Abstract
Background
En bloc resection (ERBT) is a valid alternative to piecemeal resection for non–muscle-invasive bladder cancer (NMIBC), guaranteeing pathological outcomes. However, very few studies investigated long-term oncological outcomes of ERBT.
Objective
To report long-term oncological outcome of ERBT.
Design, setting, and participants
This is a retrospective analysis of prospectively collected data. We included patients who underwent ERBT from June 2010 to February 2014, and were diagnosed with NMIBC at pathology evaluation.
Outcome measurements and statistical analysis
The primary study endpoint was recurrence-free survival at 5 yr. Secondary outcomes were presence of detrusor muscle, recurrence rate at the first follow-up cystoscopy, progression to muscle-invasive bladder cancer (MIBC) at 5 yr, and factors associated with long-term oncological outcomes. Kaplan-Meier curves were used to describe recurrence-free survival time. A univariate analysis was used to investigate factors associated with recurrence.
Results and limitations
Overall, 74 patients were included in this study. The median age was 71 (66–76) yr. Most of the patients presented with only one bladder tumor, and the median tumor diameter was 2 (interquartile range [IQR] 1–2.5) cm. After histopathological examination, eight, 35, and 31 patients were diagnosed with low-, intermediate-, and high-risk disease, respectively. All the en bloc resected tumors showed the presence of detrusor muscle. The median follow-up was 72 (IQR 66–90) mo. The recurrence rate at the first follow-up cystoscopy was 5.4% (four out of 74 patients). Overall, 57 (77%) patients were free of recurrence at 5 yr. No progression to MIBC was observed: progression-free survival was 100%. Limitations include retrospective design and small size.
Conclusions
Our findings showed that ERBT for NMIBC presents an optimal long-term oncological outcome. Further studies with larger cohorts are necessary for confirming our preliminary results and for a direct comparison with the traditional piecemeal resection.
Patient summary
In case of superficial bladder tumors, transurethral resection of the entire tumor and its base in one piece seems to provide good long-term results in terms of recurrence and progression rates.
Keywords: Non–muscle-invasive bladder cancer, High grade non–muscle-invasive bladder cancer, En bloc resection, Transurethral resection of bladder tumor, Long-term outcomes
Take Home Message
Our findings show that patients undergoing en bloc transurethral resection for non–muscle-invasive bladder cancer have very promising 5-yr recurrence- and progression-free survival rates.
1. Introduction
Transurethral resection (TUR) is a crucial primary step in the diagnosis and treatment of bladder tumors [1]. A high-quality resected specimen must include the detrusor muscle in order to allow for correct staging of the tumor and subsequent decisions regarding therapy. Especially in the case of non–muscle-invasive bladder cancer (NMIBC), a complete resection of the tumor that includes the underlying detrusor muscle is fundamental to achieve a good prognosis [2].
Several different strategies and tools have been experimented in an attempt to improve the quality of the resection [3]. Among these, en bloc resection (ERBT) is considered a valid alternative to piecemeal resection for selected exophytic tumors [1], [4], [5]. First described by Ukai and colleagues [6] almost 20 yr ago, ERBT has, in recent years, emerged as a technique able to improve the quality of resection. Indeed, several authors reported better pathological outcomes with ERBT than with traditional piecemeal resection [5], [7], [8]. ERBT provides high-quality specimens, with the presence of the muscle layer in >95% of the cases, no fragmentation of the tumor, and reduced probability of cauterization and deterioration of the tissue [4]. In addition, it can potentially minimize the amount of floating tumor cells and reduce the risk of tumor reimplantation and recurrence, by minimizing unnecessary handling and fragmentation [9].
On the contrary, there are several aspects of ERBT that still need to be clarified, the most important of which being its oncological outcome [10]. Very few data are indeed available about medium- and long-term outcomes of patients subjected to this relatively new technique. Currently, we rely mostly on surrogate endpoints, such as pathological parameters, to predict oncological outcomes. Few authors reported rates of recurrence and progression after ERBT, showing promising results [4], [5], [7]. However, most of these studies had a median follow-up of 1 or 2 yr.
