Abstract
Introduction
The aim of this study was to analyze whether differences exist in a population selected for a nerve-sparing (NS) procedure between robot-assisted (RARP) and laparoscopic radical prostatectomy (LRP), and whether they can have an impact on surgical margins (SM) status.
Material and methods
This is a single center prospective comparative trial on prostate cancer patients submitted to a RARP-NS or LRP-NS. A self-administered questionnaire on expectations before surgery, and level of satisfaction after surgery was used.
Results
A total of 134 cases were included in our analysis. A higher percentage of capsular bulging was found in the RARP group, compared to the LRP group (p = 0.077). At biopsy, the percentage of positive cores and multifocality were higher in the RARP group (p = 0.005). Positive SM (SM+) rate was higher in the RARP, than in LRP group (p = 0.046). On univariable analysis, the risk of SM+ increased 1.95 times using RARP when compared with LRP. On multivariable analysis, the surgical approach did not maintain a significant predictive role in terms of risk for SM+. Expectations before surgery were mainly focused on oncological radicality, however in the RARP group a higher percentage of cases focused on sexual function recovery. Satisfaction after surgery was lower in the RARP than in the LRP group.
Conclusions
Comparing LRP-NS with RARP-NS in a high-volume single center, the expectation/satisfaction ratio is in favor of LRP. Worse oncologic preoperative characteristics in the RARP group may influence the higher incidence of SM+. However, the surgical approach does not result as a significant and independent factor able to influence SM positivity.
Keywords: prostatic neoplasm, robot-assisted prostatectomy, laparoscopic prostatectomy, nerve-sparing
INTRODUCTION
Clinical decision in prostate cancer (PC) patients continues to depend upon serum prostate-specific antigen (PSA) levels, tumor stage, risk classes and pathologic Gleason score [1, 2, 3]. Robot-assisted radical prostatectomy (RARP) has become the most frequently used technique for the surgical management of non-metastatic PC [4]. However, despite its increasing use, the advantage of RARP over a laparoscopic (LRP) procedure remains under debate, so that patients and clinicians have to opt for one treatment in the absence of solid evidence favoring a specific approach [5–8]. Although some clinical trials have shown that RARP can offer better results than LRP in terms of potency, recovery and surgical margins (SM) in pathologically organ-confined PC [9–16], the European Urological Association (EAU) guidelines recommend to inform patients that no surgical approach (open versus LRP versus RARP) has clearly shown superiority in terms of both functional and oncologic outcomes [17]. Schroeck et al. reported that treatment satisfaction is mainly derived from perceived differences between expectations and experience and that patients who underwent RARP were more likely to be regretful and dissatisfied because of higher expectations in functional outcomes [18].
The aim of the present prospective comparative trial was to analyze whether differences exist (clinical and pathologic parameters) in a population selected for a nerve-sparing (NS) procedure between cases who underwent RARP and LRP, and whether these differences can have an impact on SM status. Preoperative expectations versus postoperative satisfaction in each surgical technique have been explored.
MATERIAL AND METHODS
This is a single institution prospective comparative non-randomized trial, analyzing a real-life setting, on PC patients submitted to a NS surgery, using RARP or LRP.
Population
Patients with a histological diagnosis of PC considered for radical prostatectomy (RP) as primary therapeutic option in our department were consecutively included in the analysis. The protocol was approved by our internal ethical committee and all patients gave their informed consent for each procedure. All diagnostic and therapeutic procedures reflected our routine clinical practice in a department with a high-volume of PC management. Inclusion criteria were: histological diagnosis of prostatic adenocarcinoma, no distant metastases at clinical staging, RP as chosen primary treatment option, estimated life-expectancy of ≥10 years. Exclusion criteria were: androgen deprivation therapies, chemotherapies, pelvic radiation therapies or treatments that could influence prostate tumor growth. A NS procedure was considered in cases with low preoperative risk of ipsilateral extracapsular disease based on clinical staging. From January 2018 to January 2020, 236 consecutive patients with PC underwent RP in our department and met our inclusion and exclusion criteria. Of these, 134 (56.8%) underwent a NS surgery and were included in the present analysis.
Clinical parameters
All cases underwent a standard random 14-core prostatic biopsy; in cases submitted to multiparametric magnetic resonance imaging (mpMRI) with Prostate Imaging – Reporting and Data System (PI-RADS) score 3-5, additional targeted samples were obtained [19, 20]. Before surgery, clinical staging and risk category (D’Amico and EAU classification) assessment was homogeneously performed using total PSA determination and imaging [mpMRI, computed tomography (CT), and bone scan)] All patients underwent a laparoscopic or robotic RP following the EAU guidelines for indications. After surgery, all patients were followed at regular intervals, in order to evaluate time to biochemical progression (BP) (confirmed total PSA progression over 0.2 ng/ml) or radiological progression (radiologically confirmed, local or distant), as recommended by the EAU guidelines [17].
A self-administered questionnaire was used in all cases before surgery, and after the procedure at 1- and 6-month intervals. Preoperatively, the questionnaire asked patients whether a preference in terms of surgical approach was present and what was their first expectation from RP; after surgery, the questionnaire asked whether patients believed that their main expectation was satisfied. Results were stratified according to the surgical approach (LRP versus RARP).
