Rezūm system) versus sham
| Outcome | Number of trials (evaluated) | Intervention | Control | Absolute risk difference (95% CI) | Relative risk (95% CI) | Quality of evidence |
|---|---|---|---|---|---|---|
| % (n/N) or mean change | % (n/N) or mean change | |||||
| Responders, based on IPSS reduction of ≥8 points | No data long term | |||||
| IPSS, mean change from baseline: long-term follow-up (>12 mo) | No data long term | |||||
| IPSS, mean change from baseline: long-term follow-up (>12 mo) | No data long term | |||||
| Need for reoperation | 1 (197) | <1 (1/135) | 0 (0/61) | 0.7% (–2.1 to 3.6) | 1.4 (0.1–33.0) | Very lowa |
| Transfusion | NR | |||||
| Urinary incontinence | NR |
CI = confidence intervals; IPSS = International Prostate Symptom Score; NR = not reported.
Downgraded based on the following:
Very wide confidence interval and few events.
One participant in the Rezūm therapy group required reoperation (open prostatectomy) due to LUTS within the double-blind period (very low CoE). Serious treatment-related harms were reported for two participants in the Rezūm therapy group, including an incidence of de novo extended urinary retention, compared with no incidence in the sham group (RR 2.3 [95% CI 0.1–46.4]; very low CoE; Table 5). There were significantly more nonserious treatment-related harms in the Rezūm therapy group than in the sham group (RR 3.9 [95% CI 1.8–8.6]; high CoE; Table 5). The nonserious treatment-related harms were transient and included dysuria, hematuria, frequency and urgency, and urinary tract infections. Anejaculation was reported for four thermal therapy group participants versus none in the sham group.
Open-label 24-mo results showed sustained improvements for the IPSS and IPSS-QoL, with scores remaining significantly improved from baseline [8]. Among the intent-to-treat population of the original 136 participants, the mean change in IPSS from baseline was –11.2 points and the mean score was 10.2 points, representing a 52% improvement from a baseline. The mean IPSS-QoL improved by 51% at 2 yr. Few harms occurred in the Rezūm therapy group between months 3 and 12, and no procedure-related AEs were reported during the 12–24-mo follow-up.
3.2. Discussion
3.2.1. Statement of principal findings
This systematic review found comparative effectiveness data of >12 mo of duration (which was the predefined primary endpoint for this review) for only two of the four newer treatment modalities, namely, PUL and PAE. Based on our findings, PUL may result in a reduced likelihood of a clinically meaningful IPSS response and a lesser reduction in the mean IPSS score than TURP. We are very uncertain about its comparative effect on QoL, need for reoperation, transfusions, and incontinence. PAE may result in a similar IPSS response, and probably results in a similar mean IPSS and QOL improvement to that with TURP. Rates of incontinence may be lower, but we are very uncertain about the effect of the need for reoperation and transfusion rates. The evidence for Aquablation versus TURP was limited to the 12-mo follow-up duration, whereas the only trial of Rezūm compared it with sham surgery only and not TURP.
3.2.2. Strengths and weaknesses of the study
This systematic review applied rigorous methodology, which included an a priori written and registered protocol that was developed with extensive stakeholder input by the members of the AUA Guideline Panel for the Surgical Treatment of Men with Lower Urinary Tract Symptoms for the updated AUA guidelines that have since been published [17], [18]. We conducted a comprehensive search of the relevant medical literature based on several databases and actively reached out to clinical experts in order to not miss relevant study reports. Clinical experts also helped inform the choice of primary endpoints as well as threshold for clinically meaningful differences. Meanwhile, subsequent study inclusion and exclusion, data abstraction, RoB assessment, and CoE ratings using GRADE were performed independently by dedicated research methodologists. Limitations of this review mainly relate to underlying evidence, notably the paucity of comparative trials providing >12 mo of follow-up, and the inherent methodological and clinical shortcomings of existing trials. We acknowledge that this review is based on randomized controlled trials only; this is in keeping with established systematic review methods. Nonrandomized studies appear unlikely to have yielded evidence other than that of very low certainty [19]. Similarly, the focus of longer-term outcomes of >12 mo was informed by stakeholder input and in accordance with our a priori protocol. Lastly, given the paucity of studies, we were unable to perform any preplanned secondary analyses.
