Abstract
Background
Intravesical bacillus Calmette-Guérin (BCG) instillation is a standard treatment for non–muscle-invasive bladder cancer (NMIBC); however, not all patients benefit from BCG therapy. Currently, no surrogate marker exists to predict BCG efficacy, and thereby, identify patients who will benefit from this treatment.
Objective
To evaluate the utility of urine Mycobacterium tuberculosis complex polymerase chain reaction (MTC-PCR) assay as a predictive marker for recurrence and progression following BCG therapy.
Design, setting, and participants
A prospective analysis was carried out for of intermediate- or high-risk NMIBC patients who received BCG instillation for the first time. Urine samples, for MTC-PCR assay, were collected at baseline and annually for up to 10 yr after the last BCG instillation, including induction and maintenance therapy. The first postoperative sample for MTC-PCR was taken at 1 yr from the last instillation.
Outcome measurements and statistical analysis
A survival analysis was performed using the Kaplan-Meier method, and risk factors for recurrence and progression after BCG treatment were assessed using Cox regression analysis.
Results and limitations
During follow-up (median: 57 mo), 468/521 samples (89.8%) were MTC-PCR positive, and 108/123 patients (87.8%) exhibited MTC-PCR positivity at least once. Five-year recurrence- and progression-free survival in patients who were not MTC-PCR positive was significantly lower than in patients who were MTC-PCR positive at least once (p < 0.001). Using multivariable Cox regression analysis, MTC-PCR positivity at least once was a significant prognostic factor for recurrence (hazard ratio [HR]: 36.782, p < 0.001) and progression (HR: 47.209, p < 0.001).
Conclusions
Patients who were not MTC-PCR positive, even once after BCG therapy, were extremely likely to exhibit recurrence and progression. Urine MTC-PCR may be an extremely useful, noninvasive surrogate marker to predict recurrence and progression following BCG therapy.
Patient summary
Urine Mycobacterium tuberculosis complex polymerase chain reaction may be a novel biomarker capable of identifying patients at risk of recurrence and progression after bacillus Calmette-Guérin (BCG) immunotherapy.
Keywords: Bladder cancer, Bacillus Calmette-Guérin, Non–muscle invasive, Mycobacterium tuberculosis complex polymerase chain reaction
Take Home Message
In intermediate- or high-risk non–muscle-invasive bladder cancer patients after intravesical bacillus Calmette-Guérin (BCG) therapy, Mycobacterium tuberculosis complex polymerase chain reaction (MTC-PCR) positivity at least once was a significant prognostic factor in recurrence. A new adequate biomarker, urine MTC-PCR, is very useful noninvasive surrogate marker to predict the recurrence after BCG therapy.
1. Introduction
Adjuvant intravesical instillation of bacillus Calmette-Guérin (BCG) has been used effectively against non–muscle-invasive bladder cancer (NMIBC) [1]. While numerous guidelines globally [2], [3], [4], [5], [6] recommend BCG for intermediate- or high-risk NMIBC patients, not every patient will benefit from BCG therapy [7]. The risk of failure of BCG treatment may be >40–50% in long-term follow-up [8], [9], and patients with NMIBC receiving BCG instillation exhibit 1- and 5-yr recurrence rates of approximately 25% and 40%, respectively [10].
Currently, markers used to predict the effects of BCG include clinicopathological [9] and molecular factors [11], but these markers lack utility in predicting BCG response [10]. As a consequence, the lack of adequate and highly specific noninvasive prognostic surrogate biomarkers that can accurately predict response to BCG remains an unmet medical need [12], [13].
Our hypothesis that long-term presence of BCG DNA in urine may predict intravesical BCG treatment responsiveness is based on reports that, while a delay in infectious BCG presentation is rare, it has been reported up to 3 yr after last BCG instillation [14], [15].
The aim of this single-center study is to examine whether urine mycobacterial DNA detected by Mycobacterium tuberculosis complex polymerase chain reaction (MTC-PCR) in NMIBC patients after BCG instillation is useful for predicting the long-term effects of BCG therapy.
2. Patients and methods
2.1. Clinical outline
Patients were eligible for inclusion in this prospective study if they presented with histologically confirmed NMIBC (intermediate or high recurrence risk according to the European Organization for Research and Treatment of Cancer [EORTC] risk table) [10], had undergone a transurethral resection of bladder tumor (TURBT), with or without random biopsies, followed by BCG. For patients with a bladder perforation during TURBT, the single immediate postoperative instillation of intravesical chemotherapy was not administered. If the detrusor muscle was not included in the primary TURBT specimen, patients with high-grade tumors underwent a second TURBT.
Bladder cancers (BCs) were staged according to the 2010 American Joint Committee on Cancer TNM classification system [16] and graded using the World Health Organization/International Society of Urologic Pathology classification system [17]. Patient characteristics, including recurrence risk classification according to EORTC recurrence risk classification [10] and Club Urologico Espanol de Tratamiento Oncologico (CUETO) recurrence risk classification [18], were documented.
All patients received intravesical instillation of BCG (Connaught strain, 3.77 × 108 colony-forming units [CFU]; Sanofi S.A., Paris, France, or Tokyo 172 strain, 48.77 × 108 CFU [19]; Japan BCG Supply, Tokyo, Japan) 6 wk after TURBT as induction therapy, given six to eight times weekly. Some patients underwent further instillations every 3–6 mo for 2 yr as maintenance therapy, which was generally three times at weekly intervals. The continuation of maintenance therapy was at the discretion of the attending physician and patient preference.
This study was performed in accordance with the Declaration of Helsinki. The internal review board and the local ethics committee authorized and approved the study (13-198). Written informed consent was obtained from each patient.
2.2. DNA extraction and urine MTC-PCR
Urine samples were collected for analysis at baseline (after TURBT and before initiation of BCG instillation), and every year after the last BCG instillation, including induction and maintenance for up to 10 yr. MTC-PCR results acquired during BCG treatment (induction and maintenance) were excluded from the data sets. MTC-PCR results acquired after the end of BCG treatment (induction or maintenance) were included in the data sets. A minimum of 50 ml freshly voided, midstream, clean catch urine was collected and stored immediately in aliquots at −80 °C until analysis. DNA was purified from the pellet using a QIAamp DNA kit (Qiagen, Hilden, Germany), and at least 2 ml of collected urine remained after centrifugation (5000 g/20 min).