Therefore, there is an unmet clinical need to investigate the long-term oncological efficacy of ERBT [10]. Aiming to fill this gap, here we report the 5-yr oncological outcomes of patients subjected to ERBT for NMIBC cancer, with a specific focus on patients with high-risk characteristics.
2. Patients and methods
2.1. Study design and population
This is a retrospective analysis of prospectively collected data from a single tertiary center. All the men and women underwent transurethral ERBT between June 2010 and February 2014, for a first diagnosis or a primary recurrence of clinical NMIBC. All patients had four or fewer lesions that were each ≤3 cm. The procedure was performed with a monopolar loop (J-electrode—Collins loop: Storz 27040 L 24 CH). The stages of the procedure are depicted in Figure 1. After detaching the lesion from the bladder wall, the tumor was extracted with an Ellick evacuator. In case of large tumors, nephroscopy sheet and laparoscopic grasp (ie, Schneider grasp) were used. Full inclusion and exclusion criteria were described previously, as well as details on the surgical technique and pathological evaluation of the specimens [11]. In this analysis, we included only patients diagnosed with urothelial NMIBC at histopathological evaluation after ERBT. Risk group stratification was performed according to the European Association of Urology (EAU) guidelines.
Fig. 1.
Stages of en bloc resection of bladder tumor. (A) Initial incision: a circular incision is performed in macroscopically “normal” mucosa surrounding the base of the tumor, maintaining a distance of approximately 5–10 mm from the tumor edge. (B) Resection of the tumor base: the incision is extended through the subepithelial connective tissue, muscularis mucosae, and muscularis propria layers. (C) Tumor traction: a gentle traction can be applied from the base of the tumor upward, in order to detach the muscle fibers. (D) Resection bed: a view of the resection bed after tumor detachment. In many cases, the procedure is virtually bloodless.
2.2. Patient management and follow-up
After ERBT, a single instillation of mitomycin (MMC) was administered within 6 h from the surgery in patients with a single lesion <1 cm in diameter. Intermediate-risk patients received MMC weekly for 6 consecutive weeks, starting within 21 d from tumor resection. We administered endovesical bacillus Calmette-Guérin (BCG) to intermediate-risk patients with three or more risk factors according to Kamat et al’s [12] criteria. Patients with high-risk features (ie, high-grade [HG] Ta/T1 tumor) underwent re-TUR within 40 d and intravesical BCG treatment according to the SWOG scheme [13]. All patients underwent a strict follow-up according to the EAU guidelines.
2.3. Outcome measure
The primary endpoint of the study was recurrence-free survival at 5 yr. Time was expressed in months. Secondary outcomes were presence of detrusor muscle, recurrence rate at the first follow-up cystoscopy (3 mo), progression to muscle-invasive bladder cancer (MIBC) at 5 yr, and factors associated with long-term oncological outcomes. We performed separate analyses for the whole cohort and for high-risk patients.
2.4. Covariates
Baseline characteristics included age at diagnosis, gender, smoking status, and comorbidities (classified using the Charlson comorbidity index). For tumor staging, we used the TNM classification system according to the American Joint Committee on Cancer, eighth edition. The Rete Oncologica Lombarda (ROL) system was used for the substaging of T1 tumors (defined as ROL1 and ROL2) [14], [15]. Grading was defined according to the 2016 World Health Organization grading classification [14].
2.5. Statistical analysis
Means and standard deviations were utilized for normally distributed continuous variables, median and interquartile ranges (IQRs) for non-normally distributed continuous variables, and frequencies and proportions for categorical variables. Time to recurrence was calculated from the date of surgery to the date of recurrence or last contact. Kaplan-Meier curves were used to describe recurrence-free survival of low-/intermediate-risk and high-risk patients. Univariate analysis was used to investigate factors associated with recurrence. Given the small sample size, multivariate analysis was not performed. All p values were two sided, and statistical significance was assumed at p < 0.05.
