ABSTRACT
Recent preclinical data suggest that alterations in the gut microbiota may be an important factor linking obesity to vascular dysfunction, an early sign of cardiovascular disease. The purpose of this study was to begin translation of these preclinical data by examining whether vascular phenotypes in humans are transmissible through the gut microbiota. We hypothesized that germ-free mice colonized with gut microbiota from obese individuals would display diminished vascular function compared to germ-free mice receiving microbiota from lean individuals.
We transplanted fecal material from obese and lean age-and sex-matched participants with disparate vascular function to germ-free mice. Using Principle Component Analysis, the microbiota of colonized mice separated by donor group along the first principle component, accounting for between 70–93% of the total variability in the dataset. The microbiota of mice receiving transplants from lean individuals was also characterized by increased alpha diversity, as well as increased relative abundance of potentially beneficial bacteria, including Bifidobacterium, Lactobacillus, and Bacteroides ovatis. Endothelium-dependent dilation, aortic pulse wave velocity and glucose tolerance were significantly altered in mice receiving microbiota from the obese donor relative to those receiving microbiota from the lean donor or those remaining germ-free.
These data indicate that the obesity-associated human gut microbiota is sufficient to alter the vascular phenotype in germ-free mice in the absence of differences in body weight or dietary manipulation, and provide justification for future clinical trials to test the efficacy of microbiota-targeted therapies in the prevention or treatment of cardiovascular disease.
KEYWORDS: vascular function, pulse wave velocity, endothelial function, obesity, microbiota, cardiovascular disease
Introduction
Cardiovascular disease (CVD) has been the leading cause of death worldwide for over a century.1 Overt clinical signs of CVD are often preceded by vascular dysfunction, particularly arterial stiffness and endothelial dysfunction, both of which are highly predictive of future cardiovascular events and mortality.2–5 Arterial stiffness and endothelial dysfunction are also commonly observed in obese individuals, and account, in part, for the increased CVD risk in this population. Although the exact processes governing the development and progression of obesity-related vascular dysfunction are unclear, emerging evidence supports a role for the gut microbiota.6
Several pre-clinical studies have demonstrated that manipulation of the gut microbiota impacts vascular outcomes. We7 and others8 have reported that diet-induced obesity in mice is characterized by gut dysbiosis and vascular dysfunction, and that suppression of dysbiotic microbiota via broad-spectrum antibiotics abrogates vascular dysfunction in these animals. Furthermore, gut microbiota transplantation of an obesity-associated microbiota from genetically obese (Ob-/-) mice to wild-type animals results in the development of vascular dysfunction in the latter;9 and supplementation with prebiotics eliminates vascular dysfunction in ApoE-/- mice.10 Taken together, these observations suggest that alterations to the gut microbiota can modify vascular function in experimental animals.
Although the potential clinical implications of these findings are clear, it remains unknown whether the human gut microbiota plays a fundamental role in the regulation of vascular function. Inter-individual differences in genetics, diet and other lifestyle factors complicate translation of these observations to human disease. Furthermore, significant taxonomic differences exist between the human and mouse microbiota. Therefore, establishing whether an obesity-associated human gut microbiota can influence the development of vascular dysfunction in a germ-free mouse model is the first step toward translating previous experimental observations. To this end, we colonized separate cohorts of germ-free mice with the gut microbiota of obese and lean human participants that differed in measures of arterial stiffness and endothelial function. We found that animals receiving the obesity-associated gut microbiota developed vascular dysfunction, despite being fed a standard maintenance diet. These observations suggest that the obesity-associated human gut microbiota is sufficient to initiate the development of vascular dysfunction. These findings shed light on the potential causative role of obesity-induced gut dysbiosis in the development of vascular dysfunction and associated CVD risk in humans.
Results
Lean and obese human microbiota donors
Subject characteristics of the lean and obese human donors are shown in Table 1. On average, obese microbiota donors (BMI>30) were selected based on endothelial dysfunction (RHI<1.67), as well as markedly higher arterial stiffness as determined by carotid-femoral pulse wave velocity (cfPWV) than lean individuals. Figure 1 shows taxa bar plots of the phyla-level differences between the lean and obese human donors. Mice were inoculated in two cohorts, with cohort 1 colonized by fecal donor material from a single lean or obese female and cohort 2 colonized by fecal donor material from a single lean or obese male. The obese donors for both cohorts 1 and 2 displayed greater relative abundance of Firmicutes and reduced abundance of Bacteroidetes compared to their respective lean donors (Figure 1). In cohort 1, the ratio of Firmicutes to Bacteriodetes (F:B) was 1.8 for the lean donor and 4.5 for the obese donor (Figure 1A); in cohort 2 the F:B was 5.3 for the lean donor and 20.4 for the obese donor (Figure 1B). This finding is consistent with previous studies that have demonstrated a greater F:B ratio in obese populations.11,12 Further, Actinobacteria, found in higher concentrations of obese individuals,12 were only detected in the obese male donor.
Table 1.
General and vascular characteristics of lean and obese human study population
| Cohort 1 Donors |
Cohort 2 Donors |
|||
|---|---|---|---|---|
| Lean | Obese | Lean | Obese | |
| N | 1 | 1 | 1 | 1 |
| Age (years) | 44 | 48 | 32 | 30 |
| Sex | F | F | M | M |
| BMI (kg/m2) | 23.2 | 32.6 | 22.4 | 31.2 |
| SBP (mmHg) | 115 | 129 | 121 | 124 |
| DBP (mmHg) | 66 | 75 | 63 | 77 |
| cfPWV | 5.6 | 7.4 | 6.2 | 7.1 |
| Aix | 15 | 23 | 6 | 15 |
| Aix@75 | 15 | 27 | −1 | 8 |
| RHI | 2.17 | 1.28 | 2.15 | 1.63 |
| Glu | 88 | 92 | 90 | 95 |
| HbA1c | 5.3 | 5.2 | 5.3 | 5.3 |
| CHOL (mg/dL) | 156 | 188 | 141 | 170 |
| HDL (mg/dL) | 62 | 60 | 56 | 39 |
| nHDLc (mg/dL) | 94 | 128 | 85 | 131 |
| TRIG (mg/dL) | 79 | 79 | 69 | 85 |
| TC/H | 2.5 | 3.1 | 2.5 | 4.4 |
| LDL (mg/dL) | 78 | 112 | 71 | 114 |
| VLDL (mg/dL) | 16 | 16 | 14 | 17 |
BMI Body Mass Index; BP Blood Pressure; cfPWV carotid to femoral pulse wave velocity; RHI reactive hyperemia index; Glu fasting glucose; CHOL total cholesterol; HDL high density cholesterol; nHDL non-high density cholesterol; TRIG triacylglycerols; TC/H total cholesterol to HDL ratio; LDL low density cholesterol; VLDL very low density cholesterol; n = 1/group.
Figure 1.
