Abstract
Two nomograms have been developed to predict the outcome of positron emission tomography (PET)/computed tomography (CT) imaging with68Ga-labeled ligands for prostate-specific membrane antigen (68Ga-PSMA) for patients with rising prostate-specific antigen after radical prostatectomy (RP). These nomograms quantify the ability of PSMA PET/CT to detect prostate cancer recurrences, and therefore provide critical information in determining the optimal timing for PSMA PET/CT in guiding salvage therapies. We validated the ability of these nomograms to accurately predict PET/CT outcome using another ligand tracer, 18F-DCFPyL. The external validation cohort consisted of 157 men from the Prostate Cancer Network Netherlands who underwent 18F-DCFPyL PET/CT to guide salvage therapies after RP. The nomogram of Rauscher et al (predicting a positive scan) showed accurate prediction of 50–80% (discrimination 0.68, 95% confidence interval [CI] 0.59–0.76). The nomogram of Luiting et al (predicting recurrence outside the prostatic fossa) showed accurate prediction for predicted probability values between 15% and 65%, with a small degree of overestimation for predicted probability values between 30% and 50% (discrimination 0.74, 95% CI 0.28–1.24). According to calibration curves, discrimination results, and decision curve analysis, we conclude that clinicians can use these 68Ga-PSMA–based nomograms to predict 18F-DCFPyL PET/CT outcome. These nomograms improve shared decision-making in determining the optimal time to initiate PSMA PET/CT–guided salvage therapies.
Patient summary
Prediction tools developed for prostate scans (positron emission tomography, PET) using one type of radioactive tracer (chemicals labeled with gallium-68) are also accurate in predicting scan findings with another tracer (a chemical labeled with fluorine-18). Our study confirms that these tools can be used to guide decisions on the timing of treatments for prostate cancer recurrence.
Keywords: Prostatic neoplasms, Prostate-specific membrane antigen, Positron emission tomography/computed tomography, Nomograms, Biochemical recurrence, Prostatectomy, Salvage therapy
Early visualization of prostate cancer (PCa) recurrences in men with rising prostate-specific antigen (PSA) after radical prostatectomy (RP) is relevant for optimizing salvage treatment decisions [1]. Currently, positron emission tomography/computed tomography using prostate-specific membrane antigen ligands (PSMA PET/CT) is the most sensitive imaging tool to localize PCa recurrences [1]. The detection rate of PSMA PET/CT improves with increasing PSA, whereas the efficacy of salvage radiotherapy to the prostatic fossa (SRT) decreases with increasing PSA [1], [2], [3]. Consequently, according to individual tumor characteristics, there should be an optimal PSA level for every patient after RP at which to perform PSMA PET/CT–guided SRT. Nomograms for the 68Ga-PSMA-11 tracer have been developed to assist clinicians in deciding the optimal timing for PSMA PET/CT [4], [5], [6]. The nomograms quantify the detection rate of PSMA PET/CT on the basis of patient characteristics. There is an increase in the use of 18F-labeled PSMA ligands in clinical practice because of their good (commercial) availability and potentially better imaging characteristics compared to 68Ga-labeled PSMA ligands [7]. However, the 68Ga-PSMA-11 nomograms are not validated for the 18F-labeled PSMA ligands. It is therefore questionable whether 68Ga-based nomograms can accurately predict 18F-PSMA PET/CT outcomes. We performed an external validation of the 68Ga-PSMA nomograms of Rauscher et al [4] (nomogram 1) and Luiting et al [5] (nomogram 2) to evaluate the predictive accuracy for 18F-DCFPyL PET/CT outcomes (a second-generation 18F-PSMA ligand) for men with rising PSA after RP.
