Abstract
BACKGROUND AND AIMS:
The long-term risk of disease for patients with nonalcoholic fatty liver disease (NAFLD) in the absence of elevated enzymes is unclear. We conducted a retrospective cohort study using the Corporate Data Warehouse of the Veterans Health Administration.
APPROACH AND RESULTS:
We classified patients into three groups: patients with steatosis/normal alanine aminotransferase (ALT), steatosis/elevated ALT, and no steatosis/normal ALT. We examined incidence rates for cirrhosis and hepatocellular carcinoma (HCC) and conducted cause-specific hazard models to evaluate the risk of cirrhosis and HCC. We identified 3,522 patients with steatosis/normal ALT, 15,419 patients with steatosis/elevated ALT, and 9,267 patients with no steatosis/normal ALT. The mean age in each group was 58.9, 54.7 and 59.3 years, respectively; over 90% were men. Compared to patients with hepatic steatosis/normal ALT, those with steatosis/elevated ALT were younger and more likely to be obese (both P < 0.01). In patients with steatosis/normal ALT, the incidence rates of cirrhosis and HCC were 1.22 (95% confidence interval [CI]: 0.83–1.74) and 0.20 (95% CI: 0.06–0.46) per 1,000 person-years, respectively; this was lower than in patients with steatosis/elevated ALT (cirrhosis: 3.85; 95% CI: 3.50–4.23, and HCC: 0.37; 95% CI: 0.26–0.49). Patients with steatosis/elevated ALT had a higher risk of developing cirrhosis (adjusted hazard ratio: 3.37; 95% CI: 2.34–4.86; P < 0.01) than patients with steatosis/normal ALT; they also had a higher risk of HCC, although it did not reach statistical significance (hazard ratio: 2.07; 95% CI: 0.82–5.28; P = 0.13). The risk of cirrhosis and HCC in patients with steatosis/normal ALT and those without steatosis was not significantly different.
CONCLUSIONS:
Patients with hepatic steatosis with persistently normal ALT are at lower risk for cirrhosis compared to those with steatosis and elevated ALT and not different from the risk in a clinical cohort without hepatic steatosis.
Nonalcoholic fatty liver disease (NAFLD) affects 20%−30% of the U.S. general population.(1–3) Approximately 20% of patients with NAFLD develop nonalcoholic steatohepatitis (NASH), a condition that is associated with increased risk of fibrosis, cirrhosis, hepatocellular cancer (HCC), and mortality.(4–8) In clinical practice, the evaluation of NAFLD is often triggered by the detection of abnormal liver function tests. Although liver biopsy is considered the gold standard for the diagnosis and histological assessment of NAFLD, the diagnosis is often made by the presence of steatosis or abnormal liver enzymes in the absence of other causes of liver disease (hepatitis B virus [HBV], hepatitis C [HCV], alcohol abuse, rare chronic liver disease). However, many patients with biopsy-confirmed NAFLD have persistently normal enzymes, and the entire spectrum of NAFLD—from steatosis to NASH with advanced fibrosis—has been reported in patients with normal enzymes.(9) Furthermore, with the increasing number of patients undergoing abdominal imaging for a variety of indications, incidental findings of hepatic steatosis are becoming more common. Yet, little is known about the clinical course or prognosis of incidental NAFLD detected based on abdominal imaging, especially in patients without biochemical abnormalities in liver enzymes.(10)
Most of the data on the clinical course of NAFLD in the United States come from the National Health and Nutrition Examination Survey program. Although these studies did not show an increase in mortality in patients with NAFLD with normal liver enzymes, they were limited by a lack of granular data on important risk factors and incomplete capture of relevant clinical endpoints, including cirrhosis and HCC.(11–13) There is a paucity of well-designed longitudinal studies evaluating the risk of advanced disease with large numbers of NAFLD cases with both sufficient follow-up time and relevant number of outcomes. Absence of these data is a major roadblock in developing clinical guidelines about the management of this potentially large group of patients with NAFLD seen in routine clinical practice.
We conducted a large retrospective cohort study to examine the risk of cirrhosis and HCC among patients with NAFLD and persistently normal liver enzymes who were seen in the U.S. Veterans Health Administration system, and compared them to patients with steatosis and elevated liver enzymes as well as controls without steatosis or elevated liver enzymes.
