Abstract
Background
Postoperative urinary retention (POUR) is a known complication in the postanesthesia care unit (PACU). The variations in catheterization thresholds contribute to unnecessary invasive procedures.
Objective
In the current study, we implemented an algorithm for a sterile intermittent catheterization (SIC) threshold of 800 ml with volume-dependent bladder scan intervals and compared the incidence of SIC with that of a matched patient cohort threshold of 400 ml.
Design, setting, and participants
This comparative study of two prospective historical cohorts represented two thresholds for POUR, set at 400 ml without a standardized bladder scan protocol and 800 ml with a volume-dependent bladder scan protocol.
Outcome measurements and statistical analysis
The primary outcome was the frequency of catheterization during the PACU stay. Secondary outcomes evaluated patient safety aspects in occurrence of thresholds above 400/800 ml. The study was set at the PACU under the Department of Anesthesia, Center for Cancer and Organ Diseases, Rigshospitalet, Denmark.
Results and limitations
In total, 741 patients were consecutively included, with 307 in the POUR-400 and 434 in the POUR-800 group, and with comparable group characteristics. Significantly fewer patients fulfilled the SIC/catheter a’ demeure (CAD) criteria in the POUR-800 (5.0%) versus POUR-400 (14.3%) group, equivalent to a 65.0% relative reduction in SIC.
Conclusions
Implementation of a standardized ultrasound-guided protocol with volume-dependent scan intervals and an evidence-based catheterization threshold of 800 ml decreases the need for SIC by >65%, without increasing the need for urinary catheterization at the wards.
Patient summary
In this study, we implemented an algorithm for a sterile intermittent catheterization threshold of 800 ml with volume-dependent bladder scan intervals. A marked reduction was seen in catheterization in the postanesthesia care unit, without increasing catheterization rates at the ward.
Keywords: Anesthesia, Bladder scan, Catheterization, Postoperative urinary retention, POUR
Take Home Message
An algorithm for an intermittent catheterization threshold of 800 ml, with volume-dependent bladder scan intervals during patients’ stay at the postanesthesia care unit, reduces the incidence of urinary bladder catheterization. Extensive patient-clinician communication on bladder symptomatology is a crucial prerequisite.
1. Introduction
Postoperative urinary retention (POUR) is a known complication in the postanesthesia care unit (PACU), with incidences ranging from 0% to 84% depending on the underlying pathophysiological factors including surgical procedure and anesthesiological technique [1], [2], [3]. The lack of a uniformly agreed definition has resulted in large variations in catheterization thresholds based upon assumptions rather than evidence on acceptable maximum bladder volumes, also due to the lack of large-scale studies [4], [5]. Furthermore, the general assumption that thresholds should be set at 400–500 ml stems from older rodent studies extrapolated to humans, to avoid bladder wall damage resulting in persistent voiding difficulties [6], [7], [8], [9], but with the potential for unnecessary catheterizations and damage to the urinary tract including urinary tract infections (UTIs). A study investigating early catheter removal at postoperative day 2 in patients undergoing colorectal surgery found lowered UTI risk without increasing POUR, compared with less restrictive guidelines for keeping catheters left in situ [10].
Two large randomized studies have challenged the previous assumptions on bladder volumes, proving that significant increases in catheterization thresholds resulted in significantly reduced need for catheterization without increasing persistent voiding difficulties, if applied in settings with access to frequent ultrasound assessments of urinary bladder volume [11], [12]. However, to our knowledge, no studies have investigated the clinical effect and safety of implanting the new 800 ml threshold in an algorithm including volume-dependent bladder scanning thresholds to ensure that the catheterization maximum is not exceeded.
Accordingly, we implemented a new algorithm for a sterile intermittent catheterization (SIC) threshold of 800 ml, with volume-dependent bladder scan intervals during patients’ stay at the PACU, and compared the incidence of urinary bladder catheterization with data from a matched patient cohort during the previous threshold of 400 ml.
2. Patients and methods
This was a pragmatic comparative study of two prospective cohorts representing two thresholds for POUR, set at 400 ml without standardized bladder scan intervals and 800 ml with volume-dependent bladder scan intervals.