Pathologic evaluation
All histological specimens from biopsy and RP were analyzed by our two uro-pathologists with a long experience in the PC field. Gleason score and grade group (GG) according to the World Health Organization/ International Society of Urological Pathology (WHO/ISUP) 2014 guidelines at biopsy and at surgery, pathologic staging (pTNM), SM status and perineural invasion (PNI) were routinely assessed. SM were considered positive (SM+) when carcinoma was transected by an inked SM; this could be in a setting of organ-confined or extracapsular disease.
Surgical procedure
Surgical technique was not assigned randomly. As routine clinical practice in our department, each procedure was performed by a different surgeon who had the most expertise in each approach, consistent with best practice. The RARP surgeon had >20 years of experience (>1000 procedures); the LRP surgeons had >10 years of experience (>500 procedures). A NS (intrafascial monolateral or bilateral) intraperitoneal procedure was performed at each individual surgeon’s discretion, based on clinical assessment of risk classes, risk of extracapsular disease and after discussion with the patient on the probability to maintain potency, balanced with possible harm. In particular, for either procedures: patients with clinical high-risk of ipsilateral extraprostatic extension (EPE) were excluded from a NS surgery; - extended lymph node dissection was performed in all high-risk cases, and in the intermediate-risk cases with ≥5% probability for positive nodes; intra-operative evaluation of SM was not performed; surgical intraperitoneal technique was similar using RARP or LRP.
Statistical analysis and outcomes
For statistical evaluation SPSS Statistics program was used. Descriptive statistical methods such as number of cases, mean ± standard deviation (SD), median and range were used. For the comparison of quantitative data and pairwise intergroup comparisons of variables a Mann Whitney test was performed. For comparison of qualitative data Fisher’s Exact test and Chi-square test were used. Pearson correlation analysis was also performed. Univariable and multivariable logistic regression analysis considering clinical and pathological parameters were used; a crude adjusted odds ratio (OR) and its 95% confidence interval (95%CI) were calculated. Kaplan-Meier survival curves related to surgical technique, clinical and pathological outcomes were obtained. Statistical significance was evaluated at p <0.05.
Primary outcome was to evaluate differences in SM status between cases submitted to a RARP-NS and LRP-NS procedure. Secondary outcome was to determine the independent role of differences in clinical and pathologic parameters between the two populations on oncologic postoperative outcomes (SM status and progression).
RESULTS
Baseline characteristics of the whole population (n = 236) considered for RARP and LRP are summarized in Table 1. Table 2 shows clinical and pathologic characteristics of the final population (n = 134) considered for a NS surgery according to the surgical approach, RARP (63 cases) versus LRP (71 cases). The median follow-up after surgery was 12 months (range 6–24).
Table 1.
Patients’ characteristics in the whole population [number of cases (%), mean ±SD and median (range); p value: t test or Chi-squared test]
| Variable | LRP | RARP | p-value |
|---|---|---|---|
| Patients, n° (%) | 133 (56.3) | 103 (43.7) | – |
| Age (years) mean ±SD median (range) |
65.5 ±6.2 66 (48–77) |
63.9 ±6.1 66 (47–77) |
0.054 |
| BMI mean ±SD median (range) |
26.9 ±3.6 26.2 (17.0–37.0) |
26.5 ±3.7 26.2 (19.0–27.8) |
0.503 |
| Pre-operative PSA (ng/ml) mean ±SD median (range) |
9.1 ±8.4 7.2 (1.7–24.2) |
10.3 ±8.5 7.3 (1.7–26.0) |
0.282 |
| mpMRI PI-RADS, n° (%) n° of mpMRI performed 1–2 3 4–5 |
76 (57.1) 0 (0) 15 (19.7) 61 (80.3) |
74 (71.8) 2 (2.7) 15 (20.2) 57 (77.1) |
0.348 |
| mpMRI bulging, n° (%) | 14 (18.4) | 22 (29.7) | 0.806 |
| Positivity at biopsy, n° (%) Monofocal Multifocal % of positive cores mean ±SD median (range) |
79 (59.3) 54 (40.7) 37.0 ±21.3 27.3 (2.0–70.0) |
30 (29.1) 73 (70.9) 28.9 ±21.5 29.5 (2.0–78.5) |
0.001 0.073 |
| D’Amico Risk class, n° (%) Low Intermediate High |
31 (23.3) 82 (61.6) 20 (15.1) |
18 (17.5) 68 (66.0) 17 (16.5) |
0.547 |
| Nerve-sparing RP, n° (%) No Monolateral Bilateral |
62 (46.6) 38 (28.5) 33 (24.9) |
40 (38.8) 33 (32.0) 30 (29.2) |
0.484 |
| PNI present at surgery, n° (%) | 99 (74.4) | 67 (65.0) | 0.215 |
| Pathologic stage, n° (%) pT2 pT3a pT3b pN+ |
65 (48.9) 49 (36.8) 19 (14.3) 7 (5.2) |
62 (60.1) 31 (30.1) 10 (9.8) 7 (6.8) |
0.182 |
| ISUP GG at surgery, n° (%) 1 2 3 4 5 |
26 (19.5) 62 (46.6) 27 (20.3) 9 (6.8) 9 (6.8) |
16 (15.5) 44 (42.7) 29 (28.2) 5 (4.9) 9 (8.7) |
0.577 |
| Surgical margins, n° (%) Negative Positive |
105 (75.9) 28 (21.0) |
67 (65.0) 36 (35.0) |
0.001 |
| Biochemical progression, n° (%) | 20 (15.0) | 12 (11.6) | 0.598 |
SD – standard deviation; LRP – laparoscopic radical prostatectomy; NS – nerve--sparing; RARP – robot-assisted radical prostatectomy; BMI – body mass index; PSA – prostate-specific antigen; mpMRI – multiparametric magnetic resonance imaging; PI-RADS – Prostate Imaging - Reporting and Data System; RP – radical prostatectomy; PNI – perineural invasion; ISUP – International Society of Urological Pathology; GG – grade group; n – number
Table 2.
