Abstract
Initial reports of a clinical response in patients treated with the radioligand [177Lu]-PSMA-617 for castration-resistant prostate cancer (CRPC) are promising, despite known inter- and intrapatient heterogeneity. In metastatic CRPC, we examined the association of baseline immunohistochemical (IHC) expression of prostate-specific membrane antigen (PSMA) in a single lesion and responsiveness to [177Lu]-PSMA-617 therapy, measured as the PSMA maximum standardized uptake value (SUVmax). Between 2015 and 2020, 19 patients with multiple metastases underwent single-lesion biopsy, [68Ga]-PSMA positron emission tomography (PET) imaging, and treatment with [177Lu]-PSMA-617. A monoclonal anti-PSMA antibody was used to semiquantitatively assess PSMA IHC in the biopsy specimen. Imaging evaluation of the biopsied single lesion and overall response was performed according to Positron Emission Tomography Response Criteria in Solid Tumors. The PSMA IHC histoscore correlated positively with pretreatment same-site PSMA SUVmax (rs = 0.6). Nine patients had imaging after three cycles of [177Lu]-PSMA-617 and were included in the lesion-specific analysis. Of these, five patients (55.6%) had an SUVmax response at the biopsy site, but three experienced overall progression. The histoscore was unable to predict the lesion-specific change in SUVmax (95% confidence interval [CI] −44.2 to 69.2) or PSA (95% CI−125.2 to 17.2). There was no correlation between single-lesion SUVmax and overall progression (rs = 0.1) on [68Ga]-PSMA PET imaging. Additional studies need to interrogate the clinical consequence of PSMA expression heterogeneity in metastases and the association with response to [177Lu]-PSMA-671.
Patient summary
Treatment with a radioactive binding molecule called [177Lu]-PSMA-617 for men with prostate cancer resistant to castration (CRPC) is showing promise. We investigated the association between the presence of PSMA protein in metastatic lesions at biopsy and response to [177Lu]-PSMA-617 among men with metastatic CRPC. We found that assessment of PSMA presence at biopsy is not a reliable predictor of response to [177Lu]-PSMA-617. Additional studies are needed to better determine which CRPC metastatic sites will respond to this therapy.
Keywords: Biomarker, Prostate cancer, Prostate-specific membrane antigen
Castration-resistant prostate cancer (CRPC) is a lethal disease. Prostate-specific membrane antigen (PSMA) is a particularly promising target for prostate cancer molecular imaging and therapy based on radiopharmaceuticals (eg, [68Ga]-PSMA and [18F]-PSMA). PSMA is a transmembrane receptor that functions as a folate hydrolase-carboxypeptidase that internalizes ligands on binding [1]. The level of PSMA protein expression, assessed via immunohistochemistry (IHC), correlates with parameters of prostate cancer aggressiveness, including Gleason score, propensity to metastasize, and development of castration resistance [2]. Numerous studies have shown high PSMA expression in CRPC [3]. Considering this and the generally limited amount of PSMA expression observed in benign tissues, targeting of PSMA in advanced prostate cancer has been aggressively pursued. Radionuclide therapy combining PSMA-targeted approaches with the β-emitter lutetium-177 have recently been proposed. While exciting responses have been observed in some cases, up to one-third of patients do not respond [2].
To better select patients for treatment with [177Lu]-labeled PSMA ligands, there is a need for predictive biomarkers of treatment response. Existing data suggest that platelet count, a regular need for analgesics, and the presence of visceral metastases, as well as hemoglobin, age, lactate dehydrogenase, and the [68Ga]-PSMA standardized uptake value (SUV) on positron emission tomography (PET), may serve this purpose to varying degrees [4], [5], [6]. The association of tumor PSMA IHC with response to [177Lu]-PSMA radioligand treatment is currently unknown.