This study was set at the PACU under the Department of Anesthesia, Center for Cancer and Organ Diseases, Rigshospitalet, Denmark. The study was conducted in accordance with the Helsinki declaration. Approval was obtained from the Danish Health Authority and local data handling authorities (RH-2016-304, I-Suite nr.: 04347), and ethical approval was waived by the Ethics Committee due to the observational study design. All patients provided written consent in advance that their data recorded in an anonymous form could be used for quality development.
2.1. Patients
The study population comprised a sample of unselective consecutive adults referred to various orthopedic, urological, plastic, ear-nose-throat, vascular, and abdominal procedures. No patients were excluded prior to surgery. Patients who arrived at the PACU with a urinary catheter were not included.
2.2. Data collection
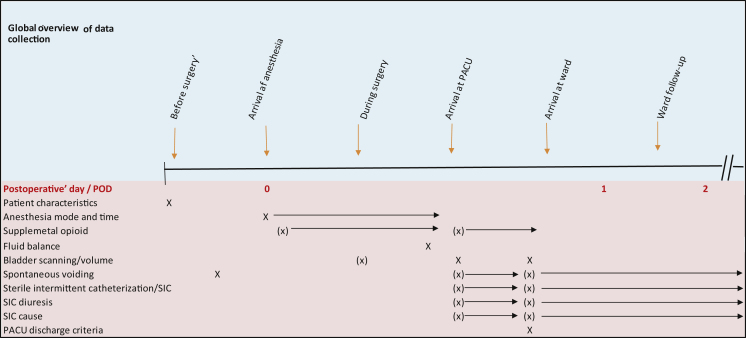
Data on outcome and relevant variables related to the two time periods (from February 3, 2016 to March 24, 2016 [POUR-400] and from April 18, 2016 to June 15, 2016 [POUR-800]) were extracted from the electronic patient records. The study observed the processes from anesthesia to PACU and transition to the surgical ward. A global overview of data collection is provided in Figure 1.
Fig. 1.
Global overview of data collection. PACU = postanesthesia care unit; POD = postoperative day; SIC = sterile intermittent catheterization.
2.2.1. Primary outcome
The primary outcome was the frequency of urinary bladder catheterization (SIC or catheter a’ demeure [CAD] during PACU stay) of patients, including those who fulfilled the criteria but refused SIC/CAD.
2.2.2. Secondary outcomes
The secondary outcomes were bladder volume thresholds above 400/800 ml and maximum bladder volumes, need for catheterization at the ward after PACU discharge, spontaneous voiding at PACU, bladder scan and volume 30 min before ward transition, spontaneous voiding, SIC/CAD, and POUR at the ward, all stratified into the two maximum thresholds.
2.2.3. Explanatory variables collected
Explanatory variables included surgical procedure, gender, age, body mass index (BMI), presurgical voiding (yes/no), anesthesia type and time (in minutes), opioid supplement during anesthesia, fluid balance at theater discharge, and opioid supplement at the PACU.
2.3. POUR threshold algorithm at the PACU
2.3.1. Control group (POUR-400 cohort)
Prior to the new 800 ml algorithm, patients underwent a bladder scan (BioCon-700; Mcube Technology Co., Korea; maximum estimated volume 999 ml) as soon as possible upon arrival at the PACU, and need for subsequent scans were individually assessed by the responsible nurse; if a bladder volume of >400 ml was detected, patients were encouraged to void. If unsuccessful, SIC was performed, irrespective of patients’ symptomatology on urinary retention.
2.3.2. Intervention group (POUR-800 cohort)
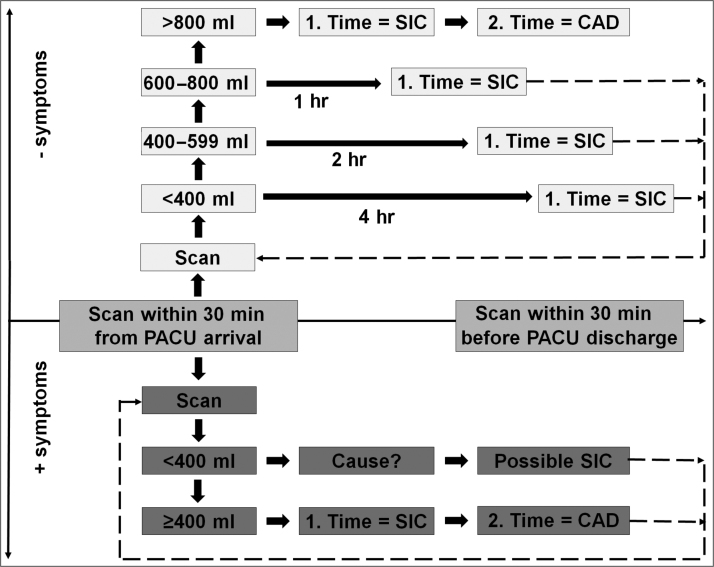
The new 800 ml threshold algorithm consists of two pathways: symptomatic and asymptomatic. All patients underwent a bladder scan within the first 30 min from arrival at the PACU. If a patient has symptoms on urinary retention (urinary urge, lower abdominal pain, or tension over the bladder) and the bladder volume is <400 ml, an attempt is made to find the cause of symptoms before any SIC is performed (Fig. 2).