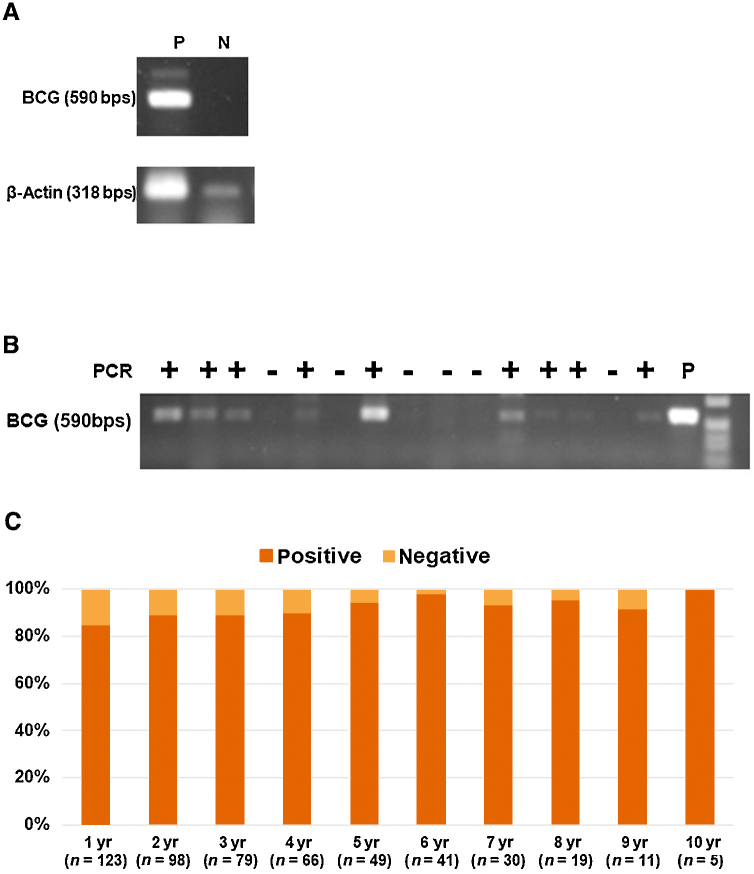
MTC-PCR was used to detect the presence of mycobacterial DNA (from M. tuberculosis complex [MTC] organisms) in urine samples, as previously described [20]. Primer pair A (5′-agagtttgatcctggctcag)/247 (5′-tttcacgaacaacgcgacaa) [21], [22] amplifies a 590 bp fragment, which contains the sequences for M. tuberculosis complex (MTC) [23]. MTC-PCR controls were prepared from urine collected 1 h after intravesical BCG instillation (positive control), and from the urine of patients without BC and urine tuberculosis on general wards (negative control; Fig. 1A).
Fig. 1.
Analysis of MTC-PCR products from DNA in urine. The products were separated by gel electrophoresis on a 1% agarose gel. (A) MTC-PCR controls were prepared from urine collected 1 h after intravesical BCG instillation (positive control), and from the urine of patients without BC on general wards (negative control). MTC-PCR for DNA coding for 168 rRNA resulted in the expected fragment of about 590 bp for the positive urine test. (B) We evaluated MTC-PCR using urine from enrolled patients. We have demonstrated that mycobacterial DNA can be detected in this way. (C) Percentage of positive and negative urine MTC-PCR results after intravesical BCG instillation therapy during the 10-yr follow-up. There was no significant difference in urine MTC-PCR positive rate for each year (p = 0.337). BC = bladder cancer; BCG = bacillus Calmette-Guérin; bps = base pairs; MTC-PCR = M. tuberculosis complex polymerase chain reaction; N = negative; P = positive; PCR = polymerase chain reaction.
2.3. Statistical analysis
Quantitative variables are shown as median and range with interquartile ranges (IQRs), and categorical variables as frequencies and proportions (percentages). Disease first recurrence was defined as the first pathologically confirmed BC relapse after adequate intravesical BCG treatment (a minimum of five or six induction therapies and two or three first maintenance therapies [6]), regardless of tumor stage and grade.
The primary endpoint of recurrence-free survival (RFS) was defined as the period between the last TURBT before BCG treatment and the date of the first pathologically confirmed recurrence.
The second endpoint was progression-free survival (PFS). Disease progression was defined according to the International Bladder Cancer Group consensus definition, in the presence of a ≥ T2 stage or lymph node positive (N+) disease or distant metastases [24].
Time to RFS and PFS was analyzed using Kaplan-Meier estimates and log rank tests. To assess the significant prognostic factors related to recurrence and progression during follow-up periods after the last intravesical BCG instillation, receiver-operating characteristic (ROC) curves, and univariate and multivariate Cox proportional hazard regression models were used to estimate hazard ratios (HR) with 95% confidence intervals (CIs).
All tests were two sided, and p < 0.05 was considered significant. All statistical tests were performed with R software v.3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Clinical and pathological characteristics
Of the 139 patients initially included in the study, 16 had missing data or their data were unavailable (Supplementary Fig. 1). Of the 123 evaluable patients (88.5%), 67 (54.5%) underwent instillation with the Connaught strain and 56 (45.5%) with the Tokyo 172 strain (Table 1). The median total number of BCG instillations was 8 (IQR: 8–9), and the overall median duration of follow-up was 57 mo (IQR: 33–100 mo).
Table 1.
