Abstract
Background
To improve and compare outcomes in healthcare, it is necessary to standardise outcome measurements. There are no widely accepted standardised outcome measures reflecting quality of care for bladder cancer (BCa) patients.
Objective
The aim of this study was to create a standardised set of outcomes for patients with muscle-invasive or metastatic BCa, using the value-based healthcare principles.
Design, setting, and participants
A multidisciplinary working group of 25 healthcare professionals and patient representatives was assembled, to develop the set.
Outcome measurements and statistical analysis
We used an online RAND-modified Delphi process to prioritise, discuss, and reach consensus regarding the outcomes, case-mix variables, and treatment factors.
Results and limitations
Recognising the heterogeneity of patients with BCa, the working group defined the scope as patients with muscle-invasive and metastatic BCa. A total of 24 outcomes, including ten patient-reported outcomes, were included in the standard set of outcomes, covering survival, complication rates, recurrence of disease, readmissions after treatment, and quality of life (QoL). Fourteen case-mix variables were included. The EQ-5D and European Organisation for Research and Treatment of Cancer quality of life (EORTC-QLQ) questionnaires were recommended to measure QoL.
Conclusions
We developed the first standardised set of patient-centred outcomes for muscle-invasive and metastatic BCa. The sue of this set enables institutions to monitor, compare, and improve the quality of BCa care, on an international level.
Patient summary
Our group of healthcare professionals and patient representatives recommended a standardised set of patient-centred outcomes to be followed during the treatment of patients with muscle-invasive or metastatic bladder cancer, in order to monitor, compare, and improve the quality of care.
Keywords: Invasive bladder cancer, Muscle invasive, Metastatic, Standard set of outcome measures, Patient-reported outcome measures
Take Home Message
We developed the first standardised set of patient-centred outcomes for muscle-invasive and metastatic bladder cancer. The use of this set enables institutions to monitor, compare, and improve the quality of bladder cancer care on an international level.
1. Introduction
Muscle-invasive bladder cancer (MIBC) and metastatic bladder cancer (mBC) are heterogeneous diseases with multifaceted disease settings, treatment options, and outcomes. Despite guideline recommendations, outcomes from bladder cancer (BCa) treatment vary widely [1], [2], suggesting a variance in adherence to guidelines and healthcare delivery [3]. The disease has a tremendous influence on quality of life (QoL), which caused an increasing focus on QoL outcomes in urological malignancies in the past 20 yr [4], [5].
To improve quality of care, value-based healthcare is increasingly being promoted. It is based on the theory of Michael Porter and Elizabeth Teisberg [6], in which creating a high value for patients, defined as the health outcomes achieved relative to the costs, is the main goal. In order to apply value-based healthcare, identification and standardisation of relevant outcome measures are required [7]. Outcomes are separated according to the three tiers of Porter [6]: health status achieved, time to health status achieved, and sustainability of health.
Standardised outcomes should be based upon evidence-based clinical practice and accepted by the urological community. A standardised set of patient-centred outcomes serves multiple purposes. First of all, the impact on health-related quality of life (HRQoL) can be evaluated and enhanced, by using patient-reported outcome measures (PROMs). Secondly, it can aid in identifying inferior or futile treatments, by comparing outcome results of different treatment modalities. Thirdly, standardised set outcome measures can be used to monitor within- and between-hospital variations in the outcome of care, as a starting point for quality improvement efforts. Lastly, the collection of standardised end-points for scientific research allows for better comparison of results between studies. In this way, standardised outcomes are able to identify and monitor best practices, and pave the way for adjustment and improvement. Moreover, it can be an important aid in shared decision-making. Shared decision-making requires insight in multidimensional treatment outcomes, in order for healthcare professional and patients to come to a solution that is most in line with the values and preferences of the patient.
Standard sets have been developed for some urological diseases, that is, localised and advanced prostate cancer [8], [9]. Nevertheless, there is no widely accepted standard set of outcome measures reflecting quality of care for BCa [10]. In the absence of a meaningful and internationally accepted standardised set of outcome measures, comparison of results and identification of best practice are hampered. The aim of this project was to create a standardised set of patient-centred outcomes for MIBC and mBC patients.
2. Patients and methods
2.1. Development of the MIBC/mBC standard set
A multidisciplinary working group was formed of experts reflecting the broad range of specialities involved in BCa care. The 24 members included clinicians (urologists, medical oncologists, radiotherapists, nuclear medicine physicians, pathologists, and clinical pharmacists), epidemiologists, nurses, and two patient representatives. Details on the membership can be found in the Supplementary material. The involved organisations were the Santeon Hospitals Consortium (consisting of seven large teaching hospitals), the University Medical Center Utrecht (UMCU), and the Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital (NCI-AVL) Amsterdam. Together, they are responsible for >15% of the Dutch volume of hospital care. The process of developing the standardised set is similar to the process used for other diseases [8], [9], [11], [12], [13], [14].
