Abstract
Background
Radiotherapy to the prostate (RTp) prolongs survival for patients with low-volume, newly diagnosed metastatic prostate cancer (ndmPC).
Objective
to evaluate whether cytoreductive radical prostatectomy (cRP) is equally beneficial as RTp in low-volume ndmPC.
Design, setting, and participants
A multicenter prospective registry was established in 2014 to observe patients with ndmPC. Eligible patients were offered cRP or RTp. For this study we selected only patients with low-volume ndmPC (n = 109). Of these, 48, 26, and 35 patients underwent cRP, RTp, and no local therapy (NLT), respectively. Median follow-up was 32 mo (interquartile range 16–49).
Intervention
cRP was compared with RTp and NLT.
Outcome measurements and statistical analysis
Overall survival (OS), cancer-specific survival (CSS), and local event–free survival (LEFS) were calculated using the Kaplan-Meier method. Factors prognostic for OS were identified using univariate and multivariate Cox regression analysis.
Results and limitations
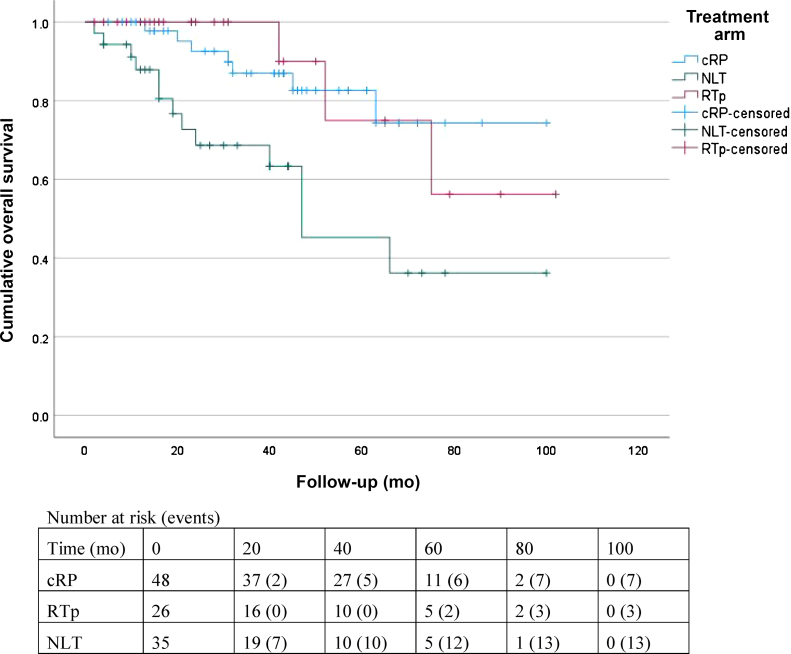
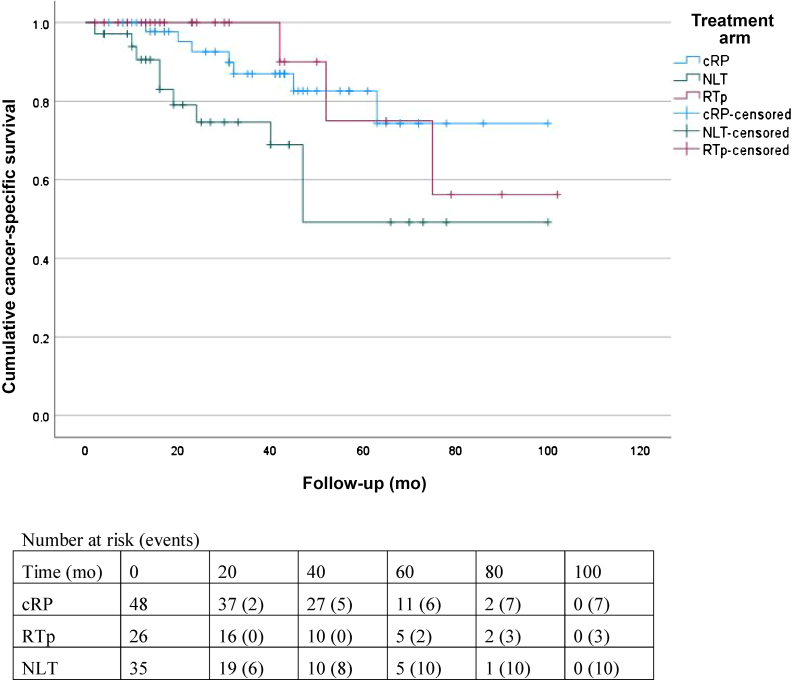
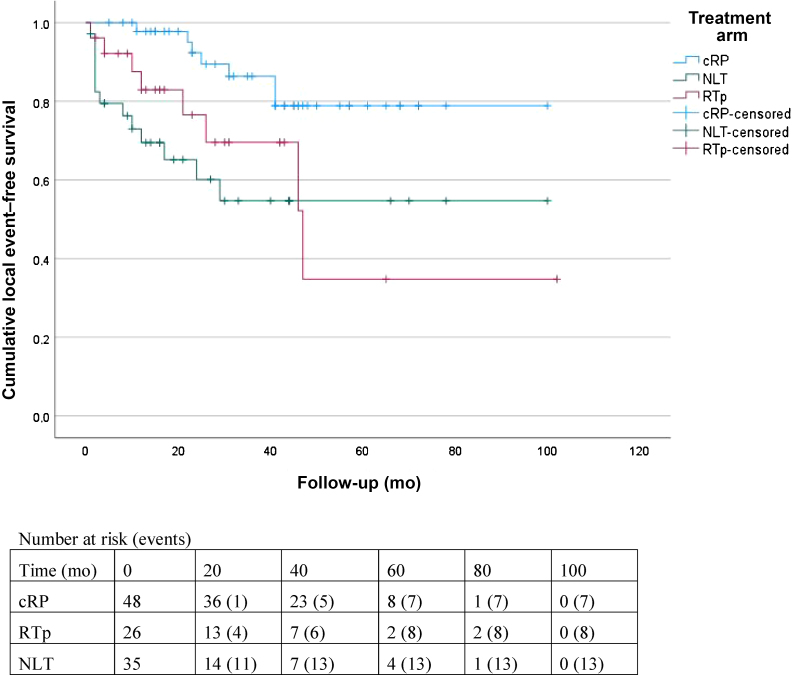
The 2-yr OS was 93%, 100%, and 69%, and 2-yr CSS was 93%, 100%, and 75% for cRP, RTp, and NLT, respectively. The cRP and RTp groups had better OS compared to NLT and there was no significant difference between cRP and RTp. The 2-yr LEFS was 92%, 77%, and 60% for cRP, RTp, and NLT, respectively. The cRP group had better LEFS compared to RTp and NLT, and there was no significant difference between RTp and NLT. Advanced tumor stage, Eastern Cooperative Oncology Group performance status ≥2, and NLT were negative prognostic factors for OS. The main limitation is selection of fitter patients with less advanced tumors for cRP and the small sample size.
Conclusions
For selected patients with low-volume ndmPC, cRP is able to achieve similar OS and CSS to RTp. cRP is effective in preventing local events due to disease progression.
Patient summary
Patients with a low volume of newly diagnosed prostate cancer that has spread beyond the prostate gland might benefit from removal of the prostate, which we found was as effective as radiotherapy to the prostate in prolonging survival. Removal of the prostate is effective in preventing urinary problems caused by cancer progression.
Keywords: Prostate cancer, Metastasis, Radical prostatectomy, Prostate, Androgen deprivation therapy
Take Home Message
In selected patients, cytoreductive radical prostatectomy is able to achieve similar overall survival and cancer-specific survival to radiotherapy.
1. Introduction
