Abstract
Background
Double J (DJ) ureteral stents are commonly inserted after ureteroscopy (URS) procedures for stone treatment. However, stent-related symptoms are still a major issue.
Objective
To determine whether a commercially available pigtail suture stent (PSS) can reduce stent-related symptoms compared to a conventional DJ stent after uncomplicated URS.
Design, setting, and participants
We designed a randomized, single-blind, parallel-group trial from January to November 2020. The inclusion criteria were stone-free URS without intraprocedural complications. Patients with distal ureteral stones were excluded.
Intervention
Insertion of a PSS or DJ stent after URS.
Outcome measurements and statistical analysis
The primary endpoint was the Urinary Symptom Index score on the Ureteral Stent Symptoms Questionnaire (USSQ) 2 wk after URS. Secondary endpoints were USSQ domain scores and responses to individual USSQ questions at 2 d and 2 wk after surgery.
Results and limitations
A total of 78 patients were randomized and treated according to protocol. The Urinary Symptom Index score (p = 0.004), overall Visual Analogue Scale (VAS) score (p = 0.022), and the percentage of patients complaining of pain (63.9% vs 86.1%, p = 0.029) were significantly in favor of PSS at both 2 d and 2 wk after URS. At 2 d, the VAS score among patients with pain (p = 0.025) and the General Health Index score (p = 0.036) were significantly better in the PSS group. No severe complications occurred in either group. Study limitations are the exclusion of patients with distal ureteral stones and the limited sample size.
Conclusions
PSS significantly reduced stent-related symptoms after URS, in particular urinary symptoms and pain, compared to conventional DJ stents, and showed a good safety profile.
Patient summary
Stents are hollow tubes placed in the passage between the kidney and the bladder (ureter). The standard stent has two coiled ends (double J stent) to keep it in place in both the kidney and the bladder. We tested a commercial stent with two strings at the bladder end (pigtail suture stent) after procedures to remove stones from the upper urinary tract and found that it caused less stent-related symptoms compared to a double J stent.
This trial is registered at Clinicaltrials.gov as NCT03344120.
Keywords: Double J stent, Lower urinary tract symptoms, Nephrolithiasis, Pain, Pigtail suture stents, Stents, Stent-related symptoms, Ureteroscopy, Urinary calculi, URS, RIRS
Take Home Message
A pigtail suture stent significantly reduced stent-related symptoms compared to a conventional double J stent, in particular urinary symptoms and pain. We suggest the use of a pigtail suture stent rather than a double J stent after uncomplicated ureteroscopy performed for renal or proximal to mid-ureteral stones.
1. Introduction
Double J (DJ) ureteral stents are widely used in urological practice and are commonly inserted after ureteroscopy (URS) procedures [1], although the role of routine stenting in uncomplicated procedures is still disputed [2]. Stent-related symptoms (SRS) represent a major issue, both for patients, complaining of a negative impact on everyday life in 92% of cases [3], and urologists, identifying patients’ tolerance as the most significant concern associated with stents [4]. Many lines of research have explored the issue with the aim of decreasing SRS, both investigating drug-therapy (such as alpha-blockers and anticholinergic medications) and engineering solutions (stent material, shape, and design) [5]. It has been proposed that the distal end of DJ stents is one of the main factors involved in stent-related urinary symptoms and pain, although previous results are conflicting [6], [7], [8]. Vogt et al [9] designed a self-made pigtail suture stent (PSS) replacing the distal pigtail with a 0.3 Fr suture reaching the bladder, showing decreased SRS and good tolerance. To date, these promising results have not been confirmed by any prospective randomized controlled trial (RCT) involving a commercial PSS. Thus, we designed a prospective, single-blind RCT to compare SRS caused by a commercially available PSS and conventional DJ stents after uncomplicated URS for stone treatment.
2. Patients and methods
2.1. Trial design and participants
From January 2020 to November 2020, patients undergoing semirigid or flexible URS for stone treatment were asked to participate in a prospective, randomized, single-blind, parallel-group trial. All patients were enrolled and underwent surgery at the Department of Urology in Città della Salute e della Scienza-Molinette University Hospital (Turin, Italy). The inclusion criteria were as follows: ureteral or renal stones <2 cm needing treatment with semirigid or flexible URS and laser lithotripsy; stone-free procedures without intraprocedural complications; World Health Organization performance status 0–2; and patients aged 18–80 yr. The exclusion criteria were as follows: distal ureteral stones (below the iliac vessels); significant residual fragments at the end of the procedure; intraprocedural complications (eg, ureteral damage); noncompliant ureter preventing stone treatment; preoperative indwelling DJ stent; acute kidney failure; urinary tract infection; urinary tract abnormalities; and pathologies or medications potentially influencing voiding pattern and pain perception (eg, α blockers and antimuscarinics).
Written informed consent was obtained from all patients. The trial was conducted according to the Declaration of Helsinki and was prospectively approved by the ethics committee of Città della Salute e della Scienza and registered at ClinicalTrials.gov (NCT03344120).