Patient characteristics
| Characteristics | Evaluable patients (n = 123) |
|---|---|
| Age (yr), median (IQR) | 68 (63–75) |
| Gender, n (%) | |
| Male | 102 (82.9) |
| Female | 21 (17.1) |
| Smoking history, n (%) | |
| Smoker | 64 (52.0) |
| Nonsmoker | 27 (22.0) |
| Unknown | 32 (26.0) |
| PS, n (%) | |
| 0 | 117 (95.1) |
| 1 | 6 (4.9) |
| Recurrence history, n (%) | |
| Primary | 60 (48.8) |
| Recurrent | 63 (51.2) |
| Second TURBT, n (%) | |
| Yes | 43 (35.0) |
| No | 80 (65.0) |
| cT, n (%) | |
| cTa | 67 (54.5) |
| cT1 | 39 (31.7) |
| cTis | 17 (13.8) |
| Tumor grade, n (%) | |
| High | 86 (69.9) |
| Low | 37 (30.1) |
| EORTC recurrence risk, n (%) | |
| Intermediate-low | 39 (31.7) |
| Intermediate-high | 56 (45.5) |
| High | 28 (22.8) |
| EORTC progression risk, n (%) | |
| Intermediate | 51 (41.5) |
| High-low | 39 (31.7) |
| High-high | 33 (26.8) |
| CUETO recurrence risk, n (%) | |
| 0–4 | 27 (22.0) |
| 5–6 | 26 (21.1) |
| 7–9 | 40 (32.5) |
| 10–16 | 30 (24.4) |
| CUETO progression risk, n (%) | |
| 0–4 | 36 (29.3) |
| 5–6 | 20 (16.3) |
| 7–9 | 25 (20.3) |
| 10–16 | 42 (34.1) |
| Immediate postoperative instillation of intravesical chemotherapy, n (%) | |
| Yes | 30 (24.4) |
| No | 93 (75.6) |
| BCG strain | |
| Connaught | 67 (54.5) |
| Tokyo 172 | 56 (45.5) |
| Maintenance BCG, n (%) | |
| Yes | 25 (20.3) |
| No | 98 (79.7) |
BCG = bacillus Calmette-Guérin; cT = clinical T disease; CUETO = Club Urologico Espanol de Tratamiento Oncologico; EORTC = European Organization for Research and Treatment of Cancer; IQR = interquartile ranges; PS = performance status; TURBT = transurethral resection of bladder tumor.
3.2. MTC-PCR
At baseline, urine MTC-PCR was negative for all patients (Fig. 1B). Of the 521 urine samples analyzed by MTC-PCR assay, 468 samples (89.8%) were positive. During the 10-yr follow-up, there was no significant difference in the urine MTC-PCR positive rate for each year (p = 0.337; Fig. 1C). During follow-up, all patients (n = 103) in the nonrecurrence group exhibited MTC-PCR positivity at least once. In the recurrence group (n = 20), 15 patients (75.0%) were negative for all MTC-PCR tests during follow-up. There was no significant difference in time period until recurrence between the negative for all MTC-PCR tests group (median: 18 mo, IQR: 13–33.5 mo) and the MTC-PCR positive at least once group (median: 27 mo, IQR: 20–33 mo, p = 0.293).
Although there was no significant difference in the total number of intravesical BCG instillations (median: 8 vs 8, p = 0.647) between the MTC-PCR positive at least once group and the MTC-PCR negative for all MTC-PCR tests group, the number of patients with an MTC-PCR positive result at least once in the Connaught strain group was significantly higher (63/67 patients, 94.0%) than that in the Tokyo 172 strain group (45/56 patients, 80.4%, p = 0.021).
3.3. Prognostic factors for recurrence after BCG instillation
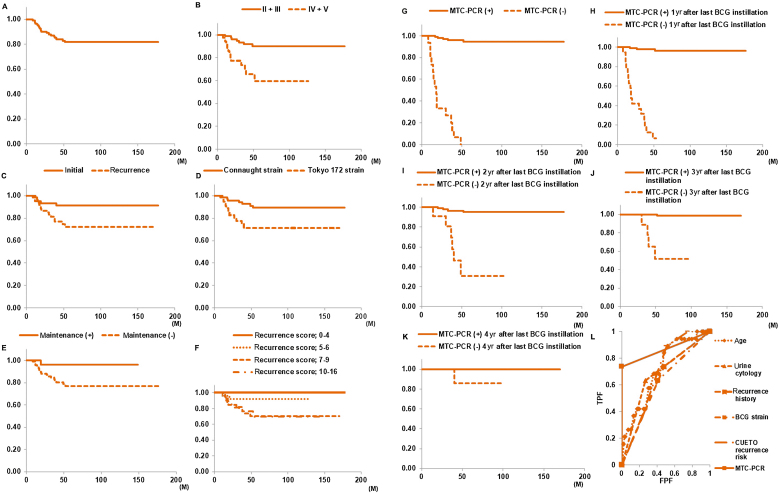
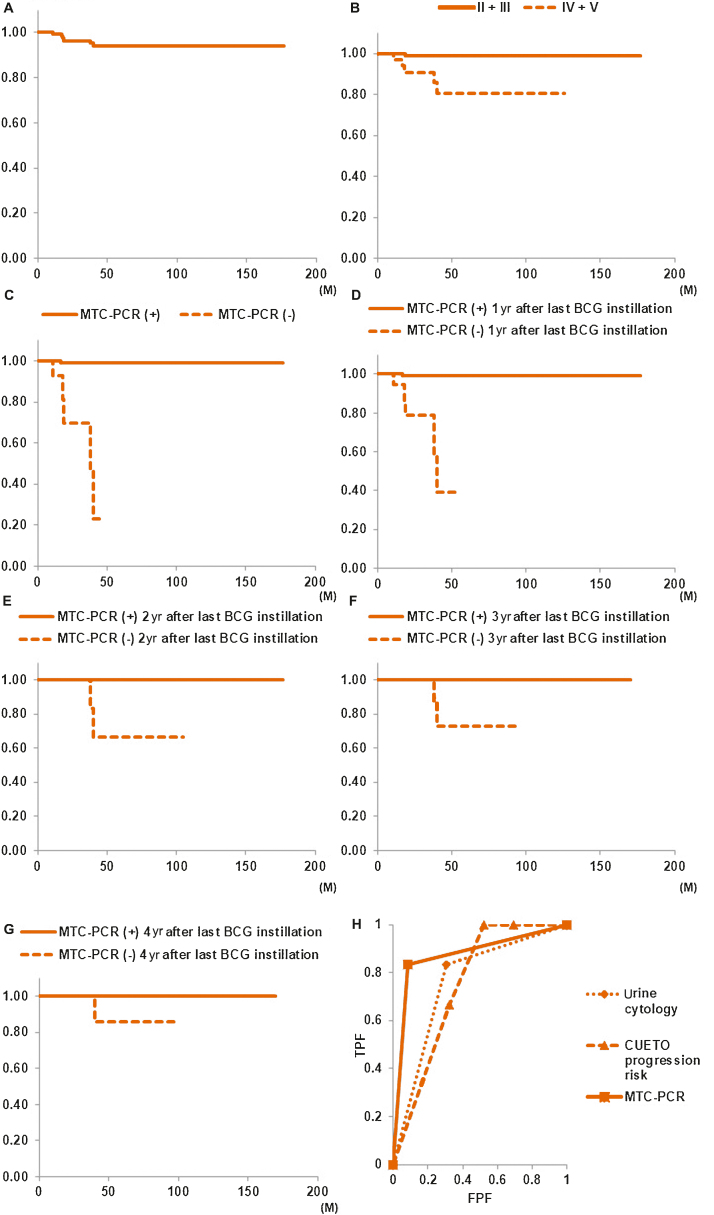
During follow-up, recurrences were observed in 20/123 patients (16.3%) after BCG, and in these patients, median time to recurrence was 19.0 mo (IQR: 14.75–34 mo). In all patients, RFS periods were 96.7% at 1 yr and 81.7% at 5 yr after the last BCG instillation (Fig. 2A).