Patients with newly diagnosed metastatic prostate cancer (ndmPC) are at high risk of succumbing to their disease [1]. Androgen deprivation therapy (ADT) is the cornerstone of systemic treatment in ndmPC [2]. Unfortunately, resistance to ADT will develop after 11–22 mo after which the disease stage is referred to as metastatic castrate-refractory prostate cancer (mCRPC) [1], [3], [4], [5]. Several systemic treatments have been introduced to improve survival in mCRPC [2]. It has been shown that initiation of some of these systemic treatments for ndmPC improves survival. These systemic treatments include docetaxel [5] and the androgen receptor–targeted agents abiraterone acetate [6], enzalutamide [7], and apalutamide [4]. Retrospective registries have suggested a survival benefit from addition of local therapy to the prostate in terms of cytoreductive radical prostatectomy (cRP) or radiotherapy to the prostate (RTp) [8], [9], [10]. In low-volume ndmPC, RTp improved survival in a prospective randomized trial [11]. Both cRP and RTp are effective in curing localized prostate cancer and are recommended in this setting [12]. As cRP and RTp are equally effective in this setting [13], the question arises as to whether cRP is effective in the treatment of low-volume ndmPC as well. The Local Treatment of Metastatic Prostate Cancer (LoMP) registry was initiated to prospectively observe the disease course for patients with ndmPC and to explore the potential benefit of local treatment (LT; cRP or RTp) in this setting. We hypothesized that cRP is equally as effective as RTp in prolonging survival in low-volume ndmPC.
2. Patients and methods
Starting in May 2014, patients with ndmPC were asked for their consent to participate in the multicenter LoMP registry (ClinicalTrials.gov NCT02138721). In this registry, LT involving either cRP or external-beam intensity-modulated RTp in addition to the contemporary standard of care (SOC) was offered. cRP was proposed for patients in whom the prostate was deemed resectable (stage ≤T3) as assessed via digital rectal examination and prostate magnetic resonance imaging. LT was initiated within 3 mo of diagnosis. The Supplementary material includes a short description of both LT modalities. The contemporary SOC was in accordance with the European Association of Urology guidelines and every decision on therapy initiation or switch was made after discussion at a multidisciplinary tumor board. Docetaxel was offered to fit patients with ndmPC starting from August 2015. Abiraterone acetate became available for high-risk ndmPC in November 2017 and apalutamide in February 2020. Starting from 2019, patients with low-volume ndmPC were offered RTp as SOC if cRP was not possible or was refused by the patient. Further treatment lines were again initiated according to contemporary SOC.
Assessment of ndmPC was via conventional imaging (thoracoabdominal computed tomography [CT] and a bone scan). Additional imaging or biopsy of a metastatic lesion was performed at the discretion of the treating physician. For this study, patients with high-volume disease were excluded, leaving only patients with low-volume disease for evaluation. High-volume disease was defined via conventional imaging as the presence of visceral metastasis and/or four or more bone lesions, with one or more lesion beyond the vertebral bodies and pelvis [5].