2.2. Intervention
Semirigid URS was performed with a 7Fr semirigid ureteroscope (Karl Storz, Tuttlingen, Germany). First, a safety guidewire was threaded through a semirigid ureteroscope. Semirigid URS was then performed with or without the use of a second guidewire, according to the caliber and anatomy of the ureter. In cases with a narrowed ureteral orifice, the railway technique was used to obtain access to the upper tract. Flexible URS was performed with a Flex-X2 (Karl Storz) or Viper (Richard Wolf, Vernon Hills, IL, USA) fiber-optic flexible ureterorenoscope. In cases with renal stones, initial semirigid URS was always performed and a 10.7/12.7Fr or 11/13Fr ureteral access sheath was inserted before flexible URS. Urinalysis and urine culture were performed before surgery. In cases with a negative urine culture, a single dose of prophylactic antibiotics was administered before the procedure. In cases with a positive urine culture, a full course of antibiotics was administered according to the antibiogram, and a second urine culture was checked for negativity before the intervention. Lithotrispy was performed using 30-W Ho:YAG laser and fragments were extracted using a 0-tip nitinol basket. A stent was always left in place at the end of the procedure and its removal was planned 2 wk after surgery via flexible cystoscopy.
Patients were prospectively randomized into two groups: the PSS group received a commercial PSS (JFil; ROCAMED, Monaco, MC) after URS, while the DJ group received a conventional hydrophilic DJ stent (Vortek; Coloplast, Humlebaek, Denmark). The JFil PSS consists of a 7Fr ×16 cm body, featuring a fluted beak at the distal end and two simple sutures that reach the bladder and replace the distal part of a traditional DJ stent (Fig. 1). All the PSS devices were positioned so that the suture reached the bladder, but not the urethra. The DJ stent length was between 22 and 28 cm, according to each patient’s height, while the caliber was 6Fr. All procedures in both groups were randomly assigned and performed by two experienced endourologists (A. Bosio, A. Bisconti) who perform more than 100 procedures per year.
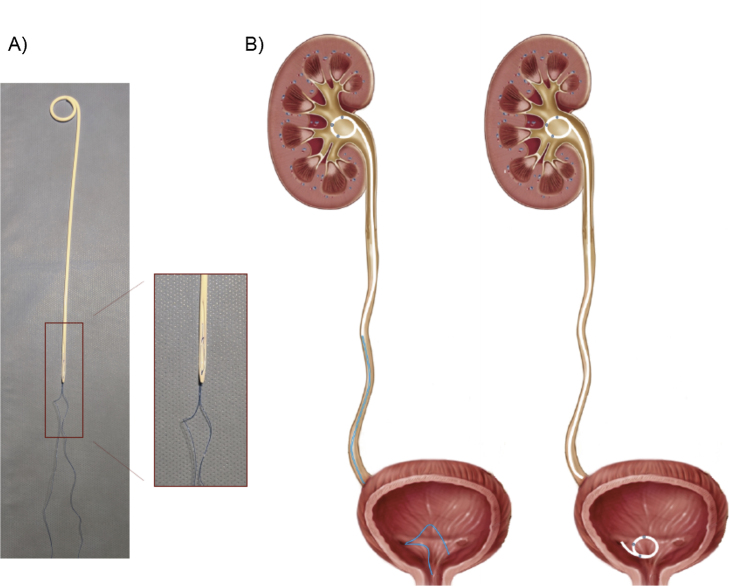
Fig. 1.
(A) Photograph and (B) drawing of the pigtail suture stent (PSS) used in our study. (A) The JFil PSS (ROCAMED, Monaco, MC) we used is a 16-cm-long 7Fr ureteral stent with a single renal pigtail. The distal part of the PSS ends in a fluted beak and extends in a 0.3Fr double surgical thread. (B) The illustration compares a PPS (on the left) with a conventional double J (DJ, on the right) stent. The DJ stent used in our study was a 22–28-cm-long (according to patient height) 6Fr Vortek stent (Coloplast, Humlebaek, Denmark). The distal end of the PSS body remains in the ureter, while the sutures (replacing the distal pigtail of a conventional DJ stent) extend into the bladder, allowing removal of the PSS via cystoscopy.
We assessed SRS using the validated Italian version [10] of the Ureteral Stent Symptoms Questionnaire (USSQ) [11]. The USSQ was presented three times to the patients: at 2 d and at 2 wk after surgery (before stent removal), and at 4 wk after stent removal (considered as the baseline assessment, 6 wk after surgery).
2.3. Outcomes
The primary endpoint was the Urinary Symptom Index score (sum of USSQ Urinary symptoms scores) in the PSS and DJ groups at 2 wk after surgery.
Secondary endpoints included USSQ domain scores at 2 d and at 2 wk after surgery, responses to individual USSQ questions at 2 d and at 2 wk after surgery, and the 2-wk USSQ domain subscores adjusted for baseline scores.
