Abstract
Introduction
The aim of this study was to determine the effects of first-line treatment posterior tibial nerve stimulation (PTNS), applied once a week for a 12 week period, as a treatment modality for patients with Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS).
Material and methods
A total of 39 female patients with IC/BPS were enrolled in the study. Patients had one 30-minute session of PTNS per week for 12 weeks and symptoms were assessed before and after the treatment sessions by the use of a voiding diary, visual analog scale (VAS) for pain, O'Leary-Sant interstitial cystitis symptom index (ICSI), and O'Leary-Sant interstitial cystitis problem index (ICPI).
Results
The mean age of the patients was 38.9 ±7.1 years. The improvements in voiding diary parameters after 12 weeks of PTNS treatment were statistically significant compared to baseline but the changes in nocturia, and average voiding volume were not statistically significant. Mean parametric improvements after 12 weeks of PTNS treatment compared to baseline included a daytime frequency decrease by 3.8 voids daily, urgency episodes decrease by 4.7 episodes daily, nocturia decrease by 0.3 voids and voided volume improvement by a mean of 8.4 ml. The difference for ICSI, ICPI and VAS between baseline and the 12th week of PTNS treatment scores demonstrated statistically significant improvements in pain severity, symptom and problem index.
Conclusions
The findings in this study demonstrated the improvements of voiding diaries, ICSI, ICPI and VAS scores in patients with IC/BPS after 12 weeks PTNS. PTNS treatment is a beneficial firs-line treatment option to IC/BPS symptom amelioration.
Keywords: posterior tibial nerve stimulation, interstitial cystitis, bladder pain syndrome, neuromodulation, electrical stimulation
INTRODUCTION
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a chronic painful bladder condition associated with lower urinary tract symptoms without the presence of another disorder [1]. As defined by the International Continence Society (ICS) and the European Society for the Study of IC/BPS (ESSIC) IC/BPS includes symptoms of chronic pelvic pain, pressure, or perceived discomfort, related to the urinary bladder, accompanied by at least one other urinary symptom like persistent urge to void or frequency with or without abnormalities on cystoscopy. The estimated prevalence of IC/BPS has risen. Some studies have reported that the prevalence of IC/BPS is 10 to 510 per 100.000 [2]. Another study found that the prevalence of IC/BPS is 0.83% to 2.71% of women [3]. Various theories have been presented involving urothelial dysfunction, mast cell activation, neurogenic inflammation, and central nervous system pain perception alteration and C-fibre neuroplasticity [4]. Current management involves only symptomatic treatment of IC/BPS, and usually does not treat the underlying multifactorial pathophysiologic causes. Different treatments, including the use of oral medications, intravesical instillations and cystoscopy with hydrodistension have been proposed. These managements generally have short lasting effects, but some patients may permanently go into remission. If patients are unlikely to benefit from pharmacological, hydrodistention and intravesical instillation treatment or cannot tolerate the side effects, repeated intramuscular injections of botulinum toxin (BTX) into the detrusor muscle or neuromodulation could be considered and have been demonstrated to lead to an improvement in IC/BPS symptoms [5, 6]. The therapeutic benefit of sacral neuromodulation (SNM) in patients with IC/BPS or chronic pelvic pain, have been suggested in various studies [7]. Posterior tibial nerve stimulation (PTNS), which is a minimally invasive neuromodulation technique, provides effective treatment for patients with neurogenic and non-neurogenic lower urinary tract symptoms (LUTS) which are unresponsive to medical treatment [8]. The positive effects of the 12-week PTNS treatment for idiopathic overactive bladder (OAB) symptoms were supported by long-term randomized controlled trials demonstrating persistence of efficacy after 12 and 24 months [8–11]. Moreover, few studies have been performed to determine the effects of PTNS in patients with IC/BPS [12–15]. Although there are a few promising studies in the treatment of IC/BPS with PTNS, the data appears to be limited and conflicting [14, 15]. The aim of this study was to determine the effects of first-line treatment PTNS which was applied once a week for a period of 12 weeks as a treatment modality for patients with IC/BPS.