Fig. 2.
POUR-800 ml algorithm. Caveats: in case of >1000 ml fluid per hour and/or diuretics, perform bladder scan hourly or place a CAD. Flowchart does not apply to patients with known bladder pathology, small bladder, <50 kg bodyweight, or >160 cm height. Symptoms include urge, lower abdominal pain, confusion, and tension over the bladder. Cause: explore other reasons for symptoms, for example, hematoma, urethral irritation, etc. Patients were always encouraged to urinate before SIC or CAD. CAD = catheter a’ demeure; PACU = postanesthesia care unit; POUR = postoperative urinary retention; SIC = sterile intermittent catheterization.
In patients without urinary urge, the algorithm provides volume-dependent timed threshold for subsequent bladder scanning and need for SIC until a threshold of 800 ml is reached. In case of subsequent need for recatheterization, a CAD is inserted (Fig. 2).
The algorithm applies only to patients who are not known with recognized small bladder capacity, have weight <50 kg, and are <160 cm tall. Patients who receive diuretics and/or intravenous fluids >1000 ml/h undergoes a bladder scan every hour or provided with a CAD. All patients are rescanned in the PACU at least 30 min before transition to the ward. No standardized questions regarding preoperative voiding difficulties were collected.
2.4. Statistical analysis
The primary outcome (urinary bladder catheterization) was presented as a percentage of all patients admitted to the PACU for undergoing catheterization, during the two study periods, and compared by analysis of variance (ANOVA) and odds ratios (ORs) with 95% confidence limits for group comparisons and risk estimates.
Data are presented by descriptive statistics median, with range and percentiles or mean with standard deviation and range. Chi-square, ANOVA, and ORs with 95% confidence limits were used for group comparisons and risk estimates. Statistical analysis was performed using IBM SPSS statistics version 25.
3. Results
During the study periods, a total of 741 patients were included in the POUR-400 (n = 307) and POUR-800 (n = 434) groups. Group characteristics are provided in Table 1. No statistically significant differences in pre- and intraoperative variables were seen between the two groups, except for surgical specialty with minor percentual differences. The majority (>99%) underwent general anesthesia. Supplemental opioid within the last hour of anesthesia or the PACU stay did not differ between groups, nor did fluid balance at surgery completion.
Table 1.
Characteristics
| POUR-400 |
POUR-800 |
||||||
|---|---|---|---|---|---|---|---|
| Number (%) | Mean (SD) | Median | Number (%) | Mean (SD) | Median | ||
| Number (n) | 307 | 434 | |||||
| Gender, female | 160 (52.1) | 242 (55.8) | |||||
| Age | 51.8 (18.2) | 53 | 50.2 (18.7) | 52 | |||
| Height | 172.6 (9.9) | 172 | 172 (10.2) | 172 | |||
| Weight | 75.7 (18.1) | 75 | 75.0 (16.6) | 73 | |||
| Body mass index | 25.3 (4.9) | 24.6 | 25.2 (4.3) | 24.7 | |||
| Surgical specialty a | |||||||
| Abdominal | 32 (10.4) | 54 (12.4) | |||||
| Orthopedic | 99 (32.2) | 134 (30.9) | |||||
| Plastic | 92 (30.0) | 158 (36.4) | |||||
| Urology | 14 (4.6) | 7 (1.6) | |||||
| TMJ/ENT | 53 (17.3) | 69 (15.9) | |||||
| Vascular | 17 (5.5) | 12 (2.8) | |||||
| Anesthesia time (min) | 109 (50.4) | 100 | 116.4 (52.3) | 110 | |||
| Anesthesia type | |||||||
| Spinal | 0 | 1 (0.2) | |||||
| Epidural | 0 | 0 | |||||
| Universal | 306 (99.7) | 431 (99.3) | |||||
| Epi + UA | 1 (0.3) | 1 (0.2) | |||||
| Local | 0 | 1 (0.2) | |||||
| Opioid suppl. end of anesthesia | 119 (38.8) | 177 (40.8) | |||||
| Opioid suppl. PACU | 188 (61.2) | 257 (59.2) | |||||
| Fluid balance b | 592 (287) | 590 | 636 (320) | 600 | |||
ENT = ear-nose-throat; Epi = epidural; PACU = postanesthesia care unit; POUR = postoperative urinary retention; SD = standard deviation; suppl. = supplement; TMJ = tooth-mouth-jaw; UA = universal anesthesia.