Nomogram 1 predicts PSMA PET/CT positivity after RP on the basis of PSA at imaging, Gleason grade group (GG) of the RP specimen, and concurrent use of androgen deprivation therapy (ADT) [4]. Nomogram 2 predicts PSMA PET/CT detection of PCa recurrences outside the prostatic fossa on the basis of PSA at imaging, GG for the RP specimen, pathological T and N stages, possible earlier SRT, and surgical margin status [5]. The validation cohort consisted of 157 men with rising PSA after RP from the Prostate Cancer Network Netherlands who underwent 18F-DCFPyL PET/CT without concurrent ADT. The imaging procedure was as previously described [8]. We evaluated the performance of the two nomograms in terms of their discrimination, calibration, and clinical utility. Missing data were imputed five times with predictive mean matching. All statistical analyses were performed with R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
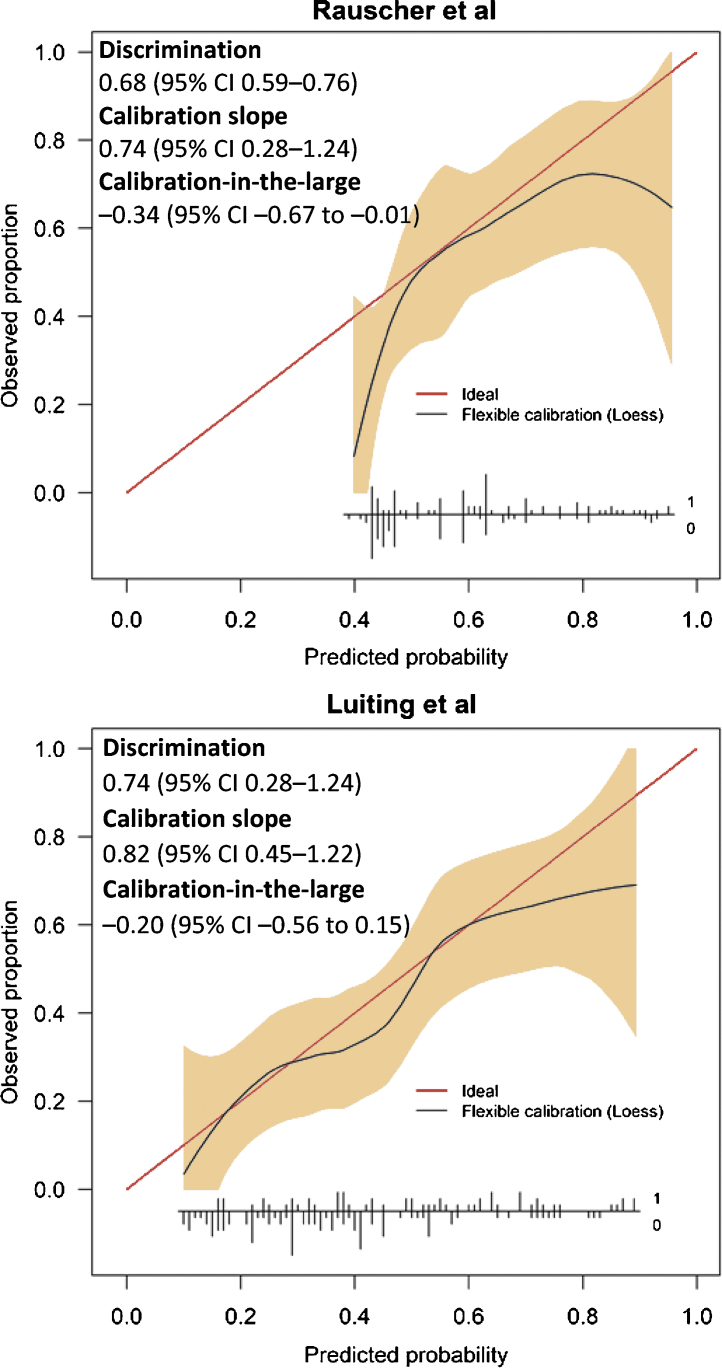
The median PSA at the time of 18F-DCFPyL PET/CT was 0.30 ng/ml (interquartile range 0.23–0.70). In total, 81 patients (51%) showed increased expression at 18F-DCFPYL PET/CT. A local PCa recurrence was detected in 21 patients (13%) and PCa recurrence outside the prostatic fossa in 60 patients (38%). The patient characteristics are listed in Supplementary Table 1. The external validation results are visualized and explained in Figure 1. The calibration curves show that nomogram 1 correctly predicted the probability of having positive 18F-DCFPYL PET/CT for predicted probability values between 50% and 80% and that nomogram 2 correctly predicted the probability of detecting metastatic PCa recurrence on 18F-DCFPYL PET/CT for predicted probability values between 15% and 65%, although there was a small degree of overestimation for predicted probability values between 30% and 50%. Decision curve analysis (DCA) showed a clinical net benefit from nomogram 1 from a predicted probability of 43% onwards (representing the minimum predicted probability) and from 20% onwards from nomogram 2 (Supplementary Fig. 1). From the calibration curves, discrimination results, and DCA, we conclude that clinicians can use the 68Ga-PSMA-based nomograms to predict 18F-DCFPyL PET/CT outcomes. However, nomogram 1 substantially overestimated the probability of detecting PCa recurrence for predicted probability values below 50%. This is most likely caused by the higher prevalence of positive PSMA PET/CT findings in the original cohort compared to the validation cohort (64.7% vs 51%) and the use of ADT in the original cohort. We know that ADT affects PSMA receptor expression on PCa cells and therefore influences the effect of other predictors such as the GG of the RP specimen on PSMA PET/CT positivity. Moreover, use of ADT at the time of the scan was the most important predictor in nomogram 1 and this validation cohort included only patients without ADT at the time of the scan. Because of the overestimation by nomogram 1, nomogram 2 is clinically most appropriate for a predicted probability range of 20–50%. Hence, nomogram 2 can be used to determine the optimal time for PSMA-guided early SRT for men with rising PSA after RP (for both 68Ga-PSMA and 18F-DCFPyL).