Fig. 2.
Kaplan-Meier analysis to evaluate RFS in patients within each subgroup. Comparisons were performed by log-rank test. (A) RFS in all patients was 96.7% at 1 yr and 81.7% at 5 yr after the last BCG instillation. (B) Before TURBT, 5-yr RFS rate in the urine cytology positive group (classes IV and V, 59.7%) was significantly lower than that in the urine cytology negative group (classes II and III, 90.1%; p < 0.001). (C) Significant difference in 5-yr RFS rate between patients with initial BC (91.2%) and patients with recurrent BC (72.1%; p = 0.017). (D) The Tokyo 172 strain group had a significantly lower 5-yr RFS rate (71.3%) than the Connaught strain group (89.1%; p = 0.009). (E) RFS rate in the maintenance group was significantly higher than that in the only induction group (p = 0.043). (F) For the CUETO recurrence risk group, there were significant differences in 5-yr RFS rate between recurrence scores 0–4 (100.0%), 5–6 (92.0%), 7–9 (70.2%), and 10–16 (70.0%; p = 0.009). (G) Compared with patients with no positive MTC-PCR results, RFS rate in the group with a positive MTC-PCR result at least once was significantly higher (p < 0.001). Compared with patients with a negative MTC-PCR result, RFS rate in the group with a positive MTC-PCR result at (H) 1, (I) 2, (J) 3, and (K) 4 yr after the last BCG instillation was significantly higher (p < 0.001). (L) ROC curve results showed the area under the curve (AUC) values of five factors that were significantly different in univariate Cox analysis. The AUC values of age, urine cytology, recurrence history, BCG strain, CUETO recurrence risk, and MTC-PCR were 0.717 (95% CI: 0.600–0.835), 0.680 (95% CI: 0.560–0.800), 0.629 (95% CI: 0.515–0.742), 0.613 (95% CI: 0.491–0.735), 0.697 (95% CI: 0.598–0.795), and 0.868 (95% CI: 0.767–0.970), respectively. The AUC of MTC-PCR was significantly higher than that of age (p = 0.044), urine cytology (p = 0.017), recurrence history (p = 0.007), BCG strain (p < 0.0001), or CUETO recurrence risk (p = 0.023). BCG = bacillus Calmette-Guérin; CI = confidence interval; CUETO = Club Urologico Espanol de Tratamiento Oncologico; FPF = false positive fraction; MTC-PCR = M. tuberculosis complex polymerase chain reaction; ROC = receiver-operating characteristic; RFS = recurrence-free survival; TPF = true positive fraction; TURBT = transurethral resection of bladder tumor.
Kaplan-Meier plots showed a significant difference in 5-yr RFS rate between the pre-TURBT urine cytology positive group (classes IV and V) and urine cytology negative group (classes II and III; p < 0.001; Fig. 2B), between initial BC (91.2%) and recurrent BC (72.1%, p = 0.017; Fig. 2C), and between the Connaught strain group (89.1%) and the Tokyo 172 strain group (71.3%, p = 0.009; Fig. 2D). The RFS rate in the maintenance group was significantly higher than that in the only induction group (p = 0.043; Fig. 2E). Although there was no significant difference in the RFS rate between EORTC recurrence risk groups (p = 0.552; Supplementary Fig. 2G), for patients grouped according to the CUETO recurrence risk classification, there were significant differences in the 5-yr RFS rate between recurrence score categories (p = 0.009; Fig. 2F).
Examining the relationship between MTC-PCR results and recurrence, 5-yr RFS rate in the group with a positive MTC-PCR result at least once (94.5%) was significantly higher than in patients with no positive MTC-PCR results (0.0%, p < 0.001; Fig. 2G). Only 4.6% of patients (5/108) with a positive MTC-PCR result at least once developed tumor recurrence within 5 yr, compared with 100% of patients with no positive MTC-PCR results during follow-up. The RFS rate in patients with a positive MTC-PCR result from 1 to 4 yr after the last BCG instillation was significantly higher than that in patients with a negative MTC-PCR result (p < 0.001; Fig. 2H–K). There were no significant differences in RFS between other clinicopathological factors, including gender, smoking habits, performance status, clinical T disease (cT), presence or absence CIS, disease grade, EORTC recurrence risk group, second TURBT, and immediate postoperative instillation of intravesical chemotherapy (Supplementary Fig. 2A–I), as determined by log-rank statistical analyses.
