Abstract
Nanogels have high tunability and stability while being able to sense and respond to external stimuli by showing changes in the gel volume, water content, colloidal stability, mechanical strength, and other physical/chemical properties. In this article, advances in the preparation of nanogels will be reviewed. The application potential of nanogels in drug delivery will also be highlighted. It is the objective of this article to present a snapshot of the recent knowledge of nanogel preparation and application for future research in drug delivery.
Keywords: Nanogels, synthesis, carriers, drug delivery, sustained release
1. Introduction
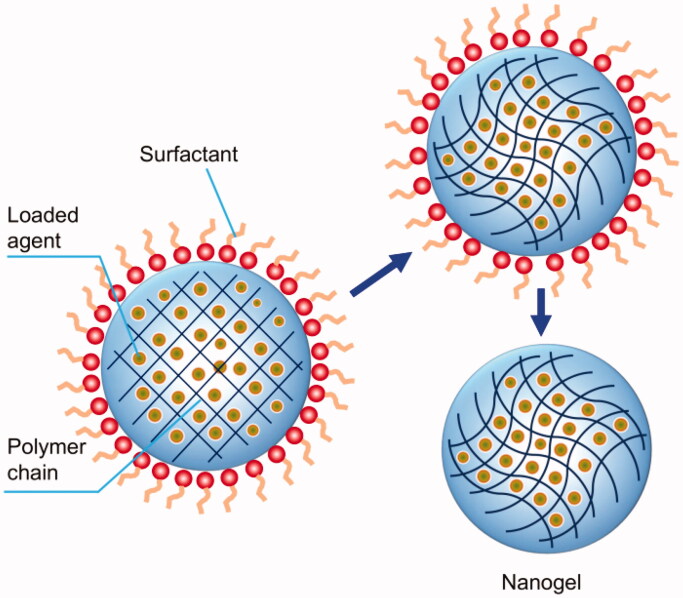
Nanomaterials can enable the controlled release of drugs and improve the stability of those agents, making a new way to disease treatment (Lang et al., 2020). Hydrogels are three-dimensional networks of polymers containing hydrophilic groups which are cross-linked by covalent bonds, hydrogen bonds, van der Waals forces or various other interactions. They can absorb and retain a substantial amount of fluids without undergoing dissolution. This unique property enables hydrogels to be used in tissue engineering and drug delivery (Peppas et al., 2006; Brandl et al., 2007; Bawa et al., 2009;; Das et al., 2021; Nai et al., 2021). Nanogels are hydrogels with the size being around 1–1000 nm (Ahmed et al., 2020). They can carry and protect the active ingredients loaded inside, and can sense and respond to external stimuli by exhibiting changes in the gel volume, water content, colloidal stability, mechanical strength, and other physical/chemical properties (Wu & Tian, 2008; He et al., 2009). Along with their high tunability and stability (Alarcón et al., 2005; Sahiner et al., 2006; Lu et al., 2021), nanogels have been used extensively in drug delivery.
Similar to biological tissues, nanogels have good biocompatibility. Meanwhile, the 3D structure enables hydrophobic or hydrophilic drugs to be encapsulated inside. This protects the drugs from degradation during storage or blood circulation (e.g. hydrolysis or enzymatic degradation) and reduces toxic side effects (Raemdonck et al., 2009; Pan et al., 2012; Maciel et al., 2013; Chen et al., 2017; Liu & Thayumanavan, 2017). Importantly, nanogels can be loaded with drugs with the drug activity retained (Vinogradov, 2010; Peng et al., 2021). In addition, the blood circulation time as well as tissue targeting capacity of nanogels can be prolonged by surface modification (Vinogradov et al., 2002). This further enhances the performance of nanogels in pharmaceutical formulation. Nanogels, therefore, have attracted the attention of many researchers.
2. Methods of nanogel preparation
Over the years, a variety of methods for the synthesis of nanogels have been developed (Nicolas & Jutta, 2010; Jiang et al., 2014; Torchilin, 2014). Depending on the raw materials adopted, nanogels can be synthesized by either having polymerization and crosslinking being held simultaneously or having polymerization being performed first followed by crosslinking.
2.1. Concomitant polymerization and crosslinking
Nanogels can be synthesized by having polymerization and crosslinking being held concomitantly. Since most of the monomers and crosslinking agents used for nanogel preparation are water-soluble, polymerization reactions are generally carried out in an aqueous medium. Based on the working mechanism, methods of nanogel preparation in which polymerization and crosslinking are held concomitantly can be divided into three types: precipitation polymerization, inversion emulsion polymerization and micro-template polymerization.
2.1.1. Precipitation polymerization
A major feature of precipitation polymerization is that the reaction system is homogeneous. In another word, all monomers, crosslinkers and initiators before the reaction are homogeneously dissolved in the same reaction medium. The length of the polymer chain increases as the polymerization reaction progresses. When the polymer chain grows to a certain length, the generated phase is separated out to form polymer colloidal particles and finally nanogels. The number of monomers, crosslinking agents and initiators all have an effect on the size of nanogels.
Precipitation polymerization is one of the first methods exploited for the generation of temperature-responsive poly(N-isopropylacrylamide) (PNIPAM) nanogels (Oh et al., 2008; Raemdonck et al., 2009). During nanogel preparation, the insertion of the degradable crosslinker enables the nanogels to have controlled drug release in tumor microenvironments (Zhang et al., 2015; Wang et al., 2016; Zhang & Tung, 2018; Liu et al., 2019; Liu et al., 2020). For example, nanogels prepared from crosslinking agents containing disulfide bonds enable accelerated drug release triggered by the high concentration of glutathione in tumor cells (Raemdonck et al., 2009; Pan et al., 2012; Maciel et al., 2013; Chen et al., 2017). Nanogels containing acetals and ketals can release the loaded drug rapidly in the tumor acidic environment (Chen et al., 2007; Liu & Thayumanavan, 2017). Chen et al. (2007) synthesized PNIPAM nanogels by using the precipitation polymerization method and tested the particle size distribution of the nanogels with a transmission electron microscope and Malvin particle size analyzer. They found that the particle size of the nanogels could be adjusted by changing the content of the surfactant sodium dodecyl sulfate. Duracher and cowrokers (Zhang et al., 2004; Ribovski et al., 2021) studied the mechanism of copolymerization reactions between N-isopropylacrylamide (NIPAM) and various other hydrophilic monomers to form PNIPAM nanogels. The aqueous solution of PNIPAM has a minimum critical solution temperature (LCST) of about 32 °C. The reaction temperature must be higher than the LCST and is thus set at 60–80 °C. When the resulting PNIPAM free radical chain grows to a certain critical chain length, the originally hydrophilic chain changes to a hydrophobic chain, resulting in ‘coil-to-globule’ conformational transformation, with the primary particles appearing in the reaction system. This is the nucleation stage of the precipitation polymerization reaction.