MATERIAL AND METHODS
This study was approved by the local ethics committee. All patients were informed of the details of all procedures and of the study. We conducted a prospective study for IC/BPS patients who presented to our hospital from January 2009 and May 2019. Written informed consent was obtained from all participants according to their own will. All patients with IC/BPS who were treated in our hospital were screened. A diagnosis of IC/BPS was determined on the basis of pain, pressure or discomfort associated with the urinary bladder, accompanied by at least one other symptom, such as daytime and/or night-time increased urinary frequency. The National Institute of Diabetes and Digestive and Kidney Disease (NIDDK) criteria for interstitial cystitis were selected for this study [16]. The inclusion criteria were patients with symptoms lasting more than 12 months with any two of the following: painful bladder filling relieved by emptying, pain (suprapubic, urethral, vaginal or perineal), decreased bladder compliance on cystometry or glomerulations and/or Hunner's lesions on cystoscopy. Patients who had not received any previous medication/treatments were included in the study. Exclusion criteria were patients less than 18 years of age, those who had symptoms lasting less than 12 months, daytime frequency less than 5 times per 12 hours, nocturia less than 2 times per night, or bladder capacity more than 400 ml. Likewise, patients with, obstructive, neurogenic, inflammatory, malignant/benign neoplastic diseases, previous urogenital surgery, pelvic irradiation, bacterial cystitis, vaginitis, cyclophosphamide induced cystitis, bladder or lower ureteral calculi, overactive bladder and chronic diseases such as diabetes mellitus, hepatic problems, peripheral neuropathy or degenerative conditions of the spinal cord were excluded. In addition, patients with pacemakers or implantable defibrillators were also excluded. A general medical history and physical examination were obtained from all patients. For the patients who participated in the study, a urinary sediment test was performed to screen for urinary tract infections before the start of the study as well as during every visit to the hospital throughout the study period. Standardized cystoscopy and biopsy were performed in all patients. The urodynamic evaluations complied with the International Continence Society (ICS) recommendations. The patients were requested to complete a 3-day voiding diary before and after the treatment of PTNS. All IC/BPS patients completed the questionnaires for the Visual analog scale for pain (VAS), O'Leary-Sant Interstitial Cystitis Symptom Index (ICSI), O'Leary-Sant Interstitial Cystitis Problem Index (ICPI) before and after PTNS [17]. PTNS was applied unilaterally with 26-gauge stainless steel needles (disposable concentric needle Medtronic, Denmark). We inserted the needle 5 cm cephalad from the medial malleolus and posterior to the edge of the tibia. According to the previous studies, electrical stimulation (Medtronic Key Point Net®, Denmark) was applied by using charge-compensated 200μs pulses with a pulse rate of 20 Hz [18, 19]. The intensity level was chosen as the intensity immediately under the threshold determining motor contraction of the toe. Electrical stimulation was triggered with a push button to determine the appropriate stimulation amplitude in order to confirm correct needle placement. The stimulation amplitude was set at the maximum tolerable level for the patient, which was usually 1.5 times the threshold for evoking plantar flexion of the toes and/or toe fanning (range: 1–5 mA).
Statistical analysis
The baseline demographic and clinical features such as age, gender, ICSI, ICPI and VAS scores were evaluated with the analysis of variance test (ANOVA). Mean values of voiding diary were evaluated for significant change using a 2-sided paired t test and median values were evaluated using a Wilcoxon signed rank test with p <0.05 considered statistically significant.