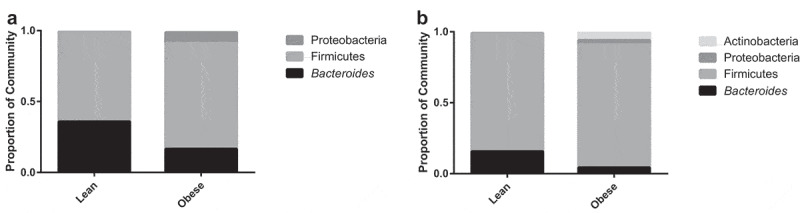
Lean and obese human donors display differences in relative abundance of the gut microbiota at the phyla level. A) Taxa Bar Plot of Cohort 1 at the Phyla Level. B) Taxa Bar Plot of Cohort 2 at the Phyla Level. n = 1/group
Mouse microbiota profiles segregate by donor phenotypes
An average of 57,269 reads/sample (min = 25,086; max = 89,654) were used to query microbiota differences between groups within each cohort. At termination, mouse microbiota profiles clustered according to their transplant donor groups. Principle Component Analysis (PCA) revealed separation of the microbiota in each cohort by donor group along the first principle component, which accounted for ~47% (cohort 1) and 89% (cohort 2) of the total variability in the dataset (Figure 2A and B). Furthermore, while several taxa appear to be driving this separation, Bacteroides ovatus and Parabacteroides diastonis were consistently associated with the mice receiving the lean microbiota (LM) in both cohorts. In cohort 1, several Bacteroides spp. and Akkermansia muciniphila were associated with the mice receiving the obese microbiota (OBM); while in Cohort 2, Clostridium hathewayi and other taxa in the Clostridiales (phyla Firmicutes) were associated with the OBM (Figure 2A and B).
Figure 2.
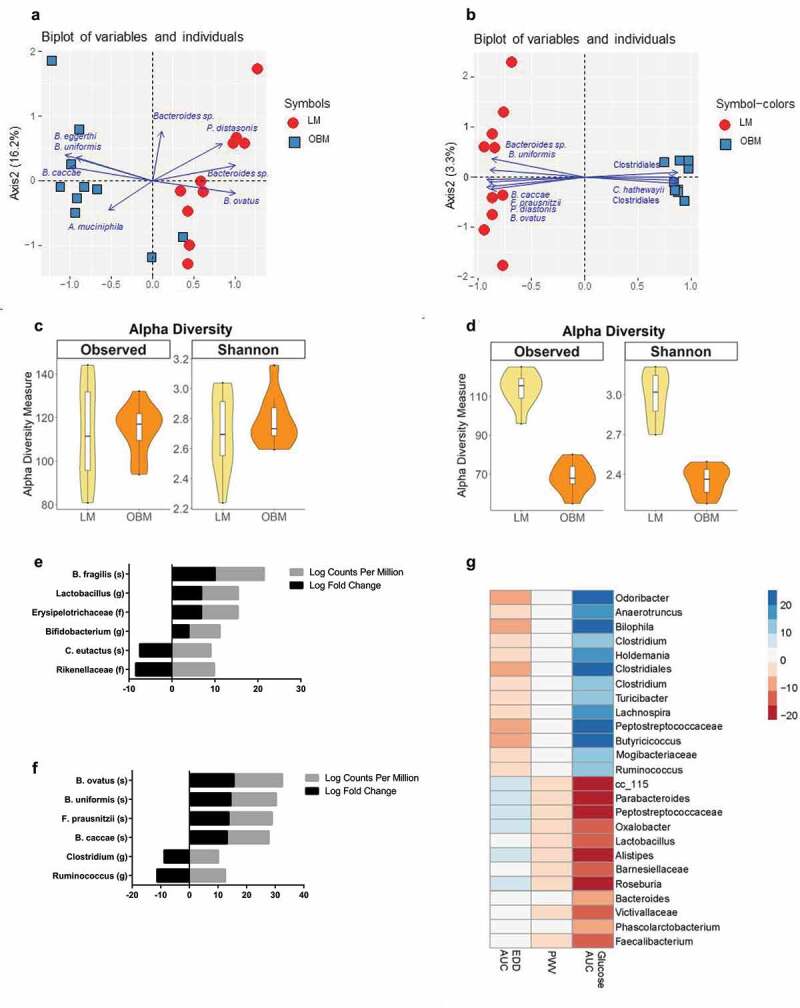
Gut microbiota transplantation from lean or obese humans results in different community structure in germ-free mice. A) Principle Component Analysis (PCA) Plot of LM vs. OBM in cohort 1. B) PCA Plot of LM vs. OBM in cohort 2. C) Alpha Diversity for Cohort 1 as measured by Observed Species and Shannon’s Diversity. D) Alpha Diversity for Cohort 2 as measured by Observed Species and Shannon’s Diversity. E) Changes in specific taxa within groups in Cohort 1, identified using a gene-wise negative binomial generalized linear model (EdgeR). F) Changes in specific taxa within groups in Cohort 2, identified using a gene-wise negative binomial generalized linear model (EdgeR). G) Sparse partial least squares (sPLS) regression of cardiometabolic parameters and bacterial taxa of all mice. FDR<0.1. EDD endothelium-dependent dilation; AUC area under the curve; PWV pulse wave velocity
Bray-Curtis distances were used to look at dissimilarity across donor groups and cohorts. Visualization by Principle Coordinates Analysis (PCoA) using Non-Metric Dimensional Scaling (NMDS; stress=0.09) revealed clustering of mouse microbiomes by donor weight status and cohort (SI Appendix, Figure 1). In particular, mice receiving LM clustered most closely together, regardless of cohort, while animals receiving OBM clustered within a cohort, but cohorts 1 and 2 clustered separately (PERMANOVA: OBM vs LM = p < .001; Cohort 1 vs Cohort 2 = p < .001; Interaction = p < .001). Because there was a significant cohort effect, the remaining microbiota analyses were conducted within cohorts.
Alpha-diversity measures differed between LM and OBM groups, but this was not consistent across cohorts. In cohort 1, there were no significant differences between LM and OBM-colonized mice in richness (Observed species; p = .87) or Shannon’s diversity (p = .48; Figure 2C). Conversely, in cohort 2, the LM had much greater richness and diversity than OBM-colonized animals (Observed species: p < .001; Shannon: p < .001; Figure 2D). Using the DiffAbund function in MyPhyloDB, we identified specific taxa that differed between donor groups in each cohort. In cohort 1, LM-colonized animals had higher levels of taxa most closely aligning to the species Bacteroides fragilis (20-fold increase), and the genera Lactobacillus (12-fold increase) and Bifidobacterium (8-fold increase) (Figure 2E). Cohort 2 displayed greater relative abundance of taxa aligning to the species Bacteroides ovatus (30-fold increase), Bacteroides uniformis (25-fold increase), Faecalibacterium prausnitzii (24-fold increase), and Bacteroides caccae (23-fold increase) when colonized with the LM (Figure 2F). Using a sparse partial least squares (sPLS) regression, we observed strong microbial associations between certain microbes and glucose area under the curve (AUC) and weaker associations between specific microbes and endothelium-dependent dilation (EDD) AUC (Figure 2G). In addition, many taxa showed opposing correlations with glucose AUC and EDD AUC, likely due to the inverse relationship between these specific outcome measures. In agreement with assessments that identified taxa associated with the LM, Lactobacillus and Parabacteroides were negatively associated with glucose AUC, while several members of the Clostridiales (drivers of OBM in cohort 1) were positively associated with glucose AUC.
Body and tissue weights did not differ between mice colonized with either lean or obese microbiota
After colonization with their respective donor fecal material, mice were fed a purified maintenance diet for 14–18 weeks. To assess the impact of LM or OBM colonization on the mice, body weight and food intake were assessed throughout the study and measures of vascular function and glucose tolerance were conducted in vivo or post-termination. General characteristics of the combined mice cohorts after termination are shown in Table 2. Average body weight and most tissue weights did not differ between the LM and the OBM. However, GF mice (animals not receiving inoculum) had significantly greater cecum weights (p < .001) and colon lengths (p < .05) compared to either the LM or OBM (Table 2).