Methods
DATA SOURCE
We used data from national Veterans Affairs (VA) Corporate Data Warehouse (CDW) and VA Central Cancer Registry (VACCR). The CDW includes radiology imaging reports, diagnosis codes (International Classification of Diseases [ICD]-9/10 codes), laboratory test results, and pharmacy data for each inpatient or outpatient encounter in the VA nationwide since 1999.(14) The CDW also contains information from annual AUDIT-C screens for alcohol use(15,16) and a Radiology Raw Data domain that houses all radiology reports performed in the VA nationwide from the CDW’s inception to present. The latter includes type of study, date, detailed report and impression, all linked through a unique patient identifier. In the VACCR, cancer registrars manually abstract case data, conforming to standards set by the North American Association of Central Cancer.(17,18) Finally, the CDW also includes vital status, which combines information from Medicare, VA, Social Security, and VA compensation and pension benefits to determine the date of death (sensitivity: 98.3%; specificity: 99.8% relative to National Death Index).(19)
STUDY POPULATION
We examined all patients aged 18 to 80 years and all of their available alanine aminotransferase (ALT) tests conducted at any of the 130 VA hospitals in the United States between January 1, 2003, and December 31, 2008, to define our study cohorts. First, we identified patients with either elevated or normal ALT tests during this time frame who did not have any evidence of other chronic liver diseases. Specifically, we classified patients as subjects with elevated ALT if they had two or more ALT values (>40 IU/mL for men and >31 IU/mL for women) in ambulatory settings and more than 6 months apart; we classified the remaining patients as subjects with normal ALT. From both groups, we excluded patients with positive serologic testing for HBV (i.e., HBV surface antigen), HCV (i.e., HCV RNA), evidence of excessive alcohol use (any AUDIT-C > 4 in men and >3 in women or any diagnosis code of alcohol use), and those with rare chronic liver diseases (hereditary hemochromatosis, primary biliary cirrhosis, primary sclerosing cholangitis, alpha-1 antitrypsin disease, or autoimmune hepatitis) defined based on ICD-9 codes. We used the date of first elevated ALT as the index date of follow-up for patients in the elevated ALT group. Second, given the potentially large size of the cohort with normal ALT, we used random sampling without replacement to match the two groups (i.e., with elevated and normal ALT) on gender, age at index date, and duration from their first VA visit to the selected ALT date (individual matching). Third, we limited the analysis to patients with an index date from January 1, 2004, to December 31, 2008, because AUDIT-C was implemented in the VA in 2004. We used 2008 as the cutoff to define study cohorts to allow a minimum 7-year follow-up for all patients. In the last step, we used the radiology domain of CDW to extract all reports from abdominal ultrasound, magnetic resonance imaging, and computed tomography scans performed any time before to 2 years after the index date in any of the 130 VA facilities for all patients meeting these criteria. We used our previously validated natural language processing algorithm that reliably identifies hepatic steatosis in abdominal imaging reports in electronic medical records (EMR).(20) To minimize the confounding by indication (for abdominal imaging), all patients had to have an abdominal imaging test to be considered for inclusion in the study cohorts. We used the 2-year window before or after the index ALT test to look for abdominal imaging, to ensure that the imaging was either before or soon after the ALT test date. This would minimize any potential survivorship bias, in which patients who survived longer would be more likely to receive abdominal imaging. However, it is plausible that by using this approach, we either misclassified or excluded some patients who did not have evidence of hepatic steatosis (or did not undergo an abdominal imaging test) during the specified time frame but developed evidence of hepatic steatosis during follow-up. Therefore, in a sensitivity analysis, we expanded the time frame for imaging tests to include all tests performed during follow-up.
The final study cohorts included a primary cohort with hepatic steatosis and persistently normal ALT, and two comparison cohorts: patients with hepatic steatosis and elevated ALT (positive control) and those without hepatic steatosis or elevated ALT (negative control).
VARIABLE SPECIFICATION
Outcomes
Our primary outcomes were cirrhosis and HCC. We defined cirrhosis as two or more outpatient or one or more inpatient ICD-9 codes for cirrhosis or its complications. We defined HCC as those patients in our cohort with two instances of ICD-9 codes for HCC (155.0 in the absence of 155.1) in the inpatient or outpatient files of the CDW data.(21) We then examined the VACCR for patients with HCC diagnosis. For discordant patients who had an ICD-9 code but were not identified as having HCC in the VACCR data, we conducted a manual review of the VA electronic medical records to determine their true HCC status. This hierarchical approach ensured high validity of all captured HCC cases. We also preformed manual chart reviews of all HCC cases to determine whether HCC developed in the setting of cirrhosis.
We excluded patients with prevalent cirrhosis or HCC whose first diagnosis date was recorded any time before to 1 year after the index date. We defined incident cases of cirrhosis or HCC as new diagnoses during follow-up beginning 1 year after the index date. The CDW did not include information on cause of death. However, we examined all-cause mortality as a secondary outcome.
Covariates
These included age at index date, gender, and race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other). We defined body mass index (BMI) by using height and weight values within any time before to 1 year after the index, choosing the value closest to the index date during the study period.(22,23) We defined diabetes, hypertension, and dyslipidemia by two or more outpatient or one or more inpatient ICD-9 codes or more than one filled prescription of diabetes medications (oral hypoglycemic medications or insulin), antihypertensive or lipid-lowering medications, respectively, any time before to 1 year after the index date. We defined health care use as the number of clinic visits and unique ALT tests performed within 1 year after the index date. We evaluated medical comorbidities associated with mortality using the Deyo comorbidity index.(24)
STATISTICAL ANALYSES
We compared demographic, key metabolic traits (diabetes, hypertension, dyslipidemia, BMI), and other comorbidities across patients in the three cohorts (hepatic steatosis with normal ALT, hepatic steatosis and elevated ALT, neither steatosis nor elevated ALT) using chi-square tests for categorical variables and appropriate parametric or nonparametric tests for continuous variables.
We followed patients from index date to development of cirrhosis, HCC, death, or December 31, 2015. We calculated and plotted the cumulative incidence functions of cirrhosis and HCC in three patient cohorts, and used Gray’s test to evaluate the differences between curves. We used the cause-specific hazard regression models to examine the differences in the risk of progression to cirrhosis or HCC, while adjusting for age, race/ethnicity, sex, comorbidity, and frequency of health care use. These analyses accounted for the competing risk for death. We examined but did not find statistically significant interactions among age, gender, race, baseline metabolic traits, and the main exposure variable (hepatic steatosis with normal ALT, hepatic steatosis with elevated ALT, and normal ALT and no steatosis).
All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Statistical significance was determined at α = 0.05, and all P values for statistical significance were two-sided.