RESULTS
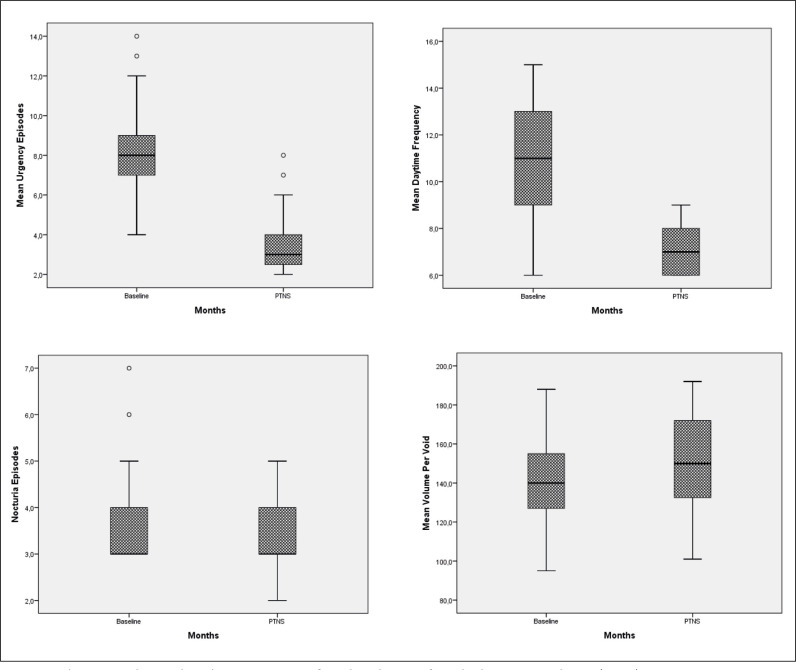
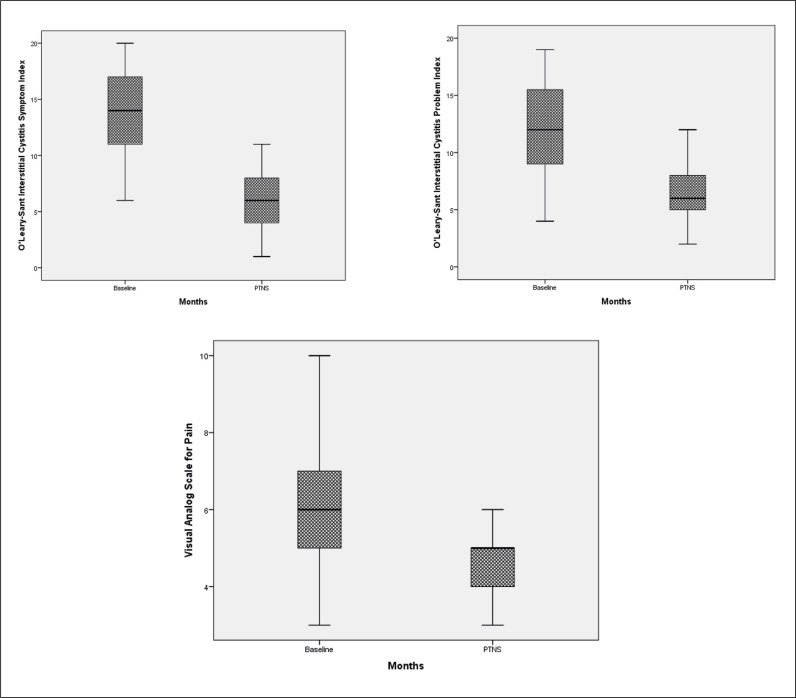
A total of 39 patients with a mean age 38.9 ±7.1 years (range 26 to 53) were enrolled in the study. Mean duration IC/BPS was 5.9 ±2.1 years. The improvements after 12 weeks of PTNS treatment within voiding diary parameters were statistically significant compared to baseline but there were no statistically significant changes in nocturia, and average voiding volume (Figure 1). Mean parametric improvements at 12 weeks of PTNS treatment from baseline included a daytime frequency decrease by 3.8 voids daily (p <0.001), urgency episodes decrease by 4.7 episodes daily (p <0.001), nocturia decrease by 0.3 voids (p >0.05) and voided volume improvement by a mean of 8.4 ml (p >0.05) (Table 1). The difference for ICSI, ICPI and VAS between the baseline and after 12 weeks of PTNS treatment scores demonstrated statistically significant improvements in pain severity, symptom and problem index (p <0.001) (Table 2, Figure 2). There were no reported treatment-related adverse events in the patients throughout the 12 weeks. Four patients reported mild to moderate pain such as in the site of the puncture, leg cramps, or tingling in the leg with an unknown relationship to the PTNS treatment.