Table 2.
General and metabolic characteristics of mice
| LM | OBM | Germ-Free | p value | |
|---|---|---|---|---|
| Bodyweight (g) | 24.9 ± 1.0 | 24.1 ± 0.7 | 24.7 ± 2.1 | 0.83 |
| Liver weight (mg) | 1241.2 ± 48.0 | 1164.9 ± 41.7 | 1178.2 ± 53.4 | 0.45 |
| Heart weight (mg) | 118.0 ± 2.9 | 113.2 ± 3.1 | 102.9 ± 4.3 | 0.039 |
| Spleen weight(mg) | 84.2 ± 4.1 | 80.2 ± 2.9 | 97.3 ± 10.4 | 0.11 |
| Epi Adipose weight (mg) | 509.0 ± 102.5 | 410.3 ± 50.3 | 336.4 ± 31.7 | 0.43 |
| SQ Adipose weight (mg) | 257.0 ± 34.6 | 228.7 ± 18.5 | 190.9 ± 11.8 | 0.42 |
| Cecum weight (mg) | 564.5 ± 28.7 | 496.7 ± 27.5 | 1645.9 ± 356.9 | <0.0001 |
| Colon length (cm) | 6.5 ± 0.2 | 6.4 ± 0.1 | 7.1 ± 0.3 | 0.05 |
| PVAT weight (mg) | 21.9 ± 2.1 | 18.9 ± 2.0 | 22.4 ± 3.9 | 0.54 |
Values are mean±SEM. LM mice receiving the lean microbiota, OBM mice receiving the obese microbiota, PVAT perivascular adipose tissue, Epi epidydimal, SQ subcutaneous, n = 6–10/group. Statistical analysis was performed using a one-way ANOVA.
Transfer of the obese human gut microbiome induces the development of vascular dysfunction in germ-free mice
To assess whether colonization with LM or OBM differentially affected vascular function in GF mice, we determined in vivo arterial stiffness and ex vivo endothelial function. Arterial stiffness, measured by PWV, was increased in mice colonized with OBM compared to GF mice (p = .007) (Figure 3A). In the individual cohorts, mice in cohort 2 colonized with OBM displayed a marked increase in arterial stiffness compared to the LM animals (p = .03; Figure 3C); however, no differences between donor groups were noted in cohort 1 (p = .44; Figure 3B).
Figure 3.
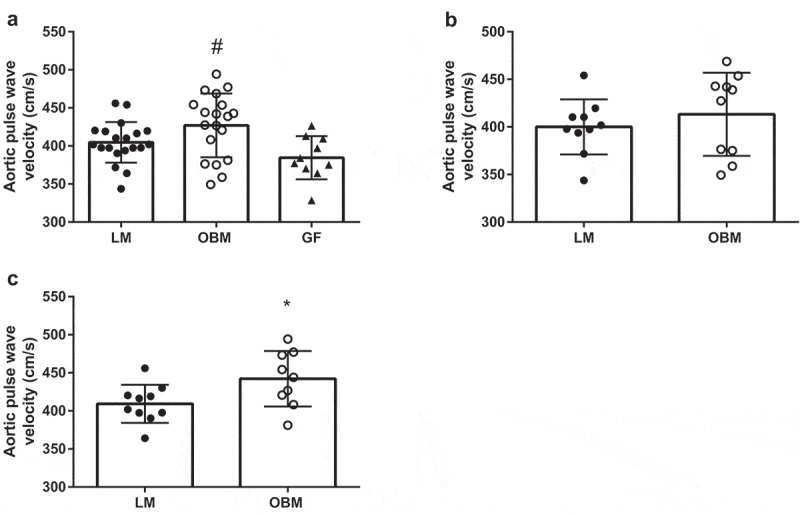
Gut microbiota transplantation increases arterial stiffness in germ-free mice. A) Arterial stiffness as measured by pulse wave velocity (PWV) in both cohorts combined. B) Arterial stiffness as measured by PWV in cohort 1. C) Arterial stiffness as measured by PWV in cohort 2. Data are expressed as mean ± SEM, n = 9–10/group for individual cohorts; n = 18–20 for both cohorts combined. Statistical analysis was performed using Student’s t-test or one-way ANOVA, where applicable. *p < .05 between LM and OBM. # p < .01 between OBM and GF
Vascular endothelial function in isolated mesenteric arteries was assessed immediately after termination via pressure myography. GF mice displayed greater constriction to the maximal dose of phenylephrine (PE) (10−5) compared to colonized mice, but there were no differences in constriction between mice colonized with LM or OBM (SI Appendix, Figure 2A and B). Conversely, EDD in response to acetylcholine was significantly impaired in OBM compared to LM (p < .05) or the GF mice (p < .01) (Figure 4A and B). In addition, this impaired dilation held true when both cohorts of animals were analyzed independently (Figure 4C–F). Endothelium-independent dilation (EID) was reduced (p < .05) in OBM compared to animals remaining GF; with no differences observed between LM and GF animals (SI Appendix, Figure 3B–F).
Figure 4.
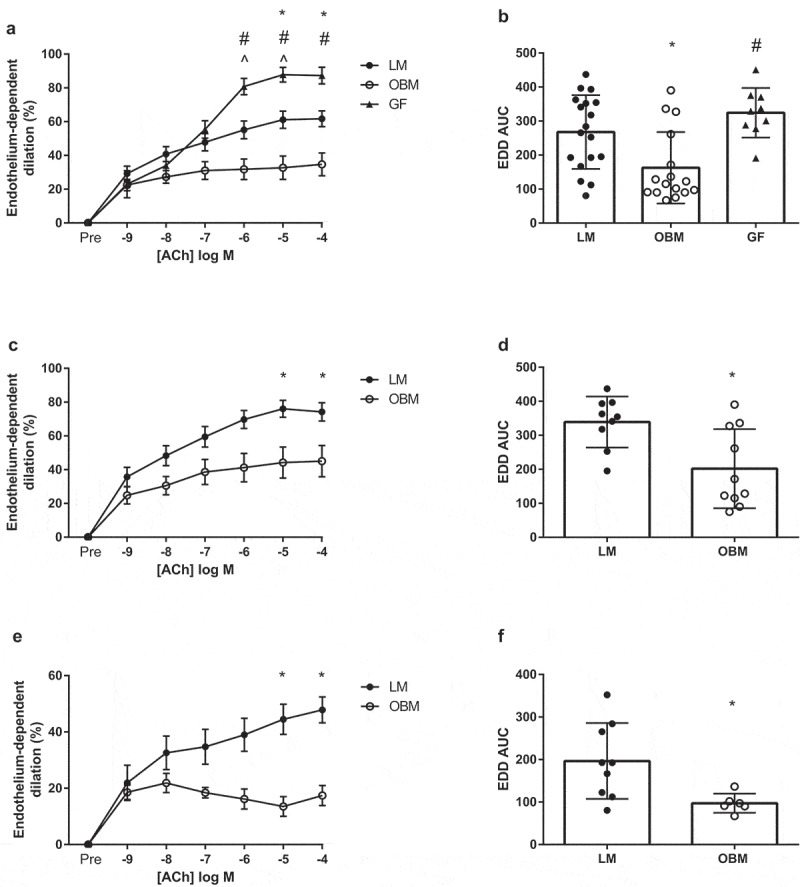
Gut microbiota transplantation from obese subjects leads to endothelial dysfunction in germ-free mice. A) Endothelium-dependent dilation (EDD) to acetylcholine (ACh) in both cohorts combined, B) EDD area under the curve (AUC) in both cohort’s combined, C) EDD to ACh in Cohort 1, D) EDD AUC in cohort 1, E) EDD to ACh in Cohort 2, F) EDD AUC in cohort 2. Data are expressed as mean ± SEM, n = 7–10/group for individual cohorts; n = 13 for both cohorts combined. Statistical analysis was performed using Student’s t-test or two-way ANOVA, where applicable. *p < .05 between LM and OBM. # p < .05 between OBM and GF
Colonization with obese human gut microbiota impairs glucose tolerance in germ-free mice
To assess metabolic effects of the transplantation, mice underwent an intraperitoneal glucose tolerance test (ipGTT). OBM displayed impaired glucose tolerance compared to LM as demonstrated by a trending glucose response curve (p = .06), and greater AUC (p = .007) (Figure 5A and B). This impairment was consistent across both cohorts of mice (Figure 5C–F). To ensure preserved GF status, we did not subject GF control mice to an ipGTT. To gain mechanistic insight into the impaired glucose tolerance, we measured phosphorylated Protein Kinase B (pAkt) in the liver and gastrocnemius. In cohort 1, the OBM displayed reduced pAkt:Akt compared to the LM (SI Appendix, Figure 4) in the gastrocnemius, but not in the liver. However, there were no differences in muscular or liver pAkt:Akt in Cohort 2, nor when data were combined. Liver or intramuscular triacylglycerols did not differ between groups in either cohort (SI Appendix, Figure 5).