Sensitivity and Secondary Analyses
In a prespecified sensitivity analysis, we expanded the time frame for imaging tests to include all tests performed during follow-up for patients and reclassified the three subgroups based on additional imaging test results. We repeated all analyses in the expanded cohorts. In the primary analysis, we classified normal versus elevated ALT groups based on two or more instances of high ALT values. Thus, our normal ALT group could have had patients with one high ALT value. We repeated analyses after excluding these patients. Finally, although our primary outcomes were progression to cirrhosis and HCC, we also conducted separate analyses in which we modeled all-cause mortality using multivariable Cox proportional hazards model, adjusting for demographic and clinical variables including the Deyo comorbidity index.
Results
PATIENT CHARACTERISTICS
We identified 3,522 patients with hepatic steatosis and normal ALT, 15,419 patients with hepatic steatosis and elevated ALT, and 9,267 patients with no steatosis and normal ALT (Fig. 1). The mean age of the cohort with hepatic steatosis and normal ALT at index was 58.9 years (SD = 10.0); 64.0% were white, 13.4% were black, and 8.8% were Hispanic. At baseline, 56.0% were obese, 41.2% had diabetes, and 77.3% had dyslipidemia (Table 1).
FIG. 1.
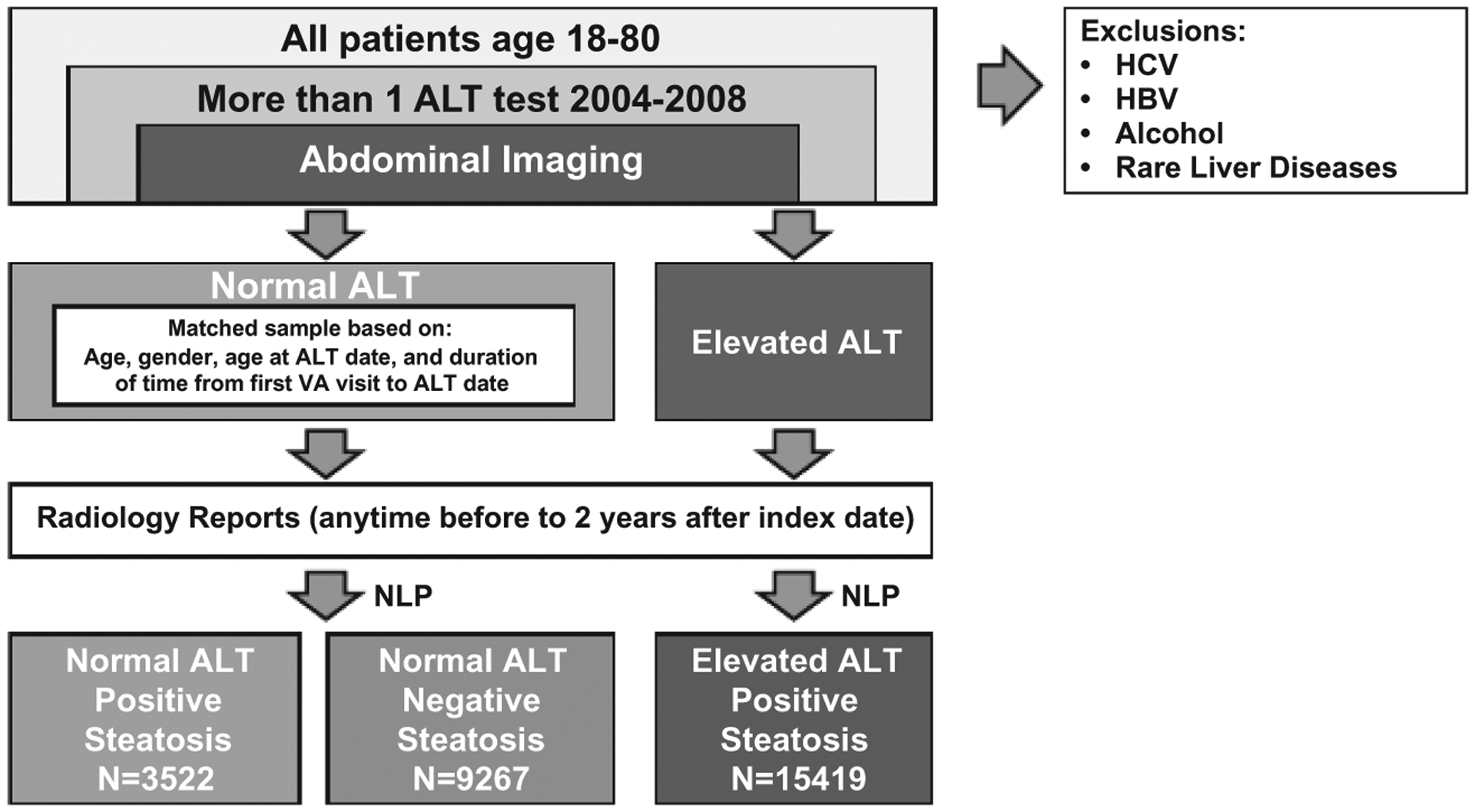
Patient selection. Abbreviation: NLP, natural language processing.
TABLE 1.