All analyses were performed using Stata (version Stata/IC 16.0; Stata Corp LLC, TX, USA). An institutional review board waiver was obtained from the IRCCS Humanitas Research Hospital.
3. Results
Overall, 87 patients underwent ERBT. At histopathological assessment, two cases harbored a nonurothelial carcinoma and 11 had MIBC; these patients were excluded from further analysis.
3.1. Baseline characteristics
The population consisted of 58 men and 16 women. The median age was 71 (66–76) yr, while 20 (65%) patients were former or current smokers. Of all patients, 68 (92%) had a comorbidity index of ≤1. Most of the patients presented with only one bladder tumor, and the median tumor diameter was 2 (IQR 1–2.5) cm. Complete baseline characteristics are shown in Table 1. After histopathological examination, eight, 35, and 31 patients were diagnosed with low-, intermediate-, and high-risk disease, respectively.
Table 1.
Baseline patient characteristics
| High-risk patients (N = 31) |
Low- and intermediate-risk patients (N = 43) |
Overall cohort (N = 74) |
||
|---|---|---|---|---|
| Age (yr), median (IQR) | 72 (67–78) | 70 (66–75) | 71 (66–76) | |
| Gender, N (%) | Male | 26 (84) | 32 (74) | 58 (78) |
| Female | 5 (16) | 11 (26) | 16 (22) | |
| Comorbidity index, N (%) | 0 | 24 (77) | 34 (79) | 58 (78) |
| 1 | 5 (16) | 5 (12) | 10 (14) | |
| ≥2 | 2 (6) | 4 (9) | 6 (8) | |
| Smoking, N (%) |
Non-smoker | 11 (35) | 18 (42) | 29 (39) |
| Former smoker | 14 (45) | 20 (47) | 34 (46) | |
| Smoker | 6 (19) | 5 (12) | 11 (15) | |
IQR = interquartile range.
3.2. Pathological and short-term outcomes
All the en bloc resected tumors showed the presence of detrusor muscle. An example of an ERBT specimen is shown in Figure 2. At histopathological evaluation, 43 patients had a low-grade Ta carcinoma, four had an HG Ta carcinoma, and 27 patients had an HG T1 carcinoma. Carcinoma in situ was detected in 13 (17%) patients. Overall, eight patients received a single MMC instillation after surgery. All the high-risk patients underwent repeat transurethral resection of bladder tumor (re-TURBT) within 40 d. A residual tumor was found in seven patients (23%); all these patients had Ta tumors. Among these, three patients had tumors in the same location of previous resections, while in four cases it was out of the field of first ERBT. No pT1 or pT2 tumors were identified at the re-TURBT. Recurrence rate at the first follow-up cystoscopy was 5.4% (four out of 74 patients).
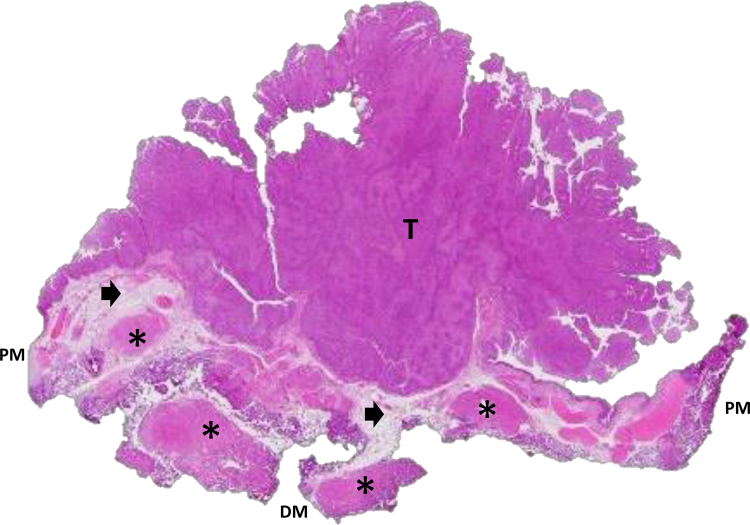
Fig. 2.