Patients with low-volume ndmPC were divided into three groups:
-
1
cRP group: the surgical technique for cRP was as previously described [14].
-
2
RTp group: the clinical target volume for external-beam intensity-modulated RTp included at least the prostate and seminal vesicles [15].
-
3
No LT (NLT) group: this group also included patients who received prostate surgery (transurethral resection of the prostate) or hypofractionated RTp because of local disease progression at a later stage in the disease. No patients in this group received LT as described for the cRP and RTp groups.
The follow-up schedule was set by the treating physician in accordance with the contemporary SOC. Dates for disease progression and the initiation and cessation dates for treatments related to prostate cancer were registered. Follow-up continued until death and the cause of death was noted. The data cutoff was November 16, 2020. Local events (LEs) were defined as any complication related to local disease progression after initiation of ADT requiring invasive treatment (including catheterization) or any invasive treatment of the urinary tract for a complication of cRP or RTp. The primary endpoint was overall survival (OS). Secondary endpoints were cancer-specific survival (CSS) and LE-free survival (LEFS).
All patients provided written informed consent and the study was approved by the local ethics committee.
2.1. Statistical analysis
Descriptive statistics were used to evaluate the study population. Categorical data are provided as the frequency and proportion. All continuous variables were non-normally distributed according to a Shapiro-Wilk test and are reported as the median with interquartile range (IQR). Categorical variables were compared among groups using Fisher’s exact test. Continuous variables were compared with the Mann-Whitney U test. Time-to-event outcomes were evaluated via the Kaplan-Meier method and groups were compared with calculation of the hazard ratio (HR) and 95% confidence interval (CI). Predictors for OS were evaluated via univariate and backward Wald multivariate Cox regression analysis, with pairwise comparisons between groups.
3. Results
The LoMP registry contains 215 patients with ndmPC, of whom 109 (50.7%) with low-volume disease comprise the present study population (Table 1). cRP, RTp, and NLT were administered in 48 (44%), 26 (24.9%), and 35 (32.1%) patients, respectively. Patients in the NLT group (n = 35) either opted not to undergo LT because of personal preference despite the option of cRP or RTp (n = 10, 28.6%), had an unresectable tumor (n = 6, 17.1%), or were unfit for surgery (n = 19, 53.4%). The median age of the entire cohort was 69 yr (IQR 60–76), with patients in the cRP group significantly younger than those in the RTp or NLT group (Table 1). The median prostate-specific antigen (PSA) at diagnosis was 27 ng/ml (IQR 11–75). Patients in the NLT group had significantly higher PSA (median 47 ng/ml, IQR 17–156) compared to those who underwent cRP (median 19 ng/ml, IQR 11–42; p = 0.008). Patients in the cRP group were more frequently diagnosed with organ-confined disease (stage T1–2) than patients in the NLT group (35.4% vs 11.4%; p = 0.02). In addition, only one patient (2.1%) who underwent cRP had a poor Eastern Cooperative Oncology Group performance score (ECOG PS 2–3), compared to six patients (17.1%) in the NLT group (p = 0.038). There were no significant differences in age, PSA, tumor stage, or ECOG PS between the RTp and NLT groups. International Society of Urological Pathology grade 4 and 5 tumors were diagnosed in 23.9% and 54.1% of cases, respectively, with no significant differences among the three groups. There were also no significant differences in regional node stage or site of metastasis among the groups. Except for the type of initial systemic treatment and age, there were no significant differences between the cRP and RTp groups. Median follow-up for the entire cohort was 32 m (IQR 16–49), with significantly longer follow-up for cRP patients.
Table 1.
Patient and tumor characteristics
| All | cRP | RTp | NLT |
p value |
|||
|---|---|---|---|---|---|---|---|
| cRP vs NLT | RTp vs NLT | cRP vs RTp | |||||
| Patients (n) | 109 | 48 | 26 | 35 | |||
| Median age, yr (IQR) | 69 (60–76) | 64 (59–72) | 70 (61–76) | 74 (69–84) | <0.001 | 0.121 | 0.023 |
| Median PSA, ng/ml (IQR) | 27 (11–75) | 19 (11–42) | 40 (15–67) | 47 (17–156) | 0.008 | 0.335 | 0.162 |
