Abstract
Atherosclerosis is a chronic inflammatory disease in which atherothrombotic complications lead to cardiovascular morbidity and mortality. At advanced stages, myocardial infarction, ischaemic stroke, and peripheral artery disease, including major adverse limb events, are caused either by acute occlusive atherothrombosis or by thromboembolism. Endothelial dysfunction, vascular smooth muscle cell activation, and vascular inflammation are essential in the development of acute cardiovascular events. Effects of the coagulation system on vascular biology extend beyond thrombosis. Under physiological conditions, coagulation proteases in blood are pivotal in maintaining haemostasis and vascular integrity. Under pathological conditions, including atherosclerosis, the same coagulation proteases (including factor Xa, factor VIIa, and thrombin) become drivers of atherothrombosis, working in concert with platelets and vessel wall components. While initially atherothrombosis was attributed primarily to platelets, recent advances indicate the critical role of fibrin clot and plasma coagulation factors. Mechanisms of atherothrombosis and hypercoagulability vary depending on plaque erosion or plaque rupture. In addition to contributing to thrombus formation, factor Xa and thrombin can affect endothelial dysfunction, oxidative stress, vascular smooth muscle cell function as well as immune cell activation and vascular inflammation. By these mechanisms, they promote atherosclerosis and contribute to plaque instability. In this review, we first discuss the postulated vasoprotective mechanisms of protease-activated receptor signalling induced by coagulation enzymes under physiological conditions. Next, we discuss preclinical studies linking coagulation with endothelial cell dysfunction, thromboinflammation, and atherogenesis. Understanding these mechanisms is pivotal for the introduction of novel strategies in cardiovascular prevention and therapy. We therefore translate these findings to clinical studies of direct oral anticoagulant drugs and discuss the potential relevance of dual pathway inhibition for atherothrombosis prevention and vascular protection.
Keywords: Factor Xa, Thrombin, Atherosclerosis, Cardiovascular, Anticoagulant
1. Introduction
Cardiovascular diseases (CVD) remain the leading cause of worldwide mortality, surpassing other communicable and non-communicable causes of death in the long term.1 While CVD is influenced by multiple risk factors and mechanisms, acute cardiovascular events are largely triggered by thrombosis. Myocardial infarction, ischaemic stroke, and complications occurring in patients with peripheral artery disease (PAD), including major adverse limb events, are caused either by acute occlusive atherothrombosis, or by thromboembolism.2 Atherothrombosis is the consequence of atherosclerosis, a chronic inflammatory arterial disease, in which haemostatic mechanisms are triggered by rupture or erosion of plaques to form a clot.3 For many years, the initiation of atherothrombosis has been thought to result from an interaction between platelets and intraplaque material; hence, antiplatelet therapy (APT) with acetylsalicylic acid (ASA), or clopidogrel, has been the mainstay of secondary prevention for decades. However, atherothrombosis is the result of a more complex process, which involves not only platelets but also coagulation proteins, other blood cells, as well as extracellular vesicles. The resulting thrombus consists of a platelet-fibrin-rich clot in close association with atherosclerotic plaque. Recognition that fibrin is an essential element in thrombus formation (both in venous and in arterial thrombosis), changed the way we think about antithrombotic therapies in CVD. It also triggered new interest in combining APT with anticoagulants, to reduce the risk of recurrent atherothrombosis.
While several years ago, we postulated that pleiotropic actions of anticoagulants would provide additional vasoprotection,4 recent results of the Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS),5 a study to Assess the Effectiveness and Safety of Rivaroxaban in Reducing the Risk of Death, Myocardial Infarction or Stroke in Participants With Heart Failure and Coronary Artery Disease Following an Episode of Decompensated Heart Failure (COMMANDER HF),6 and Efficacy and Safety of Rivaroxaban in Reducing the Risk of Major Thrombotic Vascular Events in Subjects With Symptomatic Peripheral Artery Disease Undergoing Peripheral Revascularization Procedures of the Lower Extremities (VOYAGER PAD)7 trials not only confirmed the clinical importance of this approach but raised a number of key questions related to the mechanisms of vasoprotection potentially associated with an antithrombotic regimen comprising agents with dual pathway inhibitory properties.
In this article, we discuss the impact of coagulation proteases, in particular factor (F) Xa and thrombin, on physiology and pathophysiology of the vascular endothelium. We discuss the role of these proteases in atherothrombosis, and the potential clinical consequences of dual pathway inhibition (DPI), combining platelet inhibition and anticoagulation, to achieve a stronger antithrombotic and possibly vascular-protective effect, beyond classical indications for anticoagulation.8
2. The role of coagulation proteases and protease-activated receptors in haemostasis and vascular protection
2.1. Coagulation proteins and the physiological activity of coagulation
The coagulation cascade comprises a series of linked proteins, most of which are produced by the liver: procoagulant factors (F) I–XII, and the anticoagulant proteins antithrombin, proteins C and S, and tissue factor pathway inhibitor (TFPI). Factors II (prothrombin), VII, IX, X, and proteins C and S are dependent on vitamin K for their function, the result of vitamin K oxidase reductase mediated carboxylation of these proteins.9 Some proteins like FVIII, protein S, TFPI, and thrombomodulin are also synthesized by vascular endothelial cells (ECs) in or outside the liver. Many coagulation proteins can also be produced by other, extravascular, cells in different organs, including heart, brain, bone marrow, intestines, kidney, and placenta. In the extravascular compartments, these proteins may have multiple roles in complex regulatory pathways, not related to haemostasis. An example is the effect of the thrombin–thrombomodulin–protein C pathway in directing retention and release of cells from the bone marrow.10,11 Another example is the critical functions that the ‘coagulome’ appears to fulfil in the central as well as peripheral nervous system.12–14 Discussion of most of these extravascular processes is beyond the scope of this article, with the exception of the significance of coagulation proteins within the vessel wall and in the context of atherosclerosis.
Adequate levels of coagulation proteins are essential to provide haemostasis in case of occurrence of any vascular defect. Essentially, haemostasis is important for wound healing, a process in which the platelets attract inflammatory cells such as macrophages and the fibrin matrix supports the generation of new extracellular matrices by fibroblasts.
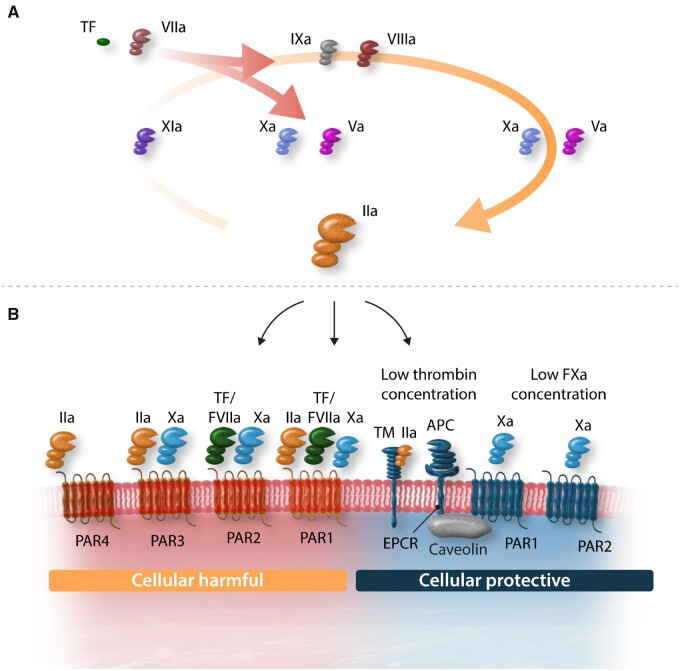
A major role of circulating coagulation proteins is to maintain an ambient level of coagulation activity, involving the generation of enzymes and the end product fibrin (Figure 1A). This process derives from controlled and limited proteolysis of coagulation proteins in a cascade model. The fact that fibrin degradation fragments including d-dimer are detected in any healthy subject, illustrates the presence of fibrin formation and cleavage under physiologic conditions, in vivo.15–17 Primate studies showed that this basal level of coagulation activity is TF/FVIIa dependent, suggesting that indeed minute amounts of TF support this coagulation activity; excess coagulation is prevented by several natural inhibitory mechanisms.18 One can imagine that for effective haemostasis a limited degree of coagulation is helpful, in order to allow a quick response to injury. Another reason may be that several coagulation proteases engage in cell signalling mechanisms, conferring protection of ECs, and their barrier function.