Baseline characteristics of the three study cohorts
| Steatosis + Normal ALT (n = 3,522) | Steatosis + Elevated ALT (n = 15,419) | P Value* | No Steatosis + Normal ALT (n = 9,267) | P Value* | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, mean (SD) | 58.9 (10.0) | 54.7 (12.3) | <0.01 | 59.3 (11.0) | 0.06 |
| Male, n (%) | 3,203 (90.9%) | 14,285 (92.7%) | <0.01 | 8,401 (90.7%) | 0.62 |
| Race, n (%) | <0.01 | <0.01 | |||
| White | 2,254 (64.0%) | 10,733 (69.6%) | 5,930 (64.0%) | ||
| Black | 473 (13.4%) | 1,364 (8.9%) | 1,728 (18.7%) | ||
| Hispanic | 311 (8.8%) | 1,423 (9.2%) | 417 (4.5%) | ||
| Other | 484 (13.7%) | 1,899 (12.3%) | 1,192 (12.8%) | ||
| Comorbidities, n (%) | |||||
| BMI ≥ 30 | 1,971 (56.0%) | 10,222 (66.3%) | <0.01 | 5,944 (64.1%) | <0.01 |
| Diabetes | 1,451 (41.2%) | 6,018 (39.0%) | 0.02 | 2,480 (26.8%) | <0.01 |
| Hypertension | 2,890 (82.1%) | 11,924 (77.3%) | <0.01 | 6,997(75.5%) | <0.01 |
| Dyslipidemia | 2,722 (77.3%) | 11,488 (74.5%) | <0.01 | 6,325 (68.3%) | <0.01 |
| Deyo score, mean (SD) | 1.2 (1.6) | 1.0 (1.6) | <0.01 | 1.2 (1.7) | 0.39 |
| Health care use | |||||
| Number of clinic visits, mean (SD) | 18.0 (17.7) | 19.6 (18.9) | <0.01 | 15.4 (12.1) | 0.43 |
| Number of ALT tests, mean (SD) | 16.5 (12.1) | 19.6 (15.8) | <0.01 | 15.4 (12.1) | <0.01 |
Compared to steatosis and normal ALT group.
Patients with hepatic steatosis and elevated ALT were younger, more likely to be Hispanic and obese but less likely to be black, have diabetes, hypertension, or dyslipidemia than patients with steatosis and normal ALT (Table 1). In contrast, patients without steatosis or elevated ALT were significantly less likely to be Hispanic, obese, have diabetes, hypertension, or dyslipidemia than patients with steatosis and normal ALT (all P < 0.01) (Table 1); there were no significant differences in age and Deyo score between the two groups.
Mean follow-up for all three cohorts was 8.4 (SD = 2.7) years. All groups had a high propensity to use health care. Table 1 lists the frequency of clinic visits and ALT tests in the three groups.
INCIDENCE OF CIRRHOSIS AND HCC
Among the 3,522 patients with steatosis and normal ALT, 31 developed cirrhosis during 25,336 person-years (PY) of follow-up at an annual incidence rate of 1.22 per 1,000 PY (95% confidence interval [CI]: 0.83, 1.74) (Table 2). In the same group, only 5 patients developed HCC during 25,441 PY of follow-up at an annual incidence rate of 0.20 (95% CI: 0.06, 0.46) per 1,000 PY. Among 15,419 patients with steatosis and elevated ALT, 435 patients progressed to cirrhosis during 112,950 PY of follow-up (incidence rate: 3.85 per 1,000 PY; 95% CI: 3.50, 4.23) and 42 patients developed HCC during 114,749 PYs of follow-up (0.37 per 1,000 PY; 95% CI: 0.26, 0.49). Among patients with neither steatosis nor elevated ALT, 61 and 4 patients were diagnosed with cirrhosis and HCC during 62,955 and 63,232 PY of follow-up at the incidence rates of 0.97 (95% CI: 0.74, 1.24) and 0.06 (95% CI: 0.02, 0.16) per 1,000 PY, respectively (Table 2).
TABLE 2.
Incidence of Cirrhosis and HCC
| Cirrhosis | HCC | ||||||
|---|---|---|---|---|---|---|---|
| Patients (n) | Incident Cases (n) | Follow-up (PY) | Cirrhosis Incidences per 1,000 PY (95% CI) | Incident Cases (n) | Follow-up (PY) | HCC Incidences per 1,000 PY (95% CI) | |
| Steatosis + normal ALT | 3,522 | 31 | 25,336 | 1.22 (0.83, 1.74) | 5 | 25,441 | 0.20 (0.06, 0.46) |
| Steatosis + elevated ALT | 15,419 | 435 | 112,950 | 3.85 (3.50, 4.23) | 42 | 114,749 | 0.37 (0.26, 0.49) |
| No steatosis + normal ALT | 9,267 | 61 | 62,955 | 0.97 (0.74, 1.24) | 4 | 63,232 | 0.06 (0.02, 0.16) |
Compared to patients with steatosis and normal ALT, patients with hepatic steatosis and elevated ALT had higher cirrhosis incidence throughout follow-up, starting 1 year after the index date and increasing over time (P < 0.01). The cumulative incidence of cirrhosis was not significantly different between patients with steatosis and normal ALT and those with neither steatosis nor elevated ALT (P = 0.19). The 5-year and 8-year cumulative incidence rates of cirrhosis were 4.0 and 7.7 per 1,000 patients in the group with steatosis and normal ALT versus 14.5 and 24.1 per 1,000 patients in the group with steatosis and elevated ALT, and 4.1 and 5.9 per 1,000 patients in those with neither steatosis nor elevated ALT (Fig. 2A).
FIG. 2.