Analgesic consumption (prescribed at discharge and taken by patients as needed), postoperative complications (reported according to the modified Clavien-Dindo classification), and adverse events were recorded according to their severity and their potential relationship with the procedure. Operative time and any difficulty in stent insertion and removal were also evaluated.
2.4. Sample size, randomization, and statistical methods
Calculation of the sample size was based on the assumption of a 20% (5 points) difference in the primary endpoint (Urinary Symptom Index score) between the two groups according to the scoring system based on the USSQ validation study [11], with an α level of 5% and 80% power. The target sample size was 39 patients per arm, allowing for a 10% dropout rate.
The randomization sequence (1:1) was created using random block sizes of 2, 4, and 6. The allocation sequence was concealed from the researchers enrolling participants. Patients, data collectors, and data analysts were kept blinded to the allocation. As blinding of the operating surgeons was not possible, they were not involved in data collection and analysis.
Results are expressed as the median and interquartile range (IQR) for continuous variables, and n (%) for categorical variables. All numerical primary and secondary outcomes were compared between treatment groups using the Mann-Whitney U test, while Pearson’s χ2 and Fisher’s exact tests were used for comparisons of categorical variables. The significance level was set to 5% (p < 0.05) for each test. All analyses were performed using STATA v12 (StataCorp LP, College Station, TX, USA). Both intention-to-treat and per-protocol analyses were performed. Since the results were totally comparable, only the intention-to-treat results are reported.
3. Results
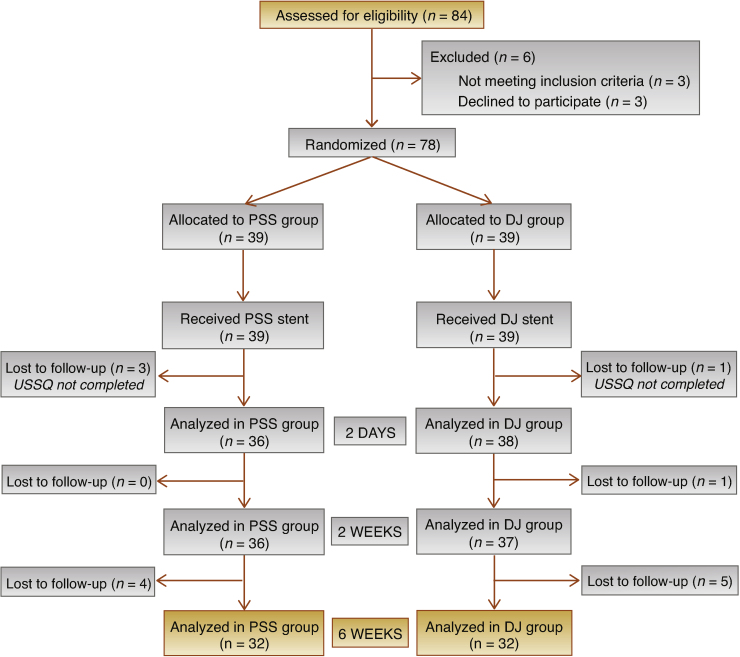
A total of 84 patients undergoing URS were assessed for eligibility. Six patients (7.1%) were excluded before treatment, leaving 78 patients who were randomized and treated (39 in the PSS and 39 in the DJ group). Three patients in the PSS group and one patient in the DJ group did not return the 2-d USSQ and were lost to follow-up, leaving data from 36 patients in the PSS group and 38 in the DJ group. One further patient in the DJ group did not return the 2-wk USSQ, so analysis was performed for 36 PSS and 37 DJ patients at this time point. Four PSS and five DJ patients did not return the 6-wk USSQ (considered as the baseline assessment), so analysis was performed for 32 patients per group.
The CONSORT flow chart is reported in Figure 2. Patient and stone characteristics are listed in Table 1.
Fig. 2.
CONSORT flow chart.
DJ = double J stent; PSS = pigtail suture stent; USSQ = Ureteral Stent Symptoms Questionnaire.
Table 1.
Patient and stone characteristics in the intention-to-treat population
| Characteristic | PSS group (N = 39) | DJ group (N = 39) |
|---|---|---|
| Median age, yr | 53 (45–65) | 57 (47–68) |
| Median height, cm | 170 (166–175) | 170 (163–176) |
| Sex | ||
| Males | 27/39 (69.2) | 29/39 (74.4) |
| Females | 12/39 (30.8) | 10/39 (25.6) |
| Median stone size, mm | 10 (7–13) | 10 (8–14) |
| Stone site | ||
| Kidney | 29/39 (74.4) | 30/39 (76.9) |
| Ureter | 10/39 (25.6) | 9/39 (23.1) |
DJ = double J stent; PSS = pigtail suture stent.
Data are presented as median (interquartile range) or n/N (%).