The development of the standardised set involved five steps, explained below, and supervised by a smaller project group (D.R., H.v.M., E.v.d.G., and P.v.d.N.). The project group guided each step, summarised the outcomes, and shared these with the working group in advance to each conference. In total, the working group convened during three conferences between May 2019 and December 2019.
2.1.1. Step 1: defining the medical condition and treatment scope
The working group discussed and defined for which patient group the standardised set of outcome measures should be designed, according to demographic factors, disease stage, and histology, including treatment modalities to cover. A trade-off was made between including a broad range of patients and maintaining homogeneity in the treatment process, in order to interpret and compare outcomes properly.
2.1.2. Step 2: identification of potential outcome domains and case-mix variables
A systematic approach was used to identify outcomes based on the three tiers of Porter [6]: tier 1—health status (survival and degree of health/recovery); tier 2—recovery process (time to recovery and disutility of care); and tier 3—sustainability of health (sustainability of health/recovery, and long-term consequences of therapy).
The project group performed a systematic literature review according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [15], through PubMed-indexed articles published between January 1, 2005 and February 13, 2019, to identify potential outcomes, including patient-reported outcomes, and case-mix variables. A saturation method was used. Initially, 100 random articles are evaluated and suitable articles are included. Subsequently, in consecutive steps, 25 new random articles are evaluated. When no new outcomes and case-mix variables were identified, a saturation point was reached, and the literature review was ended. In addition, a systematic registry search was performed.
Additionally, two patient representatives, representing a BCa patient organisation, were invited for a structured interview to complement the list of potential outcomes. Subsequently, the results were distributed to the working group, to evaluate the completeness and detect any misunderstandings, inconsistencies, and questions, before moving to the consensus process (step 3). When a member of the working group thought that an essential item was missing or an item needed fine-tuning in wording, the item was included or updated in the list. Results from all these sources were combined into a longlist of outcomes and case-mix variables.
2.1.3. Step 3: consensus process
To reach a consensus, an online two-round modified Delphi method was used following the RAND/University of California methodology [16]. Using electronic surveys, the working group was handed out voting forms of the longlist, and was asked to prioritise outcomes and case-mix variables. The project group reviewed the results of each vote and feedback from the working group. The results were summarised and discussed with the working group before the next conference.
In order for outcomes or case-mix variables to be included in the standard set, at least 70% of the working group had to vote an item as very important (a score of 7–9, on a nine-point Likert scale, in either voting round. Different criteria were used for outcomes and case-mix variables, for the working group to assess. Outcomes were assessed based on (1) impact on QoL of the patient, (2) frequency of the outcome event, (3) impact of quality of care on the outcome, and (4) feasibility of measuring the outcome. The criteria to assess variables to be used as case-mix variables were (1) relevance (impact on outcome), (2) independence, and (3) feasibility of measurement. If between 40% and 70% of the working group voted the outcome or case-mix variable as very important, the variable was discussed during the next conference. When <40% of the working group voted a variable as very important, the variable was excluded from the standard set. When differences in prioritisation emerged, they were discussed during the conferences until a consensus was reached.
2.1.4. Step 4: selection of PROMs
The patient-reported outcomes selected after prioritisation were linked in concordance with the literature and registry review results from which the outcome was retrieved, in order to select appropriate PROMs. Any additional or abbreviated forms of the PROMs mentioned in these articles were also included for evaluation. A targeted search was performed for the original studies of the instrument and, if applicable, validation studies. Subsequently, the results were discussed by the working group until a consensus was reached on the selection of PROMs. Timing at which PROMs should be evaluated was discussed during the conference.
2.1.5. Step 5: outcome definitions and measures, and data dictionary formation
After the longlist was prioritised and reduced to a shortlist of outcomes and case-mix variables, the project group drafted a data dictionary including a proposition for definitions and measures. This data dictionary was validated by the working group.
3. Results
3.1. Definition of the medical condition and treatment scope
The standard set was designed for adult patients (age ≥18 yr) with MIBC or mBC (≥T2N0M0), according to the International Classification of Diseases of Oncology (ICD-O) and tumour-node-metastasis (TNM) classification system [17]. It was decided that patients with noninvasive, but high-risk, BCa remain outside of the scope, due to difference in treatment and prognosis.
Treatment modalities included in the standard set were radical cystectomy (RC), trimodality treatment (TMT), radiotherapy (RTx), systemic therapy (STx) in the form of chemotherapy (CTx), and immunotherapy (IMTx), and best supportive care/no treatment. Combinations of treatment modalities are possible. A patient receiving neoadjuvant CTx (NAC), induction CTx (IC), or adjuvant CTx before/after RC will have data collected for RC and CTx. Similarly, a patient receiving chemoradiation will have data collected for RTx and CTx.