3.2.3. Strengths and weaknesses in relation to other studies, discussing important differences in results
A recent systematic review and network meta-analysis assessed the comparative efficacy and safety of new surgical treatments for BPH [20]. While including nine surgical treatment approaches, 109 trials, and 13 376 study participants, this review did not include the latest additions to the treatment armamentarium that were the focus of our review. Three relevant Cochrane reviews have been published, each focusing on single treatment modalities, namely, for PUL [21], Aquablation [22], and Rezūm [23]. For PUL with follow-up up to 24 mo, the review found that PUL may be less effective in relieving urinary symptoms but results in similar QoL. PUL may preserve ejaculation and have fewer unwanted effects on erections than TURP. The authors further stated that there was uncertainty or no evidence about serious unwanted side effects or the need for additional treatment after surgery [21]. For Aquablation and based on limited follow-up up to 12 mo, the authors found that the effect on urological symptoms and QoL was probably similar to that of TURP. There was major uncertainty as to major adverse events, and erectile and ejaculatory function [22]. For Rezūm, the Cochrane review found only a single, sham-controlled trial with follow-up of only 3 mo. It concluded that compared with sham, urological symptom scores and QoL appear to improve, but that the authors are very uncertain about the major adverse events. No assessment compared with TURP could be made.
A recent systematic review on PAE has also been published, with its main findings being that PAE is not as effective as established surgical therapies but has fewer side effects [24]. In contrast to ours, this review included both randomized and nonrandomized trials that the authors elected to pool indiscriminately, which we would caution against. It also rated the CoE as moderate for all patient-reported and functional outcomes, which represents a much more optimistic take than we think is warranted.
3.2.4. Meaning of the study: possible explanations and implications for clinicians and policymakers
Findings of this report have had a direct impact on clinical practice and patient care by informing an initial AUA guideline on the surgical treatment of male LUTS attributed to BPH [25] as well as a recent update [17]. Using an established framework that considers the strength of the evidence and the balance between benefits and harms [26], the panel developed three types of evidence-based recommendations (strong, moderate, and conditional) or, in the absence of sufficient evidence, provided guidance in the form of clinical principles or expert opinion. The panel made a moderate recommendation for PUL in men with a prostate volume of <80 g and verified the absence of a median lobe acknowledging a lesser degree in symptom reduction. It provided a conditional recommendation in men with particular interest in preserving erectile and ejaculatory function. Since there was no published evidence on Aquablation at the time of the initial guideline, this modality was not addressed initially. In the subsequent amendment, a conditional recommendation was made for patients with a prostate size of >30 but <80 g, presuming that they were fully informed about limited evidence about efficacy and retreatment rates [17]. Rezūm was given a conditional recommendation in both documents for men with a prostate <80 g in size with the disclaimer about limited evidence of its efficacy and long-term retreatment rates [17], [25]. Based on expert opinion, the panel recommended that PAE should not be used outside the context of a clinical trial [25]. In support, the panel cited serious safety concerns regarding radiation exposure, postembolization syndrome, vascular access, technical feasibility, and adverse events. This judgment has drew criticism by the interventional radiology community citing that major adverse events are rare, most adverse events are minor and self-limited, including postembolization syndrome, and there was no evidence to suggest that angiography increased the risk of malignancy in patients with BPH [27]. The authors further criticized the lack of interventional radiology representation. The Society of Interventional Radiology published its own guidance document [28] that describes PAE as “an acceptable minimal-invasive treatment option for appropriately selected men with BPH and moderate to severe LUTS.” Notably, this document did not follow an evidence-based guideline framework for developing these recommendations, nor was there any urology representation. Meanwhile, the National Institute for Health and Care Excellence in the UK found current evidence on the safety and efficacy of PAE to be adequate to support the use of the procedure, provided that patient selection is performed by a urologist and an interventional radiologist together, and the latter had specific training and expertise [29]. The 2019 amendment of the AUA guideline continues to reserve the use of PAE to clinical trials in order to generate better-quality evidence [17]. Guidelines of the European Association of Urology do not provide specific mention of any of these treatment modalities including PAE [30], [31].