Ribovski et al. (2021) used precipitation polymerization to prepare fluorescently labeled PNIPAM nanogels. The hardness of PNIPAM nanogels is regulated by the degree of polymer crosslinking. The effect of the hardness of PNIPAM nanogels on their interactions with the blood-brain barrier (BBB) model in vitro was studied. PNIPAM nanogels of ∼200 nm with varying degrees of stiffness were made by inclusion of 1.5 mol% (NG1.5), 5 mol% (NG5), and 14 mol% (NG14) of N,N′-methylenebis(acrylamide) (BIS) during synthesis. NG14 nanogels showed a higher level of uptake by brain endothelial cells than NG1.5 and NG5 nanogels. In addition, NG1.5 and NG5 exhibited a higher level of transcytosis compared to NG14. An increase in the size of the nanogels (up to ∼400 nm) without changing the nanogel stiffness was shown to have little influence on cellular uptake or transcytosis. All these reveal that the hardness of nanogels has an opposite effect on the uptake and endocytosis of nanogels in the BBB.
In precipitation polymerization, various colloidal particles can be used as templates or seeds, and the stimuli-responsive polymer can be used to coat the template particles to form multifunctional or stimuli-responsive composite nanogels (Zha et al., 2002). Zha et al. (2002) used the precipitation polymerization method to coat the surface-modified silica colloidal particles with PNIPAM, and then removed the silica particles to obtain the temperature-responsive nanogels with a hollow structure. The size of the hollow nanogels can be controlled by changing the size of the template. The thickness of the hollow nanogel shell can also be adjusted by changing the mass ratio of the monomer and the template. The volume phase transformation of the hollow nanogels occurs at about 32 °C. The permeability of hollow nanogel shells can be changed in response to external stimuli, and this mediates the controlled release of guest molecules. Recently, Xing et al. (2011) also adopted a similar method to synthesize pH/temperature-responsive hollow nanogels. By changing the temperature, the hollow nanogels can be loaded with drugs, with the rate of drug release being able to be controlled by changing the pH of the surrounding medium (Averick et al., 2011).
2.1.2. Inverse emulsion polymerization
Synthesis of nanogels can occur in the presence of an oil-soluble emulsifier upon the adoption of proper emulsification methods. The generated W/O inverse emulsions can undergo polymerization reactions. After removing both the organic solvent and the emulsifier, nanogels that can be stably dispersed in an aqueous medium can be obtained (Figure 1) (Wang et al., 2018). During inverse emulsion polymerization, the size of nanogels is affected by many factors, including the choice of surfactants, the concentration of the monomer and the crosslinker, and the pH of the reaction medium (Mitra et al., 2001; Peres et al., 2018). The disadvantage of this method is that an organic solvent is used as the reaction medium. The presence of emulsifiers or co-emulsifiers will bring difficulties to the purification of the generated nanogels.
Figure 1.
Generation of nanogels via the inverse emulsion polymerization method.
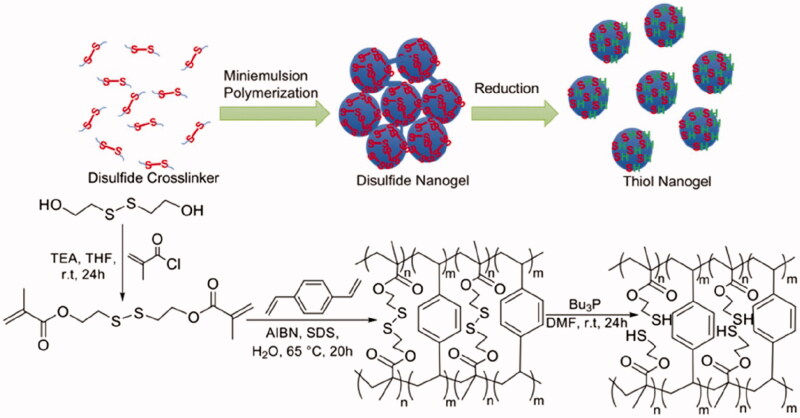
Using N, N′-methylenebis(acrylamide) (BIS) and N-acryloyl-l-glutamic acid (l-AGA), Peres et al. (2018) prepared poly(l-AGA) and poly(l-AGA-co-Bis) nanogels by inverse emulsion polymerization. The results showed that the degree of nanogel swelling increases with a change of pH. In addition, the presence of carboxylic acid groups and amide groups in the polymer network plays an important role in determining the physicochemical properties of the nanogels and in affecting the overall hydrophilicity. Deen et al. (2008) copolymerized N-acryl-N'-methylpiperazine with methyl methacrylate in an O/W reverse-phase microemulsion system to prepare pH-responsive nanogels. Nanogels swell in an acidic aqueous solution but shrink in an alkaline solution. Zhang et al. (2021) adopted the microemulsion polymerization method and introduced a high density of thiol groups into the polymer gel to obtain disulfide polymer nanogels. After forming the nanogels, the disulfide and tributyl phosphine (Bu3P) were reduced to mercaptan groups to obtain the thiol polymer nanogels. The combination of the hydrophobic benzene ring and the hydrophilic sulfhydryl group in the nanogel repeat unit makes the nanogel amphiphilic (Figure 2). The nanogel has a high sorption affinity for Hg(II) complexes and Hg-dissolved organic matter complexes found in water and for elemental (Hg0) and soluble Hg-alkyl thiol species found in hydrocarbons.
Figure 2.
A synthetic method for the generation of thiol polymer nanogels. AIBN: 2,2′-azobis(2-methylpropionitrile); Bu3P: tributylphosphine: SDS: sodium dodecyl sulfate; TEA: trimethylamine; THF: tetrahydrofuran; r.t.: room temperature; DMF: N,N-dimethylformamide. Reproduced from Zhang et al. (2021) with permission from the American Chemical Society.