3.2. Longlist of potential outcomes and case-mix variables
The literature review resulted in 776 articles (Supplementary material). The saturation point was reached after two rounds, resulting in evaluation of 150 random articles, of which 106 were included for review. Subsequently, the registry review resulted in five usable registries (Supplementary material). In total, 55 outcomes (including 25 patient reported) and 37 case-mix variables were included in the longlist to be prioritised (Supplementary material).
Results from the structured interview with patient representatives and input from the initial evaluation by the working group resulted in the inclusion of the complication ureteroenteric stenosis after RC, as it was felt to be a critical, under-reported complication.
3.3. The standard set
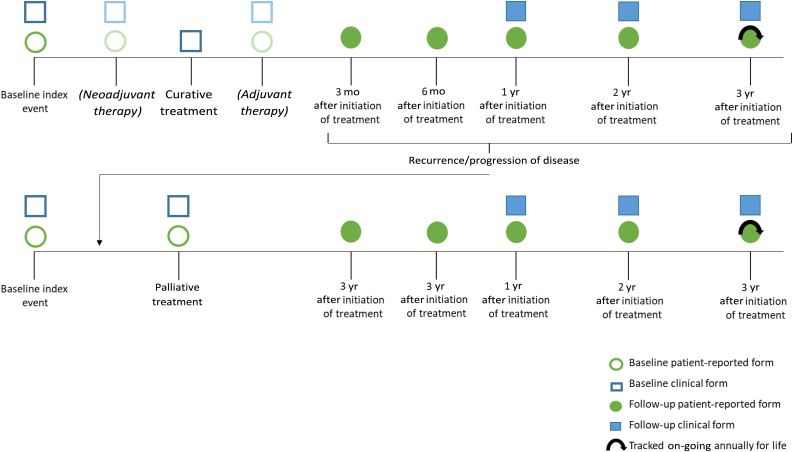
The Delphi rounds and two additional conferences resulted in a standard set consisting of 24 outcomes (Table 1) and 14 case-mix variables (Table 2). To summarise, the most relevant outcomes included survival duration and mortality cause, various QoL items, grade ≥3 complications according to the Clavien-Dindo classification or Common Terminology Criteria for Adverse Events (CTCAE), readmission rate after treatment, disease-free survival after treatment, and late-onset RTx complications (>90 d). Prioritising scores can be found in the Supplementary material. An overview of a data collection timeline is presented in Figure 1.
Table 1.
Summary of outcomes for the MIBC/mBC standard set
| Outcome set | Inclusion criteria | Details | Timing | Source |
|---|---|---|---|---|
| Tier 1a: survival | ||||
| Overall survival | All patients | Date of death | Tracked throughout | Administrative |
| Cancer-specific survival | All patients | Death attributable to bladder cancer | Tracked throughout | Administrative |
| Treatment-related mortality | All patients | Death attributable to bladder cancer treatment <30/90/120 d | Tracked throughout | Administrative |
| Tier 1b: degree of health/recovery | ||||
| Quality of Life | All patients | Tracked via EUROQOL-5D-5L | Baseline; 3 mo after initiation of treatment; 6 mo after initiation of treatment; 1 yr after initiation of treatment; Tracked on-going annually for life | Patient reported |
| Cancer-specific quality of life | All patients | Tracked via EORTC QLQ-C30 & BLM30 | ||
| Bladder cancer–specific quality of life | All patients | Tracked via EORTC QLQ-C30 & BLM30 | ||
| Pain | All patients | Tracked via EORTC QLQ-C30 & BLM30 | ||
| Physical functioning | All patients | Tracked via EORTC QLQ-C30 & BLM30 | ||
| Urinary symptoms | All patients | Tracked via EORTC QLQ-C30 & BLM30 | ||
| Fatigue | All patients | Tracked via EORTC QLQ-C30 & BLM30 | ||
| Activities of daily living | All patients | Tracked via EORTC QLQ-C30 & BLM30 | ||
| Symptoms during systemic therapy | All patients with STx | Tracked via EORTC QLQ-HDC29 | ||
| Health status in palliative setting | All patients in palliative setting | Tracked via EORTC QLQ-C15-PAL | ||
| Tier 2a: time to recovery | ||||
| Readmissions after treatment | All patients receiving treatment | Patients who need to be readmitted within 90 d due to a complication caused by treatment | Tracked throughout until 90 d after treatment | Clinical |
| Tier 2b: disutility of care | ||||
| Major radical cystectomy complications | All patients with RC | Presence or absence of grade ≥3 Clavien-Dindo grading while on therapy and within 1, 3, and 6 mo after initiating treatment | Tracked throughout until 6 mo after treatment | Clinical |
| Major trimodality treatment complications | All patients with TMT | Presence or absence of grade ≥3 CTCAE, including name of the adverse event while on therapy and within 1, 3, and 6 mo after initiating treatment | Tracked throughout until 3 mo after treatment | Clinical |
| Major radiation complications | All patients with RTx | |||
| Major systemic therapy complications | All patients with STx | |||
| Ureteroenteric strictures complication | All patients with RC | Development of ureteroenteric stricture after RC within 5 yr | Tracked throughout until 5 yr after treatment | Clinical |
| Tier 3a: sustainability of health/recovery | ||||
| Bladder cancer–free survival | All patients treated curatively | Time until recurrent disease after curative treatment, all forms | Tracked throughout | Clinical |
