Abstract
Introduction
The aim of this study was to assess the safety of elective urological surgery performed during the pandemic by estimating the prevalence of COVID-19-like symptoms in the postoperative period and its correlation with perioperative and clinical factors.
Material and methods
In this multicenter, observational study we recorded clinical, surgical and postoperative data of consecutive patients undergoing elective urological surgery in 28 different institutions across Italy during initial stage of the COVID-19 pandemic (between February 24 and March 30, 2020, inclusive).
Results
A total of 1943 patients were enrolled. In 12%, 7.1%, 21.3%, 56.7% and 2.6% of cases an open, laparoscopic, robotic, endoscopic or percutaneous surgical approach was performed, respectively. Overall, 166 (8.5%) postoperative complications were registered, 77 (3.9%) surgical and 89 (4.6%) medical. Twenty-eight (1.4%) patients were readmitted to hospital after discharge and 13 (0.7%) died. In the 30 days following discharge, fever and respiratory symptoms were recorded in 101 (5.2%) and 60 (3.1%) patients. At multivariable analysis, not performing nasopharyngeal swab at hospital admission (HR 2.3; CI 95% 1.01–5.19; p = 0.04) was independently associated with risk of developing postoperative medical complications. Number of patients in the facility was confirmed as an independent predictor of experiencing postoperative respiratory symptoms (p = 0.047, HR:1.12; CI95% 1.00–1.05), while COVID-19-free type of hospitalization facility was a strong independent protective factor (p = 0.02, HR:0.23, CI95% 0.07–0.79).
Conclusions
Performing elective surgery during the COVID-19 pandemic does not seem to affect perioperative outcomes as long as proper preventive measures are adopted, including nasopharyngeal swab before hospital admission and hospitalization in dedicated COVID-19-free facilities.
Keywords: coronavirus, COVID-19, infection, outcome, surgery, urology
INTRODUCTION
A pneumonia of undefined etiology, first detected in Wuhan, China, was reported by the World Health Organization (WHO) Country Office on 31 December 2019 [1]. Soon thereafter a new coronavirus was identified as the causative agent and the International Committee on Taxonomy of Viruses named the virus as ‘acute severe respiratory syndrome coronavirus 2’ (SARS-CoV-2) with the related respiratory corona virus disease (COVID-19) [2].
The COVID-19 pandemic has unequivocally brought unique challenges to the global healthcare community [3, 4, 5]. Public health guidance tried to face the overwhelming impact of the virus using the best available scientific evidence to properly inform and guide decision makers involved in the management of the pandemic [6]. Most eminent international scientific committees released recommendations for prioritizing urgent and time-sensitive surgical procedures, despite having limited and flawed supporting evidence. As a result, surgical departments have thoughtfully reviewed all scheduled procedures to minimize or postpone elective surgery so that the health care centres could eventually support a rapid increase in critical patient care needs [7–10].
We currently acknowledge paucity of data regarding perioperative outcomes of patients undergoing elective surgery during the COVID-19 pandemic [11, 12]. Indeed, the progressively growing need of assistance for COVID-19 patients will lead to fewer resources and personnel for patients seeking care for other conditions [13, 14]. Moreover, in the hypothesis of a further reduction of elective surgical activity, ethical concerns would be raised especially in the setting of oncological surgery [15–19]. Last but not least, assuming a long-lasting pandemic, we truly need strong evidence to assess safety of performing surgical procedures also for benign pathology [20].
In this light, a better understanding of the real impact of the pandemic on urological surgery and the associated perioperative outcomes represents a key unmet need and could lead towards an in-depth knowledge of the pandemic for a proper rationalization of human and logistic resources. Furthermore, we do believe our findings might eventually be transposed to other surgical specialties.
To address these needs, we retrospectively reviewed our prospectively collected clinical and surgical data of patients undergoing different types of elective urological procedures during the COVID-19 pandemic in 28 different institutions across Italy. The aim of this study was to assess the safety of elective surgery performed during the pandemic by estimating the prevalence of COVID-19-like symptoms in the postoperative period and its correlation with perioperative and clinical factors.
MATERIAL AND METHODS
Patients, dataset and selection
The AGILE group (www.agilegroup.it) is a multicenter non-profit consortium with scientific, educational and training purposes, grouping several urologists affiliated to different departments and clinics across Italy. All the centres of the consortium were asked to record and submit clinical, surgical and postoperative data with regards to patients undergoing elective urological procedures at their respective institutions between the 24th of February 2020 and the 30th of March 2020. Written informed consent was obtained from all patients before surgery. The accrual period was set basing on the first case of laboratory-confirmed COVID-19 in Italy recorded on the 21st of February, and lasted approximately one month, in order to have a picture of surgical activity performed before protocols to adequately triage patients were effectively established.