Matyjaszewski and coworkers (Zhang et al., 2021) synthesized poly(ethylene glycol) methacrylate monomethyl ether ester (OEOMA) nanogels using atomic transfer radical polymerization (ATRP) in a microemulsion system with the disulfide serving as the crosslinking point. Compared with conventional reversed microemulsion polymerization, the nanogels synthesized by using active free radicals have higher colloidal stability, narrower and more uniform distribution of size, and a more controllable structure and composition. A large number of active groups on the surface of the synthesized nanogels can be used to be conjugated with other active components (Dai et al., 2006). If this method is used to synthesize nanogels with degradable crosslinkers, the molecular weight distribution of the nanogels can be narrower, and the nanogels can be more easily metabolized in a human body. Recently, Peng et al. (2021) also designed a degradable poly(phosphorylcholine)-based (HPMPC) nanogel for drug release induced by hypoxia in tumor cells. The obtained nanogel showed a prolonged blood circulation time and led to a negligible immune response. Importantly, the nanogel was found to be effectively degraded. Drug-loaded HPMPC nanogels, therefore, showed promising tumor inhibition effects both in vivo and in vitro while having high biocompatibility.
Monodisperse nanogels can be prepared by using the membrane emulsification technology (Seiffert and Weitz, 2010). However, being limited by the pore size of the emulsion membrane, it is difficult to prepare nanogels with a particle size less than 100 nm by using this method. Microfluidic emulsification involves mixing and emulsifying the aqueous solutions containing the monomer, crosslinker, and initiator through microchannels in the presence of an organic phase to form O/W emulsions, which are then polymerized in situ to form nanogels. The shape and size of the nanogels are affected by the size of microchannels, the flow rate of the solutions and the polymerization reaction time (Oudshoorn et al., 2008).
2.1.3. Microtemplate polymerization
During microtemplate polymerization, the monomer and the crosslinking agent are first added to the microtemplate. They then undergo free radical polymerization. Finally, the hydrogel nanoparticles are separated from the microtemplate. The advantage of this method is that it can generate nanogels with different shapes (Grimaudo et al., 2019). In addition, the method allows cells or other bioactive components to be easily loaded into the nanogels; however, due to the limitation of the size of the microtemplate, it is difficult to prepare nanogels with a relatively small size. One type of microtemplate polymerization is the photolithographic microtemplate polymerization method, in which the monomer and the crosslinking agent are mixed with the photoinitiator. Upon coating on a non-wetting substrate, a layer of a non-wetting template with micro-holes is added. After illumination with a light source with a specific wavelength, free radical polymerization of the monomer and the crosslinking agent is triggered.
The most prominent feature of lithographic microtemplate polymerization is that it can precisely control the size, shape and composition of the generated nanogels. Nanogels prepared using this method can have different shapes with a particle size less than 200 nm. Yet, this method has stringent requirements on the surface of the template and the substrate. Polymethylsiloxane and photocurable perfluorinated polyether are some of the possible templates and substrate materials (Oudshoorn et al., 2007). Grooved microtemplate polymerization is similar to photolithographic microtemplate polymerization. During nanogel preparation, the monomer and the crosslinking agent are loaded into the microtemplate with grooves. The hydrogel particles with a fixed size and shape are then polymerized by light irradiation (Yeh et al., 2006; Jung et al., 2009).
2.2. Separate polymerization and crosslinking
In this category, polymers are first formed, followed by crosslinking between the polymer molecular chains to generate nanogels. This method is especially suitable for the preparation of nanogels based on natural polymers (Xia et al., 2003). Based on the mechanism of nanogel generation, the method can be of different types: precipitation/crosslinking, emulsification/crosslinking, self-assembly/crosslinking, and microtemplate forming/crosslinking.
2.2.1. Precipitation/crosslinking
The precipitation/crosslinking method is to have water-soluble polymers being precipitated out of a homogeneous aqueous solution to form nanoparticles. After that, crosslinking reactions are performed to make the polymers in the particles crosslinked to generate nanogels. Xia et al. (2003) prepared nanogels in a salt solution without using an emulsifier. The size and size distribution of the nanogels are closely related to the salt concentration and the reaction temperature. The LCST of the aqueous solution of the polymer decreased with the increase of the NaCl concentration. Then, crosslinking reactions are performed. Unfortunately, the functioning of the crosslinking agent used to prepare the nanogels requires the availability of a strongly alkaline medium, which in general is not available during the drug loading process. For this, Cai et al. (2003) first modified the molecular chain of the polymer so that its side chain was either connected to the vinyl group through the degradable ester linkage or directly connected to the acrylate group. Then the free radical polymerization reaction was initiated by heating the polymer solution above the LCST. Recently, Shen et al. (2007) prepared pH-responsive nanogels with the particle size of 70-80 nm by adding an ethylenediamine diacetic acid diacetaldehyde (EDTAA) crosslinking agent into an aqueous solution of chitosan. The nanogel has a positive charge when pH <4.8, and a negative charge when pH > 5.2. The change of pH of the surrounding medium can make the surface charge of the nanogel reverse. Nanogels can also be formed through electrostatic interactions if some polyelectrolytes with specific structures are added into an aqueous solution of chitosan (Ethirajan et al., 2008). The concentration of water-soluble polymers used to prepare nanogels by using this method should not be too high; otherwise, large gels are easily formed. The efficiency of this preparation method is, therefore, relatively low.
2.2.2. Emulsion/crosslinking
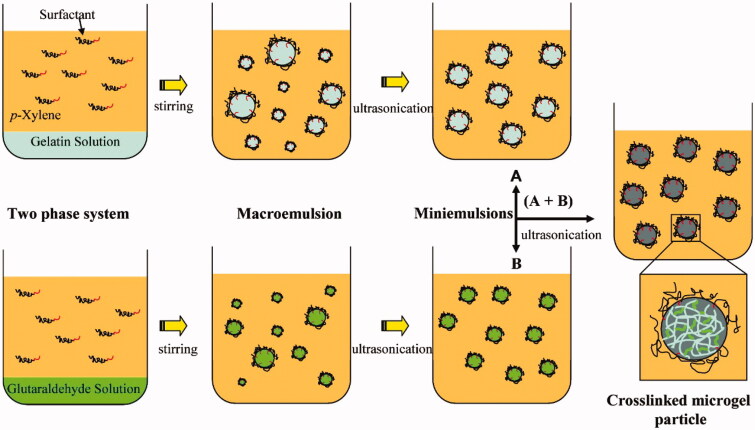
In this method, polymers are first dissolved in water. Under the action of oil-soluble surfactants, appropriate emulsion methods are applied to form an aqueous dispersion of polymers in an organic solvent to obtain W/O inverse emulsions in the presence of a surfactant. As shown in Figure 3, an aqueous solution of gelatin was emulsified into a fine emulsion under the action of an ultrasonic wave. Then glutaraldehyde was added as a crosslinking agent to generate nanogels (Mitra et al., 2001). Mitra et al. (2001) dispersed the aqueous solution of dextran-doxorubicin (DOX) conjugate and chitosan in organic solvents to form microemulsions. After adding a crosslinking agent, pH-responsive nanogels that can be used to deliver anti-cancer drugs were prepared. The crosslinking reaction between polymers occurs in tiny droplets of an aqueous solution under mild reaction conditions. Therefore, it can be used to generate nanogels loaded with fragile bioactive agents. Similar to inversion emulsion polymerization, this method involves the use of organic solvents, emulsifiers and co-emulsifiers. This brings great difficulties to the purification of nanogels.