| Metastatic-free survival | All patients treated curatively | Time until recurrent disease after curative treatment, development of metastasis | Tracked throughout | Clinical |
| Local recurrence-free survival | All patients treated curatively | Time until recurrent disease after curative treatment, development of local recurrence | Tracked throughout | Clinical |
| Tier 3b: long-term consequences of therapy | ||||
| RTOG/EORTC late radiation complications, GU | All patients with RTx | RTOG/EORTC late radiation grade 3–4 complication (domain genitourinary) after >90 d | Tracked throughout | Clinical |
| RTOG/EORTC late radiation complications, GI | All patients with RTx | RTOG/EORTC late radiation grade 3–4 complication (domain gastrointestinal) after >90 d | Tracked throughout | Clinical |
CTCAE = Common Terminology Criteria of Adverse Events; EORTC = European Organisation for Research and Treatment of Cancer; GI = gastrointestinal; GU = genitourinary; mBC = metastatic bladder cancer; MIBC = muscle-invasive bladder cancer; RC = radical cystectomy; RTOG = Radiation Therapy Oncology Group; RTx = radiotherapy treatment; STx = systemic therapy treatment; TMT = trimodality treatment.
A detailed list of definitions can be found in the reference guide, including a data dictionary for all variables, potential data sources, and recommended timelines for data collection.
Table 2.
Summary of case-mix variables for the MIBC/mBC standard set
| Measure | Inclusion criteria | Details | Source |
|---|---|---|---|
| Demographic factors | |||
| Age | All patients | Date of birth | Clinical or patient reported |
| Sex | All patients | Sex at birth | Clinical or patient reported |
| Baseline clinical factors | |||
| Comorbid conditions a | All patients | Documented or self-reported | Clinical or patient reported |
| BMI | All patients | Weight and height needed | Patient reported |
| Smoking status | All patients | Smoking status | Patient reported |
| Performance status | All patients | ECOG/WHO scale | Clinical |
| Physical status classification | All patients with RC | ASA score | Clinical |
| Creatinine clearance | All patients | Serum creatinine level at diagnosis | Clinical |
| Baseline tumour factors | |||
| Histology of tumour | All patients | Bladder cancer histology | Clinical |
| Clinical stage | All patients | As per UICC/AJCC 8th edition | Clinical |
| Grade of UCC tumour | All patients with UCC | Per WHO 1973 | Clinical |
| Location metastatic sites | All patients | Location of metastatic sites | Clinical |
| Focality of tumour | All patients | Solitary/multifocal disease | Clinical |
| Pathological (+R) stage | All patients with RC | As per UICC/AJCC 8th edition, including resection margin status | Clinical |
AJCC = American Joint Committee on Cancer; ASA = American Society of Anesthesiologists; BMI = body mass index; ECOG = Eastern Cooperative Oncology Group; mBC = metastatic bladder cancer; MIBC = muscle-invasive bladder cancer; R = surgical margin status; RC = radical cystectomy; UCC = urothelial cell carcinoma; UICC = Union for International Cancer Control; WHO = World Health Organization.
A detailed list of definitions can be found in the reference guide, including a data dictionary for all variables, potential data sources, and recommended timelines for data collection.
Comorbid conditions according to Charlson Comorbidity Index include heart disease, hypertension, vascular disease, chronic lung disease, diabetes, kidney disease, liver disease, stroke problems, nervous system diseases, other malignancies, depression, and arthritis. This can be collected by a clinician or through patient reports.
Fig. 1.
Timeline for collecting timing outcomes and case-mix variables. In this example, the outcome data collection points are visualised, for example, a patient with MIBC who is treated with curative intent. Patients can receive follow-up for life, but can be shortened when a patient is considered in full remission. When recurrence occurs, the patient becomes a palliative patient, and data collection will start. MIBC = muscle-invasive bladder cancer.
3.3.1. Outcomes
3.3.1.1. Tier 1b: degree of health/recovery
The following patient-reported outcomes were prioritised for adequate reflection of HRQoL: three global HRQoL domains (general QoL, cancer-specific QoL, and BCa-specific QoL), four physical health domains (physical functioning, activities of daily living, pain, and fatigue), and two separate domains (health status during STx treatment and health status during palliative care).