Inclusion criteria at baseline were: 1) age ≥ 18 years; 2) patients scheduled for standard open procedures, conventional laparoscopy or robotic surgery, as well as endourological procedures. COVID-19 triage through serology and/or nasopharyngeal swab was progressively introduced during the study period at different times among the various institutions, according to respective regional indications. Such data were recorded and considered in the analysis. In addition, COVID-19-like symptoms as dry cough and/or persistent fever (>3 days) within 2 months before hospitalization were investigated. Patients with non-time-sensitive urological conditions and/or laboratory confirmed COVID-19 infection were excluded.
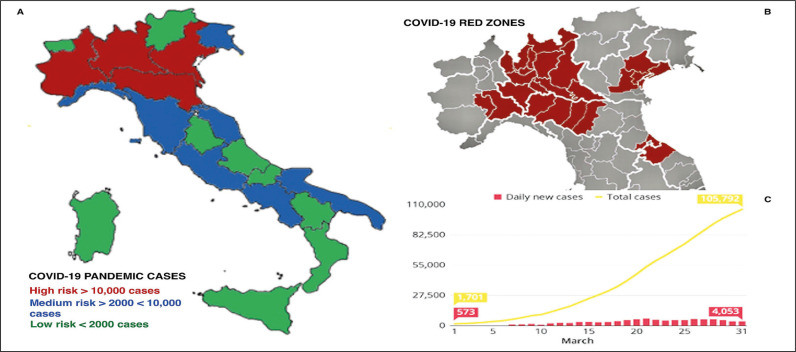
Facility was defined as any location of the included centres where healthcare was provided. Risk of contracting COVID-19 was further stratified according to patients’ geographical provenance (districts at higher disease prevalence were defined as ‘COVID-19 red zones’) as well as Italian region where surgical procedure was performed (defined as ‘risk hospital areas’). The geographical distribution of risk hospital areas and COVID-19 red zones is provided in Figure 1A and Figure 1B.
Figure 1.
A) Distribution of Italian risk areas for COVID-19 according to pandemic total cases per region in the reference timeframe. B) Distribution of COVID-19 Italian red zones; C) Italian COVID-19 daily new cases and total cases in March 2020.
Follow-up
Starting from two weeks after hospital discharge, all patients were contacted by phone to appraise whether they had developed fever and/or respiratory symptoms such as rhinitis, cough, sore throat or pneumonia during the postoperative period. Possible contacts with confirmed positive COVID-19 subjects and eventual execution of nasopharyngeal swab after discharge were also recorded.
Outcomes
The main outcomes were to estimate the prevalence of respiratory symptoms during postoperative period of patients undergoing urological elective surgery and its correlation with perioperative, geographical and clinical factors. Patients were thoroughly stratified according to preoperative and surgical features, including age, gender, geographic region, baseline comorbidities and type of surgery. Moreover, we aimed to compare asymptomatic with symptomatic patients during the postoperative period with regards to postoperative complications rate, readmission rate after discharge and mortality. Finally, geographical, clinical and surgical predictors of postoperative medical complications and respiratory symptoms were explored.
Statistical analysis
Descriptive statistics were obtained reporting medians (and interquartile ranges, IQR) for continuous variables, and frequencies and proportions for categorical variables, as appropriate. Continuous variables were compared by the Student t or the Mann-Whitney U test based on their normal or not-normal distribution, respectively (normality of variables’ distribution was tested by the Kolmogorov-Smirnov test). Categorical variables were tested with the Chi-square test. Uni- and multivariable logistic and Cox regression analyses were performed to identify predictors of postoperative respiratory symptoms and postoperative medical complications. All tests were two-sided with a significance set at p <0.05. Statistical analyses were performed using SPSS v. 24 (IBM SPSS Statistics for Mac, Armonk, NY, IBM Corp).
RESULTS
Patients characteristics and baseline features
Overall, 1943 patients were enrolled. Baseline characteristics of the entire cohort are reported in Table 1. The median age was 67 (IQR 58-74) years, median American Society of Anesthesiologists (ASA) and Charlson Comorbidity Index (CCI) score were 2 (IQR 2-3) and 3 (IQR 1-4), respectively. A urological surgical procedure was carried out due to malignant disease in 65.2% of cases.
Table 1.