Figure 3.
A schematic illustration depicting the generation of nanogels using the emulsification/crosslinking method. Reproduced from Ethirajan et al. (2008) with permission from the American Chemical Society.
The membrane emulsification method can generate a W/O invert emulsion from an aqueous solution of a polymer. The emulsion can then undergo crosslinking reactions to generate monodisperse nanogels (Wang et al., 2006). Due to the high viscosity of the polymer solution and the limitation of the pore size, this method can hardly prepare nanogels with a relatively small size. It is, however, convenient for drug loading (Zhang et al., 2007). The microfluidic emulsification technology can also be used to prepare nanogels. This was shown by Zhang et al. (2007), who used the microfluidic emulsification method to generate nanogels from alginate through crosslinking mediated by using Ca2+. The generated nanogels are stable, with the morphology and structure being easy to be controlled.
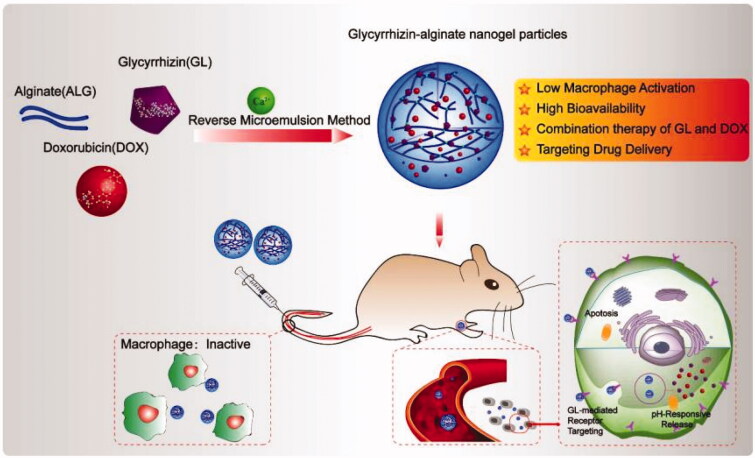
Nanogels provide a platform for drug co-delivery because they have a 3D network structure for being loaded with both hydrophilic and hydrophobic compounds. In a recent study, both glycyrrhizic acid (GL) and doxorubicin (DOX) were loaded into alginate-based nanogels by using the phase-transition temperature emulsification method. The obtained drug-loaded alginate-based nanogels exhibited not only the hepatocellular carcinoma targeting property but also the synergistic antitumor effects mediated by GL and DOX (Figure 4) (Tong et al., 2018). In addition, the alginate-based nanogels showed good biocompatibility and low toxicity to liver tissues and enabled controlled release of the drugs (Sahu et al., 2017). All these suggest that nanogels have the practical potential for use in multi-drug therapy.
Figure 4.
A schematic illustration depicting the generation and use of alginate-based nanogels co-loaded with glycyrrhizin and doxorubicin (DOX/GL-ALG NGPs) for cancer therapy. Reproduced from Wang et al. (2019) with permission from Ivyspring International Publisher.
Apart from the aforementioned, the solvent-evaporation emulsification method can be used to generate nanogels (Bagheri et al., 2021). Bagheri et al. (2021) generated nanogels from chitosan-carboxymethyl cellulose and flaxseed oil and then loaded the nanogels with atorvastatin to obtain atorvastatin-oil nanogels (ATONG). The loading efficiency, drug release sustainability, and gel stability of ATONG were tested. The results confirmed the high efficiency of drug loading and release. Cytotoxicity tests showed that ATONG can safely release atorvastatin upon cellular uptake. Recently, flaxseed oil-atorvastatin nanogels were successfully generated from chitosan, carboxymethyl cellulose and poly(vinyl alcohol). The obtained nanogels exhibited negligible toxicity and antibacterial properties.
2.2.3. Self-assembly/crosslinking
Molecular self-assembly refers to the process in which molecules spontaneously form thermodynamically stable aggregates through non-covalent interactions. Driving forces of molecular self-assembly include hydrogen bonding interactions, electrostatic interactions, hydrophobic interactions, and van der Waals forces. Water-soluble polymers with specific structures can self-assemble into nanogels driven by these interactions (Meng et al., 2009). Physical crosslinks in nanogels are not stable. Dissociation is easy to occur under high temperature or high salt concentrations. The stability of self-assembled nanogels can be significantly improved if chemical crosslinking and optical crosslinking are used instead. If disulfide bonds are involved in crosslinking, the prepared nanogels can respond to the degradation of specific reducing substances such as glutathione in cells (Sawada & Akiyoshi, 2010). The conditions for self-assembly/crosslinking during the generation of nanogels are relatively mild, so this method can be used to load the nanogels with fragile bioactive components such as proteins (Dou & Jiang, 2007). The size of the assembled nanogels can be controlled by selecting an appropriate polymer concentration or environmental parameters such as temperature, pH and ionic strength.
In fact, the self-assembly/crosslinking method is particularly suitable for the preparation of nanogels based on natural polymers (Xia et al., 2003; Rolland et al., 2005; Napier & Desimone, 2007). There are many hydroxyl groups on the molecular chains of natural polysaccharides, which can be grafted with and modified by polymers containing carboxyl groups. The copolymers generated can self-assemble through hydrogen bonding interactions, and upon further crosslinking reactions, nanogels can be obtained. Dou & Jiang (2007) grafted modified hydroxyethyl cellulose (HEC) with poly(acrylic acid) (PAA), and the generated copolymer (HEC-g-PAA) could self-assemble into nanoparticles in an aqueous medium. The molecular chains of PAA could be crosslinked with a crosslinking agent to finally generate pH-responsive nanogels.
2.2.4. Microtemplating/crosslinking
The method is to add precursors of chemical reactions into a microtemplate and then obtain nanogels upon chemical crosslinking or optical crosslinking. It is also called the ‘Particle Replication in Nonwetting Templates (PRINT)’ method. The method can generate monodisperse nanogels with a well-controlled size, shape and composition (Ito, 1999; Euliss et al., 2006; Gratton et al., 2008; Chen et al., 2013). Gratton et al. (2008) used this method to prepare nanogels with different sizes, shapes and properties. Results showed that rod-like nanogels with positive surface charge can undergo cellular uptake most efficiently. Ito (1999) also used the method to prepare pH-responsive nanogels from tetraazidoaniline-modified PAA.