Figure 5.
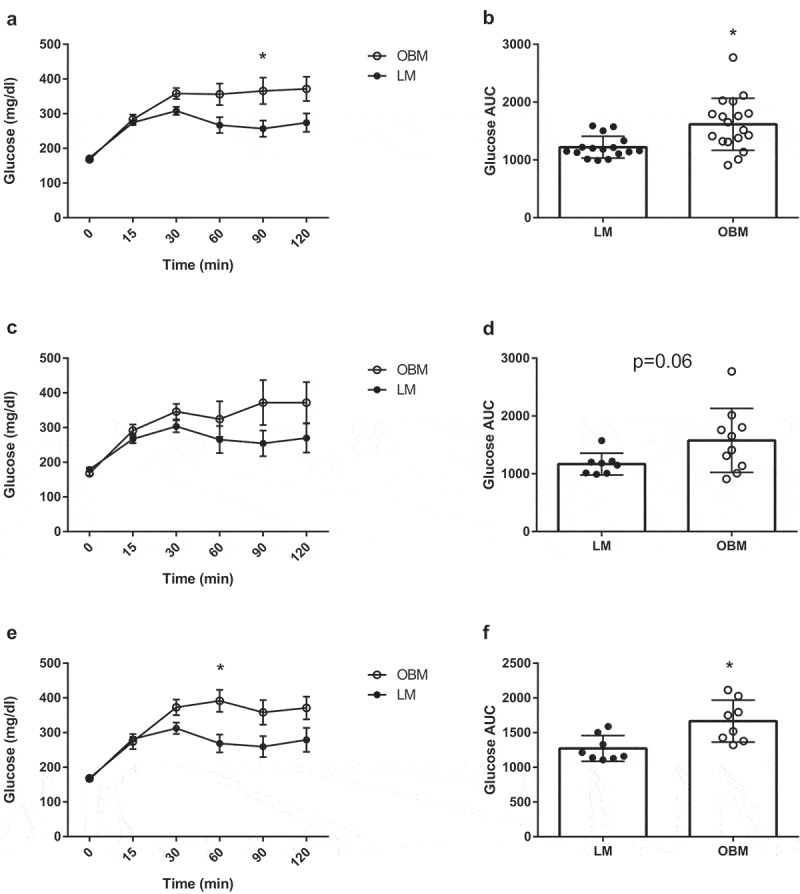
Obese gut microbiota transplantation leads to impaired glucose tolerance in germ-free mice. A) Glucose tolerance test between LM and OBM in both cohorts combined, B) Area under the curve for glucose tolerance test in both cohorts combined, C) Glucose tolerance test between LM and OBM for cohort 1, D) Area under the curve for glucose tolerance test for cohort 1, E) Glucose tolerance test between LM and OBM for cohort 2, F) Area under the curve for glucose tolerance test for cohort 2. Data are expressed as mean ± SEM, n = 9–10/group for individual cohorts; n = 18–20 for both cohorts combined. Statistical analysis was performed using two-way ANOVA. *p < .05 between LM and OBM at indicated time point
Discussion
Research over the last decade has revealed the importance of gut microbes in regulating human health and disease.13 Numerous studies have demonstrated a strong association between changes in the gut microbiota and cardiovascular disease risk, although the physiological link governing this association remains unclear. We previously demonstrated that alterations to the gut microbiota affect endothelial function and arterial stiffness, two indices of vascular function that strongly predict cardiovascular disease risk.4,5 However, inter-individual differences in genetics, diet and other lifestyle factors complicate translation of these data to human disease, and significant taxonomic differences exist between the human and mouse microbiota. Therefore, as a first step toward translating these pre-clinical data, we sought to establish whether an obesity-associated human gut microbiota would influence the development of vascular dysfunction in an experimental model. Specifically, we examined the vascular and metabolic effects of microbiota transplantations from lean and obese individuals to GF mice. We found that GF mice colonized with an obese human gut microbiota acquired the vascular phenotype of their donors and developed endothelial dysfunction, arterial stiffness, and glucose intolerance compared to non-colonized GF mice or mice colonized with a lean human gut microbiota. These changes were independent of diet or body weight and were driven by differences in gut microbial ecology (i.e. alpha diversity and differential abundance of species). These data indicate that the human gut microbiota is a fundamental and modifiable determinant of vascular function and glucose homeostasis, and provide justification for further examination in human populations and potentially for development of microbiota-targeted therapies for individuals at-risk for CVD.