Preoperative and surgical features of 1943 patients undergoing elective urological procedures in 28 different Italian centres between the 24th of February and the 30th of March 2020
| Preoperative features | |
|---|---|
| Age, median (IQR) | 67 (58–74) |
| BMI, median (IQR) | 25.6 (23.5–27.7) |
| ASA score, median (IQR) | 2 (2–3) |
| Charlson Comorbidity Index, median (IQR) | 3 (1–4) |
| Barthel Index, median (IQR) | 100 (96–100) |
| Race or ethnicity, n. (%) White/Caucasian Hispanic Black/African American Asian |
1908 (98.2%) 2 (0.1%) 23 (1.2%) 10 (0.5%) |
| Provenance from ‘COVID-19 red zones’, n. (%) No Yes |
1399 (72%) 544 (28%) |
| Risk hospital area, n (%) Low Medium High |
166 (8.5%) 891 (45.9%) 866 (44.6%) |
| History of previous neoplasm, n. (%) No Yes |
638 (32,8%) 1305 (67.2%) |
| Dry cough in the 60 days prior to hospitalization, n. (%) No Yes |
1889 (97.2%) 54 (2.8%) |
| Fever for more than 3 days in the 60 days prior to hospitalization, n. (%) No Yes |
1910 (98.3%) 33 (1.7%) |
| IgM testing in the 60 days prior to hospitalization, n. (%) No Yes |
1844 (94.9%) 99 (5.1%) |
| IgG testing in the 60 days prior to hospitalization, n. (%) No Yes |
1832 (94.3%) 111 (5.7%) |
| Nasopharyngeal swab in the 60 days prior to hospitalization, n. (%) No Yes |
1937 (99.7%) 6 (0.3%) |
| Nasopharyngeal swab at hospitalization, n. (%) No Yes |
1805 (92.9%) 138 (7.1%) |
| Preoperative haemoglobin (mg/dL), median (IQR) | 13.9 (12.7–15.1) |
| Preoperative creatinine serum level (mg/dL), median (IQR) | 0.96 (0.8–1.16) |
| Surgical indication, n (%) Benign condition Malignant condition |
676 (34.8%) 1267 (65.2%) |
n – number; IQR- interquartile range; BMI – body mass index; ASA – American Society of Anesthesiologists; IgM – immunoglobulin-M; IgG – immunoglobulin-G
Overall, 544 (28%) patients came from ‘COVID-19 red zones’. In the two months preceding hospitalization, 54 (2.8%) and 33 (1.7%) patients reported dry cough and persistent fever (>3 days), while COVID-19 specific IgM and IgG screening was performed in 5.1% and 5.7% of cases respectively. In 138 (7.1%) patients, nasopharyngeal swab was performed before hospitalization.
Surgical features and surgical complications
The surgical and postoperative features are shown in Table 2 and Table 3. In 12%, 7.1%, 21.3%, 56.7% and 2.6% of cases an open, laparoscopic, robotic, endoscopic or percutaneous surgical approach was performed, respectively. The most common surgical procedure performed was transurethral resection of bladder tumour (TURBT) in 29.4% of cases. Intraoperative medical and surgical complications were recorded in 5 (0.3%) and 15 (0.8%) cases, respectively. Overall, 166 (8.5%) postoperative complications were registered, 77 (3.9%) surgical and 89 (4.6%) medical. A laboratory confirmed COVID-19 infection during hospitalization was recorded in 10 (0.5%) patients.
Table 2.
Intraoperative features of 1943 patients undergoing elective urological procedures in 28 different Italian centres between the 24th of February and the 30th of March 2020
| Perioperative features | |
|---|---|
| Surgical approach, n (%) Open Laparoscopic Robotic Endoscopic Percutaneous |
234 (12%) 137 (7.1%) 414 (21.3%) 1107 (56.7%) 51 (2.6%) |
| Surgical procedure, n (%) Radical prostatectomy Radical prostatectomy and lymph node dissection Radical nephrectomy Partial nephrectomy Cytoreductive nephrectomy Complex radical nephrectomy (i.e. plus lymph node dissection, venous neoplastic thrombosis, etc.) Pyeloplasty Partial ureterectomy and/or ureteral reimplantation Radical cystectomy Orchifuniculectomy Retroperitoneal lymph node dissection TURBT TURP Ureteroscopy + lithotripsy Diagnostic ureteroscopy Ureteral stenting Nephrostomy placement PCNL Other |
121 (6.2%) 179 (9.2%) 90 (4.6%) 123 (6.3%) 4 (0.2%) 16 (0.8%) 19 (1%) 12 (0.6%) 78 (4%) 22 (1.1%) 2 (0.1%) 570 (29.4%) 96 (5%) 166 (8.6%) 31 (1.6%) 61 (3.2%) 16 (0.8%) 35 (1.8%) 302 (15.5%) |
| EBL (cc), median (IQR)* | 200 (100-300) |
| Intraoperative medical complications, n. (%) No Yes |
1938 (99.7%) 5 (0.3%) |
| Intraoperative surgical complications, n. (%) No Yes |
1935 (99.2%) 15 (0.8%) |
n – number; TURBT – transurethral resection of bladder tumor; TURP – transurethral resection of the prostate; PCNL – percutaneous nephrolithotomy; EBL – estimated blood loss
EBL was calculated only for open, laparoscopic and robotic procedures
Table 3.