Figure 1.
Tissue factor (TF) is the main physiological activator of the coagulation resulting in activated factors VIIa, Xa, and thrombin (IIa). Thrombin generation is further enhanced through the positive feedback loop via factors XIa, IXa, and subsequently Xa and thrombin. Through binding to thrombomodulin, thrombin activates endothelial protein C receptor (EPCR) bound protein C into activated protein C (APC). Besides the anticoagulant activity of APC on inhibition of the cofactors Va and VIII, APC has anti-inflammatory and antiapoptotic activities through activation of the protease-activated receptor 1 (PAR1). Thrombin can activate PAR1, PAR-3, and PAR-4 as well, with the notion that at low levels of thrombin generation effects are mainly cellular protective through either direct activation of PAR1 or via APC-PAR1 signalling, whereas at higher thrombin levels signalling through PAR1 is mainly cellular destructive. Factor Xa or the tissue factor: factor VIIa can activate PAR-2 and PAR-1 can be activated by the tissue factor: factor VIIa: factor Xa complex.
2.2. Coagulation proteins regulate haemostasis and vascular integrity
Specific coagulation proteins are essential for maintaining endothelial vascular integrity, as shown in knockout mice for prothrombin, TF, FVII, and protein C. Transgenic mice with homozygous deficiency in TF, FVII, prothrombin, or protein C, die in utero. For a substantial part, this is caused by severely disturbed haemostasis, resulting in overt bleeding (deficiency in TF, FVII, FX, FV, or prothrombin). In addition, in case of homozygous deficiency in TF, prothrombin, FV, or the cellular protease-activated receptor 1 (PAR1), several defects in vascular integrity, blood vessel development, and/or embryonic growth were observed (reviewed in Ref.19). Three groups independently reported that TF−/− embryos died between E8.5–10.5 due to inability to establish or maintain vascular integrity, showing defective yolk sac development.20–22 The primary defect is characterized by severely impaired vascular smooth muscle cell (VSMC)/pericyte accumulation and differentiation around ECs, resulting in loss of endothelial barrier formation and vascular leakage.20 Transgenic mice expressing a cytoplasmic tailless form of TF are rescued from lethality; FVII binding to the extracellular domain of TF, as well as catalytic function of the FVIIa/TF complex, are important for normal embryogenesis and vascular integrity.23 Low levels (±1%) of a human TF minigene rescue mice from lethality resulting in an apparently normal phenotype without signs of bleeding or loss in vascular integrity.24
One half of F7−/− mice die in utero and the remainder within 24 h due to major bleeding; there are no indications for defects in vascular development.25 Theoretically, however, survival of F7−/− mice may be the result of transfer of minute amounts of maternal FVII from the mother, as humans with FVII levels as low as 1% survive.19 F10−/− mice die mostly in utero between E11.5 and 12.5 and as far as detectable, bleeding is the major cause of death, while there are no obvious signs of impaired vascular integrity. FX deficiency is not associated with disturbed yolk sac development, while this defect was reported in F5−/− and F2−/− mice.26,27 The remarkable observation that fibrinogen−/− mice survive and develop normally28 demonstrates that abnormal blood clotting is not sufficient to explain neither the above-mentioned severe bleeding nor impaired vascular development. This raises many questions regarding the role of coagulation proteases in other cellular functions, including endothelial barrier formation.
2.3. Coagulation proteases provide protective actions through activate PARs
2.3.1. PAR-mediated cell signalling
Protease-activated receptors belong to the G-protein-coupled receptors and are unique in their activation mechanism: a protease cleaves the extracellular N-terminal domain of the receptor, thereby generating a new N-terminus (the so-called tethered ligand) which folds back and activates the receptor. Thus far, four subtypes are recognized in humans: PAR1 through 4 (Figure 1B). Early studies demonstrated that one half of PAR1 null mice (F2r−/−) die between E9.5 and 10.5.29,30 The observation that PAR1 is expressed on ECs and VSMCs, triggered further research into the link between coagulation proteases, PAR activation, and vascular properties. Many subsequent studies identified the cell signalling pathways triggered by PAR activation through thrombin, FXa, and FVIIa-TF.31,32 In a previous paper in this journal, Borissoff and colleagues33 summarized the known multiplicity in functions of thrombin, acting as an enzyme with vasoprotective properties (mediated through PAR1 activation) as well as anticoagulant functions (through activating protein C upon binding to the EC cofactor thrombomodulin). We will recapitulate some of thrombin’s properties as they link to PAR signalling and vascular integrity/permeability.
2.3.2. Thrombin activates PAR1; its cellular effects depend on thrombin concentration and cellular cofactors
Thrombin is one of the mediators of endothelial-dependent changes in tone of underlying VSMC. This PAR1-mediated effect of thrombin comprises the release of endothelial-derived relaxing factors, including nitrous oxide (NO) and prostacyclin (various in vitro studies reviewed in Ref.34). Increased activity of endothelial nitric oxide synthase3 (eNOS) induced by local thrombin or platelet release products (serotonin, ADP) can be regarded as an endothelial defense mechanism. NO-induced VSMC relaxation improves local blood flow and counteracts the locally offensive effects of platelet released vasoconstrictive mediators like thromboxane A2. Impaired flow dilation can be regarded as an early sign of EC dysfunction (see further).34
Infusion of purified thrombin preparation in a dog hindlimb model showed dose-dependent vasodilatation, likely directly related to the effects of thrombin35 producing a two-stage flow dilatational effect, which in part may be determined by platelet activation and release of mediators like ADP/ATP. Later studies in humans showed that PAR1 activation with the agonist peptide SFLLRN (tethered ligand sequence) has differential effects on venous and arterial vasculature, showing venous constriction and arterial dilation, associated with evidence of platelet activation and tissue-type plasminogen activator (tPA) release.36 Previous studies in dogs with the same agonist peptide showed that in the coronary circulation SFLLRN causes dose-dependent transient coronary dilation, followed by more sustained vasoconstriction and signs of impaired myocardial perfusion.37
Thrombin and the PAR1 activating peptide have differential effects on calcium signalling in human brain microvascular cells,38 effects that for thrombin are dependent on concentration, with a low dose (0.1 nM) preventing calcium rise, in contrast to a high dose (10 nM). The functional differences in G-protein signalling between agonist peptides and thrombin for PAR1 activation were further dissected by McLaughlin et al.39 Bae and colleagues reported that thrombin displays endothelial-protective effects through PAR1, provided that EC protein C receptor (EPCR) is associated with caveolin-1 in lipid rafts and that its occupancy by protein C/activated protein C (APC) causes dissociation from caveolin-1 and recruitment of PAR1 to a protective pertussis-toxin sensitive G(i)-protein (Figures 1B and 2). In this way, both thrombin and APC can induce protective effects via the same receptor, PAR1.40 Whether this protective effect also requires transactivation of other PARs, for instance shown for PAR1-mediated protective effects in sepsis being dependent on PAR2 transactivation,41 remains to be further established.