High-grade pTa papillary urothelial carcinoma. Example of an en bloc resection specimen in which the tumor staging results are accurate. Note that with a wall section of the tumor, the peripheral margin and the deep margin are easily identifiable (hematoxylin and eosin, original magnification 20×). The arrow indicates lamina propria and asterisk indicates muscularis propria. DM = deep margin; PM = peripheral margin; T = tumor.
3.3. Long-term oncological outcomes
Overall, the median follow-up was 72 (IQR 66–90) mo. For high-risk patients, the median follow-up was 83 (IQR 72–110) mo. Overall, 57 (77%) patients were free of recurrence at 5 yr. Among high-risk patients, we observed a slightly worse outcome. All the patients who experienced recurrence had the first episode within 3 yr from the ERBT except for one patient. Overall, recurrence-free survival at 5 yr was 77% for the whole cohort and 71% for the high-risk cohort (Fig. 3). No progression to MIBC was observed: MIBC progression-free survival was 100%. During the follow-up, none of the patients were diagnosed with an upper urinary tract tumor. A summary of the outcomes is shown in Table 2. The univariate analysis for the whole cohort is shown in Table 3. At univariate analysis performed in the high-risk subgroup, ROL2 as T1 substaging was associated with a higher probability of recurrence (odds ratio: 5.2, confidence interval 0.92–29.26, p = 0.06), although this association did not reach the set threshold for statistical significance (Table 4).
Fig. 3.
Kaplan-Meier curve examining recurrence-free survival in patients undergoing en bloc resection for non–muscle-invasive bladder cancer.
Table 2.
Tumor characteristics and oncological outcomes
| High-risk patients (N = 31) |
Low- and intermediate-risk patients (N = 43) |
Overall cohort (N = 74) |
||
|---|---|---|---|---|
| Tumor diameter (cm), median (IQR) | 2 (1.5–2.5) | 2 (1–2.5) | 2 (1–2.5) | |
| Median number of tumors (range) | 1 (1–4) | 1 (1–4) | 1 (1–4) | |
| T stage at first TURBT, N (%) | Ta | 4 (13) | 43 (100) | 47 (64) |
| ROL1 T1 | 16 (52) | – | 16 (22) | |
| ROL2 T1 | 11 (35) | – | 11 (15) | |
| Recurrence, N (%) | No recurrence | 20 (65) | 35 (81) | 55 (74) |
| Rec. within 3 months | 1 (3) | 3 (7) | 4 (5) | |
| Rec. after 3 months | 8 (26) | 5 (12) | 13 (18) | |
| Pathology report at first recurrence, N (%) | LGTa | 2 (22) | 8 (100) | 10 (53) |
| HGTa | 2 (22) | 0 (0) | 2 (11) | |
| HGT1 | 7 (78) | 0 (0) | 7 (37) | |
| Progression to MIBC, N (%) | No | 31 (100) | 43 (100) | 74 (100) |
| Yes | 0 (0) | 0 (0) | 0 (0) | |
HG = high grade; IQR = interquartile range; LG = low grade; MIBC = muscle-invasive bladder cancer; ROL = Rete Oncologica Lombarda; TURBT = transurethral resection of bladder tumor.
Table 3.
Univariable logistic regression examining factors associated with recurrence in the whole cohort
| Univariable analysis |
||||
|---|---|---|---|---|
| Odds ratio | 95% CI | p value | ||
| Age at diagnosis | 0.99 | 0.89–1.12 | 0.997 | |
| Gender | Female | 1 (Ref) | – | – |
| Male | 0.39 | 0.11–1.30 | 0.126 | |
| Risk category | Low/intermediate risk | 1 (Ref) | – | – |
| High risk | 1.79 | 0.60–5.33 | 0.296 | |
| Carcinoma in situ | Yes | 1 (Ref) | – | – |
| No | 5.67 | 1.47–21.96 | 0.012 | |
| Number of tumors | 1.35 | 0.96–1.90 | 0.085 | |
| Tumor size | 1.76 | 0.63–4.89 | 0.280 | |
CI = confidence interval; Ref = reference.