After institutional review board approval (#1217/2020), we performed a retrospective, single-center analysis of 19 patients with multifocal CRPC metastases (Supplementary Fig. 1). Patients underwent [68Ga]-PSMA-HBED-CC PET, biopsy of a single metastatic lesion, and subsequent [177Lu]-PSMA-617 therapy at 4-wk intervals from 2015 to 2020. Clinicopathological data including longitudinal PSA values were collected from patient records (Table 1). A monoclonal anti-PSMA antibody (Epitomics, Burlingame, CA, USA; rabbit monoclonal antibody, 1:50 dilution) was used to semiquantitatively assess PSMA expression via IHC. Experienced genitourinary pathologists scored immunohistochemical results as the staining intensity (on a scale from 0 to 3) and the percentage of positively stained cells to generate a histoscore between 0 and 300 according to the following equation [7]:
Table 1.
Clinicopathological and imaging data for the nine patients who underwent two consecutive [68Ga]-PSMA-PET imaging studies
| Parameter | Patient ID |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 6 | 8 | 9 | 10 | 12 | 13 | 17 | 19 | |
| Chemotherapy | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes |
| NAAI | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| ADT | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Radical prostatectomy | Yes | No | Yes | Yes | No | Yes | Yes | Yes | Yes |
| Gleason score | NA | 6 | 8 | 9 | 9 | NA | 8 | 8 | 9 |
| Platelets (109 cells/l) | 76 | 296 | 178 | 183 | 197 | 248 | 232 | 199 | 298 |
| Baseline PSA (μg/l) | 911.8 | 42.7 | 162.8 | 29.3 | 1693 | 1521 | 684 | 25.2 | 482 |
| PSMA IHC (histoscore) | 200 | 270 | 150 | 300 | 280 | 280 | 280 | 300 | 280 |
| Metastatic site | OID | L1 | OIS | L1 | OIS | OIS | OIS | L3 | OIS |
| Baseline PSA (μg/l) | 911.8 | 42.7 | 162.8 | 29.3 | 1693 | 1521 | 684 | 25.2 | 482 |
| PSA on 2nd imaging (μg/l) | 520.9 | 16.8 | 128.9 | 34.4 | 1624 | 1527 | 1475 | 8.8 | 976 |
| Change in PSA (μg/l) | -390.9 | -25,9 | -33.9 | 5.1 | -69 | 6 | 791 | -16.4 | 494 |
| PSMA SUVmax before RLT | 12.5 | 21.8 | 14.9 | 32.4 | 26.6 | 7.9 | 2.3 | 48.7 | 24.8 |
| PSMA SUVmax after RLT | 11.3 | 12.7 | 8.5 | 24.1 | 17.6 | 14.1 | 6.6 | 28.1 | 16.7 |
| Change in SUVmax (%) | −10 | −42 | −43 | −26 | −34 | 78 | 187 | −42 | −33 |
| Overall [68Ga]-PSMA-PET assessment | PR | PR | PR | PD | PD | PR | PD | PD | PD |
PET = positron emission tomography; NA = not available; NAAI = new androgen-axis inhibitor; ADT = androgen deprivation therapy; PSA = prostate-specific antigen; IHC = immunohistochemistry; PSMA = prostate-specific membrane antigen; OID = os ilium dexter; L1 = lumbar vertebra 1; OIS = os ilium sinister; L3 = lumbar vertebra 3; SUV = standardized uptake value; RLT = radioligand therapy with [177Lu]-PSMA-617; PR = partial response; PD = progressive disease.
We first assessed the association between SUV on [68Ga]-PSMA PET imaging and IHC expression of PSMA from a single CRPC metastatic site before [177Lu]-PSMA-617 therapy. Univariate linear regression analysis was used to measure the potential association between baseline PSMA on IHC and the single-lesion change in PSMA SUVmax and the PSA decline. Correlation between the single-lesion PSMA SUVmax and the overall change in PSMA SUVmax was assessed using Spearman correlation. Follow-up time was defined from treatment initiation to the date of most recent assessment or death.