2.3.3. Thrombin-mediated protein C activation at the EC surface
Early studies showed that protective effects of low doses of thrombin infusion (1 unit/kg min) in vivo are associated with rapid protein C activation.42 In addition, profibrinolytic effects were observed, caused by plasminogen activator release, but subsequent studies could not confirm a role for APC in fibrinolysis in vivo.43 In a thrombosis model in primates, a 1 h infusion of low doses of thrombin (1–2 units/kg min) reduces both arterial graft platelet deposition and fibrin incorporation in a venous-type thrombus, an effect that could be abolished by infusion of a monoclonal antibody that prevented protein C activation.44 APC formation in vivo depends on interaction between protein C and EPCR,45 while the principal driver of protein C activation is the endothelial quaternary complex of protein C bound to EPCR and thrombin, captured by thrombomodulin at the EC surface.46
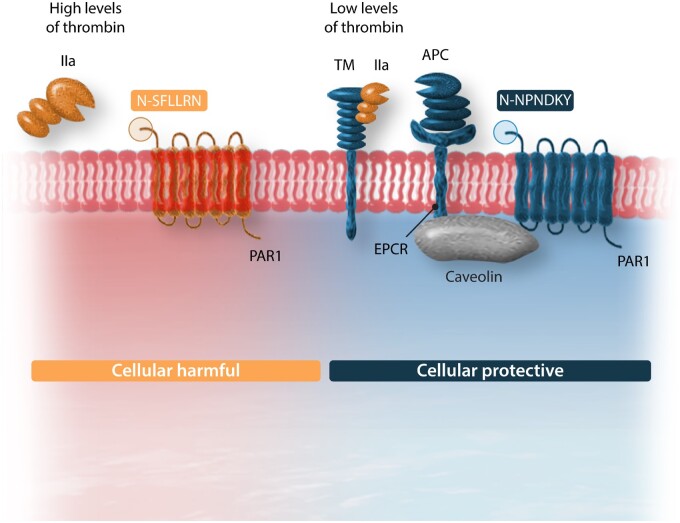
Both for thrombin and for APC, PAR1 is an important cellular signalling receptor. EC barrier function is maintained by APC in a PAR1-, as well as sphingosine 1-phosphate receptor-cross-activation, dependent manner.47 Biased stimulation of PAR1 occurs through thrombin or APC, cleaving the extracellular domain at sites that are at five amino acids distance from each other (Figure 2). While thrombin cleaves at Arg41, inducing G-protein biased signalling via extracellular signal-regulated kinases (ERK1/2) and Ras homolog family member A (RhoaA) resulting in proinflammatory effects, APC cleaves at Arg46 in a beta-Arrestin-dependent manner thereby triggering Phosphoinositide 3-kinases (PI3Ks)/protein kinase B (PKB/Akt) and Ras-related C3 botulinum toxin substrate 1 (RAC1) mediated pathways providing cytoprotective effects.48 Modifying factors include binding of thrombin to the hirudin-like sequence of PAR1, localization of PAR1 in caveolae, association with EPCR-bound protein C and dimerization with other PARs.
Figure 2.
Summary of PAR-1 biased signalling. PAR-1 can be activated by thrombin (factor IIa) or by activated protein C (APC). Thrombin cleaves PAR-1 on position R41 generating the activation sequence SFLLRN, whereas APC cleaves at position R46 revealing the tethered ligand NPNDKY. Cytoprotective effects of APC through PAR1 are mediated through Caveolin-1 interaction with EPCR in lipid rafts. APC binding to EPCR causes dissociation of Caveolin-1 and recruitment of PAR1 to a protective pertussis-toxin sensitive G(i)-protein.
Administration of APC provides cytoprotective effects in a wide range of disease models including for sepsis and ischaemic stroke,49 diabetic nephropathy,50 myocardial ischaemia-reperfusion injury,51,52 and atherosclerosis.53 A recent clinical trial with a mutated form of APC (lacking anticoagulant activity but maintaining cytoprotective properties) suggests a protective effect on the brain following ischaemic stroke.54
2.3.4. Distinct roles for factor Xa
Factor X is another key coagulation protein as it links directly to thrombin generation but also because of its active form, FXa, has independent actions through PAR activation. Under physiologic conditions, FXa may, comparably to low concentrations of thrombin, provide endothelial-protective actions, either by PAR1- or by PAR2-mediated routes (Figure 3).55 Low amounts of FXa (5 nM or higher) protect against high-dose thrombin-induced EC permeability. This effect was also seen with a PAR2 agonist peptide. Bae and colleagues56 provided further insight in the protective effects of FXa, showing that pre-treatment of EC with catalytic inactive FX (FX-S195A) allows the dissociation of EPCR from caveolin-1 (similar to protein C) and recruitment of PAR1 towards a protective pathway. In addition, FVIIa/TF-activated FX also protects via a PAR2-activated mechanism, an effect that can be mimicked by both PAR1 and PAR2 agonist peptides. Rezaie and co-workers further showed that through activation of PAR2, FXa prevented thrombin-induced EC permeability.57,58 Finally, Stavenuiter and Mosnier59 determined that FXa, like APC, also activates PAR-3, resulting in prolonged Tie-2 activation and PAR3-dependent stabilization of EC tight junctions.
Figure 3.
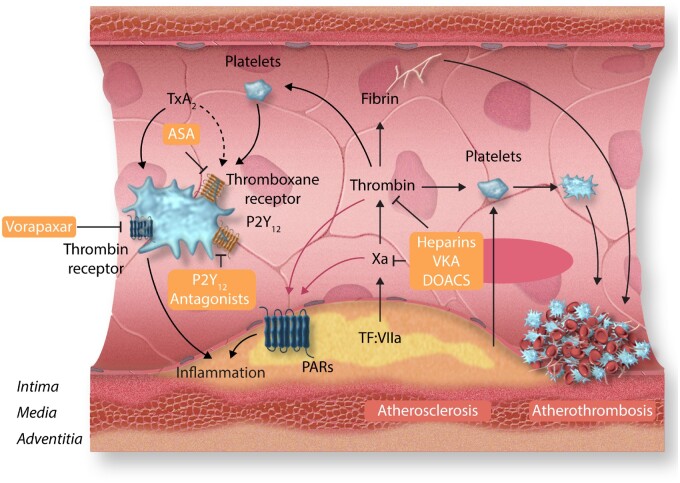
Antithrombotic and vascular-protective effects of anticoagulants and platelet inhibitors. Atherosclerosis-mediated vascular injury causes atherothrombosis through activation of coagulation and platelets. Fibrin formation can be diminished by anticoagulants including heparins, vitamin K antagonists, and direct oral anticoagulants such as the thrombin inhibitor dabigatran and the factor Xa inhibitors rivaroxaban, edoxaban, and apixaban. Platelets can be inhibited by aspirin (ASA, effecting the thromboxane A2 receptor TxA2), P2Y12 receptor antagonists clopidogrel, prasugrel, or ticagrelor, or via inhibition of the thrombin receptor PAR2 by Vorapaxar. Inhibition of thrombin, factor Xa, and platelets will diminish cellular effects through attenuated activation of the protease-activated receptors PAR1 and PAR2 on endothelial cells, vascular smooth muscle cells, and macrophages.
Summarizing, the ambient blood levels of coagulation proteins (zymogens) and their activated forms (proteases) serve at least two purposes: maintaining haemostasis and maintaining vascular patency, in particular endothelial barrier integrity.
Thrombin is a key enzyme in controlling haemostasis (platelet activation and regulating fibrin formation), while EC integrity is maintained in part by (low concentrations of) thrombin-activated PAR1-mediated vascular responses, including vasodilatation in a NO-dependent manner. More important, thrombin-mediated protein C activation yields APC-dependent cell-protective mechanisms via biased and EPCR-mediated PAR1 activation. FXa, similar to thrombin, provides cell-protective actions at relatively low concentrations in a PAR1- and PAR2-dependent manner. The presence of zymogens FX and protein C appears to enhance EC protection and the presence of EPCR provides another important protective cofactor.
3. Determinants of vascular protection: local coagulation protease concentrations and endothelial receptors EPCR and thrombomodulin
In the above vascular-protective scenarios, circulating coagulation proteins and specific cell receptors play major roles. Coagulation proteins will generally be present in sufficient amounts to serve all purposes (under physiologic conditions), although they may become rate limiting in severe deficiencies like haemophilia, where strongly reduced thrombin generation may conceptually contribute to loss in EC barrier integrity to provoke focal bleeding. However, based on the mouse studies discussed above, one may assume that such critical functions remain reasonably intact also at very low levels of clotting factors, compatible with a scenario in which low levels of FXa or thrombin activity at the EC suffice to mediate protective effects via PAR signalling, as discussed before.