Table 4.
Univariable logistic regression examining factors associated with recurrence in the high-risk population
| Odds ratio | 95% Confidence interval | p value | ||
|---|---|---|---|---|
| Age at diagnosis | 0.97 | 0.86 - 1.11 | 0.679 | |
| Gender | Male | 1 (Ref) | – | – |
| Female | 0.60 | 0.01–0.66 | 0.021 | |
| Carcinoma in situ | No | 1 (Ref) | – | – |
| Yes | 6.80 | 1.23–37.5 | 0.03 | |
| Median number of tumors | 1.43 | 0.86–2.36 | 0.168 | |
| Median tumor size | 2.59 | 0.47–14.14 | 0.272 | |
| T1 substaging | ROL1 | 1 (Ref) | – | – |
| ROL2 | 5.2 | 0.92–29.26 | 0.06 | |
Ref = reference; ROL = Rete Oncologica Lombarda.
4. Discussion
In this study, we reported the 5-yr follow-up of 74 consecutive patients undergoing ERBT for urothelial NMIBC. Our findings seem to support the notion that ERBT, together with a second TURBT, an intravesical therapy when indicated, and a strict follow-up, provides excellent long-term outcomes in this population. To the best of our knowledge, this study reported the longest follow-up currently available in the literature body. Previous studies have shown similar results, although with shorter follow-up [16]. In a retrospective study of 251 patients undergoing ERBT for NMIBC, with a mean follow-up of 40.1 mo, recurrence was almost 25% and progression to MIBC <4% [17]. Of note, almost half of the patients did not receive a second resection. In a population of 21 patients undergoing ERBT, Mandhani et al [18] observed almost the same recurrence but higher progression with a median follow-up of 40 mo. Another study comparing conventional TURBT and laser ERBT observed recurrence-free survival at 18 mo in about 94% of those undergoing ERBT [19]. Four studies analyzed 36-mo recurrence after ERBT, showing at a pooled analysis a recurrence rate of 29.6% at 36 mo [20].
Correct management of NMIBC is one of the most difficult clinical challenges a urologist has to face. First of all, every surgeon aims to provide the pathologist with high-quality specimen in order to achieve a correct diagnosis. ERBT has been proposed as an opportunity of enhancing the resection quality, compared with traditional piecemeal resection [5], [8], [21]. In the last years, several authors elaborated surgical aspects of this procedure, examining different techniques for performing ERBT [21], [22], [23]. Although it is generally felt that the ERBT procedure is more precise and controlled, high-quality data are limited to make robust recommendations on its utilization. On the contrary, a more radical resection might allow lowering of the frequency of the second TURBT, while at the same time improving clinical tumor staging and reducing recurrence rate. Our findings are in line with the literature, confirming that, when performed by experienced urologists, ERBT is able to provide high-quality specimens. In our analysis, indeed, the muscle layer was present in 100% of the specimens. This is of utmost importance to allow correct staging by the pathologist. Although we did not have any comparison group in this study, the presence of muscle layer is generally around 80% at our center when using the traditional piecemeal resection [24]. Higher-quality resections might also reduce the overall bladder cancer management cost. It has been supposed that the numbers of re-TUR could be reduced, having accurate staging at the first resection [25]. Of note, in our population, no patients were upstaged to MIBC after re-TUR, while eight patients were found to have residual disease. However, whether ERBT can help in reducing the number of re-TUR is still an open question, and prospective data are needed in order to draw conclusions in this regard.