The 2-wk USSQ responses showed significant differences in favor of the PSS group regarding Urinary Symptom Index score (p = 0.004), overall VAS score (p = 0.022), and the percentage of patients complaining of body pain and discomfort (63.9% vs 86.1%; p = 0.029). No differences between the two groups for other USSQ domains (Pain Index, General Health Index, Work Performance Index, and Sexual Matters Score) were observed. The 2-d results were comparable to the 2-wk results favoring PSS for Urinary Symptom Index score (p = 0.001), overall VAS score (p = 0.002), and the percentage of patients complaining of body pain and discomfort (74.3% vs 94.7%; p = 0.021). Furthermore, the 2-d assessment showed significantly better PSS results for VAS scores among patients complaining of body pain (p = 0.025) and General Health Index score (p = 0.036). The scores at 2 wk and 2 d are reported in detail in Table 2.
Table 2.
USSQ domain scores at 2 wk and 2 d in the intention-to-treat population
| USSQ domain | PSS group (N = 39) | DJ group (N = 39) | p value a |
|---|---|---|---|
| Responses at 2 wkb | |||
| Urinary Symptom Index score (questions U1–U11) | 24 (21–30) | 30 (25–35) | 0.004 |
| Pain Index score (questions P3–P9) c | 16 (12–22) | 18 (14–22) | 0.596 |
| Pain Intensity – VAS score (question P3) c | 4 (2–6) | 4 (3–6) | 0.248 |
| Pain Intensity – VAS score (question P3) d | 2 (0–5) | 4 (2–6) | 0.022 |
| General Health Index score (questions G1–G6) | 11 (8–15) | 12 (10–16) | 0.177 |
| Work Performance Index score (questions W5–W7) | 5 (3–9) | 6 (3–9) | 0.682 |
| Sexual Matters score (questions S3–S4) | 3 (3–4) | 3 (2–4) | 0.569 |
| Feeling about stenting in the future (question GQ) e | 4 (4–6) | 5 (4–7) | 0.051 |
| Body pain or discomfort (question P1) | 23/36 (63.9) | 31/36 (86.1) | 0.029 |
| No active sex life with the stent in situ (question S1) g | 20/35 (57.1) | 21/33 (63.6) | 0.584 |
| Sex life stopped because of the stent (question S2) g | 3/20 (15.0) | 5/21 (23.8) | 0.697 |
| Responses at 2 db | |||
| Urinary Symptom Index score (questions U1–U11) | 26 (23–33) | 33 (29–38) | 0.001 |
| Pain Index score (questions P3–P9) c | 21 (13–25) | 21 (17–25) | 0.617 |
| Pain Intensity – VAS score (question P3) c | 4 (3–6) | 6 (5–8) | 0.025 |
| Pain Intensity – VAS score (question P3) d | 3 (0–5) | 6 (3–8) | 0.002 |
| General Health Index score (questions G1–G6) | 11 (9–15) | 14 (11–17) | 0.036 |
| Work Performance Index score (questions W5–W7) | 6 (4–7) | 6 (4–8) | 0.370 |
| Sexual Matters score (questions S3–S4) | 4 (3–5) | 3 (3–4) | 0.422 |
| Feeling about stenting in the future (question GQ) e | 4 (4–6) | 5 (4–7) | 0.222 |
| Body pain or discomfort (question P1) | 26/35 (74.3) | 36/38 (94.7) | 0.021 |
DJ = double J stent; PSS = pigtail suture stent; USSQ = Ureteral Stent Symptoms Questionnaire; VAS = Visual Analogue Scale.
Mann-Whitney U test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorical variables. Bold values denote statistical significance.
Results are presented as median (interquartile range) or n/N (%).
Pain Index and VAS scores among patients with body pain (answered “yes” to question P1).
Pain Intensity – VAS scores among all the patients.
Question GQ: “In the future, if you were advised to have another stent inserted, how would you feel about it?” Answers: 4 = “Mixed feelings”; 5 = “Mostly dissatisfied”.
A few patients did not answer questions on sexual matters.
The USSQ 2-wk subscores adjusted for baseline score (6-wk assessment) showed significantly better results in favor of PSS for Urinary Symptom Index scores (p = 0.030), overall VAS scores (p = 0.004), and General Health Index scores (p = 0.014). The 2-wk subscores adjusted for baseline are reported in Table 3, together with baseline scores, which were comparable between the two groups.
Table 3.