When using systemic anticoagulation, levels of active proteases may be reduced (vitamin K antagonists), but remaining concentrations will probably still be sufficient to provide barrier protection, while even uncarboxylated proteins may retain some signalling properties.4 Pathophysiological conditions like sepsis, may dramatically change the haemostatic balance and reduced synthesis, ‘consumption’ and cell receptor shedding may deplete the reservoir of cell-protective proteins; in the most dramatic phenotype, disseminated intravascular coagulation, this can result in a systemic bleeding diathesis and vascular permeation due to massive EC barrier loss, aggravated by thrombocytopenia and platelet dysfunction, as well as disturbed fibrinolysis.60 Similarly, in anticoagulated patients the diminished coagulation protease ‘reserve’ may make the EC barrier function prone to perturbation in case of additional trauma. This may be an additional mechanism explaining the risk of major bleeding in anticoagulated patients.
Physiologically, the expression of EC receptors like EPCR and thrombomodulin is heterogeneous throughout the vascular bed and highly regulated. EPCR is detected on ECs of arteries and veins, most arterioles, and some capillary venules, but is undetectable on capillary endothelium.61 In contrast, thrombomodulin is expressed in the endothelium of large vessels as well as capillaries. Pro-inflammatory stimulation with endotoxin or thrombin induces mRNA for EPCR but also enhances receptor shedding in rodents.62 Reduced expression of endothelial EPCR and thrombomodulin is reported in severe sepsis,63 while EC are still morphologically intact. In contrast, overexpression of EPCR provides protection against endotoxemia.64 FVIIa binding to EPCR enhances protection against endotoxin-induced vascular leakage.65 Interestingly, recent studies suggest that FVIIa binding to EPCR, which also facilitates its uptake and transport to extravascular tissues where it remains catalytically active,66 induces PAR1 and β-Arrestin-1-mediated anti-inflammatory signalling pathways.67 In mice with low EPCR levels or wild-type mice treated with anti-EPCR antibodies, vascular permeability is markedly enhanced in particular in the brain, kidney, and lungs.68 EPCR deficiency can be functionally restored (APC cofactor functions) by so-called EPCR painting with caveolae-targeted GPI-coupled EPCR.69
Thrombomodulin is a cellular receptor with a C-type lectin domain at its N-terminus, six copies of the epidermal growth factor-like motif and serine/threonine-rich domain carrying a glycosaminoglycan external to the membrane. Thrombomodulin binding to thrombin changes thrombin’s actions towards anticoagulant and anti-inflammatory actions, through activation of protein C and thrombin-activatable fibrinolysis inhibitor. Thrombomodulin’s lectin domain has independent anti-inflammatory activity through its interaction with HMGB1.70,71 In addition to its cellular functions, recombinant thrombomodulin has anticoagulant and anti-inflammatory properties in pre-clinical models as well as clinical studies in sepsis.72–74
Shedding of (soluble) EPCR has been observed in patients with coronary heart disease,75 renal injury in lupus.76 In EC, EPCR shedding by cytokines is regulated by several MAP kinase signalling pathways.77 Several compounds that may downregulate EPCR shedding have been reported including epi-sesamin,78 piperlongumine,79 rosmarinic acid,80 and rutin.81 Similarly, shedding of thrombomodulin from EC occurs at low levels in culture and is increased upon inflammatory or toxic challenges.82,83 Many subsequent studies have documented increased levels of soluble thrombomodulin in various disease conditions.84–86 Loss in endothelial EPCR and thrombomodulin may enhance the risk of atherosclerosis, by reducing local protection against inflammation. Whether in atherosclerotic vessels, local levels of these receptors remain reduced or functionally impaired cannot yet be established with certainty due to the scarce evidence87,88 or vascular heterogeneity contributing to alterations in atherosclerotic lesion expressed thrombomodulin.89
Given their important roles in EC-regulated activation of protein C and in directing cell-protective effects of other coagulation proteases, it is likely that intact expression of EPCR and thrombomodulin is a very potent element of the endothelial barrier function. APC formation may be impaired in atherosclerosis, suggested by diminished anticoagulant response to thrombin infusion in primates,90 probably related to reduced expression of cofactors EPCR and thrombomodulin, at least on large arteries.46
4. Coagulation and EC dysfunction: first step in atherogenesis
Endothelial dysfunction is a hallmark of early stages of vascular pathology.91 It is characterized by loss of bioavailability of endothelium-derived protective factors, such as nitric oxide or prostacyclin.92 This not only impairs vasodilatation and promotes vascular remodelling but also enhances platelet adhesion and aggregation.93 In atherogenesis this primarily concerns the larger arteries that are susceptible to thromboinflammation, driven by flow conditions (velocity, shear stress) and anatomy, making arterial branching points the most vulnerable sites for atherosclerosis development.
In human vasculature, overproduction of superoxide anion by NADPH oxidases and eNOS, leading to rapid scavenging of NO, are primary mechanisms of endothelial dysfunction.94 Importantly, thrombin has been identified as a main activator of endothelial and VSMC NADPH oxidases.95 Thrombin has been shown to induce NADPH oxidases Nox2 and Nox5 in ECs and VSMCs and these oxidases are essential mediators of pathological responses of these cells to thrombin.96 This is important, as Nox5 expression is particularly enhanced in unstable plaques.97 These effects are heterogeneous but can be mediated by PAR1-dependent mechanisms.98 Importantly, pro-remodelling effects of thrombin on VSMCs are mediated by NADPH oxidase (Nox) 2 and Nox5 generated reactive oxygen species (ROS). Nox5-dependent effects may be related to the fact that this oxidase is calcium dependent and thrombin, acting on PAR1, increases cytosolic Ca2+ concentration in ECs.99
Factor Xa can also stimulate endothelial as well as vascular fibroblast oxidative stress via PAR2 activation and these effects appear to be primarily mediated by Nox1 homologue of NADPH oxidase.10,100 The role of PARs in mediating endothelial dysfunction has been widely discussed. In a number of conditions associated with endothelial dysfunction such as diabetes or atherosclerosis, increased expression of PAR1, PAR3, and PAR4 in the aorta has been identified.102 Interestingly, in the same study authors reported that direct thrombin inhibition by dabigatran significantly attenuated endothelial dysfunction independently of blood glucose levels. Numerous features of endothelial activation linked to endothelial dysfunction were prevented including inflammatory activation (expression of MCP-1 and ICAM-1) through NFκB activation.102 Thus, a proinflammatory coagulation-vascular circuit exists that is a major regulator of vascular tone, blood pressure, and endothelial function.103 For example, in conditions associated with upregulation of TF along with the thrombin-dependent EC dysfunction, integrin αMβ2- and platelet-dependent leukocyte adhesion to endothelium is promoted. This leads to vascular inflammation that promotes dysfunction mediated by activation of thrombin-driven FXI (FXI) feedback, independent of FXII.103
These studies indicate that targeting coagulation may represent an important therapeutic avenue for preventing endothelial dysfunction so may actually be effective at very early stages of atherosclerosis and vascular disease.
5. Inflammation and hypercoagulability in atherosclerosis and atherothrombosis
5.1. Thrombo-inflammation and atherothrombosis
Both chronic and acute inflammatory challenges challenge the blood and vessel wall compartments.104 This thrombo-inflammatory interaction contributes to endothelial dysfunction, loss in EC barrier function, while hypercoagulability due to concerted actions on inflammatory cells, platelets, extracellular vesicles (EVs), and coagulation proteases stimulate atherogenesis.105,106 In blood, evidence of coagulation cascade hyperactivity is detectable in patients with atherosclerosis; increased markers like d-dimer, prothrombin fragment (F)1.2, or thrombin-antithrombin complexes are oftentimes detected in blood from patients at risk of (recurrent) atherothrombotic events.