Postoperative features of 1943 patients undergoing elective urological procedures in 28 different Italian centres between the 24th of February 2020 and the 30th of March 2020
| Postoperative features | |
|---|---|
| Postoperative complications, n. (%) | 166 (8.5%) |
| Surgical complications, n. (%) Clavien 1 surgical complications, n. Clavien 2 surgical complications, n. Clavien 3 surgical complications, n. Clavien 4 surgical complications, n. Clavien 5 surgical complications, n. |
77 (3.9%) 26 32 18 0 1 |
| Medical complications, n. (%) Fever, n. UTI, n. Laboratory confirmed COVID-19 infections Acute renal failure, n. Respiratory complications, n. Vascular complications, n. Gastrointestinal disorders, n. Cardiologic complications, n. Metabolic disorders Delirium, n. |
89 (4.6%) 26 12 10 9 8 7 6 6 3 2 |
| Length of stay (days), median (IQR) | 3 (1-5) |
| Follow-up | |
| Rehospitalization, n (%) | 28 (1.4%) |
| Time to rehospitalization (days), median (IQR) | 11 (IQR 6–19) |
| Fever in the 30 days following discharge, n (%) | 101 (5.2%) |
| Respiratory symptoms in the 30 days following discharge, n (%) | 60 (3.1%) |
| Confirmed contact with people presenting respiratory symptoms | 68 (3.5%) |
| Confirmed contact with people infected with COVID-19 | 21 (1.1%) |
| Nasopharyngeal swab in the 30 days following discharge, n (%) Not performed Negative Positive |
1888 (97.2%) 49 (2.5%) 6 (0.3%) |
| Overall mortality, n (%) | 13 (0.7%) |
n – number; UTI – urinary tract infection; IQR – interquartile range
Follow-up
Overall, 28 (1.4%) patients were readmitted to hospital after discharge and 13 (0.7%) died. Median time to rehospitalization was 11 (IQR 6-19) days. In the 30 days following discharge, fever and respiratory symptoms were recorded in 101 (5.2%) and 60 (3.1%) patients, while 68 (3.5%) and 21 (1.1%) declared contact with people presenting respiratory symptoms or infected with COVID-19, respectively. Nasopharyngeal swab after discharge was performed in 55 (2.8%) patients, being positive in 6 of them. An insight into patients experiencing hospital readmission and death is provided in Table 5 and Table 6. A significant correlation was found between experiencing fever as well as respiratory symptoms at discharge and readmission (p = 0.01) and mortality (p = 0.001) rates.
Table 5.
A deep insight exploring correlation with geographical, clinical features in patients experiencing hospital readmission and death
| Readmission (n = 28) | p value | Mortality (n = 13) | p value | |
|---|---|---|---|---|
| Provenance from ‘COVID-19 red zones’, n. (%) No Yes |
25 (89.3%) 3 (10.7%) |
0.02 |
4 (30.8%) 9 (69.2%) |
0.001 |
| Risk hospital area Low Medium High |
3 (10.7%) 12 (42.9%) 13 (46.4%) |
0.41 |
0 (0%) 3 (23.1%) 10 (76.9%) |
0.02 |
| Admission to COVID-19-free facility, n. (%) No Yes |
18 (64.2%) 10 (35.8%) |
0.36 |
7 (53.8%) 6 (46.2%) |
0.20 |
| Nasopharyngeal swab at hospitalization, n. (%) No Yes |
24 (85.7%) 4 (14.3%) |
0.01 |
12 (92.3%) 1 (7.7%) |
0.01 |
| Postoperative surgical complications, n. (%) No Yes |
21 (75%) 7 (25%) |
0.001 |
11 (84.6%) 2 (15.4%) |
0.03 |
| Postoperative medical complications, n. (%) No Yes |
19 (67.9%) 9 (32.1%) |
0.01 |
8 (61.5%) 5 (38.5%) |
0.01 |
| Fever in the 30 days following discharge, n (%) No Yes |
17 (60.7%) 11 (39.3%) |
0.001 |
9 (69.2%) 4 (30.8%) |
0.001 |
| Respiratory symptoms in the 30 days following discharge, n (%) No Yes |
23 (82.1%) 5 (17.9%) |
0.01 |
8 (61.5%) 5 (38.5%) |
0.001 |
n – number
Table 6.