Figure 1.
Changes within voiding diary parameters from baseline to after tibial nerve stimulation (PTNS).
Table 1.
Effect of posterior tibial nerve stimulation on voiding diary parameters
| Baseline | PTNS | % change from baseline | p value | |
|---|---|---|---|---|
| Frequency Mean ±SD Min.–Max. |
10.9 ±2.4 (6–15) |
7.1 ±1.0 (6–9) |
34.8 |
<0.001 |
| Nocturia Mean ±SD Min.–Max. |
3.6 ±0.9 (3–7) |
3.3 ±0.8 (2–5) |
8.3 |
>0.05 |
| Urgency Mean ±SD Min-Max |
8.2 ±2.6 (4–14) |
3.5 ±1.4 (2–8) |
57.3 |
<0.001 |
| Voided volume Mean ±SD Min.–Max. |
140.8 ±22.1 (95–188) |
149.2 ±23.7 (101–192) |
5.9 |
>0.05 |
SD – standard deviation
Table 2.
Effect of posterior tibial nerve stimulation on VAS, ICSI and ICPI scores
| Baseline | PTNS | % change from baseline | p value | |
|---|---|---|---|---|
| VAS Mean ±SD Min–Max |
6.1 ±1.6 (3–10) |
4.6 ±0.9 (3–6) |
24.6 |
<0.001 |
| ICSI Mean ±SD Min.–Max. |
13.8 ±4.1 (6–20) |
5.9 ±2.4 (1–11) |
57.2 |
<0.001 |
| ICPI Mean ±SD Min.–Max. |
11.7 ±4.1 (4–19) |
6.5 ±2.1 (2–12) |
44.4 |
<0.001 |
SD – standard deviation; VAS – visual analag scale; ICSI – Interstitial Cystitis Symptom Index; ICPI – Interstitial Cystitis Problem Index
Figure 2.
Changes within visual analog scale (VAS) for pain, O'Leary-Sant Interstitial Cystitis Symptom Index (ICSI), O'Leary-Sant Interstitial Cystitis Problem Index (ICPI) from baseline after posterior tibial nerve stimulation (PTNS).
DISCUSSION
The treatment of patients with IC/BPS remains to be a difficult situation. Since the pathophysiology is not fully revealed, different managements have been recommended. Current management for IC/BPS proposed including lifestyle modifications as well as the use of oral pharmacotherapy such as amitriptyline, pentosane polysulphate, cimetidine, or hydroxyzine. Intravesical instillation therapy (i.e., heparin and hyaluronic acid), and intravesical Botulinum neurotoxin A (BoNT-A) injection have been recommended for pain relief, urgency and frequency in refractory BPS/IC. However, despite all of these recommended treatments, sufficient success cannot be obtained in patients with IC/BPS [20, 21]. The search for an inexpensive but effective method for IC/BPS is ongoing. Recently, neuromodulation has been presented as an alternative, minimally invasive surgery for patients with lower urinary tract symptoms unresponsive to conservative and oral medical therapy [22]. A recent meta-analysis showed that sacral neurostimulation (SNM) may be effective and safe for treating IC/BPS refractory to conventional therapies. They demonstrate marked improvements in not only pelvic pain, but also in voiding symptoms and improved symptom scores [23]. Because SNM is expensive and performed as a two-staged invasive procedure, investigating an easy method, such as posterior tibial nerve stimulation (PTNS), can provide significant benefits for IC/BPS treatment. Therefore, investigating whether PTNS is effective in treating IC/BPS can be an important step. While the underlying mechanism of the effect of PTNS in LUTS is not clear, it has been hypothesized to act by means of changes of cerebral endorphin levels, depolarization of somatic sacral and lumbar afferent fibres, activation of efferent fibres to the striated urethral sphincter and plastic reorganisation of the cortical excitability [24, 25]. Danisman et al., demonstrated a diminished mast cell count after PTNS in the bladder of female rats [26]. Chang et al suggested that PTNS could produce the effect in the rat sacral spinal cord by reducing C-fos expression (a marker of neuronal metabolic activity) after electrical stimulation of the rat’s hind leg [27]. Finazzi-Agro’ et al., studied the electrophysiological effect on supraspinal centers and found a significant increase in the amplitude of long latency