TF-driven hypercoagulability is amplified through platelet-leukocyte and EVs mediated thrombin and fibrin formation and accelerated through the contact activation system in CVD.107–109 Atherothrombosis is further determined by the potential of clots to stabilize and dissolve the net result of fibrin formation, polymerization, and fibrinolysis, as discussed elsewhere.110–113
5.2. Expression of proteins by vascular cells and the local ‘coagulome’
The inflammation-driven interaction of platelets, leukocytes, EVs, and protease-PAR signalling induced EC barrier permeation, facilitates passage of cells, EVs, and proteins to the subendothelial space. Circulating cells including monocytes also express TF, FVII, and FX.114–118 Other cells like neutrophils and anuclear platelets may either produce TF, or accumulate TF, by EVs transfer from other cells (discussed in Ref.119). In the intima, VSMCs that undergo the phenotypical switch from contractile to synthetic, also produce TF. Many coagulation proteins are expressed within the vessel wall; one of the first findings is the constitutive expression of TF by fibroblasts in the adventitia, previously referred to as a haemostatic envelope, protecting the larger arteries from bleeding upon trauma.118,120 While this primary protective function may be relevant, also other cell signalling functions of TF, triggered by binding FVII(a), may be important. Despite the well-known function of tissue factor in activation of coagulation in arterial thrombosis, the role of tissue factor signalling in development and progression of atherosclerosis remains to be elucidated. Experimental atherosclerosis is not altered by low expression (1% human level on murine deficiency or 50% murine levels) of tissue factor, sufficient to maintain haemostasis. Overall, the contribution of tissue factor on development of atherosclerosis is mainly through the signalling pathway of tissue factor: factor VIIa via PAR-2, as reviewed by Grover and Mackman.121
TF and FVII are also locally expressed by VSMC and macrophages, transformed from monocytes during their migration into the vessel wall; administration of TFPI can inhibit monocyte chemoattraction.122 This way many active coagulation components are either constitutively expressed during atherogenesis or may be induced through inflammatory stimuli, supporting local thrombin and fibrin formation, from fibrinogen transferred across the (permeable) EC barrier. Fibrin formation and cleavage into fragments is a characteristic feature of atherosclerosis and probably modulates several inflammatory mechanisms.123,124
As discussed in previous work, various interactions may take place between proteases including FXa and thrombin with PARs expressed at all cells within the arterial vessel wall. Whereas relatively low concentrations of FXa and thrombin are cytoprotective, as discussed in previous sections, increased concentrations of the same proteases may become offensive if conditions allow. These conditions include a pro-inflammatory environment and loss or downregulation of protective receptors and anticoagulant molecules as discussed for the EPCR/TM/PC system.
PAR2 is one of the key receptors involved in atherogenesis, as PAR2 null mice are protected against atherosclerosis; also, those with only haematopoietic deficiency in PAR2 (macrophages) are similarly protected.125 Activated FX or an agonist peptide based on a FXa amino acid sequence induces inflammatory molecules and stimulates lipid uptake by bone marrow-derived macrophages from wild-type mice, but not by PAR2-deficient macrophages. Genes induced by FXa-mediated degradation of the nuclear inhibitor IκBα in macrophages include an array of proinflammatory cytokines including Il-1β, IL-6, and TNFα.125 Studies from the immunology field show that tumour-associated macrophages synthesize the TF ligands FVII and FX.126,127 Recent studies show that FX produced by monocytes/macrophages activates PAR2 in a local tumour environment impeding anti-tumour activity; this reaction could be abolished by the direct FXa inhibitor rivaroxaban.128 It should be noted that while coagulation proteases are important, other enzymes can be involved and several studies showed that trypsin,129 cathepsin S,130 and tryptase131 also activate PAR2, probably in a tissue-dependent manner.
Extrapolating these data to atherosclerosis it seems likely that macrophage-derived FX also regulates proinflammatory mechanisms through PAR2 activation on these cells, as well as on VSMC. For instance, PAR2 deficiency on VSMC is associated with reduced Ccl2 and Cxcl1 mRNA expression and protein release, limiting migration of monocytes.132 Inhibition of FXa, hence thrombin generation, may be an effective way of inhibiting a variety of thromboinflammatory reactions and associated disease, including atherosclerosis, fibrosis, heart, and kidney failure.
6. Atherothrombosis
6.1 Pleiotropic nature of atherothrombosis—from plaque rupture to plaque erosion
Increasing use of statins, reduced prevalence of smoking, advances in intravascular imaging, and a better understanding of the pleiotropic nature of the process of atherothrombosis led to reports of a shift in the natural history of thrombotic events developing as complications of atherosclerosis. While plaque instability and rupture have been the focus of our diagnostic and therapeutic attention for past decades, only recently, we realized the critical importance of plaque erosion in this process.3,133–136 Erosion prone plaques are proteoglycan/glycosaminoglycan rich, have relatively small lipid cores but many smooth muscle cells with neutrophils and neutrophil extracellular traps (NETs).137 Inflammation and metabolic changes in the EC leading to endothelial–mesenchymal transition138,139 and detachment promoted by non-laminar flow in concert with basement membrane damage are the key factors contributing to plaque erosion. The rupture-prone plaque is characterized by a thin, collagen poor fibrous cap, large lipid core, macrophage-driven inflammation in plaque shoulder regions.140 Importantly, while both plaque erosion and rupture are accompanied by intense coagulation activation, chronically eroded plaques are associated with platelet-rich white thrombus whereas ruptured plaques are associated with fibrin-rich red thrombus.3
The changing insight in atherosclerosis-induced thrombosis has profound clinical implications. In the past, the most common clinical presentation of acute coronary ischaemia was ST-elevation myocardial infarction (STEMI) but this is now less common than non-STEMI (NSTEMI).3 Reflecting this shift, non-invasive therapies that target the pathology of plaque erosion pathology may become more important, including anti-inflammatory therapy with the use of canakinumab141 or colchicine,142 and DPI with the combination of APT and anticoagulant therapy.5 This is because while the thrombus is platelet rich, plaque erosion is associated with local activation of coagulation. This includes not only substances released by platelets and damaged basal membranes but also generation of NETs, which can also acquire proteins from extra-neutrophilic sources, including tissue factor (TF) that through activation of FVII and formation of FXa lead to local thrombin generation (Figure 1A).143–145
6.2 Peripheral and coronary atherothrombosis—the same or different?
A related issue of importance is that while multi-level atherosclerosis is clinically considered a more advanced form of the same disease, an increasing body of evidence suggests that in different vascular beds mechanisms of atherothrombosis may be different. In a recent histopathological study of critical limb ischaemia, it has been shown that in PAD thrombotic luminal occlusion associated with insignificant atherosclerosis is much more commonly observed than in coronary arteries.146 This links to earlier considerations of the role of plaque erosion as a primary mechanism in the peripheral circulation. Moreover, differences were observed within peripheral PAD itself, with a more prominent role of atherothrombosis in lower locations (infra-popliteal) of critical limb ischaemia. These clinical observations, while still poorly defined mechanistically, may support a greater preventive role of antithrombotic agents in PAD.147
7. Preclinical evidence for vascular protection
Many preclinical studies done in (transgenic) mice have elucidated the important role of blood coagulation proteins in atherogenesis. These studies, virtually all carried out in apoE−/− mice, showed a general pattern of increased atherosclerosis in apoE−/− mice backcrossed with animals expressing thrombophilic traits, while mice that expressed lower levels of procoagulant proteins were partially protected (discussed in Ref.148). While thrombin-triggered PAR activation may be an important mechanism, the impact of other ligands cannot be ruled out. First, as discussed, FVIIa, FXa, and thrombin each or in concert activate different PARs in a variety of cells; variations in the levels of these proteases may have considerable impact on degree and direction of PAR-mediated signalling. Second, crosstalk between the PARs and other cellular cofactors (e.g. see the important impact of thrombomodulin and EPCR expression at EC related to biased PAR signalling discussed before), is involved. Third, specific proteases may contribute unique procoagulant properties, independent from thrombin; an example is FXIa that inactivates TFPI, enhanced by platelet-derived short-chain polyphosphates.149,150 Another example is fibrinogen; its absence in null mice does not protect against atherosclerosis,151 but it can affect the phenotype of lesions in susceptible mice, probably through loss of interactions with lipoproteins.152
Inhibiting FXa or thrombin with direct inhibitors consistently attenuates atherosclerosis in apoE−/− mice.148 Studies with dabigatran revealed differences in plaque phenotypes linked to reduced or increased plaque stability,53,153 probably related to age and timing of intervention. For FXa inhibition, data are consistent with reduced atherosclerosis and increased plaque stability, although the observed effects vary per study depending on the anticoagulant dose applied.154–156 Inhibition of FXa in apoE−/− mice with existing atherosclerosis attenuates plaque volume and stabilizes plaque phenotype.156 Collectively, the data point to a substantial influence of coagulation proteases on atherosclerotic plaque formation and plaque phenotype. Inhibiting FXa is one potent therapeutic avenue to attenuate and stabilize atherosclerotic lesions. Clinical studies also provide supporting evidence for these observations although much more and robust evidence needs to be obtained.157 As recently reviewed, targeting both platelets and coagulation proteases is conceptually attractive. Platelets are key players in atherosclerosis and atherothrombosis, while the above preclinical studies indicate the importance of coagulation proteases.158 The sum of antiplatelet and anticoagulant activities is likely to amplify the potency of antithrombotic therapy aimed at preventing atherothrombosis and thromboinflammation.