p = 0.034.
Fluid balance at surgical completion.
3.1. Primary outcome: SIC or CAD
Significantly fewer patients fulfilled the SIC/CAD criteria in the POUR-800 versus POUR-400 group (22/434 [5.0%] vs 44/307 [14.3%]; OR 3.13 [95% confidence interval {CI} 1.84–5.35]; Table 2), equivalent to a 65.0% relative reduction in SIC.
Table 2.
Proportions reaching bladder volume threshold at initial scan and undergoing spontaneous voiding or SIC
| Bladder volume threshold (ml) |
||||||
|---|---|---|---|---|---|---|
| <400 | 400–800 | >800 | Total | |||
| POUR group a bladder volume | P400 | Count | 240 | 58 | 9 | 307 |
| % within max. bladder volume | 78.2 | 18.9 | 2.9 | |||
| P800 | Count | 338 | 87 | 6 | 434 | |
| % within max. bladder volume | 77.9 | 20.0 | 1.4 | |||
| Spontaneous voiding overall | Spontaneous voiding >threshold | SIC incl. SIC refusals b | ||||
| POUR group spontaneous voiding or SIC | OR 3.13 (1.84–5.35) c | |||||
| P400 | Count | 43 | 30/67 | 44/307 | ||
| % | 14 | 44.8 | 14.3 | |||
| P800 | Count | 41 | 3/6 | 22/434 | ||
| % | 9.4 | 50 | 5 | |||
OR = odds ratio; POUR = postoperative urinary retention; SIC = sterile intermittent catheterization.
Bladder volume proportions between groups (p > 0.05).
Ten patients (nine in the POUR-400 and one in the POUR-800 group) refused SIC despite fulfilling the required criteria.
Chi-square < 0.001.
Patients in the POUR-400 group predominantly underwent SIC as they reached the 400 ml threshold (37/44 [84.1%]), but without urinary urge, whereas 19/22 (86.40%) in the POUR-800 group reported urinary urge and needed SIC before reaching the 800 ml threshold, but no patients reported painful retention.
Of the patients, 28% (n = 67) In the POUR-400 group ended up with a scanned maximum bladder volume of >400 ml, including 2.9% having >800 ml, with a maximum of >999 ml. In the POUR-800 ml group, 1.4% of patients (n = 6) ended up exceeding the threshold volume, with a maximum scanned bladder volume of >999 ml (Table 2). In total, 84/741 (11.3%) patients voided spontaneously during the PACU stay, with no difference between the POUR groups (Table 2).
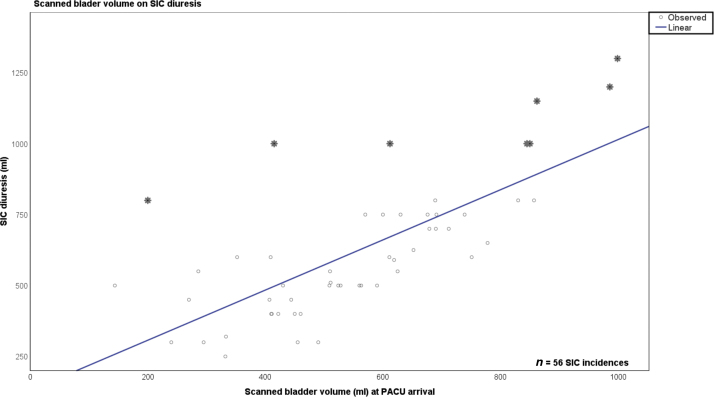
3.1.1. Bladder volume and SIC diuresis
The mean SIC diuresis was normally distributed with 625 ml (median 590 ml, interquartile range [IQR] 300 ml) and with a maximum of 1300 ml, with no significant difference between the POUR groups. A linear regression model showed a beta coefficient (β) of 0.884 (R2 = 0.525), equivalent to a relatively strong correlation between the scanned bladder volume at PACU arrival and SIC diuresis. We observed eight outliers (14%), six in the POUR-400 and two in the POUR-800 groups, exceeding the threshold of 800 ml (see Fig. 3).