Geographical, clinical and surgical details of patients experiencing postoperative death
| Age | Risk region | COVID-19-free facility | Nasopharyngeal swab before admission | Surgical procedure | Surgical approach | Cause of death | Postoperative nasopharyngeal swab |
|---|---|---|---|---|---|---|---|
| 70 | High | Yes | No | URS | Endoscopic | AMI | Positive |
| 69 | Medium | Yes | Yes | Cytoreductive nephrectomy | Open | Pneumonia | Not performed |
| 89 | Medium | No | No | TURBT | Endoscopic | Unknown | Not performed |
| 70 | Medium | Yes | No | Radical cystectomy | Open | Sepsis | Negative |
| 75 | High | No | No | Radical nephrectomy | Laparoscopic | Sarcoma end stage | Not performed |
| 43 | High | No | No | Percutaneous nephrostomy | Percutaneous | Malignant mammary tumor | Not performed |
| 81 | High | No | No | TURBT | Endoscopic | Pneumonia | Positive |
| 82 | High | No | No | Double J ureteral stenting | Endoscopic | Pneumonia | Positive |
| 82 | High | No | No | TURBT | Endoscopic | Pneumonia | Not performed |
| 79 | High | No | No | Radical nephrectomy | Open | Peritonitis and MOF secondary to duodenal lesion | Not performed |
| 85 | High | Yes | No | Double J ureteral stenting | Endoscopic | Unknown | Not performed |
| 79 | High | Yes | No | Radical nephrectomy | Laparoscopic | Gastrointestinal bleeding | Positive |
| 73 | High | Yes | No | Radical cystectomy | Robotic | Pneumonia | Positive |
AMI – acute myocardial infarction; MOF: – multiorgan failure, TURBT – transurethral resection of bladder tumor; URS – ureterorenoscopy
Stratification by risk hospital areas
Overall, 166 (8.5%), 891 (45.9%) and 866 (44.6%) patients were treated in a low, medium and high-hospital risk area, respectively. Among them, 65.3%, 90.9% and 54.3% were hospitalized in a COVID-free facility (p <0.001), while 0%, 2.2% and 53.2% came from ‘COVID-19 red zones’ (p <0.001), and 72.7%, 68% and 61.1% were hospitalized due to malignant disease (p = 0.001), respectively. A significantly lower median number of patients hospitalized in the same facility was found in low-risk areas, as compared to median and high-risk (70 vs 100 vs 120; p <0.001).
Predictors of postoperative medical complications
A significantly higher rate of perioperative medical complications was found in patients who had not performed nasopharyngeal swab at hospital admission (p = 0.003) and those treated in high-risk areas (6.3% vs 5.4% vs 3.6%; p = 0.01). No association was found between type of surgery and perioperative surgical or medical complications. At multivariable analysis after adjusting for age, gender, body mass index (BMI), comorbidity burden, type of pathology (benign vs. neoplastic), number of patients hospitalized in the same facility, patients’ geographical provenance and risk hospital area, not performing nasopharyngeal swab at hospital admission was independently associated to risk of developing postoperative medical complications (HR 2.3; CI 95% 1.01–5.19; p = 0.04) (Table 4).
Table 4.
Multivariable analysis investigating clinical and geographical predictors of postoperative medical complications and postoperative respiratory symptoms
| Covariates | Postoperative medical complications | Postoperative respiratory symptoms | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age at surgery | 0.99 | 0.96–1.04 | 0.85 | 1.02 | 0.97–1.08 | 0.12 |
| Gender Male Female |
3.94 ref |
0.52–12.16 ref |
0.38 ref |
2.14 ref |
0.72–5.69 ref |
0.15 ref |
| BMI | 1.01 | 0.95–1.08 | 0.77 | – | – | – |
| Charlson Comorbidity Index | 1.14 | 1.01–1.12 | 0.02 | 1.06 | 1.01–1.23 | 0.01 |
| Risk hospital area High Medium Low |
2.87 1.83 ref |
1.94–4.27 0.98–2.64 ref |
0.03 0.10 ref |
3.82 2.22 ref |
0.97–4.14 1.16–5.57 ref |
0.14 0.29 ref |
| Patients’ geographic provenance from ‘COVID-19 red zones’ Yes No |
1.43 ref |
1.18–6.22 ref |
0.42 ref |
0.76 ref |
0.10–6.34 ref |
0.80 ref |
| COVID-19-free facility Yes No |
0.74 ref |
0.22–1.23 ref |
0.21 ref |
0.23 ref |
0.07–0.79 ref |
0.02 ref |
| Number of patients hospitalized in the same facility | 1.65 | 0.67–4.01 | 0.28 | 1.12 | 1.00–1–05 | 0.047 |
| Nasopharyngeal swab before admission No Yes |
2.30 ref |
1.01–5.19 ref |
0.04 ref |
– – |
– – |
– – |
| Type of pathology treated Neoplastic Benign |
1.16 ref |
0.19–7.09 ref |
0.22 ref |
0.90 ref |
0.27–2.98 ref |
0.87 ref |
CI – confidence interval; BMI – body mass index
Predictors of postoperative respiratory symptoms
Univariable analysis showed a statistically significant association between respiratory symptoms and coming from ‘COVID-19 red zones’ (p = 0.016), setting of hospitalization (p = 0.03), number of patients in the facility (p <0.001), and longer length of stay (p = 0.01). At multivariable analysis, number of patients in the facility was confirmed as an independent predictor of experiencing postoperative respiratory symptoms (p = 0.047, HR:1.12; CI95% 1.00–1.05), while hospitalization in a COVID-19 free facility was a strong independent protective factor (p = 0.02, HR:0.23, CI95% 0.07–0.79) (Table 4).