8. Clinical translation: trials of the combination of rivaroxaban and aspirin in chronic atherosclerosis
Several recent trials provide strong support for the results of experimental and animal studies that combining platelet inhibition with anticoagulation not only produces a more intense antithrombotic effect but also enhances vascular protection.5–7 Previous studies evaluating the combination of long-term warfarin and aspirin suggested the potential of dual pathway therapy to reduce major adverse cardiovascular events in patients with a history of coronary artery disease.159 However, the reduction in non-fatal cardiovascular events with combination of warfarin and aspirin compared with aspirin alone was accompanied by a large excess of serious bleeding and there was no overall mortality benefit. These results dampened enthusiasm for adopting a warfarin-based dual pathway regimen for long-term management of patients with atherosclerosis.
The advent of the direct acting oral anticoagulants (DOACs) provided for the first time the potential to selectively target individual coagulation proteases with agents that produced consistent and predictable response when given in fixed doses without routine coagulation monitoring.160 Two FXa inhibitors, apixaban and rivaroxaban, have been tested on a background of APT in patients with a recent acute coronary syndrome but only rivaroxaban was shown to be effective. The APPRAISE trial tested apixaban using the same doses that were shown to be effective for stroke prevention in patients with atrial fibrillation (5 mg twice daily or 2.5 mg twice daily in those meeting prespecified criteria for dose reduction), but was stopped early because of excess bleeding.161 The ATLAS ACS 2–TIMI 51 trial tested rivaroxaban at lower doses (2.5 or 5 mg twice daily) than those shown to be effective for stroke prevention in patients with atrial fibrillation, and demonstrated a reduction in both non-fatal cardiovascular events and mortality.162 The magnitude of the benefit achieved using only a very low intensity of anticoagulation raised the possibility that the effects of rivaroxaban extended beyond effects on coagulation to provide vascular protection.
COMPASS tested rivaroxaban at the same low doses that were successfully evaluated in ATLAS, rivaroxaban 2.5 mg in combination with aspirin 100 mg once daily and rivaroxaban 5 mg twice daily alone, compared with aspirin 100 mg once daily, in patients with chronic coronary artery disease or PAD. Patients with a history of stroke ≥1 month ago could be included as long as they did not have a history of lacunar or haemorrhagic stroke.163
The trial randomized 27 395 patients from 602 centres in 33 countries, and the intent was to continue follow-up until 2200 patients had experienced a primary outcome, the composite of cardiovascular death, stroke, or myocardial infarction. However, after a mean of 23 months of follow-up, and when only just over 50% of primary outcome events had occurred, the Data Safety Monitoring Board recommended discontinuation of the antithrombotic arms of the trial because of clear evidence of benefit of the combination of rivaroxaban 2.5 mg twice daily and aspirin 100 mg once daily compared with aspirin alone.164 Rivaroxaban alone given at a dose of 5 mg twice-daily was not superior to aspirin 100 mg once-daily.
The main results of the COMPASS trial5 comparison between rivaroxaban 2.5 mg twice-daily and aspirin 100 mg once-daily are summarized in Table 1.
Table 1.
COMPASS trial: outcomes comparing the combination of rivaroxaban 2.5 mg twice daily and aspirin 100 mg once-daily with aspirin 100 mg once-daily
| Outcome | Rivaroxaban 2.5 mg twice-daily plus aspirin 100 mg once-daily vs. aspirin 100 mg once-daily N = 9152 | Aspirin 100 mg once-daily N = 9126 | HR (95% CI) |
|---|---|---|---|
| CV death, stroke, or myocardial infarction | 379 (4.1%) | 496 (5.4%) | 0.76 (0.66–0.86) |
| CV death | 160 (1.7) | 203 (2.2%) | 0.78 (0.64–0.96) |
| Stroke | 83 (0.9%) | 142 (1.6) | 0.58 (0.44–0.76) |
| Myocardial infarction | 178 (1.9%) | 205 (2.2%) | 0.86 (0.70–1.05) |
| Mortality | 313 (3.4%) | 378 (4.1%) | 0.82 (0.71–0.96) |
| Venous thromboembolism | 25 (0.3%) | 41 (0.4%) | 0.61 (0.37–1.00) |
| CV hospitalization | 1303 (14.2%) | 1394 (15.3%) | 0.92 (0.86–1.00) |
The combination of rivaroxaban and aspirin compared with aspirin reduced major adverse cardiovascular events by 24%, including a 22% reduction in cardiovascular death, 42% reduction in stroke, and a 14% reduction in myocardial infarction. Bleeding was increased by 70%, mainly from the gastrointestinal tract, and although there was a consistent pattern of increased fatal, critical organ and surgical site bleeding, these bleeds were infrequent and not significantly increased. Importantly, the combination produced a clear net clinical benefit, as evidenced by reductions in a composite outcome that included the primary efficacy outcome and serious bleeding, as well as reduction in all-cause mortality.165 The greatest benefit of the combination was in patients at highest baseline risk, including those with polyvascular disease (i.e. both coronary artery disease and PAD), heart failure, diabetes, or chronic kidney disease.166
The COMMANDER HF trial compared rivaroxaban 2.5 mg twice daily with placebo in 5022 patients with recently decompensated chronic heart failure and underlying coronary artery disease.6 The majority of patients was receiving APT (aspirin 93.1%, dual APT 34.8%). The trial involved 628 centres in 32 countries. The main results are summarized in Table 2. After a median follow-up of 21 months, rivaroxaban compared with placebo did not reduce the primary outcome, a composite of death, myocardial infarction, or stroke, and did not increase bleeding. There was a significant 34% reduction in stroke as well as a promising pattern of fewer myocardial infarctions, but in the primary composite outcome this signal was obscured by mortality which accounted for >80% of primary events and was not reduced by rivaroxaban. In a separate exploratory analysis, rivaroxaban compared with placebo significantly reduced the composite of arterial and venous events by 17%.
Table 2.