| Median follow-up, mo (IQR) | 32 (16–49) | 42 (24–57) | 26 (14–51) | 24 (12–44) | 0.018 | 0.577 | 0.116 |
| ISUP tumor grade group, n (%) a | |||||||
| Grade group 1 | 4 (3.7) | 1 (2.1) | 0 | 3 (9.4) | 0.318 | 0.189 | 0.935 |
| Grade group 2 | 6 (5.5) | 3 (6.3) | 1 (3.8) | 2 (6.3) | |||
| Grade group 3 | 11 (10.1) | 6 (12.5) | 2 (7.7) | 3 (9.4) | |||
| Grade group 4 | 26 (23.9) | 10 (20.8) | 5 (19.2) | 11 (34.4) | |||
| Grade group 5 | 59 (54.1) | 28 (58.3) | 18 (69.2) | 13 (40.6) | |||
| Tumor stage, n (%) | |||||||
| Organ-confined (T1–2) | 26 (23.9) | 17 (35.4) | 5 (19.2) | 4 (11.4) | 0.02 | 0.477 | 0.187 |
| Non–organ-confined (T3–4) | 83 (76.1) | 31 (64.6) | 21 (80.8) | 31 (88.6) | |||
| Node stage, n (%) | |||||||
| N0 | 30 (27.2) | 15 (31.3) | 4 (15.4) | 11 (31.4) | 1 | 0.23 | 0.17 |
| N1 | 79 (72.5) | 33 (68.8) | 22 (84.6) | 24 (68.6) | |||
| M stage, n (%) | |||||||
| Non-PLNs only (M1a) | 42 (38.5) | 23 (47.9) | 9 (34.6) | 10 (28.6) | 0.112 | 0.781 | 0.33 |
| Bone (M1b) | 67 (61.5) | 25 (52.1) | 17 (65.4) | 25 (71.4) | |||
| ECOG PS, n (%) | |||||||
| ECOG 0–1 | 101 (92.7) | 47 (97.9) | 25 (96.2) | 29 (82.9) | 0.038 | 0.222 | 1 |
| ECOG 2–3 | 8 (7.3) | 1 (2.1) | 1 (3.8) | 6 (17.1) | |||
| Initial systemic treatment, n (%) | |||||||
| Delayed ADT | 13 (11.9) | 8 (16.7) | 0 (0) | 5 (14.3) | 0.631 | 0.118 | 0.037 |
| Immediate ADT | 63 (57.8) | 29 (60.4) | 16 (61.5) | 18 (51.4) | |||
| Immediate ADT + docetaxel | 16 (14.7) | 7 (14.6) | 3 (11.5) | 6 (17.1) | |||
| Immediate ADT + AA | 14 (12.8) | 4 (8.3) | 4 (15.4) | 6 (17.1) | |||
| Immediate ADT + other CTx | 1 (0.9) | 0 (0) | 1 (3.8) | 0 (0) | |||
| Immediate ADT + apalutamide | 2 (1.8) | 0 (0) | 2 (7.7) | 0 (0) | |||
cRP = cytoreductive radical prostatectomy; RTp = radiotherapy to the prostate; NLT = no local treatment; IQR = interquartile range; PSA = prostate-specific antigen; ISUP = International Society of Urological Pathology; ECOG PS = Eastern Cooperative Oncology Group performance status; PLNs = pelvic lymph nodes; ADT = androgen deprivation therapy; CTx = chemotherapy.
Bold values signify that statistical significance has been reached.
Missing data for three patients.
Death was reported for seven (14.6%), three (11.5%), and 13 (37.1%) patients in the cRP, RTp, and NLT groups, respectively. Three patients in the NLT group died from other causes, while the remaining patients died from prostate cancer. The 2-yr OS (± standard error) for the entire cohort was 87 ± 4%. The 2-yr OS was 93 ± 4% for cRP versus 69 ± 9% for NLT (HR 0.28, 95% CI 0.11–0.71; p = 0.007; Fig. 1). The RTp group had 2-yr OS of 100% ± 0%, which was significantly better than for NLT (HR 0.26, 95% CI 0.07–0.91; p = 0.035). There was no significant difference in 2-yr OS between cRP and RTp (HR 1.08, 95% CI 0.27–4.29; p = 0.9; Table 2). The 2-yr CSS was 89 ± 4% for the entire cohort, 93 ± 4% for the cRP group, 100 ± 0% for the RTp group, and 75 ± 8% for the NLT group (Fig. 2). Patients undergoing cRP had significantly better 2-yr CSS compared to the NLT group (HR 0.36, 95% CI 0.14–0.94; p = 0.037) and comparable 2-yr CSS to patients undergoing RTp (HR 1.08, 95% CI 0.27–4.29; p = 0.9; Table 2).
Fig. 1.
Kaplan-Meier curves for overall survival.
cRP = cytoreductive radical prostatectomy; RTp = radiotherapy to the prostate; NLT = no local treatment.
Table 2.
Oncological outcomes in terms of OS, CSS, and LEFS
| All | cRP | RTp | NLT | cRP vs NLT | RTp vs NLT | cRP vs RTp | |
|---|---|---|---|---|---|---|---|
| Patients (n) | 109 | 48 | 26 | 35 | |||
| 2-yr OS, % (SE) | 87 (4) | 93 (4) | 100 (0) | 69 (9) | |||
| HR (95% CI) | 0.28 (0.11–0.71) | 0.26 (0.07–0.91) | 1.08 (0.27–4.29) | ||||
| p value | 0.007 | 0.035 | 0.912 | ||||
| 2-yr CSS, % (SE) | 89 (4) | 93 (4) | 100 (0) | 75 (8) | |||
| HR (95% CI) | 0.36 (0.14–0.94) | 0.33 (0.09–1.20) | 1.08 (0.27–4.29) | ||||
| p value | 0.037 | 0.091 | 0.912 | ||||
| 2-yr LEFS, % (SE) | 79 (4) | 92 (4) | 77 (10) | 60 (9) | |||
| HR (95% CI) | 0.25 (0.10–0.64) | 0.74 (0.31–1.78) | 0.31 (0.11–0.86) | ||||
| p value | 0.004 | 0.501 | 0.024 |
OS = overall survival; CSS = cancer-specific survival; LEFS = local event–free survival; cRP = cytoreductive radical prostatectomy; RTp = radiotherapy to the prostate; NLT = no local treatment; HR = hazard ratio; CI = confidence interval; SE = standard error.
Bold values signify that statistical significance has been reached.
Fig. 2.