Rather than combine the gut microbiota from large cohorts of lean and obese individuals for donor material, we selected two representative lean and obese individuals, one female and one male, that matched closely in age but differed in vascular parameters, specifically in RHI score. This approach allowed us to scrutinize the microbiota of a single donor, examining its capacity to alter cardiometabolic function in a cohort of GF mice. Donors were selected because they displayed marked differences in vascular outcomes. Specifically, obese donors displayed impaired endothelial function and increased arterial stiffness compared to lean donors, as determined by RHI and PWV.14,15 The RHI values demonstrated by obese donors were similar to those that are associated with increased future cardiovascular disease and related mortality.14,16 Obese donors also displayed numeric elevations in PWV, although absolute values were only modestly elevated, and within a single standard deviation of average PWV values for females and males of their age.17 The lack of more significant elevations in PWV in our obese donors may have been due to their relatively young age, as aging is the primary risk factor for arterial stiffness.18 Concomitant with these vascular differences, the gut microbiota of the human donors were also distinct, with obese donors displaying greater relative abundance of the phyla Firmicutes than lean donors. This finding is consistent with previous studies that have demonstrated a higher Firmicutes:Bacteroidetes (F:B) in obese individuals. Notably, Turnbaugh et al., demonstrated that GF mice inoculated with Firmicutes-enriched microbiota from obese twins increased body weight.12 Although the physiological relevance of the F:B ratio continues to be debated, the adipose-enriching effect of Firmicutes has been linked to several aspects of metabolic dysfunction.19,20
Examination of the mouse microbiota at termination revealed clustering by donor status (within cohorts), despite consumption of a standardized maintenance diet by all animal groups. Interestingly, when using Bray-Curtis distances to examine the combined cohorts, the two groups that received LM clustered by cohort, but were also very similar to one another. In contrast, the two cohorts that received OBM clustered within cohort, but were different between cohorts. This is consistent with the Anna Karenina Hypothesis, which suggests that healthy, robust microbiomes have the ability to regulate their composition, resulting in similarities, while dysbiotic microbiomes lose this regulatory capacity, resulting in various confirmations consistent with dysfunction.21 This is consistent with our previous observations in rodent models, where vascular dysfunction was more strongly correlated with loss of beneficial taxa, such as Bifidobacterium, than with introduction of specific pathogenic or pro-inflammatory organisms.9 This is further supported by our observation of reduced alpha diversity (observed richness and Shannon’s diversity) in OBM compared to LM in cohort 2. Loss of alpha diversity has also been linked to metabolic dysfunction and other diseases in humans.22,23
With regard to loss of beneficial taxa, OBM displayed reduced levels of F. prausnitzii compared to LM in cohort 2. This bacterial taxa is notably depleted in obesity, IBD, and colorectal cancers,24–28 and is being explored as a next-generation probiotic for humans.29 Using the multivariate DiffAbund function in MyPhyloDB, we found that other important taxa distinguished LM from OBM, including Bifidobacterium, Lactobacillus, and Clostridium. We have shown previously that Bifidobacterium is reduced in a diet-induced model of vascular dysfunction, and others have demonstrated that Bifidobacterium supplementation improves fasting serum insulin and hepatic triacylglycerol accumulation and reduces endotoxemia in high fat-fed mice.7,9 Collectively, these data provide scientific justification to examine the effects of Bifidobacterium supplementation in humans with vascular dysfunction. Another interesting observation was the association of several Bacteroides species, such as B. ovatus, with a lean microbiota and observation of a positive correlation between Bacteroides and EDD, and an inverse correlation to glucose AUC. Bacteroides have a well characterized pathway to synthesize menaquinones (Vit K2)30 and it was recently demonstrated that the gut microbiota regulate endothelial function and blood pressure via a vitamin K2-dependent pathway.31 It is interesting to speculate whether loss or reduction of bacterially-synthesized K2 may have driven the results observed in this study. However, it is also important to note that there were several Bacteroides sp. associated with LM in one cohort and OBM in another, and few consistent differences between LM and OBM across cohorts. This highlights the importance of examining functional, rather than taxonomic, differences in future studies to further understand the interactions between the gut microbiota and host physiology.
Mice receiving the OBM displayed reduced EDD compared to mice receiving lean microbiota or uninoculated GF mice. The magnitude of endothelial dysfunction observed in OBM was comparable to what has been observed in diet-induced models of obesity.7 Interestingly, mice receiving the lean microbiota also displayed reduced EDD compared to GF mice. Joe et al. recently demonstrated that GF mice lack the ability to adequately constrict to PE. Contrary to this finding, in the present study GF mice displayed increased constriction to a maximal dose of PE compared to both LM and OBM. One possible explanation for this difference is that Joe et al. conventionalized their rats by cohousing with other rats, whereas we colonized our GF mice with human stool transplants, thus introducing a human microbiota. OBM also displayed a modest reduction in EID compared to non-inoculated GF mice. This finding is noteworthy given that reductions in EID are associated with insulin resistance and the development of type II diabetes and CVD.32,33 In addition to endothelial dysfunction, mice receiving the obese microbiota also displayed increases in PWV, a marker of arterial stiffness, compared to non-inoculated GF mice and those receiving the lean microbiota in cohort 2. As noted earlier, our obese donors displayed only modest elevations in PWV compared to lean donors, likely due to their relatively young ages, which may account for the more modest changes in recipient mice. Still, these data support and extend previous work from our laboratory that PWV can be altered by gut microbiota transplantations, and support the supposition that the human gut microbiota is a fundamental regulator of cardiometabolic function.
In addition to impairments in vascular function, OBM also displayed reductions in glucose tolerance compared to LM, as determined by an intraperitoneal glucose tolerance test. This reduction was accompanied by a decrease in muscle pAkt:Akt in cohort 1, but not cohort 2. The reasons for the discrepancy between cohorts are unclear, but could be due to the heterogeneity of the gut microbiota or donor sex differences between the obese donors in cohort 1 and 2. Using sPLS, we observed an inverse correlation between Lactobacillus and glucose AUC. Lactobacillus has been used to improve glucose tolerance independent of weight loss in db/db mice,34 and, more recently, oral supplementation with Lactobacillus rhamnosus has reduced gluconeogenesis and improved hyperglycemia in diabetic rats.35 The reduction in glucose tolerance in recipient mice is interesting given that obese donors did not display elevations in fasting glucose or in HbA1C, a marker of chronic glucose levels. These data align with those by Vrieze et al., who demonstrated that transfer of gut microbiota from lean human donors to individuals with metabolic syndrome increased insulin sensitivity in the latter.36 Furthermore, Zoll et al. found that transplantation from high fat, high sugar-fed mice induced glucose intolerance in lean mice independent of body weight and exercise status.37 To our knowledge, data from the present study are the first to demonstrate that microbiota transplantation from obese humans, without overt metabolic derangements, can impair glucose homeostasis in GF mice. These data lead us to believe that underlying changes in the gut microbiota may occur prior to observable changes in host physiology, and can lead to the development of chronic metabolic disease, including type II diabetes and CVD.
It is important to acknowledge the potential effects of sex in our donor and recipient cohorts. The protective effects of estrogen on CVD risk have been known for many years.38 How estrogen, and its loss, impacts microbial community configurations in humans is still poorly understood, although a recent multi-cohort analysis showed that at younger ages, women have greater alpha diversity than men, and this difference was reduced with age.39 In the current study, cohort 1 animals were colonized by the female donor and cohort 2 were colonized by the male donor. Interestingly, the animals colonized with the female OBM donor were more similar to animals receiving LM than the animals colonized with the male OBM. Additionally, the alpha-diversity parameters were higher in these animals compared to the animals colonized with the male OBM. We also found differences between the cohorts in PWV, our marker of arterial stiffness. When separating out the two cohorts, only cohort 2, the mice receiving the gut microbiota transplant from the male donors, had differences in arterial stiffness between the lean and obese donors. As both female participants were pre-menopausal, estrogen may have impacted the microbiota conformations of these donors and provided a protective effect against the development of arterial stiffness in the recipient animals. Future follow up studies will be needed to confirm whether these sex differences hold up in a larger cohort of male and female donors.
Several limitations of the current work should be noted. First, we utilized GF mice as recipients because their lack of microbes eliminates the confounding and competitive effects of the baseline microbiota. However, this may slightly limit the translatability of these data to future human recipients as some studies have demonstrated variations to GF physiology including alterations in vascular phenotypes and the development of metabolic dysfunction.40–42 Second, our decision to use a single lean and obese donor in each cohort rather than gut microbiota from a mixed group may also reduce the generalizability of the data. Lastly, important questions exist regarding the amount of time that is required for transplantations to elicit physiological changes, as well the duration before those changes begin to wane. We chose a 14–18 week duration given that our previous data suggested that this duration was sufficient to elicit changes in vascular function; however, it is unclear whether these changes would be maintained over a longer period of time without additional intervention, such as introducing a high fat diet. Such details will be critical to determine as gut microbiota-targeted therapies are fully translated to human populations.