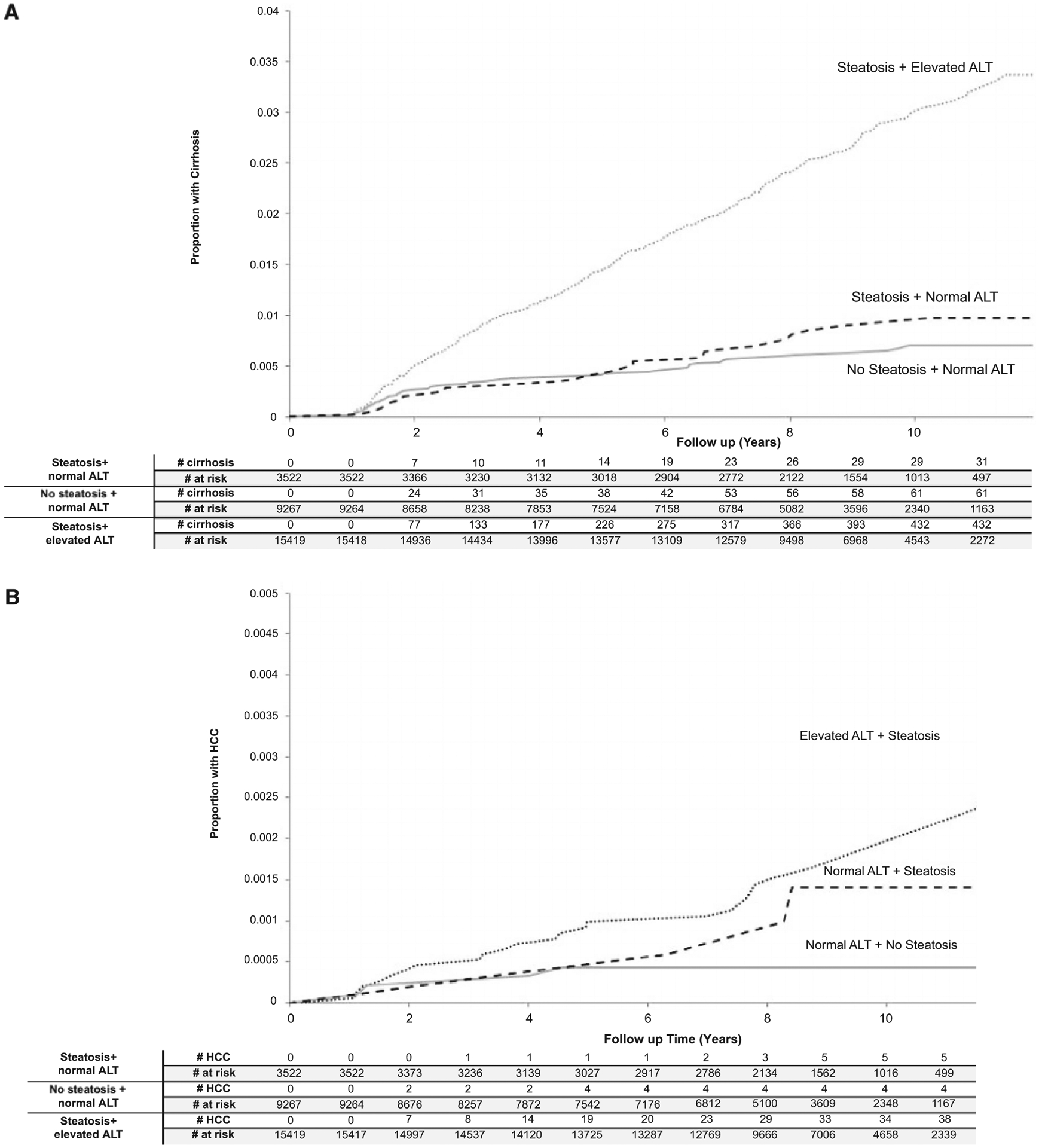
(A) Cumulative incidence of cirrhosis in the study cohorts. P value from Gray’s test using steatosis and normal ALT as the comparison group. (B) Cumulative incidence of HCC in the study cohorts. P value from Gray’s test using steatosis and normal ALT as the comparison group.
Patients with hepatic steatosis and elevated ALT had the highest cumulative incidence of HCC followed by patients with hepatic steatosis and normal ALT and those without steatosis or elevated ALT (Fig. 2B). However, these between-group differences did not reach statistical significance (steatosis and normal ALT vs. steatosis and elevated ALT, P = 0.44; steatosis and normal ALT vs. without steatosis and normal ALT groups, P = 0.16). Patients with elevated ALT and steatosis had a statistically significantly increased cumulative incidence of HCC than patients without steatosis and normal ALT (P < 0.01) (Fig. 2A,B).The 5-year and 8-year cumulative incidence rates of HCC were 0.5 and 0.9 per 1,000 patients in the group with steatosis and normal ALT versus 1.0 and 1.4 per 1,000 patients in the group with steatosis and elevated ALT, and 0.4 and 0.4 per 1,000 patients in those with neither steatosis nor elevated ALT (Fig. 2B).
These results did not change after adjusting for demographic and clinical differences among the three cohorts in a cause-specific hazard model (Table 3). Specifically, compared to patients with steatosis and normal ALT, the risk of progression to cirrhosis was significantly higher in patients with steatosis and elevated ALT (adjusted hazard ratio [HR]: 3.37; 95% CI: 2.34–4.86; P < 0.01). Patients with steatosis and elevated ALT had a higher risk of HCC than those with steatosis and normal ALT, but this trend did not reach statistical significance (adjusted HR: 2.07; 95% CI: 0.82–5.28; P = 0.13).
TABLE 3.