Besides diagnosis and staging, the main goal of the initial treatment of NMIBC is to reduce the risk of tumor recurrence and progression, and therefore also the subsequent need of additional therapies, and the morbidity and costs associated with these treatments. The probability of recurrence and progression to MIBC disease and extravesical dissemination is higher in pT1 tumors than in pTa tumors [26], [27]. To date, after re-TUR, the gold standard treatment for T1 patients is represented by intravesical BCG therapy. At the same time, patients with HG T1 bladder cancer represent a very heterogeneous population. In fact, while these individuals have an elevated probability to upstage or progress to muscle-invasive or metastatic disease, their outcomes may be highly variable and unpredictable [26]. Our results showed that ERBT guarantees excellent long-term oncological outcomes in terms of both the recurrence and the progression rate. Notably, in our series, we did not observe any progression to MIBC. Although it could be that ERBT played a role in achieving such a good outcome, other factors must be taken into account. First of all, all the patients underwent a re-TUR within 40 d from the first resection and intravesical BCG instillations according to the SWOG scheme. Despite the recommendations, often high-risk NMIBC patients did not undergo the correct management [28], [29]. Moreover, all the patients were subjected to a strict follow-up scheme, and this led to an early detection of recurrences. In addition to this, we cannot exclude the selection bias due to the retrospective nature of the study. Indeed, no strict criteria were followed to select the candidates for ERBT. However, according to our experience, patients with exophytic papillary tumor, with a diameter of <3 cm, are the best candidates. Especially in the first portion of the learning curve, ERBT should be avoided for tumors located in unfavorable locations (eg, anterior wall and ureteral orifice).
To the best of our knowledge, we presented the cohort with the longest median follow-up after ERBT. The findings we reported help in exploring one of the major unclear aspects of this technique.
Our study has several limitations. These are mainly related to its retrospective nature and to the limited number of patients. First of all, as previously mentioned, no strict criteria were used to select candidates for ERBT. Therefore, patients underwent this procedure mainly due to surgeon preference and subjective evaluation. In the study period, the en bloc technique was indeed used for a large minority of the TURBTs (<10% of the cases). Patients subjected to ERBT could have had, on average, a lower number of tumors and smaller tumors than the average candidate for TURBT. In the light of the risk of selection bias, our 0% progression rate should be interpreted with caution. Our results, although demonstrating that ERBT guarantees an overall excellent oncological control, can primarily be extended to patients who receive proper management, herein including re-TURBT full treatment with BCG when indicated, and undergo a strict follow-up schedule. In our analysis, we did not include some variables that have been demonstrated to be associated with recurrence and progression in high-risk NMIBC patients, such as body mass index, neutrophil-to-lymphocyte ratio, and glomerular filtration rate [30], [31], [32]. Finally, although we reported the longest follow-up currently available in the literature, further extension of the follow-up will be needed in order to confirm our promising results.
5. Conclusions
Our findings showed that ERBT of NMIBC presents optimal long-term recurrence- and progression-free survival. Further studies with larger cohorts in multicenter and randomized settings are mandatory for confirming our preliminary oncological results and for a proper comparison with the traditional piecemeal resection.
Author contributions: Massimo Lazzeri had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hurle, Lazzeri.
Acquisition of data: Contieri, Fasulo, Hurle, Lazzeri, Paciotti.
Analysis and interpretation of data: Hurle, Lazzeri, Paciotti.
Drafting of the manuscript: Lazzeri, Paciotti.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Fasulo, Paciotti.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Buffi, Guazzoni, Hurle, Lazzeri.
Other: None.
Financial disclosures: Massimo Lazzeri certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Acknowledgments: The authors acknowledge Nadia Lo Iacono.