Liposomes are hollow vesicles with a double-walled structure formed by the self-assembly of amphiphilic phospholipid molecules. Their cavities can be used as microtemplates to prepare nanogels. For example, Hong et al. (2008) took the liposome core as a template. They first coated the liposome core with sodium alginate, followed by the addition of an aqueous solution containing Ca2+. When the temperature of the solution exceeded the melting point of the liposome, Ca2+ in the solution entered the nucleus through the liposome shell to crosslink molecules of sodium alginate. After adding a surfactant to remove liposomes on the surface, nanogels with the particle size of 120–200 nm were obtained. The nanogels were found to respond to changes in the ionic strength of the surrounding medium.
3. Applications in drug delivery
Nanogels have been widely used as drug carriers partly because of their capacity of being loaded with agents having different properties (Table 1) (Vinogradov et al., 2002; Morimoto et al., 2006; Oh et al., 2007; Oh et al., 2008; Ashrafizadeh et al., 2019). When nanogels are developed for drug delivery purposes, monomers are selected according to the mechanism of action of the drug to be delivered (Vinogradov, 2007; Bae et al., 2008). Nanogels have good biocompatibility, high stability, and high functional flexibility. These can facilitate the use of nanogels in drug delivery (Peppas et al., 2006; Zhang et al., 2016; Vashist et al., 2018). In addition, the pores in the three-dimensional network structure of nanogels enable the inclusion of small-molecule compounds. Nanogels can control the process of drug release, shield the odor of the loaded drug, improve the therapeutic effect and reduce adverse drug reactions (Ganachaud et al., 1997; Verma & Somia, 1997; Morimoto et al., 2006). Practically, there are three ways in which non-genetic drugs are loaded into nanogels. The first one is in the form of a storage structure, in which drug molecules are concentrated in the inner layer, with the outer layer being a gel film. The second one is in the form of having the drug molecules uniformly dispersing inside the nanogel. The third one is the one in which drug molecules bind to the gel via covalent interactions. At present, research on nanogels mainly focuses on overcoming the instability of protein drugs and achieving tissue targeting. Contrary to chemical drugs, biomacromolecules such as proteins have a large molecular weight and a complex structure. Delivering them while maintaining their properties is more challenging than delivering small-molecule compounds (Li et al., 2002; Cheng et al., 2011; Thorne et al., 2011).
Table 1.
Examples of nanogels reported for drug delivery applications.
| Agent delivered | Nanogel adopted | Properties | Ref. |
|---|---|---|---|
| DOX | HPMPC nanogel | Showing good tumor inhibition effects and good biocompatibility | Peng et al., 2021 |
| Water-soluble agent | pH-responsive nanogel | Showing pH-responsive swelling properties | Jung et al., 2006 |
| Cu(II) complex | Oligo(ethylene oxide) methacrylate nanogel | Possessing a large number of active groups (which can be used for surface modification) on the nanogel surface and showing pH-responsive swelling properties | Zhang et al., 2021 |
| GL and DOX | Alginate-based nanogel | Showing tumor-targeting capacity, good tumor inhibition effects, good biocompatibility, and high drug release controllability | Tong et al., 2018 |
| Atorvastatin | Atorvastatin-oil nanogel | Having the capacity of permeating through skin layers | Kabanov & Vinogradov, 2009 |
| Water-soluble agent | PNIPAM nanogel | Having good target specificity and high drug release controllability | Duracher et al., 1999 |
| Hydrophobic and hydrophilic agents | Dual chemically and physically crosslinked anionic nanogel | Showing high efficiency in being loaded with both hydrophilic and hydrophobic agents | Bae et al., 2008 |
| Oligodeoxynucleotide | Cationic aminated latex nanogel | Having a high affinity for the oligodeoxynucleotide for nucleic acid delivery | Ganachaud et al., 1997 |
When nanogels reach the target tissue, the onset and rate of drug release have to be properly controlled. There are three major methods of achieving this: (1) drug release via gel degradation, (2) drug release via gel expansion and drug diffusion, and (3) drug release via the breakage of interactions between the drug and the nanogel. In addition, a nanogel can be designed to be either a programmed pulsed-release system (PPRS) or an intelligent pulsed-release system (IPRS). The release mode of a PPRS is achieved by the predetermined gel structure, with the lag time and duration of drug release being controlled by the polymer degradation profile. Recently, Bütün et al. (2000) designed a core-shell gel-based sustained-release system, which consisted of alternating drug-containing and non-drug-containing regions, in which the duration of drug release was determined by the gel properties and by the thickness of each layer. Besides delivering non-genetic agents, nanogels can be used to deliver nucleic acids to prevent the occurrence of problems caused by the immunogenicity and pathogenicity of viral vectors (Verma & Somia, 1997). By taking advantage of the surface charge of nanogels, nucleic acids can be effectively loaded for delivery purposes. The feasibility of this was demonstrated by Ganachaud et al. (1997), who synthesized aminated core-shell particles via soap-free emulsion polymerization of styrene and vinylbenzoamide. The particles were found to be successfully loaded with DNA. In addition to nucleic acid loading, functional groups on the surface of nanogels can be adopted so as to incorporate the nanogels with targeting ligands.
4. Concluding remarks
Nanogels have attracted extensive research interest for applications in targeted drug delivery, diagnosis, biosensing, and separation of biological substances (Li et al., 2002; Cheng et al., 2011; Thorne et al., 2011). Nanogels show the capacity of being loaded with diverse hydrophilic or hydrophobic agents, ranging from small-molecule compounds and proteins to nucleic acids. Upon proper design, nanogels can trigger controlled or sustained drug release; however, the residual surfactants or unreacted monomers after nanogel preparation can head to toxic effects. This is a problem that has to be solved in future research in order to enhance the translation of works from the laboratory to practical use. For those nanogels that are generated from synthetic polymers, more studies on the long-term safety profile, as well as the toxicological properties of metabolites of those polymers, should also be performed so as to confirm the safe use of the nanogels in treatment.