In conclusion, the current data indicate that introduction of an obesity-associated human gut microbiota are sufficient to alter the vascular phenotype and glucose tolerance in GF mice in the absence of changes in body weight or dietary manipulation. This provides evidence that the human gut microbiota is a fundamental regulator of vascular function and glucose homeostasis. The data provide justification for gut microbiota-targeted therapies for the prevention and treatment of CVD. Future studies should focus on examining functional aspects of the gut microbiota that may drive vascular impairments as well as determine whether microbiota manipulation has therapeutic potential in humans at risk for CVD and type II diabetes.
Materials and methods
Human study
Study population
Men and women 30 to 50 years of age and with a BMI (kg/m2) of 20–24.9 or 30–34.9 were recruited to participate in this study. Exclusion criteria included subjects actively trying to alter body weight; current smoking; heavy alcohol consumption (>12 drinks/week for men and >8 drinks/week for women); pregnant or breastfeeding; taking antibiotics, commercial probiotic/prebiotic supplementation, or prescription medications that may affect either the microbiota or cardiometabolic function; and previous diagnosis of hyperlipidemia, diabetes, kidney/renal or intestinal diseases.
Twenty participants who met all eligibility criteria (10 normal weight and 10 obese) then underwent vascular assessment including measures of blood pressure, arterial stiffness, and endothelial function, followed by a venous blood draw, as previously described.43 We then selected two age-matched female donors, one from the lean and one from the obese group, with disparate vascular function, to serve as gut microbiota donors for transplantation to cohort 1. Two age-matched male donors, one from the lean and one from the obese group with disparate vascular function, were chosen as donors for transplantation to cohort 2.
This trial was conducted in accordance with the Declaration of Helsinki and was approved by the Colorado State University Institutional Review Board (protocol #18-7882 H).
Study design
The human portion of the study consisted of one experimental visit lasting for ~2 hours. Prior to the experimental visit, participants were instructed to refrain for 24 hours from exercise, over-the-counter and prescription medications, smoking, alcohol or caffeine-containing beverages, and fast for 12 hours. Participants were allowed to consume water prior to the study visit.43
At the beginning of the testing visit and following 10 min of supine rest, brachial and aortic blood pressure, central hemodynamics (e.g., aortic mean arterial pressure, aortic pulse pressure) and AIx were measured in the non-dominant arm using the SphygmoCor XCEL system (AtCor Medical, Inc.) as described previously.43 Aortic stiffness was assessed by measuring carotid to femoral pulse wave velocity (cfPWV) also using the SphygmoCor XCEL as previously described.44,45 To assess vascular endothelial function, digital artery endothelium-dependent vasodilation was used via a noninvasive, reproducible plethysmographic procedure (EndoPAT2000, Itamar Medical, Ltd) in accordance with conditions specified by the manufacturer14,46–48 and as previously described.43 RHI, an index of flow-mediated dilation, was derived as previously mentioned43 and an RHI of <1.67 was defined as the cutoff value for endothelial dysfunction.49
A fasting blood sample was collected from an antecubital vein in vacutainers containing a serum clot activator (Greiner Bio-One lithium heparin tubes) and used for a comprehensive metabolic panel measured with a Piccolo Xpress analyzer (Abaxis). Hemoglobin A1c (HbA1c) levels were measured on an Alere Afinion Analyzer System using whole blood collected in EDTA tubes (Abbott).
Participants self-collected stool, which was stored at 4°C prior to processing at the clinic within 24 hours. Briefly, stool samples were suspended in reduced sterile reduced PBS with 10% glycerol and homogenized and then filtered through a 50uM filter to remove particulate matter. All samples were then stored at −80°C prior to use as animal inoculum. Inocula for the animal study were selected by categorizing individuals as lean or obese based on BMI and then selecting age and sex matched individuals that had normal and dysfunctional RHI scores.
Animal study
Experimental design
Male and female C57BL/6 J mice were bred and maintained in a germ-free facility at the University of Colorado Anschutz Gnotobiotic Facility and given ad libitum access to a standard diet (SD; 2020SX, Envigo, Indianapolis, IN). Germ-free isolators were routinely tested for sterility by culturing and pan-bacterial 16S rRNA gene PCR analysis of feces. Mice were co-housed 2–4 per cage in a temperature and humidity-controlled environment on a 12 h:12 h light-dark cycle. All animal procedures were reviewed and approved by the University of Colorado and Colorado State University Institutional Animal Care and Use Committees.
Colonization of germ-free mice occurred at 8 weeks of age and was performed using stored inocula from lean or obese human donors. Each mouse was inoculated once by oral gavage with 200 µl of inoculum under sterile conditions (n = 10 per group) and colonization was allowed to establish over a two-week period prior to any manipulations. Both male and female mice received fecal inocula from both male and female human donors. Mice received irradiated food and autoclaved drinking water. Body weight and food intake were recorded weekly for 14–18 weeks. Additionally, these analyses were also conducted on a control cohort of germ-free mice (n = 10) that did not receive inoculum.
Glucose tolerance test
One week prior to termination, mice were food fasted for 6 hours and blood glucose was determined from tail-vein blood using AlphaTRAK 2 glucose meters (Abbott Laboratories, Chicago, IL). After baseline glucose readings, mice were subjected to a glucose tolerance test, as previously described.7 We did not subject mice remaining germ-free to a glucose tolerance test to preserve germ-free status.
Arterial stiffness
Aortic pulse wave velocity (PWV) was measured at 14–18 weeks post colonization using a Doppler Flow Velocity System (Indus Instruments, Webster, TX) as previously stated.7
Animal termination and tissue collection
At 14–18 weeks post colonization, mice were anaesthetized with isoflurane and euthanized by exsanguination via cardiac puncture. To diminish the differences in study period, we euthanized an equal number of mice from either group across the 14–18 weeks. The liver and spleen were immediately excised, weighed, and flash frozen for later analyses. Next, the intestinal tract was removed and the colon and cecum were separated and remained on ice-cold PSS during mesenteric artery isolation (see Vascular Reactivity section). The heart and gastrocnemius were isolated, and flash frozen for later use.
Vascular reactivity
Endothelium-dependent and independent dilation was measured on second-order mesenteric arteries placed in pressure myograph chambers (DMT Inc., Atlanta, GA) was determined as previously described.7
Microbiota characterization
Fecal DNA was extracted using the FastDNA® Kit (MP Biomedicals, #116540400) following manufacturer’s protocol. Amplicons were generated from the V4 region (515 F-806 R) of the 16S rRNA following the Earth Microbiome Protocols.50 Negative DNA extraction controls, no template PCR controls, and the Zymo mock community were included on sequence plates as quality controls. Pooled libraries were quantified and sequenced on an Illumina MiSeq at the Next-Generation Sequencing Facility at Colorado State University.