3.2.4.1. Unanswered questions and future research
This study highlights the important shortcomings in the bodies of evidence for new treatment modalities for male LUTS, in particular the lack of an active comparator of Rezūm. Second, the short time horizon of many relevant trials of ≤12 mo appears to be inadequate to assess long-term treatment effects and retreatment rates. Third, there is inconsistent reporting of patient-important outcomes in the form of a core outcome set that all trials should report on and that is increasingly being developed in other areas of urology for conditions such as localized prostate cancer [32] and overactive bladder [33]. Fourth, methodological study limitations include unclear allocation concealment, lack of blinding that is usually feasible at least for outcome assessors, high rates of loss to follow-up, and small sample sizes paired with low event rates resulting in inadequately powered studies. Much of these issues have to do with the low evidentiary standards that the Food and Drug Administration (FDA) applies to “clear” these device-based approaches for clinical use, determining them as “substantially equivalent” to predicate devices legally marketed prior to May 1976 [34]. The device approval process in the European Union is distinct, yet similar with regard to lower evidentiary standards compared with drugs [35]. The Idea, Development, Exploration, Assessment and Long-term Follow-up (IDEAL) Group has developed guidance to better align the development and approval of drugs and devices, and could assist with future device development research in this arena [36]. Finally, we were unable to perform any predefined secondary analyses, for example, stratified by baseline symptom severity or prostate volume, which would be very informative to guide clinical practice; important prognostic variables should ideally prespecified for analyses in future trials of an adequate sample size.
4. Conclusions
The current best evidence on comparative effectiveness and harms of the newer treatments PUL, Rezūm, PAE, and Aquablation is limited to few trials with important methodological shortcomings. There is an imperative to raise evidentiary standards for new devices to guide treatment decision-making.
Author contributions: Philipp Dahm had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Wilt.
Acquisition of data: MacDonald, McKenzie, Jung.
Analysis and interpretation of data: MacDonald, Dahm, Wilt.
Drafting of the manuscript: Dahm, MacDonald.
Critical revision of the manuscript for important intellectual content: Wilt, Dahm.
Statistical analysis: MacDonald.
Obtaining funding: Wilt.
Administrative, technical, or material support: Greer.
Supervision: Wilt.
Other: None.
Financial disclosures: Philipp Dahm certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This is research project was funded by the American Urological Association Practice Guidelines Committee.
Associate Editor: Silvia Proietti
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.euros.2021.02.001.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Higgins J.P.T., Cochrane Collaboration . ed. 2. Wiley-Blackwell; Hoboken, NJ: 2020. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 2.Cochrane Collaboration . The Nordic Cochrane Center, The Cochrane Collaboration; Copenhagen, Denmark: 2008. Review Manager (Revman) [Google Scholar]
- 3.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 4.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canfield S.E., Dahm P. Rating the quality of evidence and the strength of recommendations using GRADE. World J Urol. 2011;29:311–317. doi: 10.1007/s00345-011-0667-2. [DOI] [PubMed] [Google Scholar]
- 6.Roehrborn C.G., Gange S.N., Shore N.D. The prostatic urethral lift for the treatment of lower urinary tract symptoms associated with prostate enlargement due to benign prostatic hyperplasia: the L.I.F.T. study. J Urol. 2013;190:2161–2167. doi: 10.1016/j.juro.2013.05.116. [DOI] [PubMed] [Google Scholar]
- 7.Gratzke C., Barber N., Speakman M.J. Prostatic urethral lift vs transurethral resection of the prostate: 2-year results of the BPH6 prospective, multicentre, randomized study. BJU Int. 2017;119:767–775. doi: 10.1111/bju.13714. [DOI] [PubMed] [Google Scholar]