Univariate Cox analysis indicated that age (p = 0.003), urine cytology before TURBT (p < 0.001), tumor status (primary or recurrent; p = 0.024), BCG strain (p = 0.012), CUETO recurrence risk (p = 0.003), and urine MTC-PCR outcome (p < 0.001) were significant predictive factors for disease recurrence in NMIBC patients treated with intravesical BCG (Table 2). Multivariate Cox analysis indicated that MTC-PCR positivity at least once was an independent risk factor for recurrence (p < 0.001; Table 2). MTC-PCR positivity at least once (by ROC curve analysis) was most predictive of recurrence during follow-up periods: sensitivity, 0.750; specificity, 1.000 (Supplementary Table 1); and area under the curve (AUC), 0.868 (Fig. 2L).
Table 2.
Univariate and multivariate Cox regression analyses for the prediction of disease recurrence in NMIBC patients treated with intravesical BCG
| NMIBC patients | Univariate analyses |
Multivariate analyses |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | 1.093 | 1.030–1.159 | 0.003 | 0.991 | 0.930–1.055 | 0.769 |
| Gender (male vs female) | 1.748 | 0.405–7.533 | 0.454 | |||
| Smoking history (smoker vs nonsmoker) | 0.711 | 0.233–2.173 | 0.549 | |||
| PS (1 vs 0) | 0.862 | 0.115–6.442 | 0.885 | |||
| Cytology (IV + V vs II + III) | 4.841 | 1.897–12.355 | <0.001 | 2.692 | 0.748–9.692 | 0.130 |
| Recurrence history (recurrent vs primary) | 3.222 | 1.170–8.872 | 0.024 | 2.499 | 0.592–10.549 | 0.213 |
| Second TURBT (yes vs no) | 0.641 | 0.233–0.763 | 0.389 | |||
| cT (cT1 vs cTa) | 1.091 | 0.603–0.974 | 0.774 | |||
| Tumor grade (high vs low) | 1.122 | 0.431–2.921 | 0.813 | |||
| CIS (positive vs negative) | 1.866 | 0.743–4.685 | 0.184 | |||
| Immediate postoperative instillation of intravesical chemotherapy (yes vs no) | 1.816 | 0.690–4.779 | 0.227 | |||
| BCG strain (Connaught vs Tokyo172) | 0.304 | 0.120–0.770 | 0.012 | 0.424 | 0.142–1.267 | 0.125 |
| Maintenance BCG (yes vs no) | 0.162 | 0.022–1.210 | 0.076 | |||
| Total number of BCG instillations | 0.986 | 0.883–1.101 | 0.805 | |||
| EORTC recurrence risk | 1.388 | 0.781–2.466 | 0.265 | |||
| CUETO recurrence risk | 2.154 | 1.305–3.557 | 0.003 | 1.459 | 0.529–4.024 | 0.466 |
| MTC-PCR | 63.050 | 20.131–197.473 | <0.001 | 36.782 | 10.072–134.321 | <0.001 |
BCG = bacillus Calmette-Guérin; CI = confidence interval; CIS = carcinoma in situ; cT = clinical T disease; CUETO = Club Urologico Espanol de Tratamiento Oncologico; EORTC = European Organization for Research and Treatment of Cancer; HR = hazard ratio; MTC-PCR = urine M. tuberculosis complex polymerase chain reaction; NMIBC = non–muscle-invasive bladder cancer; PS = performance status; TURBT = transurethral resection of bladder tumor.
3.4. Prognostic factors for progression after BCG instillation
During follow-up, progression was observed in six of 123 (4.9%) patients after BCG, and three patients exhibited an increase in T stage or grade. The 5-yr PFS rate was 94.2% (Fig. 3A).
Fig. 3.
(A) PFS in all patients was 99.2% at 1 yr and 94.2% at 5 yr after BCG instillation. (B) PFS rate in the pre-TURBT urine cytology positive group (classes IV and V) was significantly lower than that in the urine cytology negative group (classes II and III; p = 0.002). (C) PFS rate in the group with positive MTC-PCR results at least once was significantly higher than in patients with no positive MTC-PCR results (p < 0.001). Compared with patients with a negative MTC-PCR result, RFS rate in the group with a positive MTC-PCR result at (D) 1, (E) 2, (F) 3, and (G) 4 yr after the last BCG instillation was significantly higher (p < 0.001). (H) ROC curve results showed the area under the curve (AUC) values of three factors that were significantly different in univariate Cox analysis. The AUC values of urine cytology, CUETO progression risk, and MTC-PCR were 0.765 (95% CI: 0.596–0.934), 0.752 (95% CI: 0.631–0.874), and 0.875 (95% CI: 0.710–1.041), respectively. There were no significant differences between these factors. BCG = bacillus Calmette-Guérin; CI = confidence interval; CUETO = Club Urologico Espanol de Tratamiento Oncologico; FPF = false positive fraction; MTC-PCR = M. tuberculosis complex polymerase chain reaction; PFS = progression-free survival; ROC = receiver-operating characteristic; RFS = recurrence-free survival; TPF = true positive fraction; TURBT = transurethral resection of bladder tumor.
In the pre-TURBT urine cytology positive group (classes IV and V), PFS rate was significantly lower than that in the urine cytology negative group (classes II and III, p = 0.002; Fig. 3B). The PFS rate in the positive MTC-PCR results at least once group was significantly higher than that in patients with no positive MTC-PCR results (p < 0.001; Fig. 3C). Progression occurred in five of 15 patients (33.3%) in the no positive MTC-PCR results group, compared with just in one of 108 patients (0.9%) in the positive MTC-PCR result at least once group. The PFS rate in patients with a positive MTC-PCR result from 1 to 4 yr after the last BCG instillation was significantly higher than that in patients with a negative MTC-PCR result (p < 0.001; Fig. 3D–G). There were no significant differences in PFS for other clinicopathological factors (Supplementary Fig. 3A–M), as determined by log-rank analyses. Univariate Cox analyses demonstrated that urine cytology before TURBT (p = 0.017), CUETO progression risk (p = 0.049), and urine MTC-PCR outcome (p < 0.0001) were significant predictive factors of PFS (Table 3). Multivariate Cox analyses indicated that urine MTC-PCR outcome was a significant predictive factor of PFS (p < 0.001; Table 3). MTC-PCR positivity at least once (by ROC curve analysis) was most predictive of progression: sensitivity, 0.833; specificity, 0.915 (Supplementary Table 2), and AUC, 0.875 (Fig. 3H).