Funding Statement
This work was supported by the Chinese University of Hong Kong (Shenzhen) [PF01001421, UF0200142, and UDF01001421].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ahmed S, Alhareth K, Mignet N. (2020). Advancement in nanogel formulations provides controlled drug release. Int J Pharm 584:119435. [DOI] [PubMed] [Google Scholar]
- Alarcón C, Pennadam S, Alexander C. (2005). Stimuli responsive polymers for biomedical applications. Chem Soc Rev 34:276–85. [DOI] [PubMed] [Google Scholar]
- Ashrafizadeh M, Tam K, Javadi A, et al. (2019). Synthesis and physicochemical properties of dual-responsive acrylic acid/butyl acrylate cross-linked nanogel systems. J Colloid Interface Sci 556:313–23. [DOI] [PubMed] [Google Scholar]
- Averick S, Magenau A, Simakova A, et al. (2011). Covalently incorporated protein-nanogels using AGET ATRP in an inverse miniemulsion. Polym Chem 2:1476–8. [Google Scholar]
- Bae K, Mok H, Park T. (2008). Synthesis, characterization, and intracellular delivery of reducible heparin nanogels for apoptotic cell death. Biomaterials 29:3376–83. [DOI] [PubMed] [Google Scholar]
- Bagheri F, Darakhshan S, Mazloomi S, et al. (2021). Dual loading of Nigella sativa oil-atorvastatin in chitosan-carboxymethyl cellulose nanogel as a transdermal delivery system. Drug Dev Ind Pharm 47:569–78. [DOI] [PubMed] [Google Scholar]
- Bawa P, Pillay V, Choonara Y, et al. (2009). Stimuli-responsive polymers and their applications in drug delivery. Biomed Mater 4:022001. [DOI] [PubMed] [Google Scholar]
- Boddohi S, Moore N, Johnson P, et al. (2009). Polysaccharide-based polyelectrolyte complex nanoparticles from chitosan, heparin, and hyaluronan. Biomacromolecules 10:1402–9. [DOI] [PubMed] [Google Scholar]
- Brandl F, Sommer F, Goepferich A. (2007). Rational design of hydrogels for tissue engineering: impact of physical factors on cell behavior. Biomaterials 28:134–46. [DOI] [PubMed] [Google Scholar]
- Bütün V, Wang X, Banez M, et al. (2000). Synthesis of shell cross-linked micelles at high solids in aqueous media. Macromolecules 33:1–3. [Google Scholar]
- Cai T, Hu Z, Ponder B, et al. (2003). Synthesis and study of and controlled release from nanoparticles and their networks based on functionalized hydroxypropylcellulose. Macromolecules 36:6559–64. [Google Scholar]
- Chacko R, Ventura J, Zhuang J, et al. (2012). Polymer nanogels: a versatile nanoscopic drug delivery platform. Adv Drug Deliv Rev 64:836–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Gu Y, Hu Y. (2007). Synthesis and characterization of pH and temperature-sensitive nano-hydrogels. J China Med Univ 38:25–9. [Google Scholar]
- Chen W, Zou Y, Zhong Z, et al. (2017). Cyclo (RGD)-decorated reduction-responsive nanogels mediate targeted chemotherapy of integrin overexpressing human glioblastoma in vivo. Small 13:1601997. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ballard N, Bon S. (2013). Moldable high internal phase emulsion hydrogel objects from non-covalently crosslinked poly(N-isopropylacrylamide) nanogel dispersions. Chem Commun 49:1524–6. [DOI] [PubMed] [Google Scholar]
- Cheng R, Feng F, Meng F, et al. (2011). Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Control Release 152:2–12. [DOI] [PubMed] [Google Scholar]
- Dai H, Chen Q, Qin H, et al. (2006). A temperature-responsive copolymer hydrogel in controlled drug delivery. Macromolecules 39:6584–9. [Google Scholar]
- Das D, Alhusaini Q, Kaur K, et al. (2021). Enzyme-responsive biopolymeric nanogel fibers by extrusion: engineering of high-surface-area hydrogels and application in bacterial enzyme detection. ACS Appl Mater Interfaces 13:12928–40. [DOI] [PubMed] [Google Scholar]
- Deen G, Gan L. (2008). Study of microemulsion polymerization conditions on the preparation of “stimuli” responsive copolymer nanogels of N‐acryloyl‐N′‐methyl piperazine and methyl methacrylate. J Dispers Sci Technol 29:431–5. [Google Scholar]
- Dou H, Jiang M. (2007). Fabrication, characterization and drug loading of pH‐dependent multi‐morpho‐logical nanoparticles based on cellulose. Polym Int 56:1206–12. [Google Scholar]
- Dou H, Yang W, Tao K, et al. (2010). Thermal sensitive microgels with stable and reversible photoluminescence based on covalently bonded quantum dots. Langmuir 26:5022–7. [DOI] [PubMed] [Google Scholar]
- Duracher D, Elaissari A, Pichot C. (1999). Preparation of poly (N‐isopropylmethacrylamide) latexes kinetic studies and characterization. J Polym Sci 37:1823–37. [Google Scholar]
- Eckmann D, Composto R, Tsourkas A, et al. (2014). Nanogel carrier design for targeted drug delivery. J Mater Chem B 2:8085–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethirajan A, Schoeller K, Musyanovych A, et al. (2008). Synthesis and optimization of gelatin nanoparticles using the miniemulsion process. Biomacromolecules 9:2383–9. [DOI] [PubMed] [Google Scholar]
- Euliss L, Dupont J, Gratton S, et al. (2006). Imparting size, shape, and composition control of materials for nanomedicine. Chem Soc Rev 35:1095–104. [DOI] [PubMed] [Google Scholar]
- Ganachaud F, Elaïssari A, Pichot C, et al. (1997). Adsorption of single-stranded DNA fragments onto cationic aminated latex particles. Langmuir 13:701–7. [Google Scholar]
- Gratton S, Ropp P, Pohlhaus P, et al. (2008). The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA 105:11613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaudo M, Concheiro A, Alvarez-Lorenzo C. (2019). Nanogels for regenerative medicine. J Control Release 313:148–60. [DOI] [PubMed] [Google Scholar]
- He J, Tong X, Zhao Y. (2009). Photoresponsive nanogels based on photocontrollable cross-links. Macromolecules 42:4845–52. [Google Scholar]
- Hendrickson G, Smith M, South A, et al. (2010). Design of multiresponsive hydrogel particles and assemblies. Adv Funct Mater 20:1697–712. [Google Scholar]
- Hong J, Vreeland W, Lacerda S, et al. (2008). Liposome-Templated Supramolecular Assembly of Responsive Alginate Nanogels. Langmuir 24:4092–6. [DOI] [PubMed] [Google Scholar]
- Ito Y. (1999). Photolithographic synthesis of intelligent microgels. J Intell Mater Syst Struct 10:541–7. [Google Scholar]