Kaplan-Meier curves for cancer-specific survival.
cRP = cytoreductive radical prostatectomy; RTp = radiotherapy to the prostate; NLT = no local treatment.
Seven (14.6%) cRP, eight (30.8%) RTp, and 13 (37.1%) NLT patients experienced an LE after initiation of systemic treatment (Table 3). The estimated 2-yr LEFS was 79 ± 4% for the entire cohort, 92 ± 4% for the cRP group, 77 ± 10% for the RTp group, and 60 ± 9% for the NLT group (Table 2). Patients who underwent cRP had a significantly lower LE risk compared to those who underwent RTp (HR 0.31, 95% CI 0.11–0.86; p = 0.024) and those with NLT (HR 0.25, 95% CI 0.10–0.64; p = 0.004). The LE risk was not significantly lower for RTp compared to NLT (HR 0.74, 95% CI 0.31–1.78; p = 0.5; Fig. 3).
Table 3.
Local events observed by type
| Patients, n (%) |
||||
|---|---|---|---|---|
| All | cRP | RTp | NLT | |
| Patients | 109 | 48 | 26 | 35 |
| No local event | 81 (74.3) | 41 (85.4) | 18 (69.2) | 22 (62.9) |
| UO requiring JJ stent or nephrostomy | 4 (3.7) | 1 (2.1) | 2 (7.7) | 1 (2.9) |
| UO requiring JJ stent + urinary retention requiring CIC | 1 (0.9) | 0 (0) | 0 (0) | 1 (2.9) |
| Urinary retention | 16 (14.7) | 1 (2.1) | 5 (19.2) | 10 (28.6) |
| Need for temporary transurethral catheter | 9 | 0 | 3 | 6 |
| Need for CIC | 3 | 1 | 0 | 2 |
| Need for a permanent suprapubic catheter | 3 | 0 | 1 | 2 |
| Need for transurethral resection of the prostate | 1 | 0 | 1 | 0 |
| Hematuria requiring cystocoagulation | 1 (0.9) | 0 (0) | 0 (0) | 1 (2.9) |
| Urinary incontinence requiring AUS/male | 2 (1.8) | 2 (4.2) | 0 (0) | 0 (0) |
| Erectile dysfunction requiring a penile prosthesis | 1 (0.9) | 1 (2.1) | 0 (0) | 0 (0) |
| Urethral stricture requiring an internal urethrotomy | 3 (2.8) | 2 (4.2) | 1 (3.8) | 0 (0) |
cRP = cytoreductive radical prostatectomy; RTp = radiotherapy to the prostate; NLT = no local treatment; UO = ureteric obstruction; CIC = clean intermittent catheterization; AUS = artificial urinary sphincter.
Fig. 3.
Kaplan-Meier curves for local event–free survival.
cRP = cytoreductive radical prostatectomy; RTp = radiotherapy to the prostate; NLT = no local treatment.
In the entire cohort, factors prognostic for OS in univariate analysis were non–organ-confined tumor (HR 4.5, 95% CI 1.03–18.81; p = 0.045), ECOG PS ≥2 (HR 7.09, 95% CI 2.74–18.36; p < 0.001), and LT (HR 0.27, 95% CI 0.12–0.63; p = 0.002). In multivariate analysis, ECOG PS ≥2 (HR 4.85, 95% CI 1.78–13.21; p = 0.002), and LT (HR 0.36, 95% CI 0.15–0.85; p = 0.02) remained significant prognostic factors (Table 4). For comparison of patients who underwent NLT versus cRP, non–organ-confined tumor (HR 4.69, 95% CI 1.08–20.35; p = 0.039), ECOG PS ≥2 (HR 6.44, 95% CI 2.44–17.02; p < 0.001), and cRP (HR 0.28, 95% CI 0.11–0.71; p = 0.007) were factors prognostic for OS. In multivariate analysis, ECOG PS ≥2 (HR 4.74, 95% CI 1.73–12.97; p = 0.002) and cRP (HR 0.36, 95% CI 0.14–0.94; p = 0.037) remained significant. For comparison of patients who underwent NLT versus RTp, ECOG PS ≥2 (HR 9.87, 95% CI 3.28–29.71; p < 0.001) and RTp (HR 0.26, 95% CI 0.07–0.93; p = 0.037) were prognostic for OS in univariate analysis, but only ECOG PS ≥2 remained significant in multivariate analysis (HR 7.08, 95% CI 2.21–22.72; p = 0.001).
Table 4.