Fig. 3.
Linear regression of scanned bladder volume versus SIC diuresis. PACU = postanesthesia care unit; SIC = sterile intermittent catheterization.
3.1.2. Univariate risk factors for SIC/CAD
SIC was performed in 35/296 (11.8%) patients given supplemental opioids in the PACU compared with that performed in 35/445 (7%) of patients who did not receive supplemental opioid (p = 0.023). For all other explanatory factors, no significant differences were found in SIC/CAD incidence between the POUR groups. Anesthesia duration did not differ between groups (SIC: mean 128 min [95% CI 113–142] vs no SIC: mean 112 min [95% CI 108–116]); neither fluid balance at the end of surgery influenced whether patients were having SIC (+687 ml, 95% CI 602–772) or not having SIC (+612 ml, 95% CI 599–635), nor gender, age, or supplemental opioid use during surgery (n = 576, 77.7%) affected SIC incidence or differed between the POUR groups (p > 0.05 for all).
3.1.3. Discharge from PACU
Two out of 35 patients (5.7%) in the POUR-400 group were placed with a permanent indwelling CAD at discharge from the PACU. None of the nine patients in the POUR-400 group, who reached the bladder threshold volume at PACU discharge and refused to have SIC, underwent catheterization at the ward. Five other patients underwent SIC at the ward.
In the POUR-800 group, two out of 21 patients who had SIC and three other patients (mean =219 ml, minimum =60 ml, maximum =318 ml) without SIC in the PACU were placed with a CAD. Five additional POUR-800 patients underwent subsequent SIC at the ward.
Accordingly, the total OR for being placed with a CAD was 2.09 (95% CI 1.27–3.46) times higher in the POUR-400 group (n = 40/307, 13.0% excluding nine refusals) than in the POUR-800 group (n = 29/434, 6.7% excluding one refusal) during the entire perioperative period. In patient records, no serious events were reported among patients with transient high bladder volume or those undergoing procedures of SIC/CAD.
By considering different bladder volume thresholds and number of patients having SIC or CAD during the entire observation period (postoperative days 0–1), in total 40/307 (13.0%) in the POUR-400 group and 26/434 (6.0%) in the POUR-800 group may be classified as having POUR.
3.2. Secondary outcomes
At anesthesia arrival, 435 (59.9%) patients had documented spontaneous voiding within an hour before surgery. Twenty-seven (3.7%) patients had no voiding, and in 275 cases (37.1%) no documentation on presurgery voiding could be obtained from the patient records. No POUR group difference was observed on these patterns.
At PACU arrival, 160 patients (21.8%) had a bladder volume of >400 ml (Table 2), thus exceeding the institution’s acceptable maximum of an intraoperative bladder volume of 400 ml. The initial bladder scan revealed that those documented with no voiding ahead of surgery had a significantly higher mean bladder volume (Table 3). A sensitivity analysis, where we assumed that the patients with unknown status all either had voided or had not, was still statistically significant, favoring patients who had documented voiding ahead of surgery (p = 0,003). Additional findings showed that patients in the POUR-400 group with no voiding ahead of surgery were more likely to reach the maximum bladder volume threshold (57.1%) than those who had voided (24.6%) or had unknown voiding status (25.4%).
Table 3.
Voiding before surgery and bladder volume at PACU arrival
| N | Mean (ml) | Standard deviation | |
|---|---|---|---|
| Voiding 1 h before surgery a | |||
| Yes | 435 | 245.8 | 194.4 |
| No | 27 | 376.1 | 235.4 |
| Unknown | 273 | 281.7 | 201.4 |
| Total | 735 | 263.9 | 200.3 |
ANOVA = analysis of variance; PACU = postanesthesia care unit.