somatosensory evoked potentials recorded 24 hours after the end of a 12 session PTNS program. This finding could reflect a modification in elaboration mechanisms of sensory stimuli possibly suggesting a reorganization of cortical excitability after PTNS [28]. PTNS was first described by McGuire et al. in patients with incontinence, using a transcutaneous electrode over the common peroneal or posterior tibial nerve and a contralaterally placed ground electrode over the same nerve [29]. Later, Stoller et al. adjusted this method by using a percutaneous needle electrode and placing the ground electrode on the ipsilateral extremity [30]. Since then, many studies have been done to evaluate PTNS as a treatment in patients who presented with symptoms of OAB. All of these studies have demonstrated good results and improvements on urodynamic parameters after PTNS treatment. A statistically significant decrease was observed in leakage episodes, the number of pads used, voiding frequency and nocturia and an equal increase in the mean and smallest volumes voided [6, 9, 11]. In their study Finazzi-Agro’ et al. have reported the effects of PTNS in 35 women with refractory, idiopathic detrusor overactivity (DO). They showed that of the patients treated with PTNS, 71% had a reduction of urge incontinence episodes greater than 50% (p <0.001) [8]. We have also previously demonstrated the effects of PTNS with acute urodynamic parameters on NDO in MS and PD patients. Our studies revealed an increase of involuntary detrusor contraction volume and cystometric capacity in NDO patients [18, 31]. Also, in another study in which we enquired about the effect of PTNS in MS patients with NDO, we revealed improvements in both urodynamic and voiding parameters after 3 months of PTNS treatment [32]. In our previous randomized sham-controlled study we reported the efficacy of PTNS with chronic nonbacterial prostatitis (CP)/chronic pelvic pain syndrome (CPPS) patients. Also, we showed improvements in VAS for pain, and National Institutes of Health-Chronic Prostatitis Symptom Index (NIH-CPSI) scores after 12 weeks of PTNS in 18 patients (40%), and partial response in 27 patients (60%), 30 (66.6%) patients, 15 (33.3%) patients respectively. Mean NIH-CPSI scores, VAS for pain, VAS for urgency significantly changed from 23.6 ±6.3 at baseline to 10.2 ±3.6, 7.6 ±0.8 at baseline to 4.3 ±0.6, 5.7 ±0.8 at baseline to 3.4 ±0.7, respectively. Mean NIH-CPSI scores, VAS for pain, VAS for urgency did not change in the placebo treatment group [33]. To date, few studies have been done to evaluate the effectiveness of PTNS in IC/BPS patients [12, 14, 15, 34]. Available data on PTNS are limited and sometimes appear to be inconsistent for promising results of studies investigating the effect of PTNS in IC/BPS treatment. Zhao et al. reported no significant clinical effect of 30 minutes of PTNS therapy once a week for 10 weeks in resistant IC/PBS patients [14]. The same authors also concluded that 30 minutes of PTNS therapy twice a week for 10 weeks had a positive effect on symptoms in 44% of patients and might be an alternative form of therapy [15]. In another study, it was reported that there was no statistically significant difference between 12-week PTNS treatment in IC / BPS patients between mean VAS, day-time frequency, nocturia, mean void volume, and ICPI scores [34]. Sudol et al. reported their results for 10 patients who completed 12 weeks of PTNS treatment, in which the GRA response rate was 40% at 6 weeks and 30% at 12 weeks. They reported that they could not find a statistically significant difference in Global Response Assessment (GRA), VAS, ICSI and ICPI scores [12]. When the results of the few studies pertaining to PTNS treatment for IC/BPS in the literature were