Factors Associated With the Risk of Progression to Cirrhosis and HCC: Results of Cause-Specific Hazard Model
| Cirrhosis | HCC | |||
|---|---|---|---|---|
| Characteristics | Adjusted HR (95% CI) | P Value* | Adjusted HR (95% CI) | P Value* |
| Study cohorts | ||||
| Hepatic steatosis/normal ALT | Reference | Reference | ||
| Hepatic steatosis/elevated ALT | 3.37 (2.34, 4.86) | <0.01 | 2.07 (0.82, 5.28) | 0.13 |
| No hepatic steatosis/normal ALT | 0.86 (0.56, 1.34) | 0.53 | 0.36 (0.10, 1.35) | 0.12 |
| Age | 1.02 (1.02, 1.03) | <0.01 | 1.10 (1.06, 1.13) | <0.01 |
| Race/ethnicity, ref = non-Hispanic white | ||||
| Hispanic | 0.91 (0.66, 1.24) | 0.54 | 0.63 (0.19, 2.04) | 0.44 |
| Non-Hispanic black | 0.69 (0.50, 0.95) | 0.02 | 0.19 (0.03, 1.38) | 0.10 |
| Other | 0.77 (0.57, 1.04) | 0.09 | 1.09 (0.49, 2.45) | 0.83 |
| Gender, ref = male | ||||
| Female | 0.76 (0.52, 1.11) | 0.16 | 0.42 (0.06, 3.12) | 0.40 |
| BMI, ref < 30 | ||||
| ≥30 | 0.98 (0.81, 1.19) | 0.86 | 1.68 (0.89, 3.20) | 0.11 |
| Diabetes, ref = no | 1.96 (1.58, 2.44) | <0.01 | 2.24 (1.10, 4.57) | 0.03 |
| Hypertension, ref = no | 1.12 (0.86, 1.49) | 0.39 | 0.88 (0.35, 2.23) | 0.78 |
| Dyslipidemia, ref = no | 0.62 (0.50, 0.77) | <0.01 | 0.35 (0.19, 0.66) | <0.01 |
| Deyo score, ref = 0 | ||||
| 1 | 1.35 (1.06, 1.72) | 0.01 | 1.69 (0.79, 3.59) | 0.17 |
| 2 | 1.47 (1.10, 1.98) | 0.01 | 0.20 (0.03, 1.56) | 0.13 |
| ≥3 | 1.46 (1.09, 1.94) | 0.01 | 1.70 (0.70, 4.13) | 0.25 |
Models adjusted for Deyo score and health care use.
There were no statistically significant differences in the risk of cirrhosis and HCC in patients with normal ALT with or without evidence of steatosis (adjusted HR: cirrhosis: 0.86; 95% CI: 0.56, 1.34; P = 0.53, and HCC: HR: 0.36; 95% CI: 0.10, 1.35; P = 0.12).
We performed manual chart reviews of patients with HCC to evaluate whether HCC occurred in the setting of cirrhosis. Of the five HCC cases in patients with steatosis and normal ALT, two occurred in the setting of cirrhosis, one occurred in the setting of stage 3 fibrosis, and the other two cases occurred in patients without cirrhosis. In the patients with no steatosis and normal ALT, one patient developed HCC without cirrhosis and the remaining patients developed HCC in in the setting of cirrhosis.
SENSITIVITY AND SECONDARY ANALYSES
Expanding the timeframe for abdominal imaging in our sensitivity analyses resulted in a substantial increase in the sizes of the three cohorts: 11,112 patients with steatosis and normal ALT, 42,282 patients with steatosis and elevated ALT, and 23,495 patients with neither steatosis nor elevated ALT. The associations were stronger in this sensitivity analysis (Supporting Tables S1 and S2; Supporting Figs. S1 and S2). Specifically, compared to patients with steatosis and normal ALT, those with steatosis and elevated ALT had statistically significantly higher risks of cirrhosis (adjusted HR: 2.63; 95% CI: 2.20, 3.14; P < 0.01) and HCC (adjusted HR: 4.35; 95% CI: 1.90, 9.94; P < 0.01). The risk of cirrhosis and HCC was not significantly different between patients with steatosis and normal ALT and those with neither steatosis nor elevated ALT (adjusted HR: 0.95; 95% CI: 0.77–1.17, and adjusted HR: 1.30; 95% CI: 0.51–3.32, respectively) (Supporting Table S2).
In total, 127 (3.6%) and 173 (1.9%) patients had one ALT value that was higher than the specified cutoff in the groups with steatosis and normal ALT and those without steatosis and normal ALT, respectively; all other ALT values were below the cutoff for these patients. Removing these patients in a sensitivity analysis did not change the direction or magnitude of our results (see Supporting Table S3).
Compared to patients with steatosis and normal ALT, the risk of all-cause mortality was higher in patients without steatosis or elevated ALT. The risk of all-cause mortality was similar in the two groups with steatosis (adjusted HR normal vs. elevated ALT; HR: 0.94; 95% CI: 0.87, 1.01) (Supporting Tables S4 and S5).
Discussion
We found that patients with NAFLD and persistently normal liver enzymes had a lower risk of progression to cirrhosis compared to those with NAFLD and elevated ALT; the risk of progression to cirrhosis in this group was not different from the risk among a control group of patients without steatosis or elevated ALT. The absolute risk of progression was low throughout the 8.4 years of average follow-up. Overall, among the 3,522 patients with hepatic steatosis and persistently normal liver enzymes, cirrhosis developed in 31 patients at an annual incidence of 1.22 per 1,000 person-years. Compared to patients with steatosis and normal ALT, the risk of progressing to cirrhosis was 3-fold higher in patients with elevated ALT.
We also found that patients with steatosis and normal ALT had a low, but not a nonexistent, risk of HCC. In our primary analysis, 5 patients with steatosis and normal ALT progressed to HCC at an annual incidence of 0.20 per 1,000 PY. The absolute risk of HCC was numerically lower than the risk in patients with NAFLD and elevated ALT. However, this trend did not reach statistical significance, likely due to the limited number of events in the steatosis and normal ALT group. Indeed, the differences in the annual and cumulative risks of HCC became more evident and were statistically significant in the sensitivity analysis that expanded the three groups based on additional imaging data during follow-up (Supporting Fig. S2 and Supporting Tables S1 and S2).