COMMANDER HF trial: overall results comparing rivaroxaban 2.5 mg twice-daily and with placebo on a background of standard care
| Outcome | Rivaroxaban 2.5 mg twice-daily | Placebo twice-daily | HR (95% CI) |
|---|---|---|---|
| N = 2507 | N = 2515 | ||
| Death, myocardial infarction, or stroke | 626 (25%) | 658 (26.2%) | 0.94 (0.84–1.05) |
| Death | 546 (21.8%) | 556 (22.1%) | 0.98 (0.87–1.10) |
| Myocardial infarction | 98 (3.9%) | 118 (4.7%) | 0.83 (0.63–1.08) |
| Stroke | 51 (2.0%) | 76 (3.0%) | 0.66 (0.47–0.95) |
| Composite of thromboembolic events: myocardial infarction, ischaemic stroke, sudden/unwitnessed deaths, symptomatic PE, symptomatic DVT | 328 (13.1%) | 390 (15.5%) | 0.83 (0.72–0.96) |
The VOYAGER PAD trial compared rivaroxaban 2.5 mg twice daily with placebo on background of standard APT (aspirin alone or dual APT) in 6564 patients with PAD who had recently undergone a lower limb revascularization procedure.7 The trial was performed in 542 sites in 34 countries.
The main results are summarized in Table 3. After a median follow-up of 28 months, rivaroxaban compared with placebo reduced the primary outcome, a composite of acute limb ischaemia, major amputation for vascular causes, myocardial infarction, ischaemic stroke, or CV death, by 15%, including a 33% reduction in acute limb ischaemia. There was no reduction in mortality and no significant increase in TIMI major bleeding although ISTH major bleeding was increased by 42%. Results were consistent when separately examined in patients receiving single compared with dual APT.
Table 3.
VOYAGER PAD trial: overall results comparing rivaroxaban 2.5 mg twice-daily with placebo on a background of antiplatelet therapy
| Outcome | Rivaroxaban 2.5 mg twice-daily | Placebo twice-daily | HR (95% CI) |
|---|---|---|---|
| N = 3286 | N = 3278 | ||
| Acute limb ischaemia, major amputation for vascular causes, myocardial infarction, ischaemic stroke, or CV death | 508 (15.5%) | 584 (17.8%) | 0.85 (0.76–0.96) |
| Acute limb ischaemia | 155 (4.7%) | 227 (6.9%) | 0.67 (0.55–0.82) |
| Major amputation for vascular causes | 103 (3.1%) | 115 (3.5%) | 0.89 (0.68–1.16) |
| Myocardial infarction | 131 (4.0%) | 148 (4.5%) | 0.88 (0.70–1.12) |
| Ischaemic stroke | 71 (2.2%) | 82 (2.5%) | 0.87 (0.63–1.19) |
| CV death | 199 (6.1%) | 174 (5.3%) | 1.14 (0.93–1.40) |
| Mortality | 321 (9.8%) | 297 (9.1%) | 1.08 (0.92–1.27) |
| Venous thromboembolism | 25 (0.8%) | 41 (1.3%) | 0.61 (0.37–1.00) |
It is not possible to establish with certainty from the results of these trials whether the benefits of combination therapy are explained solely by increased intensity of antithrombotic therapy or whether the combination also provides vascular protection through an effect on the vascular endothelium. The reduction in venous thromboembolism obtained by using rivaroxaban on top of APT in both the COMPASS and the VOYAGER trials is strongly suggesting of a direct antithrombotic effect as this result is unlikely to be explained by ‘vascular protection’. However, the context of previously summarized experimental and animal research, as well as the results of the ATLAS ACS 2–TIMI 51trial,162 the unexpectedly large reduction in major adverse cardiovascular events of adding a low dose of rivaroxaban to aspirin in the COMPASS trial and the consistent benefits of low-dose rivaroxaban in other trials is consistent with the conclusion that rivaroxaban has effects beyond inhibition of coagulation.
9. Conclusions and future perspectives
Atherothrombotic complications of atherosclerotic vascular disease can at least be limited by a strategy of DPI, aimed at both platelets and coagulation proteases, in particular FXa (and consequent thrombin formation) (Figure 2). The fact that this strategy provides substantial clinical benefit may be due to a combination of factors: (i) patients with atherosclerosis have signs of active thrombo-inflammation, not only in blood but also in the arterial vessel wall compartment; (ii) pre-clinical data provide robust evidence for a causal association between hypercoagulability and atherosclerosis towards an unstable phenotype, mediated by PAR directed cell signalling; (iii) attenuating thrombo-inflammation likely dampens plaque instability and in doing so, may actually reverse the risk of atherothrombotic complications; and (iv) DOACs inhibit or even reverse severity of atherosclerosis in experimental mouse models through attenuating thrombo-inflammatory mechanisms.
It is an important question whether higher doses of DOACs such as used to prevent ischaemic stroke in AF also provide such atheroprotective effects? Although some studies suggest this may be the case, such extrapolation may not be obvious. Maybe low concentrations of anticoagulants are just sufficient to dampen thromboinflammation, while higher doses prevent thrombosis but may theoretically undermine some of the protein C activating mechanisms that are so critical in host defense. An additional advantage of DOACs over VKA may be the fact that while VKA also lowers functional activity of proteins C and S, DOACs do not directly affect these natural anticoagulants.
Preventing atherothrombosis through a potent anticoagulant action remains obviously a key property of any anticoagulant and irrespective of the differences, DOAC and VKA are equally potent in that regard. Potency however comes at a price of bleeding and while this may relate to haemostasis impairment, undermining the vascular-protective properties of coagulation and in particular the protein C system, may be an additional reason for the dose dependent increases in bleeding and the difference in organ specific bleeds, between DOACs and VKA.
Given the complexity of the coagulation-PAR-EC-vessel wall interactions, where even a limited number of proteases and cell receptors may have sheer infinite different signalling interactions, depending on systemic and local conditions, simple answers to some of the raised questions are impossible to give. Still, appreciating the complexity of coagulation proteases and their cellular effects provides a starting point for better targeted and tailored vascular-protective medication, ultimately removing bleeding side effects that remain critically linked to any type of antithrombotic strategy.
Acknowledgements
H.t.C. and H.M.H.S. receive grant support by the Netherlands Heart Foundation (CVON2014-09), Reappraisal of Atrial Fibrillation: Interaction between HyperCoagulability, Electrical Remodeling, and Vascular Destabilisation in the Progression of Atrial Fibrillation (RACE V), and from REG-MED XB: Cardiovascular Moonshot. H.t.C. was supported by a fellowship of the Gutenberg University Mainz. J.E. holds a mid-career award from the Heart and Stroke Foundation and a foundation grant from the Canadian Institutes for Health Research.
Conflict of interest: H.t.C. and H.M.H.S. received funding for research from Bayer and Pfizer and they are stockholders in Coagulation Profile. H.t.C. is consultant for Alveron. J.E. has received honoraria and/or research support from Bayer, BI, BMS, DSI, Janssen, and Pfizer.