ANOVA between group difference (p = 0.001; missing values = 6).
An explorative linear regression model (R2 = 0.046 [adjusted R2 = 0.039]) suggested that anesthesia duration (p < 0,001) and BMI (p = 0.037) were significantly associated with bladder volume at PACU arrival. Fluid balance at the end of surgery was not associated with bladder volume, nor was gender or age (p > 0.05).
4. Discussion
The current study showed that implementation of an ultrasound-guided POUR protocol significantly reduced the need for SIC/CAD in the PACU, without increased SIC/CAD at the wards. Cases of bladder volumes severely exceeding thresholds were also reduced despite the increased threshold. Thus, we observed a 65% reduction in catheterization at the PACU, equivalent to approximately 1000 fewer SIC procedures being performed annually.
The POUR incidence of 9% observed is relatively low compared with reports ranging from 5% to 70%, but marginally higher than seen in a comparable Danish study having a POUR threshold of 600 ml and an incidence of 5% [9]. However, the highest risk for SIC or CAD in our study was not POUR but was related to the defined bladder volume threshold algorithm of 400 ml, suggesting this limit to be less sensitive toward true POUR, patient (dis)comfort, and potential bladder complications. Accordingly, our finding based on a pragmatic prospective surveillance of two cohorts without intended need of intraoperative catheterization confirms the findings from randomized controlled trials (RCTs) suggesting lower SIC incidences without compromising patient safety, when elevating the bladder volume threshold to 800 ml or using an individualized index [11], [12].
The risk of traumatic and spontaneous bladder rupture is rare, which has predominantly been seen as a severe complication in hospitalized patients undergoing surgical and endoscopic procedures [13], or in cases where obvious signs of urinary retention have been ignored, resulting in severe bladder function damage [14]. However, patients with POUR may be vulnerable for developing bladder atrophy, urinary incontinence, and hypertension [1], [4]. Catheterization associated with hospitalization and surgery not in the least constitutes a significant risk of UTI, which is the most prevalent cause of all nosocomial infections [15], [16]. The present study, based on clinical evidence, demonstrate that underlying guidelines may independently affect clinical outcomes of catheterization even though clinical circumstances did not differ between the groups.
The present study suggests that a transient high bladder volume may be tolerated during short-term surgery, when taking several coherent preventative factors into account. First, patients should be able to collaborate adherently to presurgery fast track procedures, including voiding 1 h in advance of surgery [17], [18]. One should not ignore bladder complaints and symptoms during the perioperative course, which forms the fundamental concept of the applied POUR-800 algorithm. We found that patients who had micturition urge or bladder symptoms represented a clear majority (>80%) of those having SIC in the 800 ml threshold group, thus indicating the patients’ ability to cooperate in the PACU. Importantly, we found that among eight patients having SIC (six from the POUR-400 and two from the POUR-800 group), diuresis was extensively higher (on average 30.5% and with a maximum bladder volume of 1300 ml) than measured on the bladder scan, underscoring the crucial importance of involving patients and symptoms in clinical judgment. Furthermore, we also found that a documented no presurgery voiding was significantly associated with an increased bladder volume at the PACU for all patients, which was similar to previous findings [19]. Thus, we recommend that more efforts should be made by the staff to secure this crucial presurgical procedure, bearing in mind that environmental factors at the operating theater and time for patient education remain the challenges during the presurgical course [20].
The POUR algorithm includes regular bladder scans performed by the nurses at PACU and occasionally during the intraoperative course. The procedure was fully implemented at our institution, and <1% of patients of the total cohort were not scanned at PACU arrival. Among them, two patients expressed bladder complaints and were having SIC, underscoring the importance of a complementary patient communicative and objective approach ahead of SIC decision.
The obvious advantage of implementing a standard bladder scan is the avoidance of long-term excessive bladder volumes. SIC diuresis was equal in both groups, indicating no prolonged period of POUR in the 800 ml group. Accordingly, we strongly recommend regular bladder scan as a prerequisite in urinary bladder damage, POUR prevention, and reducing unnecessary catheterization.