USSQ domain scores at 2 wk adjusted for baseline and at 6 wk (baseline) in the intention-to-treat population
| USSQ domain | PSS group (N = 39) | DJ group (N = 39) | p value a |
|---|---|---|---|
| Responses at 2 wk adjusted for baselineb | |||
| Urinary Symptom Index score (U1–U11) | 5 (1–8) | 10 (3–19) | 0.030 |
| Pain Index score (P3–P9) c | 2 (1–4) | 2 (0–7) | 0.828 |
| Pain Intensity – VAS score (P3) d | 0 (0–2) | 4 (0–5) | 0.004 |
| General Health Index score (G1–G6) | 0 (0–3) | 4 (0–6) | 0.014 |
| Sexual Matters score (S3–S4) | 0 (0–1) | 0 (0–1) | 0.679 |
| Feeling about stenting in the future (GQ) e | 0 (0–1) | 0 (0–2) | 0.197 |
| Responses at 6 wk (baseline)b | |||
| Urinary Symptom Index score (U1–U11) | 19 (14–22) | 19 (15–24) | 0.415 |
| Pain Index score (P3–P9) c | 13 (10–20) | 14 (12–21) | 0.354 |
| Pain Intensity – VAS score (P3) c | 3 (2–6) | 3 (2–6) | 0.975 |
| Pain Intensity – VAS score (P3) d | 0 (0–3) | 0 (0–2) | 0.808 |
| General Health Index score (G1–G6) | 8 (7–11) | 7 (6–11) | 0.759 |
| Work Performance Index score (W5–W7) | 3 (3–3) | 3 (3–3) | 0.820 |
| Sexual Matters score (S3–S4) | 3 (2–3) | 3 (2–3) | 0.580 |
| Feeling about stenting in the future (GQ) e | 4 (3–6) | 4 (3–6) | 0.555 |
DJ = double J stent; PSS = pigtail suture stent; USSQ = Ureteral Stent Symptoms Questionnaire; VAS = Visual Analogue Scale.
Mann-Whitney U test. Bold values denote statistical significance.
Results are presented as median (interquartile range).
Pain Index and VAS scores among patients with body pain (who answered “yes” to question P1).
Pain Intensity – VAS scores among all the patients.
Question GQ: “In the future, if you were advised to have another stent inserted, how would you feel about it?” Answers: 4 = “Mixed feelings”; 5 = “Mostly dissatisfied”.
Analysis of responses to individual questions revealed that scores for urinary frequency, feeling of incomplete bladder emptying, and burning at voiding were significantly lower in the PSS group at both 2 d and 2 wk after surgery. The same finding applies to pain during micturition and interfering with everyday life. Furthermore, a significantly higher percentage of the DJ group needed health professional help for SRS (31.4% vs 11.1%; p = 0.045) during the first 2 wk after surgery, before stent removal. Sites of pain did not significantly differ between the two groups (p = 0.482). Results for 2-d and 2-wk responses to individual USSQ questions are reported in Table 4.
Table 4.
Responses to individual USSQ questions at 2 d and 2 wk in the intention-to-treat population
| Question | Responses at 2 d |
Responses at 2 wk |
||||
|---|---|---|---|---|---|---|
| PSS group | DJ group | p value a | PSS group | DJ group | p value a | |
| Urinary symptom questionsb | ||||||
| Urinary frequency score (U1) | 3 (3–4) | 4 (4–5) | <0.001 | 3 (2–4) | 4 (3–4) | 0.028 |
| Nocturia score (U2) | 2 (2–4) | 2 (2–4) | 0.647 | 2 (2–3) | 2 (2–3) | 0.711 |
| Urgency score (U3) | 2 (2–3) | 2 (2–4) | 0.234 | 2 (2–3) | 2 (2–3) | 0.374 |
| Urge incontinence score (U4) | 1 (1–2) | 1 (1–2) | 0.435 | 1 (1–2) | 1 (1–1) | 0.147 |
| Incontinence without urge score (U5) | 1 (1–1) | 1 (1–1) | 0.861 | 1 (1–1) | 1 (1–1) | 0.306 |
| Incomplete emptying score (U6) | 2 (1–2) | 2 (2–3) | 0.005 | 2 (1–2) | 3 (2–4) | <0.001 |
| Burning at voiding score (U7) | 3 (1–4) | 4 (3–5) | 0.007 | 2 (1–3) | 3 (2–5) | 0.002 |
| Macroscopic hematuria score (U8) | 2 (1–3) | 3 (2–4) | 0.072 | 1 (1–2) | 2 (1–3) | 0.093 |
| Grade of hematuria score (U9) | 2 (1–2) | 2 (2–3) | 0.134 | 2 (1–2) | 2 (1–2) | 0.220 |
| Current state is a problem score (U10) | 2 (2–4) | 3 (2–4) | 0.110 | 2 (1–4) | 3 (2–4) | 0.054 |
| Rest of life like this score (U11) c | 5 (4–6) | 6 (5–7) | 0.003 | 5 (4–6) | 6 (5–7) | 0.003 |
| Body pain or discomfortb,d | ||||||
| Pain while passing urine (P6) | 17/35 (48.6) | 33/38 (86.8) | <0.001 | 15/36 (41.7) | 26/35 (74.3) | 0.005 |
| Renal pain while passing urine (P7) | 10/35 (28.6) | 6/37 (16.2) | 0.208 | 6/36 (16.7) | 4/35 (11.4) | 0.735 |
| Pain requiring painkillers (P8) | 19/35 (54.3) | 26/38 (68.4) | 0.215 | 17/36 (47.2) | 17/35 (48.6) | 0.909 |
| Pain interfering with life (P9) | 22/35 (62.9) | 34/38 (89.5) | 0.007 | 21/36 (58.3) | 29/35 (82.9) | 0.024 |
| Additional problemsb | ||||||
| Feeling of urinary tract infection (A1) | 14/35 (40.0) | 22/38 (57.9) | 0.127 | 13/36 (36.1) | 20/35 (57.1) | 0.076 |
| Need for antibiotics (A2) | 7/35 (20.0) | 7/37 (18.9) | 0.908 | 5/36 (13.9) | 10/35 (28.6) | 0.155 |
| Need for health professional help (A3) | 3/35 (8.6) | 8/36 (22.2) | 0.189 | 4/36 (11.1) | 11/35 (31.4) | 0.045 |
| Need to visit the hospital (A4) | 1/35 (2.9) | 3/37 (8.1) | 0.615 | 2/36 (5.6) | 5/35 (14.3) | 0.260 |
Mann-Whitney U test for continuous variables, and Pearson’s χ2 test or Fisher’s exact test for categorical variables. Bold values denote statistical significance.