“Urinary symptoms” remained inconclusive after the final voting but was included after patient representatives gave it a high rating of importance. They felt that these symptoms (eg, stoma/catheter concerns, severe increased urinary frequency, dysuria, and haematuria) impact their HRQoL greatly, are BCa patient specific, and should be taken into consideration.
3.3.1.2. Tier 2b: disutility of care or treatment process
The Clavien-Dindo classification was selected for complications after RC [18]; after TMT, RTx, and STx (CTx and IMTx), the CTCAE would be used [19]. Registration of complications received high prioritisation, but there was a debate whether adequate registration was feasible due to the procedure being time consuming and the variety in definitions. A consensus was reached to collect only grade ≥3 complications, since grade 1–2 complications are difficult to identify and register. Collection in general categories was deemed most feasible. To diminish registration burden, for both complication classification systems, it was decided that registering only grade ≥3 complications would be sufficient and no distinction would be made between grades 3 and 4. Categories for both the Clavien-Dindo and the CTCAE classification can be found in the data dictionary (Supplementary material).
3.3.2. Case-mix variables
Comorbidity conditions are assessed and collected based on the Charlson Comorbidity Index (CCI) using the Self-administered Comorbidity Questionnaire developed by Sangha et al [20], which has been shown to correlate with a physician-reported CCI [21]. The index score is calculated without the BCa diagnosis. The patient’s performance status expressed in Eastern Cooperative Oncology Group/World Health Organization scale was found to be a sufficient and internationally widely accepted indicator for patient’s level of functioning in terms of their ability to care for themselves, daily activity, and physical ability. Included in the standard set was the American Society of Anesthesiologists classification for the subset of patients undergoing an RC, in order to assess a patient’s preanaesthesia medical comorbidity, predict perioperative risk, and make evaluation of treatment mortality possible.
The Union for International Cancer Control/American Joint Committee on Cancer eighth edition will be used for both TNM classification and stage groups [17], [22]. For patients undergoing RC, additional pathological TNM stage including surgical margin status is an important item to collect, in order to evaluate factors such as treatment effect of NAC/IC, adequate preoperative staging of disease by imaging modalities, as well as prognostic factor for survival.
During the conferences and prioritisation process, the working group felt that there are treatment factors (that do not fit the definition of a case-mix variable or an outcome) that are important and indispensable in the evaluation of BCa care. These treatment factors (such as regimen of STx used, whether RC is performed in open or robotic fashion, etc.) can support interpretation of the outcomes. Therefore, we advise collection of treatment factors as an additional subset of variables (Supplementary material).
3.4. Selection of PROMs
Regarding the selection of PROMs, the aim was to select generic PROM(s) with good psychometric performance that would favourably capture more than one of the selected outcome measures on HRQoL, to diminish patient and administrative burden. Moreover, in order to maintain a possible comparison with other diseases/disorders, the selection should not include only BCa-specific PROMs. There was a preference for PROMs that are widely used internationally and validated in multiple languages, in order to make international implementation feasible. In total, 36 PROMs were identified and reviewed (Supplementary material). Subsequently, the results were discussed by the working group and five PROMs were selected (Table 1). Our considerations on the selection of PROMs are explained in the Supplementary material. The frequency at which PROMs should be evaluated were set at baseline and 3, 6, and 12 mo after the initiation of treatment, and subsequently tracked annually.
3.5. Outcome measure definitions and data dictionary formation
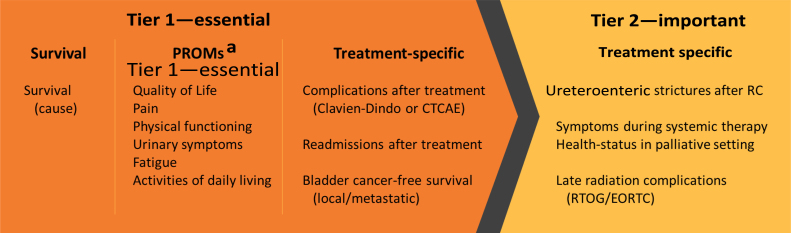
A data dictionary, on which the working group reached a consensus, describing each measure, with definition, timing of collection, inclusion/exclusion criteria, and possible data sources, can be found in the Supplementary material. Owing to concerns regarding the standard set’s length and the difficulties of implementation, the outcomes included in the set were grouped into two tiers according to the methodology of Verberne et al [11]: first an essential tier that included the PROMs and subsequently an important tier (Fig. 2). When healthcare providers implement the set, there should be focus on monitoring the outcomes from the essential tier, whereas important outcomes can be implemented when feasible.
Fig. 2.