Patient characteristics in the nerve-sparing procedures [number of cases (%), mean ±SD and median (range); p value: t test or Chi-squared test]
| Variable | LRP NS | RARP NS | p-value |
|---|---|---|---|
| Patients, n° (%) | 71 (53.0) | 63 (47.0) | – |
| Age (years) mean ±SD median (range) |
64.7 ±6.5 65 (48–74) |
63.9 ±6.8 64 (47–75) |
0.119 |
| BMI mean ±SD median (range) |
26.6 ±3.5 25.9 (17.0–34.0) |
26.6 ±3.6 25.9 (19.0–38.8) |
0.898 |
| Pre-operative PSA (ng/ml) mean ±SD median (range) |
7.1 ±3.1 6.6 (1.7–19.0) |
7.3 ±3.3 7.0 (2.8–17.0) |
0.544 |
| mpMRI PI-RADS, n° (%) n° of mpMRI performed 1–2 3 4–5 |
44 (62.0) 0 (0) 9 (20.5) 35 (79.5) |
53 (84.1) 2 (3.8) 9 (17.0) 42 (79.2) |
0.403 |
| mpMRI bulging, n° (%) | 6 (13.6) | 11 (21.6) | 0.077 |
| Clinical T stage, n° (%) cT1c cT2a cT2b cT2c cT3a |
36 (50.7) 12 (16.9) 9 (12.7) 8 (11.3) 6 (8.4) |
37(58.7) 4 (6.3) 3 (4.8) 8 (12.7) 11 (17.5) |
0.427 |
| Positivity at biopsy, n° (%) Monofocal Multifocal % of positive cores mean ±SD median (range) |
42 (59.1) 29 (40.9) 27.6 ±21.4 25 (2.0–78.5) |
22 (34.9) 41 (65.1) 32.2 ±21.2 25 (2.0–70.0) |
0.005 |
| D’Amico Risk class, n° (%) Low Intermediate High |
27 (38.1) 43 (60.5) 1 (1.4) |
18 (28.6) 44 (69.8) 1 (1.6) |
0.512 |
| Nerve-sparing RP, n° (%) Monolateral Bilateral |
38 (53.5) 33 (46.5) |
33 (52.4) 30 (47.6) |
0.895 |
| PNI present at surgery, n° (%) | 50 (70.4) | 31 (49.2) | 0.016 |
| Pathologic stage, n° (%) pT2 pT3a pT3b pN+ |
45 (63.4) 25 (32.2) 1 (1.4) 1 (1.4) |
48 (76.2) 13 (20.6) 2 (3.2) 1 (1.6) |
0.153 |
| ISUP grade at surgery, n° (%) 1 2 3 4 5 |
23 (32.4) 37 (52.1) 10 (14.1) 1 (1.4) 0 (0) |
16 (25.4) 37 (58.7) 9 (14.3) 1 (1.6) 0 (0) |
0.585 |
| Surgical margins, n° (%) Negative Positive pT2 pT3 Posterior-lateral Apex EPE, (mm) median (range) Grade 3 Grade 4 |
57 (80.3) 14 (19.7) 5/45 (11.1) 9/26 (34.6) 9/14 (64.3) 5/14 (35.7) 2.0 (1.0–5.0) 12/14 (85.7) 2/14 (14.3) |
43 (68.3) 20 (31.7) 12/48 (25.0) 8/15 (53.3) 14/20 (70.0) 6/20 (30.0) 2.0 (1.0–5.0) 17/20 (85.0) 3/20 (15.0) |
0.046 |
SD – standard deviation; LRP – laparoscopic radical prostatectomy; NS – nerve- -sparing; RARP – robot- assisted radical prostatectomy; BMI – body mass index; PSA – prostate-specific antigen; mpMRI – multiparametric magnetic resonance imaging; PI-RADS – Prostate Imaging - Reporting and Data System; RP – radical prostatectomy; PNI – perineural invasion; ISUP – International Society of Urological Pathology; EPE – extraprostatic extention; n – number
Differences in clinical and pathologic parameters between RARP-NS and LRP-NS
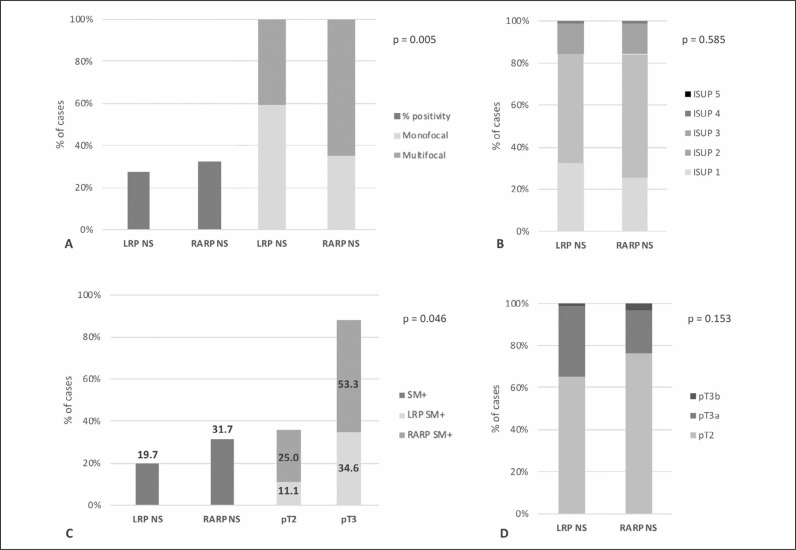