DISCUSSION
The current COVID-19 pandemic has critically underlined the importance of a mindful employment of financial and human resources. Indeed, after the outbreak of the novel coronavirus in China, Europe and the USA soon thereafter were hit with the highest vehemence, emphasizing the shortage of available qualified professionals and assistance facilities. If on one hand preserving resources and manpower is paramount in healthcare, likewise on the other it is pivotal to ensure the ability of surgeons and specialized professionals to keep providing the highest assistance through the pandemic [21, 22, 23]. Several opinion leaders worldwide have proposed recommendations for the triage of elective urologic surgeries [24–29]. However, we must point out that many ‘elective’ procedures are not truly elective but only scheduled and not only medically necessary, are time-sensitive. Furthermore, in the hypothesis of a long-lasting pandemic, we acknowledge a lack of non-biased evidence to support the feasibility and safety of resuming elective surgery also for benign disorders. The exact burden of COVID-19 on perioperative outcomes after different urological procedures is still poorly investigated. In our opinion, the exact assessment of COVID-19 impact and its correlation with perioperative and clinical factors represents the first step towards a more comprehensive appraisal of the trustworthiness of recent recommendations and how those may affect every day urologic practice. To fill this gap, herein we provided a real-life overview of the Italian situation during the COVID-19 pandemic.
The first key finding of our study is that performing elective surgery during the COVID-19 pandemic is safe as long as proper preventive measures are adopted. This is a paramount finding, since high complication rates and risks have been hypothesized for surgical procedures performed during the pandemic. In this regard, in line with our findings, Soytas et al. [10] recently reported that urological procedures applied with appropriate infrastructure and protocols during the pandemic can be safely performed. Indeed, setting of hospitalization now more than ever can meaningfully affect postoperative outcomes [30]. In our experience, the number of patients hospitalized and the non-COVID-19-free type of facility were independently associated with the onset of postoperative respiratory symptoms. Considering the unfeasibility of performing a control swab to every subject developing respiratory symptoms after discharge, we could not ascertain the COVID-19 origin of symptoms. However, we could speculate that patients developing postoperative respiratory symptoms might have been unintentionally scheduled for surgery during their incubation period or have contracted the infection during their hospital stay. This result strongly underlines the need to set specific areas for the management of patients not affected by COVID-19, by identifying facilities capable of providing higher assistance in compliance with the safety for both patients and health care providers.
A second key point of our study is that not performing nasopharyngeal swab at hospital admission was confirmed as an independent predictor of developing postoperative medical complications. Indeed, nowadays an indispensable condition for the patient to be candidate for surgical treatment is being tested with nasopharyngeal swab for COVID-19 before entering the facility. However, how and how long before admission is meaningfully changeable, being extremely depending on the internal logistics of the hospital and the time needed to obtain the result of the swab. We truly believe that such precautionary measures nowadays represent the mainstay for disease control and should be thoroughly pursued whenever any kind of elective surgery is performed.
Third, it is reasonable to assume that COVID-19 might have influenced peri- and postoperative outcomes. Lei et al. suggested that surgical stress can exacerbate disease progression and severity in patients recently undergoing surgery [11, 31]. Since less than 10% of our cohort was tested with nasopharyngeal swab before admission, we cannot precisely establish the percentage of patients in their incubation period at the time of surgery and those who have contracted the infection during hospital stay. However, most importantly, herein we provided a comparison between asymptomatic patients and those developing postoperative respiratory symptoms after surgery, showing a significant correlation between experiencing fever and respiratory symptoms at discharge and readmission and mortality rates.