Outcomes of the MIBC/mBC standard set divided into two tiers: an essential tier and an important tier. When healthcare providers implement the set, there should be focus on monitoring the outcomes from the essential tier, whereas the important outcomes can be implemented when feasible (according to a methodology based on the study of Verberne et al [11]). CTCAE = Common Terminology Criteria of Adverse Events; EORTC = European Organisation for Research and Treatment of Cancer; PROM = patient-reported outcome measure; RC = radical cystectomy; RTOG = Radiation Therapy Oncology Group. a Collected with the use of the EUROQOL-5D-5L, EORTC QLQ-C30, and BLM30.
4. Discussion
A standardised set of outcome measures was developed for MIBC and mBC, following value-based healthcare principles. The implementation of a standardised set of outcome measures in daily clinical practice in institutions worldwide enables the possibility of comparing outcomes between various treatments and institutions. Ultimately, the most suited treatment for patients and the best practices can be identified. Incorporation of patient-reported outcomes will result in not only a reflection of survival and complication outcomes, but also patients’ QoL and functional outcomes. This can lead to improved shared decision-making in, for example, situations where the patient is considering treatment with a curative RC versus chemoradiation, or palliative CTx versus best supportive care. Therefore, the use of the outcomes of a standardised set will result in a high value for the patient. This set of outcome measures was developed as a multidisciplinary one to capture what is valued most by the patient. By including PROMs, it captures certain patient-specific outcomes, which are generally still not collected routinely. Although regional, national, and international registries exist, there is still no consensus on definition, timing, and PROMs in BCa care. With the development of this set, we hope to contribute to conformity in outcome measurements of BCa patients.
We acknowledge that this set does not contain all outcomes that could matter to patients, but our aim was to create a minimum standard set relevant to BCa patients, whilst keeping in mind the patient and registration burden of data collection. This standard set of outcomes should not limit physicians and institutions in collecting a broader range of outcomes or case-mix variables. Similarly, there is a high variety of PROMs available. There were several considerations that resulted in the PROMs included in the set.
Owing to the heterogeneity of the population of patients with MIBC/mBC, the working group preferred a set of PROMs based on a “core” questionnaire, which can be supplemented by a range of tumour-, treatment-, symptom-, or setting-specific “modules” as required. In the field of cancer-specific PROMs, there are two providers of such questionnaires that are most widely used, and translated and validated in multiple languages [23]. The European platform of cancer research, European Organisation for Research and Treatment of Cancer (EORTC), has developed questionnaires such as the QLQ-C30 for cancer patients and the QLQ-BLM30 for MIBC patients. In contrast, there are Functional Assessment of Cancer Therapy (FACT) questionnaires, such as FACT-G for cancer patients, FACT-G7 as an abbreviated instrument, and FACT-Bl specifically for BCa patients. The EORTC questionnaires are preferred to the FACT questionnaires, which we explained in the Supplementary material [23], [24]. The EORTC questionnaires have a superior system of core and module questionnaires, better suited for domain coverage in concordance with our results, and additional coverage of domains that received high prioritisation but not sufficient to be included in the standard set.
Similarly, for general health/overall QoL domain coverage, there are two major contenders with widespread use, the EQ-5D from the EuroQol group and the Medical Outcomes Study (MOS) group Short Forms (SF), offered in various sizes such as the SF-12, SF-20, and SF-36 (formerly the RAND-36). As the EORTC “core” and “module” questionnaires contain at least 60 items for all patients and cover multiple domains, we recommend the EQ-5D questionnaire based on its shortness. We recognise that the SF-12 questionnaire would have been a viable choice as well.
The PROM preference varies widely around the world, and each PROM is believed to have its own merits and limitations. Which set of PROMs is best suited for BCa patients is an on-going debate in literature, on which numerous studies have been based. The goal of this study was not to answer that question. It was decided that only a selection of PROMs would be recommended. In the future and with revision of the standard set, different or additional PROMs may be considered.
The durability and sustainability of this standard set depend not only on widespread implementation, but also on the data registration infrastructure that will be required. This may be challenging for many organisations, due to the investments that might be required for data collection or the infrastructure development around it (including PROMs). Electronic health records provide an instrument to collect data directly from clinical care and allow for easier acquisition of data from a large number of patients [25]. However, most electronic health records keep relying on unstructured free text fields of essential information such as disease status, treatment rationale, or treatment outcomes. Often, prospectively maintained databases still rely on manual data collection. Therefore, the data dictionary that we developed is designed for manual data registry. As the development of electronic health records advances, so do data collection processes. In concordance, this standard set requires to be updated. Therefore, we acknowledge the importance of an annual review, the convening of new evidence and literature, and the continuous refinement of this standard set of outcomes to keep it up to date, as suggested by others [14].