evaluated, there were methodological differences such as the insufficient number of patients included in the study, the use of different diagnostic criteria, the non-standard PTNS protocol, the variations in the evaluation criteria and the conditions for starting PTNS treatment. Therefore, in this study, we tried to evaluate a standard PTNS protocol by including patients diagnosed with the general criteria recommended for IC/BPS diagnosis. The main goal of our study was to determine the overall symptom improvement rate using validated measurements after the completion of 12 weekly PTNS sessions as the first-line treatment for IC/BPS patients. The final results of our study demonstrated that PTNS has improvements on voiding parameters, VAS scores and symptom/problem index in IC/BPS patients. The voiding diary parameters of daytime frequency and urgency episodes all significantly improved from baseline but there were no statistically significant changes in nocturia, and average voiding volume. Consistent with objective results, the ICSI, ICPI and VAS scores further confirm improvement after 12 weeks of treatment thus reflecting the clinical significance of changes for patients. Power analysis and sample size rationale are complementary parts of the study design of research in which an inference will be made. Determining the appropriate sample size is challenging because there are unknown parameters that need to be estimated. Power analysis is recommended for studies planned to be 80–90% power, if at all possible. In our study, power was calculated as 90.6% according to the number of 39 patients included in the study [35]. The limitation of this study is the lack of randomized control or sham arm. However, the results of our study have suggested that PTNS is safe and simple to perform, and patients readily accept this method. Future studies with larger patients’ series are required; however, the results of our study encourage the use of PTNS as an off-label treatment for first-line treatment IC/BPS.
CONCLUSIONS
The findings in this study demonstrated the improvements of daytime frequency, urgency episodes, ICSI, ICPI and VAS scores in IC/BPS patients after 12 weeks of PTNS. PTNS treatment is a beneficial first-line treatment option to IC/BPS symptom amelioration and should be taken into consideration as a well tolerated treatment for IC/BPS patients However, we believe that these results must be confirmed by prospective multicenter randomized controlled studies to evaluate the exact role of PTNS in these indications and to assess the long-term durability of the treatment.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.McLennan MT. Interstitial cystitis: epidemiology, pathophysiology, and clinical presentation. Obstet Gynecol Clin North Am. 2014;41:385–395. doi: 10.1016/j.ogc.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Jones CA, Nyberg L. Epidemiology of interstitial cystitis. Urology. 1997;49:2–9. doi: 10.1016/s0090-4295(99)80327-6. [DOI] [PubMed] [Google Scholar]
- 3.Clemens JQ, Link CL, Eggers PW, Kusek JW, Nyberg LM, Jr, McKinlay JB, BACH Survey Investigators Prevalence of painful bladder symptoms and effect on quality of life in black, Hispanic and white men and women. J Urol. 2007;177:1390–1394. doi: 10.1016/j.juro.2006.11.084. [DOI] [PubMed] [Google Scholar]
- 4.Rahnama'i MS, Marcelissen T, Apostolidis A, et al. The efficacy of botulinum toxin A and sacral neuromodulation in the management of interstitial cystitis (IC)/bladder pain syndrome (BPS), what do we know? ICI-RS 2017 think thank, Bristol. Neurourol Urodyn. 2018;37:99–107. doi: 10.1002/nau.23493. [DOI] [PubMed] [Google Scholar]