Many patients with NAFLD have normal liver enzymes, and this group is steadily increasing.(25) Our study shows that the presence of hepatic steatosis in the absence of elevated liver enzymes per se may be largely inconsequential; these individuals have similar liver outcomes as those without NAFLD. Indeed, the American Association for the Study of Liver Disease recommends against routine evaluation of individuals (even those with incidental finding of hepatic steatosis) unless they have abnormal liver biochemistries, metabolic risk factors, or evidence of progressive disease, (i.e., those who have clinically relevant NAFLD).(26,27) Studies also show a direct relationship between ALT (and aspartate aminotransferase) levels and the degree of liver fibrosis in NAFLD; elevated ALT is also associated with increased liver-related mortality in individuals with NAFLD (as well as in the general population). (28–31) Collectively, these data suggest that patients with hepatic steatosis and persistently normal ALT may not require ongoing follow-up and monitoring for liver outcomes. In contrast, our results highlight the importance of focusing on individuals with NAFLD and evidence of ALT elevation—a large group that remains unrecognized in routine practice.(32)
Both groups with steatosis had a higher proportion of patients with diabetes, dyslipidemia, and hypertension than those in the group with neither steatosis nor elevated ALT, thus pointing toward a possible underlying metabolic syndrome. However, patients with hepatic steatosis yet normal ALT appeared to be phenotypically different than those with both steatosis and elevated ALT. For example, although metabolic traits were common in both groups with steatosis, the prevalence of diabetes, hypertension, and dyslipidemia was higher in patients with steatosis and normal ALT; the latter group was also older at the index date. It is possible that an earlier age of onset for NAFLD is associated with higher likelihood of elevated ALT and worse prognosis.
Our study has few limitations. Some individuals in our control cohort may have unrecognized risk factors (including NAFLD in patients with neither steatosis nor elevated ALT); thus, we may have overestimated cirrhosis and HCC risk in the control cohort. Indeed, 2 of the 5 patients with HCC in this group had metabolic traits during our explicit EMR review. This could have biased the difference between steatosis and normal ALT versus the control group toward null. The all-cause mortality risk was higher in our control group without steatosis than the group with steatosis and normal ALT: adjusted HR versus steatosis and normal ALT: 1.28 (95% CI: 1.18–1.38). These findings might be a function of our selection criteria that relied on the availability of an abdominal imaging test. It is plausible that patients in the control group (without known liver disease or elevated ALT) likely underwent abdominal imaging for another competing significant health condition, resulting in unmeasured confounding in the mortality analysis.(33) However, we believe that our approach that modeled death as a competing risk minimized any potential bias introduced by differential risk of death in the three groups in the primary analyses (for cirrhosis and HCC outcomes). Although the cirrhosis algorithm was highly predictive of the presence of cirrhosis diagnosis in EMR in our previous study, we might have missed patients with cirrhosis, especially if cirrhosis was not recognized (and coded as such) as part of routine care.(34) However, our previous work suggests that ascertainment bias in cirrhosis definition is likely small and nondifferential. Of the patients with elevated liver enzymes (and likely NAFLD), only 36.4% had imaging done within 2 years. We limited our study to patients who had undergone at least one imaging test, to minimize the possibility of confounding by indication and to enhance the internal validity of our findings. However, because this might have limited the generalizability of our data, we repeated the analysis using a broader time frame for imaging and found similar results. Finally, our study is limited to veterans who were mostly men; therefore, the results may not be generalizable to women or individuals with NAFLD seen in other health care systems.(35)
In conclusion, individuals with steatosis and normal ALT, while having several features of the metabolic syndrome, have a low risk of cirrhosis and HCC that is no different from patients without hepatic steatosis and with normal ALT. Individuals with hepatic steatosis and normal ALT may not need recurring evaluation for liver-related outcomes, at least in the short and intermediate term (5–8 years). In contrast, patients with steatosis and elevated ALT are at increased risk for progression of liver disease and should be monitored closely.
Supplementary Material
Acknowledgments
This material is supported by a grant from the Cancer Prevention & Research Institute of Texas (RP150587 to F.K.) and a Baylor Junior Faculty Seed Award (to Y.N.). This work is also supported by the Center for Gastrointestinal Development, Infection, and Injury (NIDDK P30 DK 56338), the Veterans Administration Center for Innovations in Quality, Effectiveness, and Safety (CIN 13-413), and the National Cancer Institute (NCI U01 CA230997-01).
Abbreviations:
- ALT
alanine aminotransferase
- BMI
body mass index
- CDW
Corporate Data Warehouse
- CI
confidence interval
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- ICD
International Classification of Diseases
- PY
person-year
- VA
Veterans Affairs
- VACCR
VA Central Cancer Registry
Footnotes
The opinions and assertions contained herein are the sole views of the authors and are not to be construed as official or as reflecting the views of the Department of Veterans Affairs or the official views of the National Institutes of Health.
Supporting Information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.31157/suppinfo.
Potential conflict of interest: Dr Natarajan received grants from Gilead and Allergan.