Lesion-specific changes in PSMA SUVmax were determined by an experienced nuclear medicine physician (S.R.) and classified as responsive (SUVmax ≤30%), progressive (SUVmax ≥30%), or stable (lesions not meeting the criteria for response or progression) [8]. [68Ga]-PSMA PET assessment was quantified according to Positron Emission Tomography Response Criteria in Solid Tumors (PERCIST) [8]. PSA response was defined as an absolute negative change in PSA from baseline to second imaging [9].
A PSMA histoscore could be determined for 12 of the 19 patients (median histoscore 280, interquartile range [IQR] 175–290). Baseline characteristics are shown in Supplementary Table 1. PSMA expression on IHC correlated with pretreatment same-site SUVmax (rs = 0.6). Of these 12 patients, nine underwent a second PSMA PET evaluation after three cycles of [177Lu]-PSMA-617 therapy and were included in the lesion-specific analysis. Clinicopathological and imaging data for these patients are shown in Table 1. Among the nine patients, the median number of previous systemic CRPC therapies was three (IQR 3–4) and median pretreatment PSA was 482 μg/l (IQR 42.7–911.8). The median pretreatment biopsy-site PSMA SUVmax was 21.8 (IQR 12.5–26.6). The median interval between the imaging studies was 5.2 mo (IQR 4.9–5.6). Median radioactivity of 22 505 MBq (IQR 18 220–44 801) was delivered during radionuclide therapy. Four patients (44.4%) died during follow-up at a median time of 8.2 months (IQR 6.0–11.4) after treatment initiation.
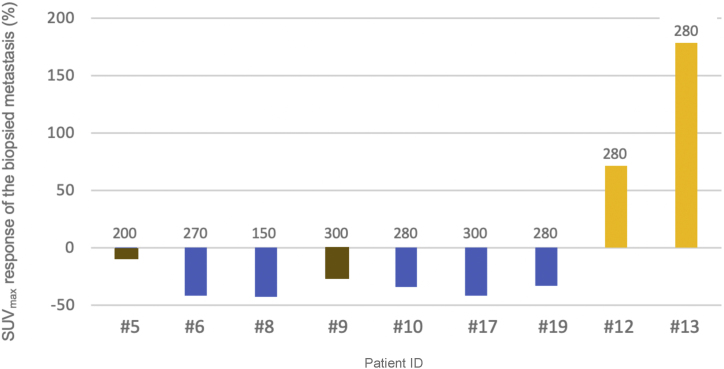
A single-lesion SUVmax response was observed in five of nine patients, while two had a stable lesion and two others experienced progression. Among the responders, the change in SUVmax for the biopsied lesion ranged from −33% to −43% on consecutive [68Ga]-PSMA PET imaging (Fig. 1). In the overall [68Ga]-PSMA PET assessment, five patients experienced progression according to PERCIST. Interestingly, three of these five patients had a single-lesion SUVmax response at the biopsy site. There was no correlation between single-lesion PSMA SUVmax and overall progression (rs = 0.1). A PSA decline was observed in four of the nine patients (44.4%). The PSMA histoscore was unable to predict the lesion-specific change in PSMA SUVmax (95% confidence interval [CI] −44.2 to 69.2; p = 0.6) or PSA decline (95% CI −125.2 to 17.2; p = 0.1) on univariate analysis. Supplementary Figure 2 shows PSMA IHC results for biopsied metastases as well as baseline and consecutive [68Ga]-PSMA PET images in three representative patients.
Fig. 1.
Percentage change in PSMA SUVmax in biopsied metastases after [177Lu]-PSMA-617 therapy for individual patients. Blue bars = lesion responded; brown bars = lesion stable; yellow bars = lesion progressed according to Positron Emission Tomography Response Criteria in Solid Tumors. The x-axis shows the individual patients with the number above the colums describing the PSMA protein expression assessed by immunohistochemistry of the investigated lesion at baseline.
PSMA = prostate-specific membrane antigen; SUVmax = maximum standardized uptake value on [68Ga]-PSMA positron emission tomography imaging.