Table 3.
Univariate and multivariate Cox regression analyses for the prediction of disease progression in NMIBC patients treated with intravesical BCG
| NMIBC patients | Univariate analyses |
Multivariate analyses |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | 1.109 | 0.993–1.240 | 0.067 | |||
| Cytology (IV + V vs II + III) | 13.785 | 1.605–18.402 | 0.017 | 2.814 | 0.211–37.549 | 0.434 |
| Recurrence history (recurrent vs primary) | 2.167 | 0.396–11.852 | 0.373 | |||
| Second TURBT (yes vs no) | 0.389 | 0.045–3.328 | 0.389 | |||
| cT (cT1 vs cTa) | 1.918 | 0.703–5.239 | 0.204 | |||
| Tumor grade (high vs low) | 0.954 | 0.175–5.211 | 0.957 | |||
| CIS (positive vs negative) | 3.411 | 0.687–16.942 | 0.133 | |||
| Immediate postoperative instillation of intravesical chemotherapy (yes vs no) | 0.836 | 0.096–7.244 | 0.871 | |||
| BCG strain (Connaught vs. Tokyo172) | 0.576 | 0.114–2.899 | 0.503 | |||
| Maintenance BCG (yes vs no) | 0.615 | 0.072–5.286 | 0.658 | |||
| Total number of BCG instillations | 1.109 | 0.959–1.281 | 0.162 | |||
| EORTC progression risk | 1.768 | 0.666–4.694 | 0.253 | |||
| CUETO progression risk | 3.259 | 1.003–10.588 | 0.049 | 1.884 | 0.452–7.864 | 0.385 |
| MTC-PCR | 71.399 | 10.058–506.870 | <0.001 | 47.209 | 5.013–444.550 | <0.001 |
BCG = bacillus Calmette-Guérin; CI = confidence interval; CIS = carcinoma in situ; cT = clinical T disease; CUETO = Club Urologico Espanol de Tratamiento Oncologico; EORTC = European Organization for Research and Treatment of Cancer; HR = hazard ratio; MTC-PCR = urine M. tuberculosis complex polymerase chain reaction; NMIBC = non–muscle-invasive bladder cancer; TURBT = transurethral resection of bladder tumor.
4. Discussion
Although BCG therapy is widely accepted as standard therapy after TURBT, nearly 5% of patients develop systemic BCG infection [15], and there is a high rate of tumor recurrence and progression [6]. Furthermore, some patients do not benefit from BCG therapy [25], and we are currently unable to predict which patients respond to this treatment [26]. We report here for the first time that for patients who were not MTC-PCR positive even once after BCG therapy, this was a significant predictive factor for post-BCG recurrence and progression. While previous studies have shown an association between a positive UroVysion fluorescent in situ hybridization (FISH) assay with an increased risk of recurrence among patients who have undergone BCG therapy [25], these studies had limitations [27], [28], [29], [30]. FISH assays are generally more cumbersome to perform than polymerase chain reaction (PCR) tests and are also more expensive; PCR testing is a simpler assay to perform in a clinical setting.
The urine MTC-PCR assay used in this study to determine the response to BCG therapy is based on the amplification of the mycobacterial DNA fragment from the MTC [20]. The presence of long-term residual BCG organisms in urine has previously been reported. Bowyer et al [14] found persistent acid-fast bacilli in the urine of some patients after intravesical BCG therapy, and the presence of long-lasting and persistent BCG DNA in the bladder, measured by PCR, has also been demonstrated [31], [32]. The authors previously investigated urine culture for acid-fast bacilli for the detection of BCG DNA in urine, but only three of 58 (5.2%) MTC-PCR–positive patients had a positive urine culture after BCG instillation (data not shown), so this avenue was not pursued.
Our hypothesis was that the expression of BCG DNA in urine is crucial to sustain and determine the BCG treatment effect following therapy; MTC-PCR positivity persisted in many samples for a very long time after the last BCG instillation. There was no significant difference in the total number of BCG instillations in the MTC-PCR positive result at least once group, compared with the MTC-PCR negative group. Therefore, BCG instillation period does not appear to affect MTC-PCR positivity. However, the number of patients who were MTC-PCR positive at least once in the Connaught strain group was significantly higher than in the Tokyo 172 strain group. These findings may be the reason why a previous study by Niwa et al [33] suggested that the Connaught strain may be more effective than the Tokyo 172 strain.
Using ROC curve analyses and multivariate Cox analyses, MTC-PCR outcome provided a good predictive factor for recurrence and progression after BCG treatment. Although BCG is difficult to differentiate from other MTC bacteria using conventional methods, including regular PCR [34], all patients in this study had no history or signs of urogenital tuberculosis, as well as a negative MTC-PCR result before BCG instillation. Taking these findings into consideration, we propose that this method can adequately detect long-term urinary BCG DNA in urine. Ongoing local antigen stimulation may have an important role to play in the mode of action of BCG; thus, the continuous presence of BCG in urine may stimulate the immune system to generate an antitumor response.
From these data, it is not possible to predict the usefulness of BCG from MTC-PCR before the start of treatment, and the optimal timing, frequency, and period of urine MTC-PCR positivity after the last BCG instillation are not fully known. While surveillance with cystoscopy and cytology for detecting the recurrence and progression of BC is essential standard of care, MTC-PCR tests may help predict post-treatment recurrence and progression in a timely manner. The recurrence and progression rates of the MTC-PCR positive from 1 to 4 yr group were significantly lower than the recurrence rates for patients in the MTC-PCR negative from 1 to 4 yr group (p < 0.001).
Study limitations included the relatively low number of patients (all from a single center), the low number of BCG bladder instillations (median number of doses, n = 8), and <20% of patients receiving maintenance therapy. Patients who discontinued BCG instillation were also included in the analyses. As the aim of the study was to examine the usefulness of the MTC-PCR assay in predicting the efficacy of BCG after the end of BCG administration, it was considered that stopping BCG administration early would not affect MTC-PCR assay results. Another limitation was the necessity to examine MTC-PCR results as a predictive factor according to the type of BCG failure category. Recommendations from specialists and professional guidelines devote significant attention to the definition of BCG failure [3], [5], [6], [35], [36], [37], [38], and recent evidence suggests that BCG-relapsing disease is associated with better outcomes than BCG-refractory disease [39]. All patients with recurrence in this study were in the BCG-relapse group.