Forward and reverse sequence reads were imported into QIIME2 version 2019.10 for analysis as previously described.51 Briefly, paired end sequences were concatenated and denoised using the DADA2 plugin in QIIME2. The feature table was used as input for calculation of alpha diversity parameters. Taxonomies were generated using the Greengenes reference database. Resulting taxonomy files and alpha diversity data were imported into R using the phyloseq package. Transplantation efficiency was visualized via Principle Component Analysis (PCA) plots. Alpha diversity was analyzed using non-phylogenetic (Observed and Shannon’s) measures. Differentially abundant taxa were determined using a negative binomial GLM (DiffAbund function) in MyPhyloDB v.1.2.0.52 Beta diversity was determined by Bray Curtis distance and visualized by Principle Coordinate Analysis (PCoA) in MyPhyloDB version v.1.2.0. Sparse partial least squares (sPLS) regression using Spearman’s correlations in MyPhyloDB v.1.2.0 was used to look for predictors in the microbiota of specific outcomes. Sequence reads and associated metadata are publicly available on MyPhyloDB (https://myphylodb.azurecloudgov.us/myPhyloDB/home) under the project name: GutMicrobes2021.
Statistics
Data are expressed as mean ± SEM. For comparisons between more than two groups, statistical analyses were performed using either a one-way or two-way ANOVA (GraphPad Prism, La Jolla, CA). When a significant main effect was observed, a Tukey’s post-hoc test was performed to determine specific pairwise differences. For comparisons between the mice receiving an inocula, an unpaired two-tailed Student’s t-test was used. A p-value of <0.05 was considered statistically significant; while p = .051–0.08 was used to signify a trend. Two sample t-test was used to determine statistical significance of alpha diversity measures Significance of Bray-Curtis distances were determined using PERMANOVA with 1000 iterations. A FDR<0.1 was used to determine which taxa contribute significantly to differences between groups.
Supplementary Material
Acknowledgments
This research was supported through the Co-Pilot mechanism of NIH/NCATS Colorado CTSA Grant Number UL1 TR002535 (TLW, SPC, and CLG); by the National Heart, Lung, and Blood Institute Grant RO1 HL 14411 (CLG, TLW, SAJ) and by NIDDK R01 DK104713 (SPC); and the American Heart Association grant 18TPA34170585 (CLG and TLW).
We would also like to thank Cassandra Levens from the Gnotobiotic Core Service Center (University of Colorado, Anschutz Medical Campus). Additionally, we thank Michael Sumpter and Gaby Brown for assistance with data collection.
Funding Statement
This work was supported by the American Heart Association [18TPA34170585]; National Heart, Lung, and Blood Institute [1R01HL144611]; National Institute of Diabetes and Digestive and Kidney Diseases [1R01DK104713]; NIH/NCATS Colorado CTSA [UL1 TR002535].
Author contributions
S.R.J.T., D.M.L., S.A.J., S.P.C., C.L.G., T.L.W. conceived and designed research; S.R.J.T., D.M.L., K.E.E., S.D.W., K.N.T., M.L.B., Y.W., C.L.G., and T.L.W. performed experiments; S.R.J.T., D.M.L., K.E.E., S.D.W., C.L.G. and T.L.W. analyzed data; S.R.J.T., D.M.L., S.A.J., C.L.G., and T.L.W. interpreted results of experiments; S.R.J.T., D.M.L. prepared figures; S.R.J.T., C.L.G., and T.L.W drafted the manuscript; S.R.J.T., D.M.L., S.A.J., K.A.K., S.P.C., C.L.G., and T.L.W. edited and revised manuscript; S.R.J.T., D.M.L., K.E.E., S.D.W., K.N.T., M.L.B., Y.W., S.A.J., K.A.K., S.P.C., C.L.G. and T.L.W. approved final version of manuscript.
Disclosure Statement
The authors declare no competing interests.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129(3):e28–16. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ.. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavalcante JL, Lima JA, Redheuil A, Al-Mallah MH. Aortic stiffness: current understanding and future directions. J Am Coll Cardiol. 2011;57(14):1511–1522. doi: 10.1016/j.jacc.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106(16):2085–2090. doi: 10.1161/01.CIR.0000033824.02722.F7. [DOI] [PubMed] [Google Scholar]
- 5.Widlansky ME, Gokce N, Keaney JF Jr., Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42(7):1149–1160. doi: 10.1016/S0735-1097(03)00994-X. [DOI] [PubMed] [Google Scholar]
- 6.Battson ML, Lee DM, Weir TL, Gentile CL. The gut microbiota as a novel regulator of cardiovascular function and disease. J Nutr Biochem. 2018;56:1–15. [DOI] [PubMed] [Google Scholar]
- 7.Battson ML. Suppression of gut dysbiosis reverses Western diet-induced vascular dysfunction. Am J Physiol Endocrinol Metab. 2018;314(5):E468–E477. doi: 10.1152/ajpendo.00187.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vikram A, Kim Y-R, Kumar S, Li Q, Kassan M, Jacobs JS, Irani K. Vascular microRNA-204 is remotely governed by the microbiome and impairs endothelium-dependent vasorelaxation by downregulating Sirtuin1. Nat Commun. 2016;7(1):12565. doi: 10.1038/ncomms12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battson ML, Lee DM, Li Puma LC, Ecton KE, Thomas KN, Febvre HP, Chicco AJ, Weir TL, Gentile CL. Gut microbiota regulates cardiac ischemic tolerance and aortic stiffness in obesity. Am J Physiol Heart Circ Physiol. 2019;317(6):H1210–H1220. doi: 10.1152/ajpheart.00346.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catry E, Bindels LB, Tailleux A, Lestavel S, Neyrinck AM, Goossens J-F, Lobysheva I, Plovier H, Essaghir A, Demoulin J-B. Targeting the gut microbiota with inulin-type fructans: preclinical demonstration of a novel approach in the management of endothelial dysfunction. Gut. 2018;67(2):271–283. doi: 10.1136/gutjnl-2016-313316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, Griffin NW, Lombard V, Henrissat B, Bain JR, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science. 2018;362(6416):776–780. doi: 10.1126/science.aau5812. [DOI] [PubMed] [Google Scholar]
- 14.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31(9):1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 15.Mattace-Raso FU, Van Der Cammen TJM, Hofman A, van Popele NM, Bos ML, Schalekamp MADH, Asmar R, Reneman RS, Hoeks APG, Breteler MMB. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113(5):657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 16.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 17.Diaz A, Galli C, Tringler M, Ramirez A, Cabrera Fischer EI. Reference values of pulse wave velocity in healthy people from an urban and rural argentinean population. International Journal of Hypertension. 2014;2014:653239. doi: 10.1155/2014/653239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benetos A. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens. 2002;15(12):1101–1108. doi: 10.1016/S0895-7061(02)03029-7. [DOI] [PubMed] [Google Scholar]
- 19.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT, et al. Metabolic syndrome and altered gut microbiota in mice lacking toll-like receptor 5. Science. 2010;328(5975):228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 21.Zanevelt J, McMinds R, Thurber R. Stress and stability: applying the Anna Karenina principle to animal microbiomes. Nat Microbiol. 2017;2(9):17121. doi: 10.1038/nmicrobiol.2017.121. [DOI] [PubMed] [Google Scholar]
- 22.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500(7464):585–588. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]