Univariate and multivariate Cox regression analyses to identify factors prognostic for overall survival
| Univariate |
Multivariate |
||||
|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | ||
| Age in years | 1.03 (0.99–1.07) | 0.168 | |||
| PSA in ng/ml | 1 (0.99–1.01) | 0.638 | |||
| Tumor grade | 1.92 (0.94–3.92) | 0.074 | |||
| Tumor stage (non-OC vs OC) | 4.5 (1.03–18.81) | 0.045 | 3.2 (0.73–14.09) | 0.123 | |
| Nodal stage (N1 vs N0) | 1.83 (0.62–5.39) | 0.272 | |||
| Metastatic site (M1b vs M1a) | 1.47 (0.62–3.47) | 0.383 | |||
| ECOG PS (≥2 vs 0–1) | 7.09 (2.74–18.36) | <0.001 | 4.85 (1.78–13.22) | 0.002 | |
| Initial STX (ADT alone a vs ADT + STx) | 1.97 (0.75–5.19) | 0.17 | |||
| Local treatment (cRP or RTp vs NLT) | 0.27 (0.12–0.63) | 0.002 | 0.36 (0.15–0.85) | 0.021 | |
| cRP and NLT groups | |||||
| Age in years | 1.02 (0.98–1.07) | 0.307 | |||
| PSA in ng/ml | 1 (0.99–1.01) | 0.637 | |||
| Tumor grade | 1.77 (0.86–3.65) | 0.12 | |||
| Tumor stage (non-OC vs OC) | 4.69 (1.08–20.35) | 0.039 | 2.91 | 0.64–13.31 | 0.168 |
| Nodal stage (N1 vs N0) | 2.49 (0.73–8.5) | 0.146 | |||
| Metastatic site (M1b vs M1a) | 1.96 (0.75–5.1) | 0.171 | |||
| ECOG PS (≥2 vs 0–1) | 6.44 (2.44–17.02) | <0.001 | 4.74 | 1.73–12.97 | 0.002 |
| Initial STX (ADT alone a vs ADT + STx) | 2.28 (0.86–6.05) | 0.098 | |||
| Local treatment (cRP or RTp vs NLT) | 0.28 (0.11–0.71) | 0.007 | 0.36 | 0.14–0.94 | 0.037 |
| RTp and NLT groups | |||||
| Age in years | 1.03 (0.97–1.08) | 0.33 | |||
| PSA in ng/ml | 1 (0.99–1.01) | 0.459 | |||
| Tumor grade | 1.82 (0.82–4.01) | 0.141 | |||
| Tumor stage (non-OC vs OC) | 26.5 (0.08–8735) | 0.268 | |||
| Nodal stage (N1 vs N0) | 1 (0.32–3.12) | 0.998 | |||
| Metastasis stage (M1b vs M1a) | 1.03 (0.35–2.98) | 0.962 | |||
| ECOG PS (≥2 vs 0–1) | 9.87 (3.28–29.71) | <0.001 | 7.08 (2.21–22.72) | 0.001 | |
| Initial STX (ADT alone a vs ADT + STx) | 1.99 (0.55–7.17) | 0.294 | |||
| Local treatment (cRP or RTp vs NLT) | 0.26 (0.07–0.93) | 0.037 | 0.42 (0.11–1.62) | 0.206 | |
cRP = cytoreductive radical prostatectomy; RTp = radiotherapy to the prostate; NLT = no local treatment; HR = hazard ratio; CI = confidence interval; PSA = prostate-specific antigen; OC = organ-confined; ECOG PS = Eastern Cooperative Oncology Group performance status; STx = systemic treatment; ADT = androgen deprivation therapy.
Either immediate or delayed ADT.
4. Discussion
Treatment of the primary tumor has been established in the metastatic setting for several cancer as it improves OS and might palliate local symptoms [16], [17]. It has been hypothesized that a reduction in tumor volume improves response to systemic treatment [18] and that control of the primary tumor slows down metastatic spread and disease progression [19].
In prostate cancer, an analysis of the Surveillance, Epidemiology and End Results (SEER) database showed significantly better 5-yr OS of 67% with LT (cRP or prostate brachytherapy) versus 23% with NLT [8]. An analysis using the National Cancer Data Base (NCDB) also showed a survival advantage for LT (cRP, brachytherapy, or external-beam RT) compared to NLT [10] with 3-yr OS of 69% vs 54% (p < 0.001). These findings are in line with the present analysis, which also demonstrated a survival benefit in favor of LT. An important observation for the SEER and NCDB databases was that younger and fitter patients with lower PSA and lower tumor burden were selected for LT. Therefore, the question remains whether the survival benefit is due to this selection bias or whether there is a true survival benefit linked to LT. In our series, LT was a favorable prognostic factor for OS in both univariate and multivariate analysis, with a relative reduction in mortality of 64% for the groups undergoing LT (cRP or RTp). However, when interpreting these results the important selection bias of our study needs to be taken into account. It is possible that any difference in survival would be less obvious in randomized series.
The SEER and NCDB studies were retrospective studies. More recently, RTp has been evaluated in two randomized controlled trials (HORRAD [20] and STAMPEDE [11]). In evaluating patients with low- or high-volume disease, neither the HORRAD nor the STAMPEDE trial was able to demonstrate a gain in OS in favor of RTp. However, patients in the HORRAD trial with fewer than five bone metastases (as a surrogate for low-volume disease) had a 32% lower risk of death (HR 0.68, 95% CI 0.42–1.10) with RTp [20]. More robust data are provided by the STAMPEDE trial, for which a prespecified subanalysis for low-volume disease did demonstrate significantly better OS for patients undergoing RTp compared to NLT (HR 0.68, 95% CI 0.52–0.90) [11]. Our data corroborate these findings, as we also observed a significant survival benefit for low-volume disease in favor of RTp (HR 0.26, 95% CI 0.07–0.91). This further strengthens the current strong recommendation to offer RTp in addition to ADT for patients with low-volume ndmPC [2].