Opioid use at PACU was significantly associated with an increased incidence of SIC, with no between-group differences observed. Systemic opioid analgesia has been established as a risk factor for POUR due to a direct impact on the detrusor muscle function by inhibiting the release of acetylcholine from the parasympathetic sacral neurons that control detrusor contractility [4], [21]. On the contrary, pain relief is one of the primary cornerstones in fast track surgery that may enhance early mobilization [22]. Mobilization at PACU has been documented to reduce POUR frequency in patients undergoing surgery for cervical or lumbar disc herniation [23].
4.1. Limitations
Our study was not an RCT comparing the two thresholds and not blinded, potentially allowing a bias. However, data were prospectively recorded under similar circumstances in two adjacent time periods, without other institutional changes regarding POUR or related factors. Thus, the cohorts were comparable, and the study aim was to assess the effect of the POUR algorithm under normal clinical circumstances, without excluding patients, thus increasing internal and external validity. Our study could have been improved by implementing assessment of long-term urinary tract complications and not just during the hospital stay (eg, the IPSS questionnaire) [24], and a more thorough assessment of preoperative voiding and bladder volume could have increased the precision of these risk factors for excessive postoperative bladder volume. Instead, we performed a sensitivity analysis, and our study shows a future area for improvement to reduce bladder volumes. However, these questions have been addressed in the RCTs forming the basis for this study.
5. Conclusions
Implementation of a standardized ultrasound-guided protocol with volume-dependent scan intervals and an evidence-based catheterization threshold of 800 ml decreases the need for SIC without increasing the need for urinary catheterization at the wards.
Author contributions: Tom Møller had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Aasvang, Engedal.
Acquisition of data: Engedal, Plum, Aasvang.
Analysis and interpretation of data: Møller, Aasvang.
Drafting of the manuscript: Møller, Aasvang.
Critical revision of the manuscript for important intellectual content: Engedal, Plum, Aasvang, Møller.
Statistical analysis: Møller, Aasvang.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: None.
Other: None.
Financial disclosures: Tom Møller certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
CRediT authorship contribution statement
Tom Møller: Formal analysis, Validation, Data curation, Writing - original draft, Visualization. Mette S. Engedal: Investigation, Resources, Visualization. Lise M. Plum: Investigation, Resources, Visualization. Eske K. Aasvang: Conceptualization, Validation, Data curation, Writing - original draft, Visualization, Supervision.
Associate Editor: Silvia Proietti
Contributor Information
Tom Møller, Email: tom.moeller@regionh.dk.
Eske K. Aasvang, Email: Eske.Kvanner.Aasvang.01@regionh.dk.
References
- 1.Wishart S.M. Decreasing the incidence of postoperative urinary retention and incontinence with total joint replacement patients after spinal anesthesia in the postanesthesia care unit: a quality improvement project. J Perianesth Nurs. 2019;34:1040–1046. doi: 10.1016/j.jopan.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Kowalik U., Plante M.K. Urinary retention in surgical patients. Surg Clin North Am. 2016;96:453–467. doi: 10.1016/j.suc.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Bjerregaard L.S., Bagi P., Kehlet H. Postoperative urinary retention (POUR) in fast-track total hip and knee arthroplasty. Acta Orthop. 2014;85:8–10. doi: 10.3109/17453674.2014.881683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldini G., Bagry H., Aprikian A., Carli F. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology. 2009;110:1139–1157. doi: 10.1097/ALN.0b013e31819f7aea. [DOI] [PubMed] [Google Scholar]
- 5.Balderi T., Mistraletti G., D’Angelo E., Carli F. Incidence of postoperative urinary retention (POUR) after joint arthroplasty and management using ultrasound-guided bladder catheterization. Minerva Anestesiol. 2011;77:1050–1057. [PubMed] [Google Scholar]