- Jiang Y, Chen J, Deng C, et al. (2014). Click hydrogels, microgels and nanogels: emerging platforms for drug delivery and tissue engineering. Biomaterials 35:4969–85. [DOI] [PubMed] [Google Scholar]
- Jung K, Lee D, Park J. (2009). Biopolymer-based microgels/nanogels for drug delivery applications. Prog Polym Sci 34:1261–82. [Google Scholar]
- Jung K, Tang C, Gao H, et al. (2006). Inverse miniemulsion ATRP: a new method for synthesis and functionalization of well-defined water-soluble/cross-linked polymeric particles. J Am Chem Soc 128:5578–84. [DOI] [PubMed] [Google Scholar]
- Kabanov A, Vinogradov S. (2009). Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed Engl 48:5418–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang X, Wang T, Sun M, et al. (2020). Advances and applications of chitosan-based nanomaterials as oral delivery carriers: a review. Int J Biol Macromol 154:433–45. [DOI] [PubMed] [Google Scholar]
- Lee E, Kim D, Youn Y, et al. (2008). A virus-mimetic nanogel vehicle. Angew Chem Int Ed Engl 47:2418–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, van Nostrum C, Mastrobattista E, et al. (2017). Nanogels for intracellular delivery of biotherapeutics. J Control Release 259:16–28. [DOI] [PubMed] [Google Scholar]
- Li W, Wang W, Li R, et al. (2002). Synthesis and bio-medical application of the intelligent nanogels. Nucl Tech 25:624–30. [Google Scholar]
- Liu R, An Y, Jia W, et al. (2020). Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. J Control Release 321:589–601. [DOI] [PubMed] [Google Scholar]
- Liu R, Hu C, Yang Y, et al. (2019). Theranostic nanoparticles with tumor-specific enzyme-triggered size reduction and drug release to perform photothermal therapy for breast cancer treatment. Acta Pharm Sin B 9:410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Thayumanavan S. (2017). Substituent effects on the pH sensitivity of acetals and ketals and their correlation with encapsulation stability in polymeric nanogels. J Am Chem Soc 139:2306–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Jia D, Ma X, et al. (2021). Reduction-responsive chemo-capsule-based prodrug nanogel for synergistic treatment of tumor chemotherapy. ACS Appl Mater Interfaces 13:8940–51. [DOI] [PubMed] [Google Scholar]
- Maciel D, Figueira P, Xiao S, et al. (2013). Redox-responsive alginate nanogels with enhanced anticancer cytotoxicity. Biomacromolecules 14:3140–6. [DOI] [PubMed] [Google Scholar]
- Marty J, Oppenheim RC, Speiser P. (1978). Nanoparticles – a new colloidal drug delivery system. Pharm Acta Helv 53:17–23. [PubMed] [Google Scholar]
- Meng F, Hennink W, Zhong Z. (2009). Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials 30:2180–98. [DOI] [PubMed] [Google Scholar]
- Mitra S, Gaur U, Ghosh P, et al. (2001). Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J Control Release 74:317–23. [DOI] [PubMed] [Google Scholar]
- Morimoto N, Nomura S, Miyazawa N, et al. (2006). Nanogel engineered designs for polymeric drug delivery. Polym Matrices Drug Particle Eng 924:88–101. [Google Scholar]
- Nai J, Rajput R, Narkhede J, et al. (2021). Synthesis and evaluation of UV cross-linked poly (acrylamide) loaded thymol nanogel for antifungal application in oral candidiasis. J Polym Res 28:15. [Google Scholar]
- Napier M, Desimone J. (2007). Nanoparticle drug delivery platform. Polymer Revs 47:321–7. [Google Scholar]
- Nicolas S, Jutta R. (2010). Synthesis of nanogels/microgels by conventional and controlled radical crosslinking. Polym Chem 1:965–77. [Google Scholar]
- Nosrati H, Abbasi R, Charmi J, et al. (2018). Folic acid conjugated bovine serum albumin: an efficient smart and tumor targeted biomacromolecule for inhibition folate receptor positive cancer cells. Int J Biol Macromol 117:1125–32. [DOI] [PubMed] [Google Scholar]
- Nukolova N, Oberoi H, Cohen S, et al. (2011). Folate-decorated nanogels for targeted therapy of ovarian cancer. Biomaterials 32:5417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Siegwart D, Matyjaszewski K. (2007). Synthesis and biodegradation of nanogels as delivery carriers for carbohydrate drugs. Biomacromolecules 8:3326–31. [DOI] [PubMed] [Google Scholar]
- Oh JK, Drumright R, Siegwart DJ, Matyjaszewski K. (2008). The development of microgels/nanogels for drug delivery applications. Prog Polym Sci 33:448–77. [Google Scholar]
- Oudshoorn M, Penterman R, Rissmann R, et al. (2007). Preparation and characterization of structured hydrogel microparticles based on cross-linked hyperbranched polyglycerol. Langmuir 23:11819–25. [DOI] [PubMed] [Google Scholar]
- Oudshoorn M, Penterman R, Rissmann R, et al. (2008). Fabrication of uniformly shaped hydrogel microparticles based on crosslinked hyperbranched polyglycerol by micromolding and photolithographic methods. J Control Release 132:e31–32. [Google Scholar]
- Pan Y, Chen Y, Wang D, et al. (2012). Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials 33:6570–9. [DOI] [PubMed] [Google Scholar]
- Peng S, Ouyang B, Xin Y, et al. (2021). Hypoxia-degradable and long-circulating zwitterionic phosphorylcholine-based nanogel for enhanced tumor drug delivery. Acta Pharm Sin B 11:560–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppas N, Hilt J, Khademhosseini A, et al. (2006). Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater 18:1345–60. [Google Scholar]
- Peres L, dos Anjos R, Tappertzhofen L, et al. (2018). pH-responsive physically and chemically cross-linked glutamic-acid-based hydrogels and nanogels. Eur Polym J 101:341–9. [Google Scholar]
- Raemdonck K, Demeester J, De S. (2009). Advanced nanogel engineering for drug delivery. Soft Matter 5:707–15. [Google Scholar]
- Ribovski L, Jong E, Mergel O, et al. (2021). Low nanogel stiffness favors nanogel transcytosis across an in vitro blood-brain barrier. Nanomedicine 34:102377. [DOI] [PubMed] [Google Scholar]
- Rolland J, Maynor B, Euliss L, et al. (2005). Direct fabrication and harvesting of monodisperse, shape-specific nanobiomaterials. J Am Chem Soc 127:10096–100. [DOI] [PubMed] [Google Scholar]
- Sahiner N, Godbey W, McPherson G, et al. (2006). Microgel, nanogel and hydrogel-hydrogel semi-IPN composites for biomedical applications: synthesis and characterization. Colloid Polym Sci 284:1121–9. [Google Scholar]