Clinical parameters, such as age and preoperative PSA, were not significantly different between the two groups (p >0.50), as well as the distribution of PC risk classes (p = 0.512). In cases who underwent mpMRI, the distribution of PI-RADS 3-5 scores was similar between the two groups (p = 0.403), but a higher percentage of capsular bulging was found in the RARP group (21.6%), when compared to the LRP group (13.6%) (p = 0.077). Although differences were not statistically significant, a higher percentage of clinical stage cT2a and cT2b in the LRP group, as well as a higher percentage of cT3a in the RARP group was present (p = 0.427) (Table 2). At biopsy, the percentage of positive cores and multifocality incidence were significantly higher in the RARP group (p = 0.005) (Table 2 and Figure 1A).
Figure 1.
Bar-chart graphs showing the percentage of patients in the LRP-NS and RARP-NS groups according to: A) prostatic biopsy results (monofocal versus multifocal and % of positive cores; chi-square = 0.005); B) pathological ISUP grading (chi-square = 0.585); C) surgical margins (SM), total and stratified on the basis of pathologic T stage (p = 0.046); D) pathologic T stage (chi-square = 0.153).
ISUP – International Society of Urological Pathology; LRP – laparoscopic radical prostatectomy; NS – nerve-sparing; RARP – robot-assisted radical prostatectomy
In all cases an intrafascial NS technique was performed, with a similar distribution of monolateral and bilateral procedures between LRP and RARP cases (p = 0.895) (Table 2).
The distribution of ISUP grading and pathological T (pT) stage were not significantly different between the two groups (p = 0.584 and 0.153, respectively) (Table 2, Figure 1B and 1D). Positive SM (SM+) rate was higher with RARP, when compared to LRP (31.7% versus 19.7%, respectively) (p = 0.046) (Table 2, Figure 1C). The site for SM+ was posterior-lateral in 70.0% and 64.3% of cases with RARP and LRP, respectively. EPE of SM+ was similar between the two groups [median 2 mm (range 15) in both groups]. Stratifying cases with SM+ by pT stage, in pT2 cases the percentage of SM+ was 11.1% and 25.0% using LRP and RARP, respectively, whereas in pT3 cases SM+ were present in 34.6% and 53.3% of cases, respectively (Table 2, Figure 1C). In both groups, a Gleason grade 3 was predominantly present in SM+ (85.7% and 85.0% in LRP and RARP groups, respectively).
Correlation among SM status or progression and the other clinical and pathologic parameters in NS procedures: oncologic outcomes at follow-up
Pearson correlation analysis showed a statistically significant correlation between SM status and surgical approach (r = 0.192; p = 0.013) or pT stage (r = 0.170; p = 0.025) (Table 5). No other significant correlations were found. BP was significantly correlated only with PNI status (r = -0.157; p = 0.040) (Table 5).
Table 5.