- 8.Roehrborn C.G., Barkin J., Gange S.N. Five year results of the prospective randomized controlled prostatic urethral L.I.F.T. study. Can J Urol. 2017;24:8802–8813. [PubMed] [Google Scholar]
- 9.Sonksen J., Barber N.J., Speakman M.J. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol. 2015;68:643–652. doi: 10.1016/j.eururo.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Abt D., Hechelhammer L., Mullhaupt G. Comparison of prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: randomised, open label, non-inferiority trial. BMJ. 2018;361:k2338. doi: 10.1136/bmj.k2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carnevale F.C., Iscaife A., Yoshinaga E.M., Moreira A.M., Antunes A.A., Srougi M. Transurethral resection of the prostate (TURP) versus original and PErFecTED prostate artery embolization (PAE) due to benign prostatic hyperplasia (BPH): preliminary results of a single center, prospective, urodynamic-controlled analysis. Cardiovasc Intervent Radiol. 2016;39:44–52. doi: 10.1007/s00270-015-1202-4. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y.A., Huang Y., Zhang R. Benign prostatic hyperplasia: prostatic arterial embolization versus transurethral resection of the prostate—a prospective, randomized, and controlled clinical trial. Radiology. 2014;270:920–928. doi: 10.1148/radiol.13122803. [DOI] [PubMed] [Google Scholar]
- 13.Abt D., Mordasini L., Hechelhammer L., Kessler T.M., Schmid H.P., Engeler D.S. Prostatic artery embolization versus conventional TUR-P in the treatment of benign prostatic hyperplasia: protocol for a prospective randomized non-inferiority trial. BMC Urol. 2014;14:94. doi: 10.1186/1471-2490-14-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilling P.J., Barber N., Bidair M. Randomized controlled trial of Aquablation versus transurethral resection of the prostate in benign prostatic hyperplasia: one-year outcomes. Urology. 2019;125:169–173. doi: 10.1016/j.urology.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 15.McVary K.T., Gange S.N., Gittelman M.C. Minimally invasive prostate convective water vapor energy ablation: a multicenter, randomized, controlled study for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2016;195:1529–1538. doi: 10.1016/j.juro.2015.10.181. [DOI] [PubMed] [Google Scholar]
- 16.Roehrborn C.G., Gange S.N., Gittelman M.C. Convective thermal therapy: durable 2-year results of randomized controlled and prospective crossover studies for treatment of lower urinary tract symptoms due to benign prostatic hyperplasia. J Urol. 2017;197:1507–1516. doi: 10.1016/j.juro.2016.12.045. [DOI] [PubMed] [Google Scholar]
- 17.Foster H.E., Dahm P., Kohler T.S. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA Guideline Amendment 2019. J Urol. 2019;202:592–598. doi: 10.1097/JU.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 18.Foster H.E., Dahm P., Kohler T.S. Re: Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA Guideline Amendment 2019. J Urol. 2020;203:1219. doi: 10.1097/JU.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 19.Schunemann H.J., Tugwell P., Reeves B.C. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Res Synth Methods. 2013;4:49–62. doi: 10.1002/jrsm.1078. [DOI] [PubMed] [Google Scholar]
- 20.Huang S.W., Tsai C.Y., Tseng C.S. Comparative efficacy and safety of new surgical treatments for benign prostatic hyperplasia: systematic review and network meta-analysis. BMJ. 2019;367:l5919. doi: 10.1136/bmj.l5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung J.H., Reddy B., McCutcheon K.A. Prostatic urethral lift for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database Syst Rev. 2019;5 doi: 10.1002/14651858.CD012832.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang E.C., Jung J.H., Borofsky M., Kim M.H., Dahm P. Aquablation of the prostate for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database Syst Rev. 2019;2 doi: 10.1002/14651858.CD013143.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang T.W., Jung J.H., Hwang E.C., Borofsky M., Kim M.H., Dahm P. Convective radiofrequency water vapour thermal therapy for lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database Syst Rev. 2020;3 doi: 10.1002/14651858.CD013251.