Results are reported as the median score (interquartile range) or n/N (%).
Question U11: “If you were to spend the rest of your life with the urinary symptoms, if any, associated with the stent just the way they are, how would you feel about it?” Answers: 5 = “Mostly dissatisfied”; 6 = “Unhappy”.
Pain score rates are among all the patients, with or without pain.
3.1. Complications and adverse events
Complications and adverse events occurred in seven of the 78 patients (8.97%), including three patients with severe hematuria (Clavien-Dindo grade I, all in the DJ group) and four patients with fever >38 °C (Clavien-Dindo grade II, 3 PSS and 1 DJ). No cases of Clavien-Dindo grade ≥ III complications, urosepsis, stent dislodgment, or worsening hydronephrosis after stent removal were recorded in either group. No significant differences were observed between the groups. Complications and adverse events are reported in Table 5.
Table 5.
Complications and adverse events in the intention-to-treat population
| Clavien-Dindo grade | Complication | Patients (n) |
p value a | |
|---|---|---|---|---|
| PSS group (N = 39) | DJ group (N = 39) | |||
| I | Severe hematuria | 0 | 3 | 0.240 |
| II | Fever >38°°C | 3 | 1 | 0.358 |
| III | Stent dislodgment | 0 | 0 | |
| IV | Urosepsis | 0 | 0 | |
| Total | 3 | 4 | 1.000 | |
Fisher’s exact test.
No difficulties were encountered in positioning the PSS and DJ stents. The mean operative time did not differ between the PSS (69.10 ± 10.17 min) and DJ (69.69 ± 9.81 min) groups (p = 0.795). All DJ stents were easily removed with a flexible cystoscope, while it was necessary to remove two PSS devices via rigid cystoscopy because of a poor grip on the sutures with the flexible cystoscope forceps.
4. Discussion
Our prospective, randomized, single-blind trial proved that PSS significantly reduced urinary symptoms, body pain, and discomfort at both 2 d and 2 wk after URS compared to a conventional DJ stent. For the first time, we have shown that a commercial PSS has a better tolerance profile than a conventional DJ stent, significantly reduces SRS, improves patient quality of life, and has a comparable safety profile.
The need for routine stent insertion after URS is widely debated [12]; a recent Cochrane review failed to provide practical advice owing to the limitations of the studies considered [2]. However, results from previous surveys showed that urologists tend to insert a ureteral stent after uncomplicated URS procedures, and this approach appears to have remained stable over time [1], [4], [11]. SRS are a major concern; one series showed a high percentage of patients complaining of SRS after URS for stone treatment, including urinary symptoms (reported as a problem by 88.4% of patients) and pain (83.2%), with a significant impact on many aspects of everyday life, such as general health, working life, and sexual activity [3].
Wiseman et al [13] demonstrated a reduction in urinary symptoms and pain using silicone stents. However, overall SRS were still relevant and the benefits regarding pain in the silicone stent group was not significant before 3 wk after stent placement, a timeframe that exceeds the stenting duration favored by most urologists [14].
The distal end of DJ stents has been investigated as one of the main factors influencing SRS, as it is involved in the vesicoureteral reflux mechanism [15] and lower urinary tracts symptoms, owing to its direct contact with the bladder mucosa. On the basis of these considerations, Vogt et al [9] developed a self-made PSS in which the distal part of a DJ stent was replaced with a 0.3Fr suture reaching the bladder to avoid a material impact on the distal part of the ureter and the bladder mucosa, with the aim of achieving a better-tolerated device. Their observational study showed a decrease in USSQ scores among 24 patients strongly complaining of SRS who had a DJ stent replaced with a PSS. Two recent RCTs of self-manufactured PSS showed a reduction in SRS [16], [17]. However, these studies are of limited use because of poor reproducibility and potential risks related to self-modification of a medical device, severely limiting their application to clinical practice.