- 23.Asnicar F, Berry SE, Valdes AM, Nguyen LH, Piccinno G, Drew DA, Leeming E, Gibson R, Le Roy C, Khatib HA, et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat Med. 2021;27(2):321–332. doi: 10.1038/s41591-020-01183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux -J-J, Blugeon S, Bridonneau C, Furet J-P, Corthier G, et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. PNAS. 2008;105(43):16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neish AS. microbes in gastrointestinal health and disease. Gastroenterology. 2009;136(1):65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Palma G, Nadal I, Collado MC, Sanz Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br J Nutr. 2009;102(8):1154–1160. doi: 10.1017/S0007114509371767. [DOI] [PubMed] [Google Scholar]
- 27.Furet JP, Kong L-C, Tap J, Poitou C, Basdevant A, Bouillot J-L, Mariat D, Corthier G, Dore J, Henegar C, et al. Differential adapatation of human gut microbiota to bariatric surgery-induced weight loss. Diabetes. 2010;59(12):3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajilic-Stojanovic M, Biagi E, Heilig HGHJ, Kajander K, Kekkonen RA, Tims S, de Vos WM. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrom. Gastroenterology. 2011;141(5):1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 29.Martin R, Miquel S, Benevides L, Bridonneau C, Robert V, Hudault S, Chain F, Berteau O, Azevedo V, Chatel JM. Functional characterization of novel faecalibacterium prausnitzii strains isolated from healthy volunteers: a step forward in the use of f. prausnitzii as a next-generation probiotic. Front Microbiol. 2017;8. doi: 10.3389/fmicb.2017.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walther M, Chollet M. Menaquinones, bacteria, and foods: vitamin K2 in the diet. In J.O. Gordeladze (Ed.), Vitamin K2 - Vital for health and wellbeing, InTech: London, UK (2017), pp. 63–84). [Google Scholar]
- 31.Liu TH, Tao W-C, Liang Q-E, Tu W-Q, Xiao Y, Chen L-G. Gut microbiota-related evidence provides new insights into association between activating transcription factor 4 and development of salt-induced hypertension in mice. Front Cell Dev Biol. 2020;8. doi: 10.3389/fcell.2020.585995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 33.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899–1906. doi: 10.1161/01.CIR.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 34.Yun SI, Park HO, Kang JH. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol. 2009;107(5):1681–1686. doi: 10.1111/j.1365-2672.2009.04350.x. [DOI] [PubMed] [Google Scholar]
- 35.Farida E, Nuraida L, Giriwono PE, Jenie BSL. lactobacillus rhamnosus reduces blood glucose level through downregulation of gluconeogenesis gene expression in streptozotocin-induced diabetic rats. Int J Food Sci. 2020:6108575. doi: 10.1155/2020/6108575. PMID: 32399477; PMCID: PMC7201496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, Dallinga–Thie GM, Ackermans MT, Serlie MJ, Oozeer R. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–916 e917. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 37.Zoll J, Read MN, Heywood SE, Estevez E, Marshall JPS, Kammoun HL, Allen TL, Holmes AJ, Febbraio MA, Henstridge DC, et al. Fecal microbiota transplant from high caloric-fed donors alters glucose metabolism in recipient mice, independently of adiposity or exercise status. Am J Physiol Endocrinol Metab. 2020;319(1):E203–E216. doi: 10.1152/ajpendo.00037.2020. [DOI] [PubMed] [Google Scholar]
- 38.Hayward CS, Kelly RP, Collins P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc Res. 2000;46(1):28–49. doi: 10.1016/S0008-6363(00)00005-5. [DOI] [PubMed] [Google Scholar]
- 39.de la Cuesta-Zuluaga J, Kelley ST, Chen Y, Escobar JS, Mueller NT, Ley RE, McDonald D, Huang S, Swafford AD, Knight R,et al. 2019. Age- and sex-dependent patterns of gut microbial diversity in human adults. mSystems 4:e00261-19. 10.1128/mSystems.00261-19 [DOI] [PMC free article] [PubMed]
- 40.Kaden-Volynets V, Basic M, Neumann U, Pretz D, Rings A, Bleich A, Bischoff SC. Lack of liver steatosis in germ-free mice following hypercaloric diets. Eur J Nutr. 2019;58(5):1933–1945. doi: 10.1007/s00394-018-1748-4. [DOI] [PubMed] [Google Scholar]
- 41.Joe B, McCarthy CG, Edwards JM, Cheng X, Chakraborty S, Yang T, Golonka RM, Mell B, Yeo J-Y, Bearss NR. Microbiota introduced to germ-free rats restores vascular contractility and blood pressure. Hypertension. 2020;76(6):1847–1855. doi: 10.1161/HYPERTENSIONAHA.120.15939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edwards JM, Roy S, Tomcho JC, Schreckenberger ZJ, Chakraborty S, Bearss NR, Saha P, McCarthy CG, Vijay-Kumar M, Joe B, et al. Microbiota are critical for vascular physiology: germ-free status weakens contractility and induces sex-specific vascular remodeling in mice. Vascul Pharmacol. 2020;125-126:106633. doi: 10.1016/j.vph.2019.106633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Litwin NS, Van Ark HJ, Hartley SC, Michell KA, Vazquez AR, Fischer EK, Melby CL, Weir TL, Wei Y, Rao S, et al. Impact of red beetroot juice on vascular endothelial function and cardiometabolic responses to a high-fat meal in middle-aged/older adults with overweight and obesity: a randomized, double-blind, placebo-controlled, crossover trial. Curr Dev Nutr. 2019;3(11):nzz113. doi: 10.1093/cdn/nzz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hwang MH, Yoo J-K, Kim H-K, Hwang C-L, Mackay K, Hemstreet O, Nichols WW, Christou DD. Validity and reliability of aortic pulse wave velocity and augmentation index determined by the new cuff-based sphygmocor xcel. J Hum Hypertens. 2014;28(8):475–481. doi: 10.1038/jhh.2013.144. [DOI] [PubMed] [Google Scholar]
- 45.Wilkinson IM, Schillaci CM, Boutouyrie G, Segers P, Donald P, Chowienczyk A. ARTERY society guidelines for validation of non-invasive haemodynamic measurement devices: part 1, arterial pulse wave velocity. Artery Res. 2010;4(2):34–40. doi: 10.1016/j.artres.2010.03.001. [DOI] [Google Scholar]
- 46.Brant LC, Barreto SM, Passos VM, Ribeiro AL. Reproducibility of peripheral arterial tonometry for the assessment of endothelial function in adults. J Hypertens. 2013;31(10):1984–1990. doi: 10.1097/HJH.0b013e328362d913. [DOI] [PubMed] [Google Scholar]
- 47.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117(19):2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCrea CE, Skulas-Ray AC, Chow M, West SG. Test-retest reliability of pulse amplitude tonometry measures of vascular endothelial function: implications for clinical trial design. Vasc Med. 2012;17(1):29–36. doi: 10.1177/1358863X11433188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Syvanen K, Korhonen P, Partanen A, Aarnio P. Endothelial function in a cardiovascular risk population with borderline ankle-brachial index. Vasc Health Risk Manag. 2011;7:97–101. doi: 10.2147/VHRM.S17249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee DM, Ecton KE, Trikha SRJ, Wrigley SD, Thomas KN, Battson ML, Wei Y, Johnson SA, Weir TL, Gentile CL, et al. Microbial metabolite indole-3-propionic acid supplementation does not protect mice from the cardiometabolic consequences of a Western diet. Am J Physiol. 2020;319(1):G51–G62. doi: 10.1152/ajpgi.00375.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manter DK, Korsa M, Tebbe C, Delgado JA. (2016) myPhyloDB: a local web server for the storage and analysis of metagenomic data. Database (Oxford). 2016;2016:baw037. doi: 10.1093/database/baw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