- 5.Chiu B, Tai HC, Chung SD, Birder LA. Botulinum toxin A for bladder pain syndrome/interstitial cystitis. Toxins. 2016;8:E201. doi: 10.3390/toxins8070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Chen Y, Chen J, Zhang G, Wu P. Sacral neuromodulation for refractory bladder pain syndrome/interstitial cystitis: a global systematic review and meta-analysis. Sci Rep. 2017;7:11031. doi: 10.1038/s41598-017-11062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcelissen T, Jacobs R, van Kerrebroeck P, de Wachter S. Sacral neuromodulation as a treatment for chronic pelvic pain. J Urol. 2011;186:387–393. doi: 10.1016/j.juro.2011.02.2694. [DOI] [PubMed] [Google Scholar]
- 8.Finazzi-Agro E, Petta F, Sciobica F, et al. Pasqualetti P, Musco S, Bove P. Percutaneous tibial nerve stimulation effects on detrusor overactivity incontinence are not due to a placebo effect: a randomized, double-blind, placebo controlled trial. J Urol. 2010;184:2001–2006. doi: 10.1016/j.juro.2010.06.113. [DOI] [PubMed] [Google Scholar]
- 9.Peters KM, Carrico DJ, Perez-Marrero RA, et al. Randomized trial of percutaneous tibial nerve stimulation versus sham efficacy in the treatment of overactive bladder syndrome: Results from the SUmiT Trial. J Urol. 2010;183:1438–1443. doi: 10.1016/j.juro.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 10.MacDiarmid SA, Peters KM, Shobeiri SA, et al. Long-term durability of percutaneous tibial nerve stimulation for the treatment of overactive bladder. J Urol. 2010;183:234–240. doi: 10.1016/j.juro.2009.08.160. [DOI] [PubMed] [Google Scholar]
- 11.Peters KM, Carrico DJ, MacDiarmid SA, et al. Sustained therapeutic effects of percutaneous tibial nerve stimulation: 24-month results of the STEP study. Neurourol Urodyn. 2013;32:24–29. doi: 10.1002/nau.22266. [DOI] [PubMed] [Google Scholar]
- 12.Sudol NT, Guaderrama N, Adams-Piper E, Whitcomb E, Lane F. Percutaneous tibial nerve stimulation for the treatment of interstitial cystitis/bladder pain syndrome: a pilot study. Int Urogynecol J. 2020 doi: 10.1007/s00192-020-04481-4. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Ragab MM, Tawfik AM, El-enen MA, et al. Evaluation of Percutaneous Tibial Nerve Stimulation for Treatment of Refractory Painful Bladder Syndrome. Urology. 2015;86:707–711. doi: 10.1016/j.urology.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J, Nordling J. Posterior tibial nerve stimulation in patients with intractable interstitial cystitis. BJU Int. 2004;94:101–104. doi: 10.1111/j.1464-410X.2004.04909.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Bai J, Zhou Y, Qi G, Du L. Posterior tibial nerve stimulation twice a week in patients with interstitial cystitis. Urology. 2008;71:1080–1084. doi: 10.1016/j.urology.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 16.Gillenwater JY, Wein AJ. Summary of the National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases Workshop on Interstitial Cystitis, National Institutes of Health. J Urol. 1988;140:203–206. doi: 10.1016/s0022-5347(17)41529-1. [DOI] [PubMed] [Google Scholar]
- 17.Esen B, Obaid K, Suer E, et al. Turkish Versions of the Interstitial Cystitis Symptom Index (ICSI) and Interstitial Cystitis Problem Index (ICPI): Linguistic and Psychometric Validation. ICS Congress abstract 633, 2019. [Google Scholar]
- 18.Kabay SC, Kabay S, Yucel M, Ozden H. Acute urodynamic effects of percutaneous posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with Parkinson’s disease. Neurourol Urodyn. 2009;28:62–67. doi: 10.1002/nau.20593. [DOI] [PubMed] [Google Scholar]
- 19.Van Balken MR, Vandoninck V, Gisolf KW, et al. Posterior tibial nerve stimulation as neuromodulative treatment of lower urinary tract dysfunction. J Urol. 2001;166:914–918. doi: 10.1097/00005392-200109000-00025. [DOI] [PubMed] [Google Scholar]
- 20.Hanno PM, Erickson D, Moldwin R, Faraday MM, American Urological Association Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193:1545–1553. doi: 10.1016/j.juro.2015.01.086. [DOI] [PubMed] [Google Scholar]