- 6.Choi S., Awad I. Maintaining micturition in the perioperative period: strategies to avoid urinary retention. Curr Opin Anaesthesiol. 2013;26:361–367. doi: 10.1097/ACO.0b013e32835fc8ba. [DOI] [PubMed] [Google Scholar]
- 7.Scholten R., Kremers K., van de Groes S.A.W., Somford D.M., Koeter S. Incidence and risk factors of postoperative urinary retention and bladder catheterization in patients undergoing fast-track total joint arthroplasty: a prospective observational study on 371 patients. J Arthroplasty. 2018;33:1546–1551. doi: 10.1016/j.arth.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Hollman F., Wolterbeek N., Veen R. Risk factors for postoperative urinary retention in men undergoing total hip arthroplasty. Orthopedics. 2015;38:e507–11. doi: 10.3928/01477447-20150603-59. [DOI] [PubMed] [Google Scholar]
- 9.Dreijer B., Møller M.H., Bartholdy J. Post-operative urinary retention in a general surgical population. Eur J Anaesthesiol. 2011;28:190–194. doi: 10.1097/EJA.0b013e328341ac3b. [DOI] [PubMed] [Google Scholar]
- 10.Ghuman A. Urinary retention in early urinary catheter removal after colorectal surgery. Am J Surg. 2018;215:949–952. doi: 10.1016/j.amjsurg.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Bjerregaard L.S. Postoperative urinary catheterization thresholds of 500 versus 800 ml after fast-track total hip and knee arthroplasty: a randomized, open-label, controlled trial. Anesthesiology. 2016;124:1256–1264. doi: 10.1097/ALN.0000000000001112. [DOI] [PubMed] [Google Scholar]
- 12.Brouwer T.A. Postoperative bladder catheterization based on individual bladder capacity: a randomized trial. Anesthesiology. 2015;122:46–54. doi: 10.1097/ALN.0000000000000507. [DOI] [PubMed] [Google Scholar]
- 13.Phillips B. Trauma to the bladder and ureter: a review of diagnosis, management, and prognosis. Eur J Trauma Emerg Surg. 2017;43:763–773. doi: 10.1007/s00068-017-0817-3. [DOI] [PubMed] [Google Scholar]
- 14.Joelsson-Alm E., Nyman C.R., Svensén C., Ulfvarson J. Micturition problems after bladder distension during hospitalization in Sweden: "I’m not ill, just damaged for the rest of my life". Nurs Res. 2014;63:418–425. doi: 10.1097/NNR.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 15.Parker D. Nursing interventions to reduce the risk of catheter-associated urinary tract infection. Part 1: catheter selection. J Wound Ostomy Continence Nurs. 2009;36:23–34. doi: 10.1097/01.WON.0000345173.05376.3e. [DOI] [PubMed] [Google Scholar]
- 16.Iacovelli V. Nosocomial urinary tract infections: a review. Urologia. 2014;81:222–227. doi: 10.5301/uro.5000092. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y. Application of fast track surgery in routine nursing for patient with colorectal cancer. Saudi J Biol Sci. 2017;24:1939–1942. doi: 10.1016/j.sjbs.2017.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hjort Jakobsen D., Rud K., Kehlet H., Egerod I. Standardising fast-track surgical nursing care in Denmark. Br J Nurs. 2014;23:471–476. doi: 10.12968/bjon.2014.23.9.471. [DOI] [PubMed] [Google Scholar]
- 19.Hansen B.S., Soreide E., Warland A.M., Nilsen O.B. Risk factors of post-operative urinary retention in hospitalised patients. Acta Anaesthesiol Scand. 2011;55:545–548. doi: 10.1111/j.1399-6576.2011.02416.x. [DOI] [PubMed] [Google Scholar]
- 20.Kruzik N. Benefits of preoperative education for adult elective surgery patients. AORN J. 2009;90:381–387. doi: 10.1016/j.aorn.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Petros J.G., Mallen J.K., Howe K., Rimm E.B., Robillard R.J. Patient-controlled analgesia and postoperative urinary retention after open appendectomy. Surg Gynecol Obstet. 1993;177:172–175. [PubMed] [Google Scholar]
- 22.Kehlet H., Wilmore D.W. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. 2008;248:189–198. doi: 10.1097/SLA.0b013e31817f2c1a. [DOI] [PubMed] [Google Scholar]
- 23.Hansen Ab, Olsen Ks. The number of in-out catheterisations is reduced by mobilising the postoperative patient with bladder needs to the toilet in the recovery room: A randomised clinical trial. Eur J Anaesthesiol. 2015;32:486–492. doi: 10.1097/EJA.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 24.Bjerregaard L.S. Incidence of and risk factors for postoperative urinary retention in fast-track hip and knee arthroplasty. Acta Orthop. 2015;86:183–188. doi: 10.3109/17453674.2014.972262. [DOI] [PMC free article] [PubMed] [Google Scholar]