The development of this standard set of outcome measures had some limitations. Although it included physicians and other healthcare professionals of multiple disciplines and from institutions across the entire country of the Netherlands, it reflects the opinion of a selected group of experts and patient representatives, and lacks international representation. This could have resulted in a bias. For example, one major limitation that occurred was refraining from implementing the collection of grade ≤2 complications. The possibility of collecting grade ≤2 complications was discussed extensively by the working group during the conferences. In the Netherlands, some parts of post-treatment patient care are performed outside of the hospital by a general practitioner. To adequately collect data on all grade complications, it is mandatory that either the patient receives the full BCa treatment and follow-up process in the same institute, or adequate communication between multiple institutions must be present (eg, the general practitioner and the hospital). In the Netherlands, both are absent. As a result, it is difficult to adequately collect data on all grade complications, without the risk of missing a significant amount of data. Thus, this was not implemented in the standard set. However, when deemed feasible and reliable, the collection of grade ≤2 complications could be performed during implementation of this standard set. Furthermore, with the inclusion of the disciplines covering the key treatment modalities for patients (eg, urological surgeons for RC, radiotherapists for RTx, and oncologists for CTx and IMTx), we aimed to develop and structure the standard set so that it can be implemented worldwide. Undoubtedly, there are differences in BCa patient care due to international differences in healthcare systems, regional preferences for treatment modalities, patients’ access to healthcare systems and insurance, or other cultural differences that will influence the outcome results or the collection of outcomes. A goal of the standard set is to identify best practices and thus reveal these differences in patient care, but results must always be interpreted in concordance with international differences. Future efforts in international evaluation of the set and evaluation by a large international patient cohort could aid in global coverage and support for this standard set of outcome measures.
5. Conclusions
With a multidisciplinary group of physicians, epidemiologists, registry specialists, scientists, nurses, and patient representatives, we developed the first patient-centred standardised set of outcome measures in patients with MIBC and mBC. Collection of standardised outcomes can aid institutions and physicians worldwide in the identification of best practices, and pave the way for adjustment and improvement. Addition of treatment-related costs can supply all ingredients that are required for a value-based healthcare approach. PROMs to identify treatment impact on HRQoL can aid in shared decision-making.
Author contributions: Daan J. Reesink had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Reesink, van de Garde, Los, van Melick, van der Nat.
Acquisition of data: Reesink, van de Garde, Somford, Meijer, Los, van Melick, van der Nat.
Analysis and interpretation of data: Reesink, van de Garde, Somford, Meijer, van Melick, van der Nat.
Drafting of the manuscript: Reesink, van de Garde, van Melick, van der Nat.
Critical revision of the manuscript for important intellectual content: Reesink, van de Garde, Somford, Meijer, Los, Biesma, Horenblas, van Melick, van der Nat.
Statistical analysis: Reesink, van de Garde, van Melick, van der Nat.
Obtaining funding: Reesink, van Melick, van der Nat.
Administrative, technical, or material support: Reesink, van de Garde, van Melick, van der Nat.
Supervision: van de Garde, Biesma, Horenblas, van Melick, van der Nat.
Other: The Santeon MIBC Study Group functioned as the multidisciplinary group that prioritised, discussed, and voted for the outcomes and case-mix variables.
Financial disclosures: Daan J. Reesink certifies that all conflicts of interest including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received or pending), are the following: None.
Funding/Support and role of the sponsor: This research received a grant from Roche Nederland B.V. to perform this study (grant number: ML40374; Daan J. Reesink). The funders had no role in the study design, data collection, analysis, and decision regarding where to publish this manuscript.
Ethics statement: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Data sharing: Data dictionary is available in the Supplementary material, and obtainable through request.
CRediT authorship contribution statement
Daan J. Reesink: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualization, Project administration. Ewoudt M.W. van de Garde: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualization, Project administration. Diederik M. Somford: Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing. Richard P. Meijer: Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing. Maartje Los: Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing. Douwe H. Biesma: Supervision, Writing - review & editing. Simon Horenblas: Supervision, Writing - review & editing. Harm H.E. van Melick: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition. Paul B. van der Nat: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration. for the Santeon MIBC Study Group: Resources.