Correlation coefficients among surgical margin status or biochemical progression and the other parameters in nerve-sparing procedures (Pearson coefficient)
| Correlation | Coefficient | P-value |
|---|---|---|
| SM – Risk class | 0.041 | 0.321 |
| SM – mpMRI PI-RADS | 0.065 | 0.216 |
| SM – pathological stage | 0.170 | 0.025 |
| SM – pathological grading | 0.040 | 0.324 |
| SM – PNI | 0.057 | 0.257 |
| SM – surgical procedure (LRP/RARP) | 0.192 | 0.013 |
| SM – NS (mono or bilateral) | 0.024 | 0.393 |
| SM – % of biopsy positivity | 0.014 | 0.437 |
| BP – risk class | 0.027 | 0.383 |
| BP – mpMRI PI-RADS | 0.054 | 0.275 |
| BP – pathological stage | 0.089 | 0.161 |
| BP – pathological grading | 0.081 | 0.184 |
| BP – PNI | 0.157 | 0.040 |
| BP – surgical procedure (LRP/RARP) | 0.054 | 0.275 |
| BP – NS (mono or bilateral) | 0.078 | 0.223 |
| BP – biopsy positivity (%) | 0.037 | 0.339 |
SM – surgical margins; mpMRI – multiparametric magnetic resonance imaging; PI-RADS – Prostate Imaging - Reporting and Data System; PNI – perineural invasion; LRP – laparoscopic radical prostatectomy; RARP – robot-assisted radical prostatectomy; NS – nerve-sparing; BP – biochemical progression
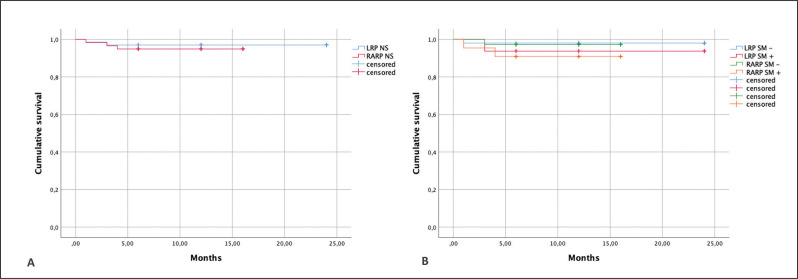
At a median follow-up of 12 months, BP was detected in 1.4% and 4.8% of cases in the LRP and RARP groups, respectively. Figure 2A depicts Kaplan-Meier curves on BP-free survival according to surgical procedure (Log rank-p = 0.552). Stratifying cases also by SM status, the RARP and SM+ group showed the lowest BP-free survival, although differences were not statistically significant (Log rank-p = 0.492) (Figure 2B).
Figure 2.
Biochemical progression-free survival. A) Kaplan Meier curves according to surgical procedure (LRP-NS versus RARP-NS). Log rank (Mantel-Cox): Chi-square = 0.353; p = 0.552. B) Kaplan-Meier curves according to surgical procedure (LRP-NS versus RARP-NS) stratified on the basis of surgical margins (SM) status (SM – versus SM +). Log rank (Mantel-Cox): Chi-square = 2.411; p = 0.492.
LRP – laparoscopic radical prostatectomy; NS – nerve-sparing; RARP – robot-assisted radical prostatectomy
Logistic regression analysis: predictors for SM status and progression at follow-up
Table 3 shows a logistic regression analysis carried out to identify predictors of SM+ in our population undergoing a NS procedure. On univariable analysis, the risk of SM+ increased 1.95 times using RARP when compared with LRP (95%CI 0.93–4.12). Similarly, the risk of SM+ increased 2.09 times in pT3a cases when compared to pT2 (95%CI 0.94–4.63). On multivariable analysis, the surgical approach did not show a significant independent predictive role in terms of risk for SM+ (p = 0.22).
Table 3.
Logistic regression analysis for the identification of predictors for positive surgical margins after surgery in nerve-sparing procedures
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Risk class low intermediate high |
1.0 1.15 0.48 |
– 0.53–2.52 0.01–20.97 |
– 0.72 0.70 |
1.0 2.44 14.63 |
– 0.75–7.91 0.01–189.13 |
– 0.14 0.84 |
| mpMRI PI–RADS 1–2 3 4–5 |
1.0 0.38 0.45 |
– 0.20–7.40 0.03–7.55 |
– 0.53 0.58 |
1.0 2.21 2.45 |
– 0.02–256.85 0.02–265.24 |
– 0.74 0.71 |
| Surgical procedure LRP RARP |
1.0 1.95 |
– 0.93–4.12 |
– 0.08 |
1.0 1.86 |
– 0.69–5.03 |
– 0.22 |
| NS procedure monolateral bilateral |
1.0 1.27 |
– 0.61–2.66 |
– 0.52 |
1.0 2.14 |
– 0.74–6.19 |
– 0.16 |
| Pathologic T stage pT2 pT3a pT3b |
1.0 2.09 1.44 |
– 0.94–4.63 0.12–16.57 |
– 0.07 0.77 |
1.0 2.60 1.75 |
– 0.86–7.81 0.12–24.59 |
– 0.09 0.68 |
| ISUP at surgery 1 2 3 4 5 |
1.0 1.20 0.94 0.83 7.44 |
– 0.52–2.80 0.28–3.19 0.01–80.49 0.08–724.61 |
– 0.67 0.92 0.93 0.39 |
1.0 1.11 1.03 1.63 17.41 |
– 0.32–3.86 0.20–5.36 0.01–191.91 0.02–14615.21 |
– 0.87 0.97 0.84 0.41 |
| PNI at surgery absent present |
1.0 0.89 |
– 0.42–1.88 |
– 0.76 |
1.0 1.04 |
– 0.37–2.94 |
– 0.94 |
OR – odds ratio; CI – confidence interval; mpMRI – multiparametric magnetic resonance imaging; PI-RADS – Prostate Imaging -Reporting and Data System; LRP – laparoscopic radical prostatectomy; RARP – robot-assisted radical prostatectomy; NS – nerve-sparing; ISUP – International Society of Urological Pathology; PNI – perineural invasion
Results from the self-administered questionnaire
Results of the self-administered questionnaire are described in Table 4. A high percentage of cases (67.9%) preferred a single approach (RARP) as surgical procedure. Overall, before surgery, the main expectation from RP was the oncologic radicality (64.2%). However, in the RARP group a higher percentage of cases described as first expectation a complete and rapid sexual function recovery (28.6% in RARP versus 9.9% in LRP, respectively). At the 1-month interval after surgery, a higher percentage of cases submitted to LRP believed that their main expectation was achieved (80.3% in LRP versus 69.8% in RARP, respectively). At the 6-month interval, the percentage of satisfied cases remained stable in the LRP group, whereas it slightly increased (74.6%) in the RARP group.