To the best of our knowledge, our study is the first RCT to investigate an innovative commercial PSS and to provide fully reproducible and exploitable results. The technique for PSS positioning is comparable to that for a conventional DJ stent and can be performed either with or without a cystoscope. Our data showed a significant reduction in urinary symptoms and VAS, improvement in general health, and a >20% reduction in the number of patients complaining of pain. Moreover, PSS showed a good safety profile: complications and adverse events were comparable in the two groups, while a significantly lower percentage of patients in the PSS group needed help from a health professional, potentially reducing stent-related costs. These results can justify extensive use of PSS after URS, possibly representing a paradigm change in consolidated stenting management and providing a more comfortable postoperative course after upper tract endoscopic procedures.
As urologists seem to consider ureteral stents as “a comfort blanket allowing them, if not the patient, to sleep easier after the procedure” [12] and appear to be reluctant to leave patients stentless after URS, PSS may represent a step forward.
The results from our study might also help to shed light on SRS pathogenesis: material reduction not only in the bladder but also in the distal ureter could play a key role in decreasing some SRS, such as urinary frequency. Reduced pain during micturition may be linked to material reduction in the intramural ureter as well as the absence of vesicoureteral reflux. The role of the distal part of the ureter in SRS highlighted by our results, and little considered so far, is consistent with the efficacy of α blockers in reducing SRS [18], [19] and with previous studies assessing the beneficial effect of complete intraureteral loop-tail stent placement compared to conventional placement [20]. Moreover, a possible explanation for the significant decrease in symptoms may be a reduction in edema and inflammation, as observed at the ureteral orifice with PSS in a porcine model [21].
The positive results for this innovative device should lead to further investigation of its potential applications, benefits, and possible risks. In particular, future studies should explore any patient characteristics influencing PSS-related symptoms and PSS applicability in different settings, such as distal ureteral lithiasis. Moreover, the PSS safety profile should be confirmed with more extensive use of the device. Finally, the particular commercial PSS used in our study could be further improved to facilitate its removal, which was less easy than removal of a conventional DJ stent. Further investigations might also specifically address the issue of PSS removal in terms of comfort and duration of the procedure, which was not specifically evaluated in our study.
Although innovative, our RCT has some limitations. First, the study design, which excluded patients with distal ureteral stones and residual fragments after URS, does not allow us to recommend PSS use in these settings, or for an indwelling time longer than 2 wk. Second, the limited sample size calculated for the primary endpoint may have prevented observation of other significant differences, such as in the use of painkillers. Thus, use of this device on a wider scale may provide further information and associations, including potential benefits or drawbacks.
5. Conclusions
In conclusion, our RCT involving a commercial device proved that PSS significantly reduces SRS, in particular urinary symptoms and body pain, compared to a conventional DJ stent after URS, and showed a comparable safety profile. On the basis of these results, PSS insertion can be recommended after uncomplicated URS performed for renal or proximal to mid-ureteral stones in place of a conventional DJ cases, at least in cases for which the urologist decides against performing a stentless procedure.
Author contributions: Andrea Bosio had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bosio.
Acquisition of data: Agosti, Vitiello, Vercelli, Bisconti, Piana.
Analysis and interpretation of data: Fop.
Drafting of the manuscript: Bosio, Alessandria.
Critical revision of the manuscript for important intellectual content: Bosio, Alessandria.
Statistical analysis: Fop.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Gontero.
Other: None.
Financial disclosures: Andrea Bosio certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Data sharing statement: Data are available for bona fide researchers on request from the authors.
Ethical considerations: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Acknowledgments: The authors are very grateful to Prof. Julian Hoskins for editing of the manuscript.
CRediT authorship contribution statement
Andrea Bosio: Conceptualization, Investigation, Methodology, Project administration, Writing - original draft, Writing - review & editing, Visualization. Eugenio Alessandria: Writing - original draft, Writing - review & editing, Visualization. Simone Agosti: Investigation. Federico Vitiello: Investigation. Eugenia Vercelli: Investigation. Alessandro Bisconti: Investigation. Paolo Piana: Investigation. Fabrizio Fop: Data curation, Formal analysis. Paolo Gontero: Supervision.