Associate Editor: Axel Bex
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.euros.2021.01.014.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Brausi M., Collette L., Kurth K. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol. 2002;41:523–531. doi: 10.1016/s0302-2838(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 2.Hounsome L.S., Verne J., McGrath J.S., Gillatt D.A. Trends in operative caseload and mortality rates after radical cystectomy for bladder cancer in England for 1998–2010. Eur Urol. 2015;67:1056–1062. doi: 10.1016/j.eururo.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Leow J.J., Bedke J., Chamie K. SIU-ICUD consultation on bladder cancer: treatment of muscle-invasive bladder cancer. World J Urol. 2019;37:61–83. doi: 10.1007/s00345-018-2606-y. [DOI] [PubMed] [Google Scholar]
- 4.Porter M.P., Penson D.F. Health related quality of life after radical cystectomy and urinary diversion for bladder cancer: a systematic review and critical analysis of the literature. J Urol. 2005;173:1318–1322. doi: 10.1097/01.ju.0000149080.82697.65. [DOI] [PubMed] [Google Scholar]
- 5.Large M.C., Malik R., Cohn J.A. Prospective health-related quality of life analysis for patients undergoing radical cystectomy and urinary diversion. Urology. 2014;84:808–814. doi: 10.1016/j.urology.2014.05.046. [DOI] [PubMed] [Google Scholar]
- 6.Porter M.E., Teisberg E.O. Redefining health care: Creating value-based competition on results. Harvard Business School Press; Boston: 2006. (ISBN: 9781591397786) [Google Scholar]
- 7.Porter Me, Larsson S., Lee Th. Standardizing patient outcomes measurement. N Engl J Med. 2016;374:504–506. doi: 10.1056/NEJMp1511701. [DOI] [PubMed] [Google Scholar]
- 8.Morgans A.K., Van Bommel A.C.M., Stowell C. Development of a standardized set of patient-centered outcomes for advanced prostate cancer: an international effort for a unified approach. Eur Urol. 2015;68:891–898. doi: 10.1016/j.eururo.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Martin N.E., Massey L., Stowell C. Defining a standard set of patient-centered outcomes for men with localized prostate cancer. Eur Urol. 2015;67:460–467. doi: 10.1016/j.eururo.2014.08.075. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery J.S., Miller D.C., Weizer A.Z. Quality indicators in the management of bladder cancer. JNCCN J Natl Compr Cancer Netw. 2013;11:492–500. doi: 10.6004/jnccn.2013.0061. [DOI] [PubMed] [Google Scholar]
- 11.Verberne W.R., Das-Gupta Z., Allegretti A.S. Development of an international standard set of value-based outcome measures for patients with chronic kidney disease: a report of the International Consortium for Health Outcomes Measurement (ICHOM) CKD Working Group. Am J Kidney Dis. 2019;73:372–384. doi: 10.1053/j.ajkd.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 12.McNamara R.L., Spatz E.S., Kelley T.A. Standardized outcome measurement for patients with coronary artery disease: consensus from the International Consortium for Health Outcomes Measurement (ICHOM) J Am Heart Assoc. 2015;4:1–9. doi: 10.1161/JAHA.115.001767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ong W.L., Schouwenburg M.G., Van Bommel A.C.M. A standard set of value-based patient-centered outcomes for breast cancer: the International Consortium for Health Outcomes Measurement (ICHOM) initiative. JAMA Oncol. 2017;3:677–685. doi: 10.1001/jamaoncol.2016.4851. [DOI] [PubMed] [Google Scholar]
- 14.Daeter E.J., Timmermans M.J.C., Hirsch A. Defining and measuring a standard set of patient-relevant outcomes in coronary artery disease. Am J Cardiol. 2018;121:1477–1488. doi: 10.1016/j.amjcard.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 16.Fitch K., Bernstein S.J., Aguilar M.D. RAND Corporation; 2001. The RAND-UCLA appropriateness method user’s manual. [Google Scholar]
- 17.Sobin L.H., Gospodarowicz M.K., Wittekind C. ed. 7. Wiley-Blackwell; 2010. TNM classification of malignant tumours. [Google Scholar]
- 18.Dindo D., Demartines N., Clavien P.-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services Common Terminology criteria for adverse events (CTCAE) version 5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- 20.Sangha O., Stucki G., Liang M.H., Fossel A.H., Katz J.N. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 21.Habbous S., Chu K.P., Harland L.T.G. Validation of a one-page patient-reported Charlson comorbidity index questionnaire for upper aerodigestive tract cancer patients. Oral Oncol. 2013;49:407–412. doi: 10.1016/j.oraloncology.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 22.American Joint Committee on Cancer . Springer; New York, NY: 2017. Bladder. [Google Scholar]
- 23.Luckett T., King M.T., Butow P.N. Choosing between the EORTC QLQ-C30 and FACT-G for measuring health-related quality of life in cancer clinical research: Issues, evidence and recommendations. Ann Oncol. 2011;22:2179–2190. doi: 10.1093/annonc/mdq721. [DOI] [PubMed] [Google Scholar]
- 24.Lien K., Zeng L., Nguyen J. Comparison of the EORTC QLQ-C15-PAL and the FACIT-Pal for assessment of quality of life in patients with advanced cancer. Expert Rev Pharmacoeconomics Outcomes Res. 2011;11:541–547. doi: 10.1586/erp.11.64. [DOI] [PubMed] [Google Scholar]
- 25.Bertagnolli M.M., Anderson B., Quina A., Piantadosi S. The electronic health record as a clinical trials tool: opportunities and challenges. Clin Trials. 2020;17:237–242. doi: 10.1177/1740774520913819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.