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zumstein V., Betschart P., Vetterlein M.W. Prostatic artery embolization versus standard surgical treatment for lower urinary tract symptoms secondary to benign prostatic hyperplasia: a systematic review and meta-analysis. Eur Urol Focus. 2019;5:1091–1100. doi: 10.1016/j.euf.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Foster H.E., Barry M.J., Dahm P. Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia: AUA guideline. J Urol. 2018;200:612–619. doi: 10.1016/j.juro.2018.05.048. [DOI] [PubMed] [Google Scholar]
- 26.Faraday M., Hubbard H., Kosiak B., Dmochowski R. Staying at the cutting edge: a review and analysis of evidence reporting and grading; the recommendations of the American Urological Association. BJU Int. 2009;104:294–297. doi: 10.1111/j.1464-410X.2009.08729.x. [DOI] [PubMed] [Google Scholar]
- 27.Bagla S., Piechowiak R., McClure T.D., Isaacson A. Re: Surgical management of lower urinary tract symptoms attributed to benign prostatic hyperplasia. H. E. Foster, M. J. Barry, P. Dahm, M. C. Gandhi, S. A. Kaplan, T. S. Kohler, L. B. Lerner, D. J. Lightner, J. K. Parsons, C. G. Roehrborn, C. Welliver, T. J. Wilt and K. T. McVary J Urol 2018; 200: 612–619. J Urol. 2019;201:400–403. doi: 10.1016/j.juro.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 28.McWilliams J.P., Bilhim T.A., Carnevale F.C. Society of Interventional Radiology multisociety consensus position statement on prostatic artery embolization for treatment of lower urinary tract symptoms attributed to benign prostatic hyperplasia: from the Society of Interventional Radiology, the Cardiovascular and Interventional Radiological Society of Europe, Societe Francaise de Radiologie, and the British Society of Interventional Radiology: endorsed by the Asia Pacific Society of Cardiovascular and Interventional Radiology, Canadian Association for Interventional Radiology, Chinese College of Interventionalists, Interventional Radiology Society of Australasia, Japanese Society of Interventional Radiology, and Korean Society of Interventional Radiology. J Vasc Intervent Radiol. 2019;30 doi: 10.1016/j.jvir.2019.02.013. 627–637.e1. [DOI] [PubMed] [Google Scholar]
- 29.National Institute for Health and Care Excellence (NICE) NICE guidance—prostate artery embolisation for lower urinary tract symptoms caused by benign prostatic hyperplasia: © NICE (2018) Prostate artery embolisation for lower urinary tract symptoms caused by benign prostatic hyperplasia. BJU Int. 2018;122:11–12. doi: 10.1111/bju.14404. [DOI] [PubMed] [Google Scholar]
- 30.Oelke M., Bachmann A., Descazeaud A. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol. 2013;64:118–140. doi: 10.1016/j.eururo.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Gravas S., Cornu J.N., Gacci M. Management of non-neurogenic male LUTS. Eur Assoc Urol. 2019 [Google Scholar]
- 32.MacLennan S., Bekema H.J., Williamson P.R. A core outcome set for localised prostate cancer effectiveness trials: protocol for a systematic review of the literature and stakeholder involvement through interviews and a Delphi survey. Trials. 2015;16:76. doi: 10.1186/s13063-015-0598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foust-Wright C., Wissig S., Stowell C. Development of a core set of outcome measures for OAB treatment. Int Urogynecol J. 2017;28:1785–1793. doi: 10.1007/s00192-017-3481-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tradewell M.B., Albersheim J., Dahm P. Use of the IDEAL framework in the urological literature: where are we in 2018? BJU Int. 2019;123:1078–1085. doi: 10.1111/bju.14676. [DOI] [PubMed] [Google Scholar]
- 35.Van Norman G.A. Drugs and devices: comparison of European and U.S. approval processes. JACC Basic Transl Sci. 2016;1:399–412. doi: 10.1016/j.jacbts.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sedrakyan A., Campbell B., Merino J.G., Kuntz R., Hirst A., McCulloch P. IDEAL-D: a rational framework for evaluating and regulating the use of medical devices. BMJ. 2016;353:i2372. doi: 10.1136/bmj.i2372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.