As both cRP and RTp are equally effective treatment options for nonmetastatic prostate cancer [13], the question arises as to whether cRP would be as beneficial as RTp in low-volume ndmPC as well. The feasibility and safety of cRP in newly diagnosed HSPC have previously been demonstrated [14], [21]. Nevertheless, cRP might provoke substantial harm in some patients and at present cRP is only recommended for ndmPC within the framework of a clinical trial [2]. Several prospective studies of cRP in this setting are currently ongoing, each of which has its own peculiarities [22]. Knipper et al [23] evaluated cRP outcomes for patients corresponding to the eligibility criteria for STAMPEDE arm H in a prospective, nonrandomized registry. As seen in Kaplan-Meier analyses for this cohort, 2-yr OS was similar to what we found in our patient group (3-yr OS 91%), suggesting good local and distant disease control.
Retrospective studies evaluating cRP have previously been published. A SEER-Medicare analysis observed 3-yr OS of 73% for cRP versus 34% for NLT (HR 0.43, 95% CI 0.26–0.72), but the study also included patients with high-volume disease, was not matched, and involved an important selection bias (see below) [9]. Heidenreich et al [24] evaluated the potential benefit of cRP in addition to SOC for selected patients with a maximum of three osseous metastases, representing a study population more comparable with the low-volume ndmPC in our series. In this case-control study, patients were matched for age, performance status, and tumor stage and grade. After median follow-up of approximately 3 yr, OS was 91% for cRP versus 79% for NLT (p = 0.048). This 3-yr OS is in line with the 87% rate in our series. The 3-yr OS of 69% for NLT in our series is somewhat poorer compared to the cohort of Heidenreich et al [24]. However, our NLT cohort is not matched to the cRP cohort and these patients have lower ECOG PS, older age, and higher PSA, and present more frequently with a non–organ-confined tumor. Without matching, selection bias is nearly inevitable, as only fit patients whose tumor is deemed resectable are candidates for cRP, as clearly demonstrated in our series. The same observation was made in the SEER-Medicare analysis [9].
As an alternative to RTp, cRP should only be implemented in low-volume ndmPC if there is an equally effective survival benefit. The SEER-Medicare analysis already suggested that cRP and RTp using intensity-modulated RT (IMRT) offer a similar survival benefit. However, conformal RT failed to improve survival compared to NLT [9]. In our series, all patients receiving RTp were treated with IMRT, and we observed that cRP and RTp were associated with better OS compared to NLT. The main advantage of cRP appears to be the reduction in LEs. Patients with ndmPC are at risk of developing LEs due to local disease progression (mainly bladder outlet obstruction and ureteric obstruction). Won et al [25] reported that for patients who progress to mCRPC the LE risk is as high as 54.6% in the NLT setting. The LE rate was significantly lower for cRP (20%) but not for RTp (46.7%) [25]. In our series we observed the same trend, with significantly better LEFS for the cRP group compared to RTp and NLT. There was no significant difference in LEFS between RTp and NLT. Surgical removal of the local tumor bulk appears to be the best modality for reducing local complications, but at the risk of stress urinary incontinence. Conversely, RTp can be offered to patients unfit for cRP and/or in whom the local tumor is not safely resectable. In this series, patients undergoing RTp were indeed older than those undergoing cRP. When interpreting LEFS, one must take into account the important selection bias, whereby only patients with resectable tumor were offered cRP. The higher LE rate with RTp and NLT might be attributable, at least in part, to the more advanced local tumor stage.
4.1. Study limitations
Inevitably, this study has several limitations. First, the patient groups are rather small and only 109 patients were included in the analysis. However, since the introduction of PSA screening, the number of patients who present with ndmPC has been decreasing. Second, the follow-up is relatively short, which might have an important impact on the survival data observed. Third, although a prospective registry, it is not a randomized trial and therefore we cannot draw any definitive conclusions. Because of the treatment allocation process, there is an imbalance for several patient and tumor characteristics. Univariate and multivariate analyses were conducted to identify possible confounding factors. However, it is possible that some other, still unknown important confounders are not included in the analysis and might have had an impact on the results. To overcome this registry selection bias, the Local Treatment of Metastatic Prostate Cancer 2 (LOMP-2) trial has been initiated in which patients are randomized to cRP + SOC or SOC alone (NCT03655886). This registry was only analyzed for low-volume disease because the number of patients undergoing cRP in high-volume disease is too low to draw meaningful conclusions. We used conventional imaging to determine whether disease was of low or high volume. It remains unknown how introduction of the more sensitive prostate-specific membrane antigen (PSMA)-based positron emission tomography (PET)/CT imaging might affect this definition [26]. At present, there is no reason to deny patients LT if they have low disease volume on conventional imaging but high disease volume on PSMA PET/CT.
Since this was a nonrandomized, patient-preference analysis, the study is unable to answer the question of whether LT is an alternative to additional systemic treatment or whether it adds value to ADT plus additional systemic treatment. However, this is a hypothesis-generating study that supports ongoing randomized trials evaluating the effect of cRP in the treatment of metastatic prostate cancer.
5. Conclusions
For selected patients, cRP is able to achieve similar OS and CSS to RTp. cRP is effective in preventing local events. Only fit patients with resectable local tumors are eligible for cRP and in these patients the risk of a local event is low.
Author contributions: Nicolaas Lumen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lumen, Ost.
Acquisition of data: All authors.
Analysis and interpretation of data: Lumen, Ost.
Drafting of the manuscript: Lumen, Ost, De Bleser.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Lumen, Ost.
Obtaining funding: De Bleser, Lumen, Ost.
Administrative, technical, or material support: None.
Supervision: Lumen, Ost.
Other: None.