Table 4.
Preoperative and postoperative self-administered questionnaire completed by 134 cases (LRP – 71 cases; RARP – 63 cases) submitted to nerve-sparing procedures. Number of cases (%)
| 1. Do you prefer a single approach for your surgical procedure of radical prostatectomy? | |||||
| YES, LRP | YES, RARP | NO, I have not preferences | |||
| 0 (0) | 91 (67.9) | 43 (32.1) | |||
| 2. Which is your first expectation from this procedure of radical prostatectomy? | |||||
| Oncologic radicality | Rapid and complete urinary continence recovery | Rapid and complete sexual function recovery | |||
| Answers from all cases | 86 (64.2) | 23 (17.2) | 25 (18.6) | ||
| Answers from LRP cases | 51 (71.8) | 13 (18.3) | 7 (9.9) | ||
| Answers from RARP cases |
35 (55.5) |
10 (15.9) |
18 (28.6) |
||
| 3. At 1-month interval from radical prostatectomy do you believe that your main expectation has been successfully satisfied? | |||||
| YES | NO | ||||
| Answers from all cases | 97 (72.4) | 37 (27.6) | |||
| Answers from LRP cases | 57 (80.3) | 14 (19.7) | |||
| Answers from RARP cases | 44 (69.8) | 19 (30.2) | |||
| 4. At 6-month interval from radical prostatectomy do you believe that your main expectation has been successfully satisfied? | |||||
| YES | NO | ||||
| Answers from all cases | 105 (78.4) | 29 (21.6) | |||
| Answers from LRP cases | 60 (84.5) | 11 (15.5) | |||
| Answers from RARP cases | 47 (74.6) | 16 (25.4) | |||
LRP – laparoscopic radical prostatectomy; RARP – robot-assisted radical prostatectomy
DISCUSSION
Over the last decades RARP has gained widespread acceptance within urologic surgical practice. This technique aims to achieve less perioperative morbidity, less intraoperative bleeding and faster recovery time [21]. The expectation from RARP is also that this technique would allow a better preservation of neurovascular structures involved in erection [9, 11, 12, 22, 23, 24]. However, several systematic reviews, meta-analyses and randomized trials have demonstrated that the different approaches to RP have yielded similar oncologic and functional results [4, 5, 25–28]. Therefore, as of now no surgical approach can be recommended over another [17].
As underlined by some authors, treatment satisfaction is mainly derived from perceived differences between expectations and experience [18, 29]. Therefore, pretreatment patient information and counseling are crucial elements for the decision process. For patients selected to the RP procedures it is important to properly discuss preoperative expectations in order to positively influence postoperative satisfaction.
In the present analysis, we focused on cases selected for a NS procedure. The self-administered questionnaire revealed that a high percentage of cases showed a preoperative preference in terms of surgical approach, favoring RARP. Although patients’ main preoperative expectation was the oncologic radicality, in the RARP group a higher percentage of cases described as first expectation a complete and rapid sexual function recovery, when compared to the LRP group (28.6% versus 9.9%, respectively). This result highlights that patients who undergo RARP could have a higher expectation from this technique in terms of functional results. After surgery, the oncological results showed a higher SM+ rate with RARP, when compared to LRP (31.7% versus 19.7%, respectively). With regards to functional outcomes, at 6-month interval of follow-up the time and rate of urinary continence recovery were comparable in the two groups, whereas those for potency recovery were higher in the RARP group. Probably, the different expectation, more than differences in terms of oncological and function results between RARP versus VLRP groups, may lead to a different patient satisfaction after surgery; indeed, during the follow-up, a higher percentage of cases in the LRP group believed that their main expectation was satisfied, when compared to RARP groups. Schroeck et al., comparing open retropubic versus RARP, reported that 84% of cases were satisfied after treatment, yet cases undergoing RARP were more likely to be regretful, possibly due to the higher expectation from a robotic innovative procedure [18].