The current study was not devoid of several limitations. Only a limited percentage of patients underwent the specific COVID-19 confirmation test before surgery, due to the limited understanding of the pandemic situation and the shortage of laboratory kits during the study period. Similarly, a non-negligible rate of patients experiencing respiratory symptoms after discharge did not perform nasopharyngeal swab to confirm COVID-19 infection. Of course, we should consider also an undefined percentage of patients who have contracted COVID-19 during their hospital stay, but were completely asymptomatic and, thus, hardly detectable. All these issues may have introduced statistical bias, undermining trustworthiness of reported results.
Acknowledging these limitations, the current study represents the largest series thus far exploring safety of urologic elective surgery during the COVID-19 pandemic. Restructuring of the medical system represents only the first step to keep providing the highest level of care to all patients. Indeed, assuming a possible worsening of the global situation and, thus, in theory a further reduction of elective surgical activity, additional concerns would be raised, also from an ethical perspective, especially in the setting of oncologic surgery [32, 33]. Certainly, our findings could pave the way to unexplored scenarios, where resuming elective surgery does represent a feasible option also in the setting of benign pathology, as long as proper, precautionary measures are observed.
CONCLUSIONS
In our experience, performing elective surgery during the COVID-19 pandemic was safe and showed no detrimental impact on perioperative outcomes, as long as adequate, preventive measures were pursued. We hope these data might be of value for healthcare professionals and decision makers involved in the management of the COVID-19 pandemic. In the meantime, under elective conditions, we should pursue the concept of prioritizing the most urgent surgical cases and, after careful evaluation of each individual case, provide adequate preventive measures for both patients and health care providers.
CONFLICT OF INTEREST STATEMENT
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
STATEMENT OF ETHICS
Informed consent was obtained from all individual participants included in the study. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.World Health Organization (WHO) Novel Coronavirus (2019-NCoV) Situation Report. 21 January 2020. Data as reported by: 20 January 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf.
- 2.World Health Organization . Naming the coronavirus disease (COVID-19) and the virus that causes it. World Heal Organ; 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. [Google Scholar]
- 3.Hartley DM, Perencevich EN. Public Health Interventions for COVID-19: Emerging Evidence and Implications for an Evolving Public Health Crisis. JAMA. 2020;323:1908–1909. doi: 10.1001/jama.2020.5910. [DOI] [PubMed] [Google Scholar]
- 4.Autrán-Gómez AM, Favorito LA. The Social, Economic and Sanitary Impact of COVID-19 Pandemic. Int Braz J Urol. 2020;46(Suppl 1):3–5. doi: 10.1590/S1677-5538.IBJU.2020.S1ED2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milone M, Carrano FM, Letić E, et al. Surgical challenges and research priorities in the era of the COVID-19 pandemic: EAES membership survey. Surg Endosc. 2020;34:4225–4232. doi: 10.1007/s00464-020-07835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heymann DL, Shindo N. COVID-19: what is next for public health? Lancet. 2020;395:542–545. doi: 10.1016/S0140-6736(20)30374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarrintan S. Surgical operations during the COVID-19 outbreak: Should elective surgeries be suspended? Int J Surg. 2020;78:5–6. doi: 10.1016/j.ijsu.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dotzauer R, Böhm K, Brandt MP, et al. Global change of surgical and oncological clinical practice in urology during early COVID-19 pandemic. World J Urol. 2020 doi: 10.1007/s00345-020-03333-6. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzucchi E, Torricelli FCM, Vicentini FC, et al. The impact of COVID-19 in medical practice. A review focused on Urology. Int Braz J Urol. 2020;47:251–262. doi: 10.1590/S1677-5538.IBJU.2020.99.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soytas M, Boz MY, Guzelburc V, et al. Analysis of patients undergoing urological intervention amid the COVID-19: experience from the pandemic hospital. Int Urol Nephrol. 2020;52:2059–2064. doi: 10.1007/s11255-020-02553-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan VWS, Chiu PKF, Yee CH, Yuan Y, Ng CF, Teoh JYC. A systematic review on COVID-19: urological manifestations, viral RNA detection and special considerations in urological conditions. World J Urol. 2020 doi: 10.1007/s00345-020-03246-4. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porreca A, Colicchia M, D’Agostino D, et al. Urology in the Time of Coronavirus: Reduced Access to Urgent and Emergent Urological Care during the Coronavirus Disease 2019 Outbreak in Italy. Urol Int. 2020;104:631–636. doi: 10.1159/000508512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajwa P, Przydacz M, Zapała P, et al. How has the COVID-19 pandemic impacted Polish urologists? Results from a national survey. Cent European J Urol. 2020;73:252–259. doi: 10.5173/ceju.2020.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng L, Zagorac S, Stebbing J. Managing patients with cancer in the COVID-19 era. Eur J Cancer. 2020;132:5–7. doi: 10.1016/j.ejca.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yasri S, Wiwanitkit V. Cancer care under the outbreak of COVID-19. Eur J Surg Oncol. 2020;46:1188. doi: 10.1016/j.ejso.2020.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan KK, Lau J. Cessation of cancer screening: An unseen cost of the COVID-19 pandemic? Eur J Surg Oncol. 2020;46:2154–2155. doi: 10.1016/j.ejso.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawlings A, Brandt L, Ferreres A, Asbun H, Shadduck P. Ethical considerations for allocation of scarce resources and alterations in surgical care during a pandemic. Surg Endosc. 2020 doi: 10.1007/s00464-020-07629-x. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nowak Ł, Krajewski W, Kiełb P, Śliwa A, Zdrojowy-Wełna A, Zdrojowy R. Covid-19 and the urological practice: changes and future perspectives. Cent European J Urol. 2020;73:269–272. doi: 10.5173/ceju.2020.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alva Pinto AM, González MS. Endourology and benign prostatic hyperplasia in COVID-19 pandemic. Int Braz J Urol. 2020;46(Suppl 1):34–38. doi: 10.1590/S1677-5538.IBJU.2020.S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motterle G, Morlacco A, Iafrate M, et al. The impact of COVID-19 pandemic on urological emergencies: a single-center experience. World J Urol. 2020 doi: 10.1007/s00345-020-03264-2. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi SY, Kim TH. Survival strategy of urology department during the COVID-19 era. Int Urol Nephrol. 2020;52:1499–1500. doi: 10.1007/s11255-020-02515-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sosnowski R, Kamecki H, Joniau S, et al. Uro-oncology in the era of social distancing: The principles of patient-centered online consultations during the COVID-19 pandemic. Cent European J Urol. 2020;73:260–264. doi: 10.5173/ceju.2020.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stensland KD, Morgan TM, Moinzadeh A, et al. Considerations in the Triage of Urologic Surgeries During the COVID-19 Pandemic. Eur Urol. 2020;77:663–666. doi: 10.1016/j.eururo.2020.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campi R, Amparore D, Capitanio U, et al. Assessing the Burden of Nondeferrable Major Uro-oncologic Surgery to Guide Prioritisation Strategies During the COVID-19 Pandemic: Insights from Three Italian High-volume Referral Centres. Eur Urol. 2020;78:11–15. doi: 10.1016/j.eururo.2020.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diamand R, Ploussard G, Roumiguié M, et al. Timing and delay of radical prostatectomy do not lead to adverse oncologic outcomes: results from a large European cohort at the times of COVID-19 pandemic. World J Urol. 2020 doi: 10.1007/s00345-020-03402-w. [Ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esperto F, Prata F, Civitella A, et al. Implementation and strategies to ensure adequate coordination within a Urology Department during the COVID-19 pandemic. Int Braz J Urol. 2020;46(Suppl 1):170–180. doi: 10.1590/S1677-5538.IBJU.2020.S122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shabbir A, Menon RK, Somani J, et al. ELSA recommendations for minimally invasive surgery during a community spread pandemic: a centered approach in Asia from widespread to recovery phases. Surg Endosc. 2020;34:3292–3297. doi: 10.1007/s00464-020-07618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francis N, Dort J, Cho E, et al. SAGES and EAES recommendations for minimally invasive surgery during COVID-19 pandemic. Surg Endosc. 2020;34:2327–2331. doi: 10.1007/s00464-020-07565-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Restivo A, De Luca R, Spolverato G, et al. The need of COVID19 free hospitals to maintain cancer care. Eur J Surg Oncol. 2020;46:1186–1187. doi: 10.1016/j.ejso.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Maida F, Antonelli A, Porreca A, Rocco B, Mari A, Minervini A. Letter to the Editor: ‘Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection’. EClinicalMedicine. 2020;22:100362. doi: 10.1016/j.eclinm.2020.100362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah UA. Cancer and Coronavirus Disease 2019 (COVID-19)-Facing the ‘C Words’. JAMA Oncol. 2020;6:1330–1331. doi: 10.1001/jamaoncol.2020.1848. [DOI] [PubMed] [Google Scholar]
- 33.Ghignone F, Mohan HM, Montroni I. Cancer surgery in a time of COVID-19: Many questions, few certainties. Eur J Surg Oncol. 2020;46:1196–1197. doi: 10.1016/j.ejso.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]