Fig. 1.
Calibration curves for the compact nomogram of Rauscher et al [4] (nomogram 1) predicting the probability of positive prostate-specific membrane antigen–based positron emission tomography/computed tomography and the nomogram of Luiting et al [5] (nomogram 2) predicting the probability of detecting prostate cancer recurrence outside the prostatic fossa. Discrimination was quantified in terms of the area under the receiver operating characteristic curve. Calibration was quantified via calibration-in-the-large and the calibration slope. The red line denotes perfect prediction (predicted probability is equal to observed proportion). The black line denotes to the actual observed proportion as a function of the predicted probability. When the black line is below the red line, the nomogram gives an overprediction. When the black line is above the red line, the observed proportion is higher than the predicted probability. The gray area denotes the 95% confidence interval (CI) of the flexible calibration. Vertical inset lines indicate the frequency distribution of predicted probabilities and if there were either positive findings/showed metastasis (marked as 1) or negative findings/showed no metastasis (marked as 0).
For men with rising PSA after RP, the decision must be made whether and when to administer early SRT. A recently published meta-analysis showed that early SRT for men with localized or locally advanced PCa is noninferior to adjuvant radiotherapy following RP [9]. Consequently, administering SRT to all patients with detectable (ultrasensitive) PSA after RP will most likely represent overtreatment and unnecessary toxicity. First, a high percentage of disease recurrence is located beyond the prostatic fossa at PSMA PET/CT in men with rising PSA after RP, and thus outside the standard SRT field. Second, a substantial percentage of patients with PSA <0.05 ng/ml after RP will not progress to PSA ≥0.2 ng/ml within 3 yr and thus would never benefit from SRT. Therefore, the European Association of Urology (EAU) guidelines recommend a “wait and see” strategy for patients with favorable factors, such as time to biochemical recurrence >3 yr, ≤pT3a, and GG ≤2/3 (EAU low-risk biochemical recurrence) [1]. SRT administration for patients whose PCa recurrence is located outside the prostatic fossa will cause unnecessary toxicity, so ideally these patients should be identified. PSMA PET/CT can be used to better identify these patients with increasing PSA, although our results also highlight the importance of the risk characteristics of the primary tumor. To elaborate, if a clinician aims to prevent approximately 20–30% of all misdirected SRT by using PSMA PET/CT, patients with high-risk characteristics can undergo PSMA PET/CT at low PSA of 0.1–0.2 ng/ml (Table 1). These are the patients who are also most likely to benefit from early SRT. Besides preventing misdirected SRT by detecting metastatic PCa recurrences, PSMA PET/CT can detect local recurrence. Detection of a local recurrence is a positive predictor for SRT efficacy [8]. Up to now, PSMA PET/CT has mainly been used to exclude metastatic disease because of radioactivity considerations for the bladder, whereas local recurrences often remain undetected. Further research is warranted to improve detection of local recurrences and confirm the benefit of this detection.
Table 1.