Since our study represents a prospective analysis based on real-life management of PC cases, surgical technique was not assigned randomly; each procedure was performed by a different surgeon with the most expertise in each approach, and a NS technique was performed at each individual surgeon’s discretion and after a discussion with the patient. We tried to define whether the indication for a NS RP significantly changed between the LRP and the RARP groups, and whether these differences could have an impact on SM status. Of note, the RARP group showed worse clinical oncologic characteristics, although differences were not always statistically significant. In particular, a higher percentage of capsular bulging at mpMRI, as well as a significantly higher percentage of positive cores and multifocality at biopsy were detected in the RARP group, when compared to the LRP group. Therefore, in our real-life setting, there was slight heterogeneity in the indication for a NS procedure, as demonstrated by the worse clinical oncologic conditions found in the RARP group, in comparison to LRP. Considering pathologic results at surgery, the distribution of pT stage and ISUP grading was similar between the two groups, whereas a significant difference was reported in terms of SM status (p = 0.046). SM+ rate was higher in the RARP-NS group than in the LRP-NS (31.7% versus 19.7%, respectively), whereas the site, EPE, and Gleason grade for SM+ were similar. SM positivity was higher with RARP, either in pT2 or in pT3 cases, however it did not result in a significantly higher incidence of BP at 12 months. On univariable analysis, the risk of SM+ increased 1.95 times using RARP when compared with LRP (95%CI 0.93–4.12), and 2.09 times from pT2 to pT3a cases (95%CI 0.94–4.63). However, on multivariable analysis, the surgical approach did not represent a significant independent predictor of risk for SM+. Villamil et al. in a single institution retrospective experience on 300 cases undergoing different approaches to RP, showed no statistically significant differences regarding SM+ when a NS procedure was performed, although percentages were higher with RARP (23.4%), compared to LRP (17.6%) [21]. Our study prospectively represents a real-life comparative non-randomized situation in a high-volume and high experience single center for the management of PC where either a laparoscopic or a robotic-assisted procedure is offered to patients. Limitations of our analysis are mainly due to the limited follow-up after surgery that affected the ability to obtain significant results also in terms of longer-term outcomes (i.e., biochemical and clinical recurrence).
CONCLUSIONS
When comparing LRP-NS to RARP-NS in a high-volume single center, the expectation/satisfaction ratio seems to be in favor of the former procedure. On the other hand, the type of procedure does not seem to have an independent impact on the risk of positive surgical margins.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Sciarra A, Voria G, Monti S, et al. Clinical understaging in patients with prostate adenocarcinoma submitted to radical prostatectomy: predictive value of serum chromogranin A. Prostate. 2004;58:421–428. doi: 10.1002/pros.10347. [DOI] [PubMed] [Google Scholar]
- 2.Sciarra A, Maggi M, Del Proposto A, et al. Impact of uni- or multifocal perineural invasion in prostate cancer at radical prostatectomy. Transl Androl Urol. 2021;10:66–76. doi: 10.21037/tau-20-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flammia S, Frisenda M, Maggi M, et al. Cribriform pattern does not have a significant impact in Gleason Score ≥7/ISUP Grade ≥2 prostate cancers submitted to radical prostatectomy. Medicine (Baltimore) 2020;99:e22156. doi: 10.1097/MD.0000000000022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novara G, Ficarra V, Mocellin S, et al. Systematic review and meta-analysis of studies reporting oncologic outcomes after robot-assisted radical prostatectomy. Eur Urol. 2012;62:382–404. doi: 10.1016/j.eururo.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 5.Ficarra V, Novara G, Artibani W, et al. Retropubic, laparoscopic and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–1063. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Maggi M, Gentilucci A, Salciccia S, et al. Psychological impact of different primary treatments for prostate cancer: A critical analysis. Andrologia. 2019;51:e13157. doi: 10.1111/and.13157. [DOI] [PubMed] [Google Scholar]
- 7.Rapoport L, Yossepowitch O, Shpot E, et al. Radical prostatectomy performed via robotic, transperitoneal and extraperitoneoscopic approaches: functional and early oncological outcomes. Cent European J Urol. 2018;71:378–385. doi: 10.5173/ceju.2018.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A, Gupta P, Kumar S, et al. 3-D transperitoneal laparascopic radical prostatectomy in locally advanced high-risk prostate cancer: a prospective evaluation. Cent European J Urol. 2019;72:218–219. doi: 10.5173/ceju.2019.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asimakopoulos A, Miano R, Di Lorenzo N, Spera E, Vespasiani G, Mugnier C. Laparoscopic versus robotic-assisted bilateral nerve-sparing radical prostatectomy: comparison of pentafecta rates for a single surgeon. Surg Endosc. 2013;27:4297–4304. doi: 10.1007/s00464-013-3046-9. [DOI] [PubMed] [Google Scholar]