5. Conclusions
This study reports the significantly low likelihood of tumor recurrence and progression among patients with at least one MTC-PCR positive result after BCG therapy, compared with that among patients with a negative MTC-PCR result for all follow-up tests. MTC-PCR test results may, therefore, be related to BCG therapeutic response and may influence prognosis. Post-BCG urine MTC-PCR analysis allows urologists the possibility to stratify patients according to the likelihood of relapse and progression after BCG therapy.
Author contributions: Satoru Muto had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Muto.
Acquisition of data: Lu, Saito, Kitamura, Noma, Koyasu, Hirano, Ashizawa.
Analysis and interpretation of data: Ide.
Drafting of the manuscript: Muto.
Critical revision of the manuscript for important intellectual content: Isotani, Nagata.
Statistical analysis: Muto.
Obtaining funding: Muto.
Administrative, technical, or material support: Yamaguchi.
Supervision: Horie.
Other: None.
Financial disclosures: Satoru Muto certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
CRediT authorship contribution statement
Satoru Muto: Conceptualization, Methodology, Writing - original draft. Yan Lu: Methodology. Hisamitsu Ide: Validation, Writing - review & editing. Raizo Yamaguchi: Formal analysis. Keisuke Saito: Investigation. Kousuke Kitamura: Resources. Yasuhiro Noma: Data curation. Hiroki Koyasu: Data curation. Hisashi Hirano: Data curation. Takeshi Ashizawa: Data curation. Shuji Isotani: Project administration. Masayoshi Nagata: Visualization. Shigeo Horie: Supervision.
Associate Editor: Guillaume Ploussard
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.euros.2021.02.005.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Boehm B.E., Cornell J.E., Wang H., Mukherjee N., Oppenheimer J.S., Svatek R.S. Efficacy of bacillus Calmette-Guérin strains for treatment of nonmuscle invasive bladder cancer: a systematic review and network meta-analysis. J Urol. 2017;198:503–510. doi: 10.1016/j.juro.2017.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang S.S., Boorjian S.A., Chou R. American Urological Association Education and Research, Inc.; 2020. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline 2016, amended 2020. [Google Scholar]
- 3.Babjuk M., Burger M., Compérat E.M. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)—2019 Update. Eur Urol. 2019;76:639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Flaig T.W., Spiess P.E., Agarwal N. Bladder cancer, version 3. 2020, NCCN clinical practice guideline in oncology. J Natl Compr Canc Netw. 2020;18:329–345. doi: 10.6004/jnccn.2020.0011. [DOI] [PubMed] [Google Scholar]
- 5.Monteiro L.L., Witjes J.A., Agarwal P.K. ICUD‑SIU International Consultation on Bladder Cancer 2017: management of non‑muscle invasive bladder cancer. World J Urol. 2019;37:51–60. doi: 10.1007/s00345-018-2438-9. [DOI] [PubMed] [Google Scholar]
- 6.Kamat A.M., Sylvester R.J., Böhle A. Definitions, end points, and clinical trial designs for non-muscle-invasive bladder cancer: recommendations from the International Bladder Cancer Group. J Clin Oncol. 2016;34:1935–1944. doi: 10.1200/JCO.2015.64.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witjes J.A. Management of BCG failures in superficial bladder cancer: a review. Eur Urol. 2006;49:790–797. doi: 10.1016/j.eururo.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Sylvester R.J., van der Meijden A.P., Lamm D.L. Intravesical bacillus Calmette-Guérin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–1970. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 9.Kamat A.M., Li R., O’Donnell M.A. Predicting response to intravesical bacillus Calmette-Guérin immunotherapy: are we there yet? A systematic review. Eur Urol. 2018;73:738–748. doi: 10.1016/j.eururo.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Cambier S., Sylvester R.J., Collette L. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance bacillus Calmette-Guérin. Eur Urol. 2016;69:60–69. doi: 10.1016/j.eururo.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 11.Cai T., Nesi G., Dal Canto M. Loss of heterozygosis on IFN-alpha locus is a prognostic indicator of bacillus Calmette-Guérin response for nonmuscle invasive bladder cancer. J Urol. 2010;183:1738–1743. doi: 10.1016/j.juro.2009.12.105. [DOI] [PubMed] [Google Scholar]
- 12.Kamat A.M., Hegarty P.K., Gee J.R. ICUD-EAU international consultation on bladder cancer 2012: screening, diagnosis, and molecular markers. Eur Urol. 2013;63:4–15. doi: 10.1016/j.eururo.2012.09.057. [DOI] [PubMed] [Google Scholar]
- 13.Zuiverloon T.C.M., Nieuweboer A.J.M., Vékony H., Kirkels W.J., Bangma C.H., Zwarthoff E.C. Markers predicting response to bacillus Calmette-Guérin immunotherapy in high-risk bladder cancer patients: a systematic review. Eur Urol. 2012;61:128–145. doi: 10.1016/j.eururo.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Bowyer L., Hall R.R., Reading J., Marsh M.M. The persistence of bacille Calmette-Guérin in the bladder after intravesical treatment for bladder cancer. Br J Urol. 1995;75:188–192. doi: 10.1111/j.1464-410x.1995.tb07309.x. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-Jacoiste Asín M.A., Fernández-Ruiz M., López-Medrano F. Bacillus Calmette-Guérin (BCG) infection following intravesical BCG administration as adjunctive therapy for bladder cancer: incidence, risk factors, and outcome in a single-institution series and review of the literature. Medicine. 2014;93:236–254. doi: 10.1097/MD.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edge S.B., Byrd D.R., Compton C.C. Urinary bladder. In: Edge S.B., Byrd D.R., Compton C.C., editors. AJCC cancer staging manual. 7th ed. Springer; New York, NY: 2010. pp. 497–505. [Google Scholar]