REFERENCES
- 1).Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and non-alcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–131. [DOI] [PubMed] [Google Scholar]
- 2).Caballería L, Pera G, Arteaga I, Rodríguez L, Alumà A, Morillas RM, et al. High prevalence of liver fibrosis among European adults with unknown liver disease: a population-based study. Clin Gastroenterol Hepatol 2018;16:1138–1145.e5. [DOI] [PubMed] [Google Scholar]
- 3).Le P, Chaitoff A, Rothberg MB, McCullough A, Gupta NM, Alkhouri N. Population-based trends in prevalence of nonalcoholic fatty liver disease in US adults with type 2 diabetes. Clin Gastroenterol Hepatol 2019;17:2377–2378. [DOI] [PubMed] [Google Scholar]
- 4).Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol 2009;24: 248–254. [DOI] [PubMed] [Google Scholar]
- 5).Dam-Larsen S, Becker U, Franzmann MB, Larsen K, Christoffersen P, Bendtsen F. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol 2009;44:1236–1243. [DOI] [PubMed] [Google Scholar]
- 6).Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006;43:682–689. [DOI] [PubMed] [Google Scholar]
- 7).Pelusi S, Cespiati A, Rametta R, Pennisi G, Mannisto V, Rosso C, et al. Prevalence and risk factors of significant fibrosis in patients with nonalcoholic fatty liver without steatohepatitis. Clin Gastroenterol Hepatol 2019;17:2310–2319.e6. [DOI] [PubMed] [Google Scholar]
- 8).Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol 2019;17:748–755.e3. [DOI] [PubMed] [Google Scholar]
- 9).Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003;37:1286–1292. [DOI] [PubMed] [Google Scholar]
- 10).Hagström H, Nasr P, Ekstedt M, Stål P, Hultcrantz R, Kechagias S. Accuracy of noninvasive scoring systems in assessing risk of death and liver-related endpoints in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019;17:1148–1156.e4. [DOI] [PubMed] [Google Scholar]
- 11).Lazo M, Hernaez R, Bonekamp S, Kamel IR, Brancati FL, Guallar E, et al. Non-alcoholic fatty liver disease and mortality among US adults: prospective cohort study. BMJ 2011;343:d6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013;57:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, et al. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD). Dig Dis Sci 2013;58:3017–3023. [DOI] [PubMed] [Google Scholar]
- 14).Health Services Research & Development VA Informatics and Computing Infrastructure (VINCI). 2017.
- 15).Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The audit alcohol consumption questions (audit-c): an effective brief screening test for problem drinking. Arch Intern Med 1998; 158:1789–1795. [DOI] [PubMed] [Google Scholar]
- 16).Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, Kivlahan DR. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am J Manag Care 2006;12:597–606. [PubMed] [Google Scholar]
- 17).Jackson GL, Melton LD, Abbott DH, Zullig LL, Ordin DL, Grambow SC, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol 2010;28:3176–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Zullig LL, Jackson GL, Dorn RA, Provenzale DT, McNeil R, Thomas CM, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med 2012;177:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Sohn M-W, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Redman JS, Natarajan Y, Hou JK, Wang J, Hanif M, Feng H, et al. Accurate identification of fatty liver disease in data warehouse utilizing natural language processing. Dig Dis Sci 2017;62:2713–2718. [DOI] [PubMed] [Google Scholar]
- 21).Kanwal F, Kramer JR, Mapakshi S, Natarajan Y, Chayanupatkul M, Richardson PA, et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 2018;155:1828–1837.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Lee Y, Doumouras AG, Yu J, Brar K, Banfield L, Gmora S, et al. Complete resolution of nonalcoholic fatty liver disease after bariatric surgery: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2019;17:1040–1060.e11. [DOI] [PubMed] [Google Scholar]
- 23).Kim Y, Chang Y, Cho YK, Ahn J, Shin H, Ryu S. Obesity and weight gain are associated with progression of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2019;17:543–550.e2. [DOI] [PubMed] [Google Scholar]
- 24).Deyo R Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45: 613–619. [DOI] [PubMed] [Google Scholar]
- 25).Alazawi W, Mathur R, Abeysekera K, Hull S, Boomla K, Robson J, et al. Ethnicity and the diagnosis gap in liver disease: a population-based study. Br J Gen Pract 2014;64:e694–e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 27).Carter A, Mann JP. The clinical relevance of differentiating non-alcoholic steatohepatitis from simple steatosis. Clin Gastroenterol Hepatol 2018;16:596. [DOI] [PubMed] [Google Scholar]
- 28).Schneider JE Jr, Tabatabaie T, Maidt L, Smith RH, Nguyen X, Pye Q, et al. Potential mechanisms of photodynamic inactivation of virus by methylene blue. I. RNA-protein crosslinks and other oxidative lesions in Q beta bacteriophage. Photochem Photobiol 1998;67: 350–357. [PubMed] [Google Scholar]
- 29).Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865–873. [DOI] [PubMed] [Google Scholar]
- 30).Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 2010;51: 595–602. [DOI] [PubMed] [Google Scholar]
- 31).Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology 2009;136:477–485.e11. [DOI] [PubMed] [Google Scholar]
- 32).Blais P, Husain N, Kramer JR, Kowalkowski M, El-Serag H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol 2015;110:10–14. [DOI] [PubMed] [Google Scholar]
- 33).Stefan N Nonalcoholic fatty liver disease and mortality. Clin Gastroenterol Hepatol 2018;16:1043–1045. [DOI] [PubMed] [Google Scholar]
- 34).Walker M, El-Serag HB, Sada Y, Mittal S, Ying J, Duan Z, et al. Cirrhosis is under-recognised in patients subsequently diagnosed with hepatocellular cancer. Aliment Pharmacol Ther 2016;43:621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Ye Z-L, Guo W-Q, Li L. Sex-based differences in the association between nonalcoholic fatty liver disease and mortality. Clin Gastroenterol Hepatol 2019;17:211–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