- Sahu P, Kashaw S, Jain S, et al. (2017). Assessment of penetration potential of pH responsive double walled biodegradable nanogels coated with eucalyptus oil for the controlled delivery of 5-fluorouracil: in vitro and ex vivo studies. J Control Release 253:122–36. [DOI] [PubMed] [Google Scholar]
- Sawada S, Akiyoshi K. (2010). Nano-encapsulation of lipase by self-assembled nanogels: induction of high enzyme activity and thermal stabilization . Macromol Biosci 10:353–8. [DOI] [PubMed] [Google Scholar]
- Seiffert S, Weitz D. (2010). Controlled fabrication of polymer microgels by polymer-analogous gelation in droplet microfluidics. Soft Matter 6:3184–90. [Google Scholar]
- Shen X, Zhang L, Jiang X, et al. (2007). Reversible surface switching of nanogel triggered by external stimuli. Angew Chem Int Ed Engl 46:7104–7. [DOI] [PubMed] [Google Scholar]
- Soni K, Desale S, Bronich T. (2016). Nanogels: an overview of properties, biomedical applications and obstacles to clinical translation. J Control Release 240:109–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J, Vine G, Snowden M. (2011). Microgel applications and commercial considerations. Colloid Polym Sci 289:625–46. [Google Scholar]
- Tong X, Zhao F, Ren Y, et al. (2018). Injectable hydrogels based on glycyrrhizin, alginate, and calcium for three-dimensional cell culture in liver tissue engineering. J Biomed Mater Res A 106:3292–302. [DOI] [PubMed] [Google Scholar]
- Torchilin V. (2001). Structure and design of polymeric surfactant-based drug delivery systems. J Control Release 73:137–72. [DOI] [PubMed] [Google Scholar]
- Torchilin V. (2014). Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov 13:813–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashist A, Kaushik A, Vashist A, et al. (2018). Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discov Today 23:1436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma IM, Somia N. (1997). Gene therapy – promises, problems and prospects. Nature 389:239–42. [DOI] [PubMed] [Google Scholar]
- Vinogradov S. (2007). Polymeric nanogel formulations of nucleoside analogs. Expert Opin Drug Deliv 4:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S. (2010). Nanogels in the race for drug delivery. Nanomedicine 5:165–8. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Bronich T, Kabanov A. (2002). Nanosized cationic hydrogels for drug delivery: preparation, properties and interactions with cells. Adv Drug Deliv Rev 54:135–47. [DOI] [PubMed] [Google Scholar]
- Vladisavljevic G, Williams A. (2005). Recent developments in manufacturing emulsions and particulate products using membranes. Adv Colloid Interface Sci 113:1–20. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen Q, Zhou S. (2018). Carbon-based hybrid nanogels: a synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem Soc Rev 47:4198–232. [DOI] [PubMed] [Google Scholar]
- Wang Q, Gao L, Zhu X, et al. (2019). Co-delivery of glycyrrhizin and doxorubicin by alginate nanogel particles attenuates the activation of macrophage and enhances the therapeutic efficacy for hepatocellular carcinoma. Theranostics 9:6239–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Gu Y, Zhou Z, et al. (2006). Preparation and characterization of uniform-sized chitosan microspheres containing insulin by membrane emulsification and a two-step solidification process. Colloids Surf B Biointerfaces 50:126–35. [DOI] [PubMed] [Google Scholar]
- Wang Y, Luo Y, Zhao Q, et al. (2016). An enzyme-responsive nanogel carrier based on PAMAM dendrimers for drug delivery. ACS Appl Mater Interfaces 8:19899–906. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zheng J, Tian Y, Yang W. (2015). Acid degradable poly(vinylcaprolactam)-based nanogels with ketal linkages for drug delivery . J Mater Chem B 3:5824–32. [DOI] [PubMed] [Google Scholar]
- Wu Q, Tian P. (2008). Adsorption of Cu2+ ions with poly (N-1soProPylacrylamide-co-Methaerylie Acid) miero/nanopartieles. J Appl Polym Sci 109:3470–6. [Google Scholar]
- Xia X, Tang S, Lu X, et al. (2003). Formation and volume phase transition of hydroxypropyl cellulose microgels in salt solution. Macromolecules 36:3695–8. [Google Scholar]
- Xing Z, Wang C, Yan J, et al. (2010). PH/temperature dual stimuli-responsive microcapsules with interpenetrating polymer network structure. Colloid Polym Sci 288:1723–9. [Google Scholar]
- Xing Z, Wang C, Yan J, et al. (2011). Dual stimuli responsive hollow nanogels with IPN structure for temperature controlling drug loading and pH triggering drug release. Soft Matter 7:7992–7. [Google Scholar]
- Yeh J, Ling Y, Karp J, et al. (2006). Micromolding of shape-controlled, harvestable cell-laden hydrogels. Biomaterials 27:5391–8. [DOI] [PubMed] [Google Scholar]
- Zha L, Banik B, Alexis F. (2011). Stimulus responsive nanogels for drug delivery. Soft Matter 7:5908–16. [Google Scholar]
- Zha L, Zhang Y, Yang W, et al. (2002). Monodisperse temperature-sensitive microcontainers. Adv Mater 14:1090–2. [Google Scholar]
- Zhang H, Tumarkin E, Sullan RMA, et al. (2007). Exploring microfluidic routes to microgels of biological polymers. Macromol Rapid Commun 28:527–38. [Google Scholar]
- Zhang H, Zhai Y, Wang J, et al. (2016). New progress and prospects: the application of nanogel in drug delivery. Mater Sci Eng C Mater Biol Appl 60:560–8. [DOI] [PubMed] [Google Scholar]
- Zhang W, Tung CH. (2018). Redox-responsive cisplatin nanogels for anticancer drug delivery. Chem Commun 54:8367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Malhotra S, Molina M, et al. (2015). Micro- and nanogels with labile crosslinks – from synthesis to biomedical applications. Chem Soc Rev 44:1948–73. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Garret D, Yan J, et al. (2021). Amphiphilic thiol polymer nanogel removes environmentally relevant mercury species from both produced water and hydrocarbons. Environ Sci Technol 55:1231–41. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zha L, Fu S. (2004). Kinetic analysis of poly (N-isopropylacrylamide-co-dimethylaminoethyl methacrylate) microgel latex formation. J Appl Polym Sci 92:839–46. [Google Scholar]