- 17.Eble J.N., Sauter G., Epstein J.I., editors. Pathology and genetics: tumours of the urinary system and male genital organs. IARC; Lyon, France: 2004. pp. 90–123. [Google Scholar]
- 18.Fernandez-Gomez J., Madero R., Solsona E. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guérin: the CUETO scoring model. J Urol. 2009;182:2195–2203. doi: 10.1016/j.juro.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda N., Hinda I., Yano I., Koyama A., Toida I. Bacillus Calmette-Guérin TOKYO172 substrain for superficial bladder cancer: characterization and antitumor effect. J Urol. 2005;173:1507–1512. doi: 10.1097/01.ju.0000154354.06892.ba. [DOI] [PubMed] [Google Scholar]
- 20.Richter E., Greinert U., Kirsten D. Assessment of mycobacterial DNA in cells and tissues of mycobacterial and sarcoid lesions. Am J Respir Crit Care Med. 1996;153:375–380. doi: 10.1164/ajrccm.153.1.8542146. [DOI] [PubMed] [Google Scholar]
- 21.Edwards U., Rogall T., Blöcker H., Emde M., Böttger E.C. Isolation and direct complete nucleotide determination of entire genes: characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989;17:7843–7853. doi: 10.1093/nar/17.19.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Böddinghaus B., Rogall T., Flohr T., Blöcker H., Böttger E.C. Detection and identification of Mycobacteria by amplification of rRNA. J Clin Microbiol. 1990;28:1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogall T., Wolters J., Flohr T., Böttger E.C. Towards a phylogeny and definition of species at the molecular level within the genus Mycobacterium. Int J Syst Bacteriol. 1990;40:323–330. doi: 10.1099/00207713-40-4-323. [DOI] [PubMed] [Google Scholar]
- 24.Lamm D., Persad R., Brausi M. Defining progression in non-muscle invasive bladder cancer: it is time for a new, standard definition. J Urol. 2014;191:20–27. doi: 10.1016/j.juro.2013.07.102. [DOI] [PubMed] [Google Scholar]
- 25.Kipp B.R., Karnes R.J., Brankley S.M. Monitoring intravesical therapy for superficial bladder cancer using fluorescence in situ hybridization. J Urol. 2005;173:401–404. doi: 10.1097/01.ju.0000149825.83180.a4. [DOI] [PubMed] [Google Scholar]
- 26.Ieda T., Muto S., Shimizu F. Development and validation of a novel recurrence risk stratification for initial non-muscle invasive bladder cancer in Asia. EBioMedicine. 2016;12:98–104. doi: 10.1016/j.ebiom.2016.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gopalakrishna A., Longo T.A., Fantony J.J. The diagnostic accuracy of urine-based tests for bladder cancer varies greatly by patient. BMC Urol. 2016;16:30. doi: 10.1186/s12894-016-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Aa M.N., Steyerberg E.W., Bangma C, van Rhijn BW, Zwarthoff EC, van der Kwast TH. Cystoscopy revisited as the gold standard for detecting bladder cancer recurrence: diagnostic review bias in the randomized, prospective CEFUB trial. J Urol. 2010;183:76–80. doi: 10.1016/j.juro.2009.08.150. [DOI] [PubMed] [Google Scholar]
- 29.Fantony J.J., Inman B.A. It may be time to abandon urine tests for bladder cancer. J Natl Compr Canc Netw. 2015;13:1163–1166. doi: 10.6004/jnccn.2015.0141. [DOI] [PubMed] [Google Scholar]
- 30.Kamat A.M., Briggman J., Urbauer D.L. Cytokine Panel for Response to Intravesical Therapy (CyPRIT): nomogram of changes in urinary cytokine levels predicts patient response to bacillus Calmette-Guérin. Eur Urol. 2016;69:197–200. doi: 10.1016/j.eururo.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nikaido T., Ishibashi K., Otani K. Mycobacterium bovis BCG vertebral osteomyelitis after intravesical BCG therapy, diagnosed by PCR-based genomic deletion analysis. J Clin Microbiol. 2007;45:4085–4087. doi: 10.1128/JCM.01714-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siatelis A., Houhoula D.P., Papaparaskevas J., Delakas D., Tsakris A. Detection of bacillus Galmette-Guérin (Mycobacterium bovis BCG) DNA in urine and blood specimens after intravesical immunotherapy for bladder carcinoma. J Clin Microbiol. 2011;49:1206–1208. doi: 10.1128/JCM.01595-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niwa N., Kikuchi E., Matsumoto K., Kosaka T., Mizuno R., Oya M. Purified protein derivative skin test prior to bacillus Calmette-Guérin therapy may have therapeutic impact in patients with nonmuscle invasive bladder cancer. J Urol. 2018;199:1446–1451. doi: 10.1016/j.juro.2017.12.057. [DOI] [PubMed] [Google Scholar]
- 34.Talbot E.A., Williams D.L., Frothingham R. PCR identification of Mycobacterium bovis BCG. J Clin Microbiol. 1997;35:566–569. doi: 10.1128/jcm.35.3.566-569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li R., Tabayoyong W.B., Guo C.C. Prognostic implication of the United States Food and Drug Administration—defined BCG—unresponsive disease. Eur Urol. 2019;75:8–10. doi: 10.1016/j.eururo.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 36.Kamat A.M., Flaig T.W., Grossman H.B. Consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nat Rev Urol. 2015;12:225–235. doi: 10.1038/nrurol.2015.58. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg R.L., Thomas L.J., O’Donnell M.A. Bacillus Calmette-Guérin (BCG) treatment failures in non-muscle invasive bladder cancer: what truly constitutes unresponsive disease. Bladder Cancer. 2016;1:105–106. doi: 10.3233/BLC-150015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konety B.R., Narayan V.M., Dinney C.P.N. Bacillus Calmette-Guérin salvage therapy: definitions and context. Urol Clin North Am. 2020;47:1–4. doi: 10.1016/j.ucl.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Herr H.W., Milan T.N., Dalbagni G. BCG-refractory vs. BCG-relapsing non-muscle-invasive bladder cancer: a prospective cohort outcomes study. Urol Oncol. 2015;33 doi: 10.1016/j.urolonc.2014.02.020. 108.e1–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.