For patients with prostate cancer bone lesions, we found that PSMA expression on IHC correlated with the SUVmax for the biopsied metastatic lesions at baseline (rs = 0.6). However, single-lesion PSMA expression on IHC was not an accurate predictor of response to [177Lu]-PSMA-617 therapy. Furthermore, we found that while PSMA avidity on imaging may substantially decrease in some metastatic lesions following therapy, new lesions can emerge during this time (Supplementary Fig. 3).
There are several potential explanations for our findings. These initial observations could be explained by heterogeneous PSMA expression both within and between specific tumor foci [10]. Furthermore, altered vascularity and tissue permeability, along with other factors, may alter radiopharmaceutical delivery to cancer cells. In addition, it is possible that some areas may possess features that render them resistant to β-emission–based therapy. Mixed therapeutic responses could also be reflective of tissue-specific effects of the therapy [10].
The current study has several limitations, including its retrospective approach and limited sample size. These limitations notwithstanding, such data are rare, given that biopsies of PSMA-avid CRPC metastatic lesions are not commonly performed and [177Lu]-PSMA-617 is a relatively new therapy. Thus, our study provides a unique preliminary insight into the responsiveness of metastatic prostate cancer lesions to [177Lu]-PSMA-617 therapy according to measurable pretreatment factors (ie, PSMA expression on IHC and SUVmax on [68Ga]-PSMA PET imaging). To the best of our knowledge, this is the first study to assess single-lesion PSMA IHC and response to [177Lu]-PSMA-617 therapy. These findings should motivate prospective studies to optimize patient selection and improve the efficacy of [177Lu]-PSMA-617 therapy for men with CRPC.
Author contributions: Ganesh S. Palapattu had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Stangl-Kremser, Salami, Zaslavsky, Palapattu, Rasul.
Acquisition of data: Hassler, Pozo-Salido, Steinbach.
Analysis and interpretation of data: Stangl-Kremser, Mazal.
Drafting of the manuscript: Stangl-Kremser, Zaslavsky, Tosoian.
Critical revision of the manuscript for important intellectual content: Hacker, Haug, Mitterhauser, Kramer, Shariat, Udager.
Statistical analysis: Stangl-Kremser, Tosoian.
Obtaining funding: None.
Administrative, technical, or material support: Rasul, Mitterhauser, Comperat, Mazal, Kain.
Supervision: Palapattu.
Other: None.
Financial disclosures: Ganesh S. Palapattu certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.euros.2021.06.007.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Liu H., Rajasekaran A.K., Moy P. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998;58:4055–4060. [PubMed] [Google Scholar]
- 2.Emmett L., Willowson K., Violet J., Shin J., Blanksby A., Lee J. Lutetium 177 PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci. 2017;64:52–60. doi: 10.1002/jmrs.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright G.L., Grob B.M., Haley C. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–334. doi: 10.1016/s0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 4.Heck M.M., Tauber R., Schwaiger S. Treatment outcome, toxicity, and predictive factors for radioligand therapy with 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur Urol. 2019;75:920–926. doi: 10.1016/j.eururo.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Emmett L., Crumbaker M., Ho B. Results of a prospective phase 2 pilot trial of 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clin Genitourin Cancer. 2019;17:15–22. doi: 10.1016/j.clgc.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Rasul S., Hartenbach M., Wollenweber T. Prediction of response and survival after standardized treatment with 7400 MBq 177Lu-PSMA-617 every 4 weeks in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48:1650–1657. doi: 10.1007/s00259-020-05082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stenger M. Calculating H-score. The ASCO Post. April 10, 2015. https://ascopost.com/issues/april-10-2015/calculating-h-score/.
- 8.O J.H., Lodge M.A., Wahl R.L. Practical PERCIST: a simplified guide to PET Response Criteria in Solid Tumors 1.0. Radiology. 2016;280:576–584. doi: 10.1148/radiol.2016142043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scher H.I., Morris M.J., Stadler W.M. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paschalis A., Sheehan B., Riisnaes R. Prostate-specific membrane antigen heterogeneity and DNA repair defects in prostate cancer. Eur Urol. 2019;76:469–478. doi: 10.1016/j.eururo.2019.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.