Financial disclosures: Nicolaas Lumen certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This research was supported by a grant from Kom op tegen Kanker (Stand Up to Cancer, Belgium) and funding from the Spearhead Project of Ghent University Hospital. The sponsors played a role in review of the manuscript.
Associate Editor: Guillaume Ploussard
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.euros.2021.05.006.
References
- 1.James N.D., Spears M.R., Clarke N.W. Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019) Eur Urol. 2015;67:1028–1038. doi: 10.1016/j.eururo.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 2.Cornford P., van den Bergh R.C.N., Briers E. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II—2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79:263–282. doi: 10.1016/j.eururo.2020.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong A.J., Szmulewitz R.Z., Petrylak D.P. ARCHES: a randomized, phase III study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37:2974–2986. doi: 10.1200/JCO.19.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi K.N., Agarwal N., Bjartell A. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381:13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney C.J., Chen Y.H., Carducci M. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fizazi K., Tran N., Fein L. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352–360. doi: 10.1056/NEJMoa1704174. [DOI] [PubMed] [Google Scholar]
- 7.Davis I.D., Martin A.J., Stockler M.R. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381:121–131. doi: 10.1056/NEJMoa1903835. [DOI] [PubMed] [Google Scholar]
- 8.Culp S.H., Schellhammer P.F., Williams M.B. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014;65:1058–1066. doi: 10.1016/j.eururo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Satkunasivam R., Kim A.E., Desai M. Radical prostatectomy or external beam radiation therapy vs no local therapy for survival benefit in metastatic prostate cancer: a SEER-Medicare analysis. J Urol. 2015;194:378–385. doi: 10.1016/j.juro.2015.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loppenberg B., Dalela D., Karabon P. The impact of local treatment on overall survival in patients with metastatic prostate cancer on diagnosis: a National Cancer Data Base analysis. Eur Urol. 2017;72:14–19. doi: 10.1016/j.eururo.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Parker C.C., James N.D., Brawley C.D. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392:2353–2366. doi: 10.1016/S0140-6736(18)32486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mottet N., Bellmunt J., Bolla M. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Neal D.E., Metcalfe C., Donovan J.L. Ten-year mortality, disease progression, and treatment-related side effects in men with localised prostate cancer from the ProtecT randomised controlled trial according to treatment received. Eur Urol. 2020;77:320–330. doi: 10.1016/j.eururo.2019.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Poelaert F., Verbaeys C., Rappe B. Cytoreductive prostatectomy for metastatic prostate cancer: first lessons learned from the multicentric prospective Local Treatment of Metastatic Prostate Cancer (LoMP) trial. Urology. 2017;106:146–152. doi: 10.1016/j.urology.2017.02.051. [DOI] [PubMed] [Google Scholar]
- 15.Salembier C., Villeirs G., De Bari B. ESTRO ACROP consensus guideline on CT- and MRI-based target volume delineation for primary radiation therapy of localized prostate cancer. Radiother Oncol. 2018;127:49–61. doi: 10.1016/j.radonc.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Bristow R.E., Tomacruz R.S., Armstrong D.K., Trimble E.L., Montz F.J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 17.Flanigan R.C., Mickisch G., Sylvester R., Tangen C., Van Poppel H., Crawford E.D. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171:1071–1076. doi: 10.1097/01.ju.0000110610.61545.ae. [DOI] [PubMed] [Google Scholar]
- 18.Qin X.J., Ma C.G., Ye D.W. Tumor cytoreduction results in better response to androgen ablation—a preliminary report of palliative transurethral resection of the prostate in metastatic hormone sensitive prostate cancer. Urol Oncol. 2012;30:145–149. doi: 10.1016/j.urolonc.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Psaila B., Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boeve L.M.S., Hulshof M., Vis A.N. Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: data from the HORRAD trial. Eur Urol. 2019;75:410–418. doi: 10.1016/j.eururo.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Yuh B.E., Kwon Y.S., Shinder B.M. Results of phase 1 study on cytoreductive radical prostatectomy in men with newly diagnosed metastatic prostate cancer. Prostate Int. 2019;7:102–107. doi: 10.1016/j.prnil.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranasinghe W., Chapin B.F., Kim I.Y., Sooriakumaran P., Lawrentschuk N. The cytoreductive prostatectomy in metastatic prostate cancer: what the individual trials are hoping to answer. BJU Int. 2020;125:792–800. doi: 10.1111/bju.15055. [DOI] [PubMed] [Google Scholar]
- 23.Knipper S., Beyer B., Mandel P. Outcome of patients with newly diagnosed prostate cancer with low metastatic burden treated with radical prostatectomy: a comparison to STAMPEDE arm H. World J Urol. 2020;38:1459–1464. doi: 10.1007/s00345-019-02950-0. [DOI] [PubMed] [Google Scholar]
- 24.Heidenreich A., Pfister D., Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J Urol. 2015;193:832–838. doi: 10.1016/j.juro.2014.09.089. [DOI] [PubMed] [Google Scholar]
- 25.Won A.C., Gurney H., Marx G., De Souza P., Patel M.I. Primary treatment of the prostate improves local palliation in men who ultimately develop castrate-resistant prostate cancer. BJU Int. 2013;112:E250–5. doi: 10.1111/bju.12169. [DOI] [PubMed] [Google Scholar]
- 26.Hofman M.S., Lawrentschuk N., Francis R.J. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395:1208–1216. doi: 10.1016/S0140-6736(20)30314-7. [DOI] [PubMed] [Google Scholar]