Associate Editor: Guillaume Ploussard
References
- 1.Babjuk M., Burger M., Compérat E.M. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ)— 2019 update. Eur Urol. 2019;76:639–657. doi: 10.1016/j.eururo.2019.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Brausi M., Collette L., Kurth K. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol. 2002;41:523–531. doi: 10.1016/s0302-2838(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 3.Mostafid H, Kamat AM, Daneshmand S, et al. Best practices to optimise quality and outcomes of transurethral resection of bladder tumours. Eur Urol Oncol. In press. 10.1016/j.euo.2020.06.010. [DOI] [PubMed]
- 4.Kramer M.W., Altieri V., Hurle R. Current evidence of transurethral en-bloc resection of nonmuscle invasive bladder cancer. Eur Urol Focus. 2017;3:567–576. doi: 10.1016/j.euf.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Kramer M.W., Rassweiler J.J., Klein J. En bloc resection of urothelium carcinoma of the bladder (EBRUC): a European multicenter study to compare safety, efficacy, and outcome of laser and electrical en bloc transurethral resection of bladder tumor. World J Urol. 2015;33:1937–1943. doi: 10.1007/s00345-015-1568-6. [DOI] [PubMed] [Google Scholar]
- 6.Ukai R., Kawashita E., Ikeda H. A new technique for transurethral resection of superficial bladder tumor in 1 piece. J Urol. 2000;163:878–879. [PubMed] [Google Scholar]
- 7.Mori K., D’Andrea D., Enikeev D.V., Egawa S., Shariat S.F. En bloc resection for nonmuscle invasive bladder cancer. Curr Opin Urol. 2020;30:41–47. doi: 10.1097/MOU.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 8.Liang H., Yang T., Wu K., He D., Fan J. En bloc resection improves the identification of muscularis mucosae in non-muscle invasive bladder cancer. World J Urol. 2019;37:2677–2682. doi: 10.1007/s00345-019-02672-3. [DOI] [PubMed] [Google Scholar]
- 9.Engilbertsson H., Aaltonen K.E., Björnsson S. Transurethral bladder tumor resection can cause seeding of cancer cells into the bloodstream. J Urol. 2015;193:53–57. doi: 10.1016/j.juro.2014.06.083. [DOI] [PubMed] [Google Scholar]
- 10.Babjuk M. En-bloc resection of non-muscle invasive bladder cancer: what must be answered in the future? World J Urol. 2020;38:1577–1578. doi: 10.1007/s00345-019-02710-0. [DOI] [PubMed] [Google Scholar]
- 11.Hurle R., Lazzeri M., Colombo P. “En Bloc” resection of nonmuscle invasive bladder cancer: a prospective single-center study. Urology. 2016;90:126–130. doi: 10.1016/j.urology.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Kamat A.M., Witjes J.A., Brausi M. Defining and treating the spectrum of intermediate risk nonmuscle invasive bladder cancer. J Urol. 2014;192:305–315. doi: 10.1016/j.juro.2014.02.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamm D.L., Blumenstein B.A., Crissman J.D. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000;163:1124–1129. [PubMed] [Google Scholar]
- 14.Moch H., Humphrey P.A., Ulbright T.M., Reuter V.E. ed. 4. IARC Press; Lyon, France: 2016. WHO classification of tumors of the urinary system and male genital organs. [DOI] [PubMed] [Google Scholar]
- 15.Patriarca C., Hurle R., Moschini M. Usefulness of pT1 substaging in papillary urothelial bladder carcinoma. Diagn Pathol. 2016;11:6. doi: 10.1186/s13000-016-0466-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Territo A., Bevilacqua G., Meneghetti I., Mercadé A., Breda A. En bloc resection of bladder tumors. Curr Opin Urol. 2020;30:421–427. doi: 10.1097/MOU.0000000000000737. [DOI] [PubMed] [Google Scholar]
- 17.Zhou W., Wang W., Wu W., Yan T., Du G., Liu H. Can a second resection be avoided after initial thulium laser endoscopic en bloc resection for non-muscle invasive bladder cancer? A retrospective single-center study of 251 patients. BMC Urol. 2020;20:30. doi: 10.1186/s12894-020-00599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandhani A., Sureka S., Agarwal V., Agnihotri S., Kapoor R., Srivastava A. Is en-bloc transurethral resection of bladder tumor for non-muscle invasive bladder carcinoma better than conventional technique in terms of recurrence and progression? A prospective study. Indian J Urol. 2014;30:144. doi: 10.4103/0970-1591.126887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X., Liao J., Chen L. En bloc transurethral resection with 2-micron continuous-wave laser for primary non-muscle-invasive bladder cancer: a randomized controlled trial. World J Urol. 2015;33:989–995. doi: 10.1007/s00345-014-1342-1. [DOI] [PubMed] [Google Scholar]