- 21.Engeler D, Baranowski AP, Berghmans B, et al. Chronic Pelvic Pain Chronic Pelvic Pain. EAU Guidelines; 2020. [Google Scholar]
- 22.Kessler TM, La Framboise D, Trelle S, et al. Sacral neuromodulation for neurogenic lower urinary tract dysfunction: systematic review and meta-analysis. Eur Urol. 2010;58:865–874. doi: 10.1016/j.eururo.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Chen Y, Chen J, Zhang G, Wu P. Sacral Neuromodulation for Refractory Bladder Pain Syndrome/Interstitial Cystitis: a Global Systematic Review and Meta-analysis. Sci Rep. 2017;7:11031. doi: 10.1038/s41598-017-11062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Seze M, Raibaut P, Gallien P, et al. Transcutaneous posterior tibial nerve stimulation for treatment of the overactive bladder syndrome in multiple sclerosis: results of a multicenter prospective study. Neurourol Urodyn. 2011;30:306–311. doi: 10.1002/nau.20958. [DOI] [PubMed] [Google Scholar]
- 25.Tudor KI, Seth JH, Liechti1 MD, et al. Outcomes following percutaneous tibial nerve stimulation (PTNS) treatment for neurogenic and idiopathic overactive bladder. Clin Auton Res. 2020;30:61–67. doi: 10.1007/s10286-018-0553-8. [DOI] [PubMed] [Google Scholar]
- 26.Danisman A, Kutlu O, Akkaya E, Karpuzoğlu G, Erdoğru T. Tibial nerve stimulation diminishes mast cell infiltration in the bladder wall induced by interstitial cystitis urine. Scand J Urol Nephrol. 2007;41:98–102. doi: 10.1080/00365590600911233. [DOI] [PubMed] [Google Scholar]
- 27.Chang CJ, Huang ST, Hsu K, Lin A, Stoller ML, Lue TF. Electroacupuncture decreases c-fos expression in the spinal cord induced by noxious stimulation of the rat bladder. J Urol. 1998;160:2274–2279. doi: 10.1097/00005392-199812010-00099. [DOI] [PubMed] [Google Scholar]
- 28.Finazzi-Agrò E, Rocchi C, Pachatz C, et al. Percutaneous tibial nevre stimulation produces effects on brain activity: study on the modifications of the long latency somatosensory evoked potentials. Neurourol Urodyn. 2009;28:320–324. doi: 10.1002/nau.20651. [DOI] [PubMed] [Google Scholar]
- 29.McGuire EJ, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129:78–79. doi: 10.1016/s0022-5347(17)51928-x. [DOI] [PubMed] [Google Scholar]
- 30.Stoller ML. Afferent nerve stimulation for pelvic floor dysfunction (abstract) Eur Urol. 1999;35(Suppl 2):132. https://www.karger.com/Book/Home/223566. [Google Scholar]
- 31.Kabay SC, Yucel M, Kabay S. Acute effect of posterior tibial nerve stimulation on neurogenic detrusor overactivity in patients with multiple sclerosis: urodynamic study. Urology. 2008;71:641–645. doi: 10.1016/j.urology.2007.11.135. [DOI] [PubMed] [Google Scholar]
- 32.Kabay S, Kabay SC, Yucel M, et al. The clinical and urodynamic results of a 3-month percutaneous posterior tibial nerve stimulation treatment in patients with multiple sclerosis-related neurogenic bladder dysfunction. Neurourol Urodyn. 2009;28:964–968. doi: 10.1002/nau.20733. [DOI] [PubMed] [Google Scholar]
- 33.Kabay S, Kabay SC, Yucel M, Ozden H. Efficiency of posterior tibial nerve stimulation in category IIIB chronic prostatitis/chronic pelvic paIn: a sham-controlled comparative Study. Urol Int. 2009;83:33–38. doi: 10.1159/000224865. [DOI] [PubMed] [Google Scholar]
- 34.Ragab MM, Tawfik AM, Abo El-enen M, et al. Evaluation of percutaneous tibial nerve stimulation for treatment of refractory painful bladder syndrome. Urology. 2015;86:707–711. doi: 10.1016/j.urology.2015.06.041. [DOI] [PubMed] [Google Scholar]
- 35.Mascha EJ, Vetter TR. Significance, Errors, Power, and Sample Size: The Blocking and Tackling of Statistics. Anesth Analg. 2018;126:691–698. doi: 10.1213/ANE.0000000000002741. [DOI] [PubMed] [Google Scholar]