Probability as predicted by the nomogram of Luiting et al for detection of prostate cancer recurrence outside the prostatic fossa in four different clinical scenarios stratified by PSA value
| PSA (ng/ml) | Predicted probability (%) |
|||
|---|---|---|---|---|
| pT2 pN0R1 GG2 | pT2 pN0R1 GG3 | pT2 pNxR0 GG3 | pT3b pN0R0 GG5 | |
| 0.1 | 6 | 11 | 20 | 34 |
| 0.2 | 11 | 19 | 31 | 48 |
| 0.3 | 14 | 25 | 39 | 57 |
| 0.4 | 18 | 30 | 45 | 62 |
| 0.5 | 21 | 34 | 50 | 67 |
GG = Gleason grade group; PSA = prostate-specific antigen.
An important limitation of the current study is that lesions detected by PSMA PET/CT were not confirmed by histopathology. However, the study represents current daily practice, in which PSMA PET/CT without histopathology confirmation often impacts management strategies.
In summary, our results show that 68Ga-PSMA-11–based nomograms predicting PSMA PET/CT outcome for men with rising PSA after RP also accurately predict 18F-DCFPyL PET/CT outcome. However, the overestimation by nomogram 1 until a predicted probability limits its clinical utility. The validated nomograms provide valuable information for determining the optimal time for PSMA PET/CT–guided SRT. Prospective studies evaluating SRT efficacy in the PSMA PET/CT era are warranted to increase insights into the outcomes of PSMA PET/CT–guided SRT.
Author contributions: Henk B. Luiting had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Luiting, Roobol, van Leeuwen.
Acquisition of data: Meijer, Vis, Donswijk, Oprea-Lager, Van der Poel, van Leeuwen.
Analysis and interpretation of data: Luiting, Remmers, Roobol, van Leeuwen.
Drafting of the manuscript: Luiting, Remmers, Roobol, van Leeuwen.
Critical revision of the manuscript for important intellectual content: Meijer, Vis, Donswijk, Oprea-Lager, Van der Poel, Emmett, Rauscher.
Statistical analysis: Remmers, Luiting.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Roobol, van Leeuwen.
Other: None.
Financial disclosures: Henk B. Luiting certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.euros.2021.04.002.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Mottet N., Cornford P., van den Bergh R.C.N. Arnhem, The Netherlands: European Association of Urology; 2020. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. https://uroweb.org/wp-content/uploads/EAU-EANM-ESTRO-ESUR-SIOG-Guidelines-on-Prostate-Cancer-2020.pdf
- 2.Tendulkar R.D., Agrawal S., Gao T. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol. 2016;34:3648–3654. doi: 10.1200/JCO.2016.67.9647. [DOI] [PubMed] [Google Scholar]
- 3.Fossati N., Karnes R.J., Cozzarini C. Assessing the optimal timing for early salvage radiation therapy in patients with prostate-specific antigen rise after radical prostatectomy. Eur Urol. 2016;69:728–733. doi: 10.1016/j.eururo.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Rauscher I., Duwel C., Haller B. Efficacy, predictive factors, and prediction nomograms for 68Ga-labeled prostate-specific membrane antigen–ligand positron-emission tomography/computed tomography in early biochemical recurrent prostate cancer after radical prostatectomy. Eur Urol. 2018;73:656–661. doi: 10.1016/j.eururo.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Luiting H.B., van Leeuwen P.J., Remmers S. Optimal timing for prostate specific membrane antigen positron emission tomography/computerized tomography in patients with biochemical recurrence after radical prostatectomy. J Urol. 2020;204:503–510. doi: 10.1097/JU.0000000000001012. [DOI] [PubMed] [Google Scholar]
- 6.Ceci F., Bianchi L., Borghesi M. Prediction nomogram for 68Ga-PSMA-11 PET/CT in different clinical settings of PSA failure after radical treatment for prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47:136–146. doi: 10.1007/s00259-019-04505-2. [DOI] [PubMed] [Google Scholar]
- 7.De Visschere P.J.L., Standaert C., Futterer J.J. A systematic review on the role of imaging in early recurrent prostate cancer. Eur Urol Oncol. 2019;2:47–76. doi: 10.1016/j.euo.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Meijer D., Luiting H.B., van Leeuwen P.J. Prostate specific membrane antigen positron emission tomography/computerized tomography in the evaluation of initial response in candidates who underwent salvage radiation therapy after radical prostatectomy for prostate cancer. J Urol. 2021;205:1100–1109. doi: 10.1097/JU.0000000000001437. [DOI] [PubMed] [Google Scholar]
- 9.Vale C.L., Fisher D., Kneebone A. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet. 2020;396:1422–1431. doi: 10.1016/S0140-6736(20)31952-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.