- 20.Zhang D., Yao L., Yu S. Safety and efficacy of en bloc transurethral resection versus conventional transurethral resection for primary nonmuscle-invasive bladder cancer: a meta-analysis. World J Surg Oncol. 2020;18:4. doi: 10.1186/s12957-019-1776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bach T., Muschter R., Herrmann T.R.W. Technical solutions to improve the management of non-muscle-invasive transitional cell carcinoma: summary of a European Association of Urology Section for Uro-Technology (ESUT) and Section for Uro-Oncology (ESOU) expert meeting and current and future pers. BJU Int. 2015;115:14–23. doi: 10.1111/bju.12664. [DOI] [PubMed] [Google Scholar]
- 22.Hurle R., Casale P., Lazzeri M. En bloc re-resection of high-risk NMIBC after en bloc resection: results of a multicenter observational study. World J Urol. 2020;38:703–708. doi: 10.1007/s00345-019-02805-8. [DOI] [PubMed] [Google Scholar]
- 23.Gakis G., Karl A., Bertz S. Transurethral en bloc submucosal hydrodissection versus conventional resection for resection of non-muscle invasive bladder cancer (HYBRIDBLUE): a randomized, multicentre trial. BJU Int. 2020;126:509–519. doi: 10.1111/bju.15150. [DOI] [PubMed] [Google Scholar]
- 24.Colombo R., Hurle R., Moschini M. Feasibility and clinical roles of different substaging systems at first and second transurethral resection in patients with T1 high-grade bladder cancer. Eur Urol Focus. 2018;4:87–93. doi: 10.1016/j.euf.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Soria F., D’Andrea D., Moschini M. Predictive factors of the absence of residual disease at repeated transurethral resection of the bladder. Is there a possibility to avoid it in well-selected patients? Urol Oncol. 2020;38 doi: 10.1016/j.urolonc.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Hurle R., Buffi N., Lista G. Long-term outcomes of high-grade T1 bladder cancer treated with intravesical bacillus Calmette-Guérin: experience of a single center. Minerva Urol Nefrol. 2018;70:501–508. doi: 10.23736/S0393-2249.18.03042-4. [DOI] [PubMed] [Google Scholar]
- 27.Cambier S., Sylvester R.J., Collette L. EORTC nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1–3 years of maintenance bacillus Calmette-Guérin. Eur Urol. 2016;69:60–69. doi: 10.1016/j.eururo.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 28.Gendy R., Delprado W., Brenner P. Repeat transurethral resection for non-muscle-invasive bladder cancer: a contemporary series. BJU Int. 2016;117(Suppl 4):54–59. doi: 10.1111/bju.13265. [DOI] [PubMed] [Google Scholar]
- 29.Schraml J., Silva J.D.C., Babjuk M. Current concept of transurethral resection of bladder cancer. Curr Opin Urol. 2018;28:591–597. doi: 10.1097/MOU.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 30.Vartolomei M.D., Ferro M., Cantiello F. Validation of neutrophil-to-lymphocyte ratio in a multi-institutional cohort of patients with T1G3 non–muscle-invasive bladder cancer. Clin Genitourin Cancer. 2018;16:445–452. doi: 10.1016/j.clgc.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Ferro M., Vartolomei M.D., Russo G.I. An increased body mass index is associated with a worse prognosis in patients administered BCG immunotherapy for T1 bladder cancer. World J Urol. 2019;37:507–514. doi: 10.1007/s00345-018-2397-1. [DOI] [PubMed] [Google Scholar]
- 32.Blute M.L., Kucherov V., Rushmer T.J. Reduced estimated glomerular filtration rate (eGFR <60 mL/min/1.73 m2 ) at first transurethral resection of bladder tumour is a significant predictor of subsequent recurrence and progression. BJU Int. 2017;120:387–393. doi: 10.1111/bju.13904. [DOI] [PubMed] [Google Scholar]