- 10.Park B, Kim W, Jeong BC, et al. Comparison of oncological and functional outcomes of pure versus robotic-assisted laparoscopic radical prostatectomy performed by a single surgeon. Scand J Urol. 2013;47:10–18. doi: 10.3109/00365599.2012.696137. [DOI] [PubMed] [Google Scholar]
- 11.Ploussard G, de la Taille A, Moulin M, et al. Comparison of perioperative, functional and oncological outcomes after robot-assisted versus pure extraperitoneal laparoscopic radical prostatectomy. Eur Urol. 2014;65:610–619. doi: 10.1016/j.eururo.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 12.Ficarra V, Novara G, Fracalanza S, et al. A prospective non randomized trial comparing robot-assisted laparoscopic and retropubic radical prostatectomy in one European Institution. BJU. 2009;104:534–539. doi: 10.1111/j.1464-410X.2009.08419.x. [DOI] [PubMed] [Google Scholar]
- 13.Alessandro S, Alessandro G, Susanna C, et al. Laparoscopic versus open radical prostatectomy in high prostate volume cases: impact on oncological and functional results. Int Braz J Urol. 2016;42:223–233. doi: 10.1590/S1677-5538.IBJU.2015.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goßler C, May M, Rosenhammer B, et al. Obesity leads to a higher rate of positive surgical margins in the context of robot-assisted radical prostatectomy. Results of a prospective multicenter study. Cent European J Urol. 2020;73:457–465. doi: 10.5173/ceju.2020.0265.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kania P, Salagierski M, Mieleszko R, et al. Simplified single suture posterior reconstruction and modified urethrovesical anastomosis during 3D laparoscopic radical prostatectomy. Cent European J Urol. 2020;73:573–574. doi: 10.5173/ceju.2020.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kania P, Wośkowiak P, Salagierski M. Preservation of continence in radical prostatectomy patients: a laparoscopic surgeon's perspective. Cent European J Urol. 2019;72:32–38. doi: 10.5173/ceju.2019.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mottet N, van den Bergh RCN. Prostate Cancer: European Association of Urology (EAU) guidelines 2019. https://uroweb.org/guideline/prostate-cancer.
- 18.Schroeck FR, Krupski TL, Sun L, et al. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;54:785–793. doi: 10.1016/j.eururo.2008.06.063. [DOI] [PubMed] [Google Scholar]
- 19.Sciarra A, Panebianco V, Cattarino S, et al. Multiparametric Magnetic Resonance Imaging of the Prostate Can Improve the Predictive Value of the Urinary Prostate Cancer Antigen 3 Test in Patients With Elevated Prostate-Specific Antigen Levels and a Previous Negative Biopsy. BJU Int. 2012;110:1661–1665. doi: 10.1111/j.1464-410X.2012.11146.x. [DOI] [PubMed] [Google Scholar]
- 20.Maggi M, Panebianco V, Mosca A, et al. Prostate Imaging Reporting and Data System 3 Category Cases at Multiparametric Magnetic Resonance for Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol Focus. 2020;6:463–478. doi: 10.1016/j.euf.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 21.Villamil W, Billordo Peres N, Martinez P, et al. Incidence and location of positive surgical margins following open, pure laparoscopic and robotic assisted radical prostatectomy and its relation with neurovascular preservation: a single institution experience. J Robot Surg. 2013;7:21–27. doi: 10.1007/s11701-012-0335-6. [DOI] [PubMed] [Google Scholar]
- 22.Allan C, Ilic D. Laparoscopic verus robotic-assisted radical prostatectomy for the treatment of localized prostate cancer: a systematic review. Urol Int. 2016;96:373–378. doi: 10.1159/000435861. [DOI] [PubMed] [Google Scholar]
- 23.Coelho RF, Rocco B, Patel MB, et al. Retropubic, laparoscopic and robot-assisted radical prostatectomy: a critical review of outcomes reported by high-volume centers. J Endourol. 2010;24:2003–2015. doi: 10.1089/end.2010.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haglind E, Carlsson S, Stranne J, et al. Urinary incontinence and erectile dysfunction after robotic versus open radical prostatectomy: a prospective controlled nonrandomized trial. Eur Urol. 2015;68:216–225. doi: 10.1016/j.eururo.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 25.Dell’Oglio P, Mottrie A, Mazzone E. Robot-assisted radical prostatectomy versus open radical prostatectomy: latest evidences on perioperative functional and oncological outcomes. Curr Opin Urol. 2019;30:73–78. doi: 10.1097/MOU.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 26.Ilic D, Evans SM, Allan CA, Jung JH, Murphy D, Frydenberg M. Laparoscopic and robotic-assisted versus open radical prostatectomy for the treatment of localized prostate cancer: a Cochrane systematic review. BJU. 2017;121:845–853. doi: 10.1111/bju.14062. [DOI] [PubMed] [Google Scholar]
- 27.Yaxley JW, Coughlin GD, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomized controlled phase 3 study. Lancet. 2016;388:1057–1066. doi: 10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
- 28.Coughlin GD, Yaxley JW, Chambers SK, et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomized controlled study. Lancet. 2018;19:1051–1060. doi: 10.1016/S1470-2045(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 29.Bock D, Angenete E, Bjartell A, et al. Agreement between patient reported outcomes and clinical reports after radical prostatectomy - a prospective longitudinal study. BMC Urol. 2019;19:1–10. doi: 10.1186/s12894-019-0467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]