Associate Editor: Silvia Proietti
References
- 1.Pereira J.F., Bower P., Jung E. Ureteral stenting practices following routine ureteroscopy: an international survey. World J Urol. 2019;37:2501–2508. doi: 10.1007/s00345-019-02660-7. [DOI] [PubMed] [Google Scholar]
- 2.Ordonez M., Hwang E.C., Borofsky M., Bakker C.J., Gandhi S., Dahm P. Ureteral stent versus no ureteral stent for ureteroscopy in the management of renal and ureteral calculi. Cochrane Database Syst Rev. 2019;2019 doi: 10.1002/14651858.CD012703.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bosio A., Alessandria E., Dalmasso E. How bothersome double-J ureteral stents are after semirigid and flexible ureteroscopy: a prospective single-institution observational study. World J Urol. 2019;37:201–207. doi: 10.1007/s00345-018-2376-6. [DOI] [PubMed] [Google Scholar]
- 4.Auge B.K., Sarvis J.A., L’Esperance J.O., Preminger G.M. Practice patterns of ureteral stenting after routine ureteroscopic stone surgery: a survey of practicing urologists. J Endourol. 2007;21:1287–1291. doi: 10.1089/end.2007.0038. [DOI] [PubMed] [Google Scholar]
- 5.Sali G.M., Joshi H.B. Ureteric stents: overview of current clinical applications and economic implications. Int J Urol. 2020;27:7–15. doi: 10.1111/iju.14119. [DOI] [PubMed] [Google Scholar]
- 6.Lingeman J.E., Preminger G.M., Goldfischer E.R., Krambeck A.E. Comfort Study Team. Assessing the impact of ureteral stent design on patient comfort. J Urol. 2009;181:2581–2587. doi: 10.1016/j.juro.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawahara T., Ito H., Terao H. Changing to a loop-type ureteral stent decreases patients’ stent-related symptoms. Urol Res. 2012;40:763–767. doi: 10.1007/s00240-012-0500-4. [DOI] [PubMed] [Google Scholar]
- 8.Bosio A., Alessandria E., Agosti S. Loop-tail stents fail in reducing stent-related symptoms. Results of a prospective randomized controlled trial. BJU Int. 2021 doi: 10.1111/bju.15395. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Vogt B., Desgrippes A., Desfemmes F.N. Changing the double-pigtail stent by a new suture stent to improve patient’s quality of life: a prospective study. World J Urol. 2015;33:1061–1068. doi: 10.1007/s00345-014-1394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giannarini G., Keeley F.X., Jr, Valent F. The Italian linguistic validation of the Ureteral Stent Symptoms Questionnaire. J Urol. 2008;180:624–628. doi: 10.1016/j.juro.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Joshi H.B., Newns N., Stainthorpe A., MacDonagh R.P., Keeley F.X., Jr, Timoney A.G. Ureteral Stent Symptom Questionnaire: development and validation of a multidimensional quality of life measure. J Urol. 2003;169:1060–1064. doi: 10.1097/01.ju.0000049198.53424.1d. [DOI] [PubMed] [Google Scholar]
- 12.Hughes B., Wiseman O.J., Thompson T. The dilemma of post-ureteroscopy stenting. BJU Int. 2014;113:184–185. doi: 10.1111/bju.12482. [DOI] [PubMed] [Google Scholar]
- 13.Wiseman O., Ventimiglia E., Doizi S. Effects of silicone hydrocoated double loop ureteral stent on symptoms and quality of life in patients undergoing flexible ureteroscopy for kidney stone: a randomized multicenter clinical study. J Urol. 2020;204:769–777. doi: 10.1097/JU.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 14.Mottet N., Cornford P., van den Bergh R.C.N. Arnhem, The Netherlands: European Association of Urology; 2020. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. https://uroweb.org/wp-content/uploads/EAU-EANM-ESTRO-ESUR-SIOG-Guidelines-on-Prostate-Cancer-2020.pdf
- 15.Mosli H.A., Farsi H.M., al-Zimaity M.F., Saleh T.R., al-Zamzami M.M. Vesicoureteral reflux in patients with double pigtail stents. J Urol. 1991;146:966–969. doi: 10.1016/s0022-5347(17)37976-4. [DOI] [PubMed] [Google Scholar]
- 16.Bostanci Y., Mercimek M.N., Gulsen M., Ozden E., Yakupoglu Yk, Sarikaya S. Clinical effectiveness of single pigtail suture stent on patient comfort: a double-blind prospective randomized trial. J Laparoendosc Adv Surg Tech A. 2020;30:1183–1188. doi: 10.1089/lap.2020.0127. [DOI] [PubMed] [Google Scholar]
- 17.Betschart P, Piller A, Zumstein V, et al. Reduction of stent-associated morbidity by minimizing stent material: a prospective, randomized, single-blind superiority trial assessing a customized ‘suture stent’. BJU Int. In press. 10.1111/bju.15290. [DOI] [PubMed]
- 18.Johnson L.J., Davenport D., Venkatesh R. Effects of alpha-blockade on ureteral peristalsis and intrapelvic pressure in an in vivo stented porcine model. J Endourol. 2016;30:417–421. doi: 10.1089/end.2015.0251. [DOI] [PubMed] [Google Scholar]
- 19.Lamb A.D., Vowler S.L., Johnston R., Dunn N., Wiseman O.J. Meta-analysis showing the beneficial effect of α-blockers on ureteric stent discomfort. BJU Int. 2011;108:1894–1902. doi: 10.1111/j.1464-410X.2011.10170.x. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida T., Inoue T., Taguchi M. Efficacy and safety of complete intraureteral stent placement versus conventional stent placement in relieving ureteral stent related symptoms: a randomized, prospective, single blind, multicenter clinical trial. J Urol. 2019;202:164–170. doi: 10.1097/JU.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 21.Majdalany S.E., Aldoukhi A.H., Jung H., Mehra R., Roberts W.W., Ghani K.R. In vivo evaluation of a novel pigtail suture stent. Urology. 2021;148:83–87. doi: 10.1016/j.urology.2020.11.031. [DOI] [PubMed] [Google Scholar]