References
- 1.WHO CVD Risk Chart Working Group. World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions. Lancet Glob Health 2019;7:e1332–e1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nakahara T, Dweck MR, Narula N, Pisapia D, Narula J, Strauss HW. Coronary artery calcification: from mechanism to molecular imaging. JACC: Cardiovasc Imaging 2017;10:582–593. [DOI] [PubMed] [Google Scholar]
- 3. Libby P, Pasterkamp G, Crea F, Jang I-K.. Reassessing the mechanisms of acute coronary syndromes: the ‘vulnerable plaque’ and superficial erosion. Circ Res 2019;124:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spronk HMH, Jong AD, Crijns HJ, Schotten U, Van Gelder IC, Ten Cate H.. Pleiotropic effects of factor Xa and thrombin: what to expect from novel anticoagulants. Cardiovasc Res 2014;101:344–351. [DOI] [PubMed] [Google Scholar]
- 5. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Widimsky P, Hori M, Avezum A, Piegas LS, Branch KRH, Probstfield J, Bhatt DL, Zhu J, Liang Y, Maggioni AP, Lopez-Jaramillo P, O’Donnell M, Kakkar AK, Fox KAA, Parkhomenko AN, Ertl G, Störk S, Keltai M, Ryden L, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim J-H, Tonkin AM, Lewis BS, Felix C, Yusoff K, Steg PG, Metsarinne KP, Cook Bruns N, Misselwitz F, Chen E, Leong D, Yusuf S.. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017;377:1319–1330. [DOI] [PubMed] [Google Scholar]
- 6. Zannad F, Anker SD, Byra WM, Cleland JGF, Fu M, Gheorghiade M, Lam CSP, Mehra MR, Neaton JD, Nessel CC, Spiro TE, Veldhuisen DV, Greenberg B; COMMANDER HF Investigators. Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med 2018;379:1332–1342. [DOI] [PubMed] [Google Scholar]
- 7. Bonaca MP, Bauersachs RM, Anand SS, Debus ES, Nehler MR, Patel MR, Fanelli F, Capell WH, Diao L, Jaeger N, Hess CN, Pap AF, Kittelson JM, Gudz I, Mátyás L, Krievins DK, Diaz R, Brodmann M, Muehlhofer E, Haskell LP, Berkowitz SD, Hiatt WR.. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med 2020;382:1994–2004. [DOI] [PubMed] [Google Scholar]
- 8. Khan AA, Lip GYH.. The prothrombotic state in atrial fibrillation: pathophysiological and management implications. Cardiovasc Res 2019;115:31–45. [DOI] [PubMed] [Google Scholar]
- 9. Versteeg HH, Heemskerk JWM, Levi M, Reitsma PH.. New fundamentals in hemostasis. Physiol Rev 2013;93:327–358. [DOI] [PubMed] [Google Scholar]
- 10. Gur-Cohen S, Itkin T, Chakrabarty S, Graf C, Kollet O, Ludin A, Golan K, Kalinkovich A, Ledergor G, Wong E, Niemeyer E, Porat Z, Erez A, Sagi I, Esmon CT, Ruf W, Lapidot T.. PAR1 signaling regulates the retention and recruitment of EPCR-expressing bone marrow hematopoietic stem cells. Nat Med 2015;21:1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nguyen TS, Lapidot T, Ruf W.. Extravascular coagulation in hematopoietic stem and progenitor cell regulation. Blood 2018;132:123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luca CD, De Luca C, Virtuoso A, Maggio N, Papa M.. Neuro-coagulopathy: blood coagulation factors in central nervous system diseases. Int J Mol Sci. 2017;18:2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ren D, Giri H, Li J, Rezaie AR.. The cardioprotective signaling activity of activated protein C in heart failure and ischemic heart diseases. Int J Mol Sci 2019;20:1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gofrit SG, Shavit-Stein E.. The neuro-glial coagulonome: the thrombin receptor and coagulation pathways as major players in neurological diseases. Neural Regen Res 2019;14:2043–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bauer KA, Weiss LM, Sparrow D, Vokonas PS, Rosenberg RD.. Aging-associated changes in indices of thrombin generation and protein C activation in humans. Normative Aging Study. J Clin Invest 1987;80:1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kornberg A, Francis CW, Marder VJ.. Plasma crosslinked fibrin polymers: quantitation based on tissue plasminogen activator conversion to D-dimer and measurement in normal and patients with acute thrombotic disorders. Blood 1992;80:709–717. [PubMed] [Google Scholar]
- 17. Cadroy Y, Pierrejean D, Fontan B, Sié P, Boneu B.. Influence of aging on the activity of the hemostatic system: prothrombin fragment 1 + 2, thrombin-antithrombin III complexes and D-dimers in 80 healthy subjects with age ranging from 20 to 94 years. Nouv Rev Fr Hematol 1992;34:43–46. [PubMed] [Google Scholar]
- 18. Bauer KA, Kass BL, ten CH, Hawiger JJ, Rosenberg RD.. Factor IX is activated in vivo by the tissue factor mechanism. Blood 1990;76:731–736. [PubMed] [Google Scholar]
- 19. Aasrum M, Prydz H.. Gene targeting of tissue factor, factor X, and factor VII in mice: their involvement in embryonic development. Biochemistry 2002;67:25–32. [DOI] [PubMed] [Google Scholar]
- 20. Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van Vlaenderen I, Demunck H, Kasper M, Breier G, Evrard P, Müller M, Risau W, Edgington T, Collen D.. Role of tissue factor in embryonic blood vessel development. Nature 1996;383:73–75. [DOI] [PubMed] [Google Scholar]
- 21. Bugge TH, Xiao Q, Kombrinck KW, Flick MJ, Holmbäck K, Danton MJ, Colbert MC, Witte DP, Fujikawa K, Davie EW, Degen JL.. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci U S A 1996;93:6258–6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toomey JR, Kratzer KE, Lasky NM, Stanton JJ, Broze GJ Jr.. Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood 1996;88:1583–1587. [PubMed] [Google Scholar]
- 23. Parry GC, Mackman N.. Mouse embryogenesis requires the tissue factor extracellular domain but not the cytoplasmic domain. J Clin Invest 2000;105:1547–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Parry GC, Erlich JH, Carmeliet P, Luther T, Mackman N.. Low levels of tissue factor are compatible with development and hemostasis in mice. J Clin Invest 1998;101:560–569.9449688 [Google Scholar]
- 25. Rosen ED, Chan JC, Idusogie E, Clotman F, Vlasuk G, Luther T, Jalbert LR, Albrecht S, Zhong L, Lissens A, Schoonjans L, Moons L, Collen D, Castellino FJ, Carmeliet P.. Mice lacking factor VII develop normally but suffer fatal perinatal bleeding. Nature 1997;390:290–294. [DOI] [PubMed] [Google Scholar]
- 26. Cui J, O’Shea KS, Purkayastha A, Saunders TL, Ginsburg D.. Fatal haemorrhage and incomplete block to embryogenesis in mice lacking coagulation factor V. Nature 1996;384:66–68. [DOI] [PubMed] [Google Scholar]
- 27. Xue J, Wu Q, Westfield LA, Tuley EA, Lu D, Zhang Q, Shim K, Zheng X, Sadler JE.. Incomplete embryonic lethality and fatal neonatal hemorrhage caused by prothrombin deficiency in mice. Proc Natl Acad Sci U S A 1998;95:7603–7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suh TT, Holmbäck K, Jensen NJ, Daugherty CC, Small K, Simon DI, Potter S, Degen JL.. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev 1995;9:2020–2033. [DOI] [PubMed] [Google Scholar]
- 29. Muramatsu I, Laniyonu A, Moore GJ, Hollenberg MD.. Vascular actions of thrombin receptor peptide. Can J Physiol Pharmacol 1992;70:996–1003. [DOI] [PubMed] [Google Scholar]
- 30. McNamara CA, Sarembock IJ, Gimple LW, Fenton JW II, Coughlin SR, Owens GK.. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J Clin Invest 1993;91:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rezaie AR. Protease-activated receptor signalling by coagulation proteases in endothelial cells. Thromb Haemost 2014;112:876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Posma JJN, Posthuma JJ, Spronk HMH.. Coagulation and non-coagulation effects of thrombin. J Thromb Haemost 2016;14:1908–1916. [DOI] [PubMed] [Google Scholar]
- 33. Borissoff JI, Spronk HMH, Heeneman S, Cate HT.. Is thrombin a key player in the ‘coagulation-atherogenesis’ maze? Cardiovasc Res 2009;82:392–403. [DOI] [PubMed] [Google Scholar]
- 34. Vanhoutte PM, Shimokawa H, Feletou M, Tang EHC.. Endothelial dysfunction and vascular disease – a 30th anniversary update. Acta Physiol 2017;219:22–96. [DOI] [PubMed] [Google Scholar]
- 35. Joyner WL, Yonce LR, Iatridis PG.. Vasodilator response in the hindlimb (dog) to various thrombin preparations. Am J Physiol 1973;225:487–492. [DOI] [PubMed] [Google Scholar]
- 36. Gudmundsdóttir IJ, Megson IL, Kell JS, Ludlam CA, Fox KAA, Webb DJ, Newby DE.. Direct vascular effects of protease-activated receptor type 1 agonism in vivo in humans. Circulation 2006;114:1625–1632. [DOI] [PubMed] [Google Scholar]
- 37. Damiano BP, Cheung WM, Mitchell JA, Falotico R.. Cardiovascular actions of thrombin receptor activation in vivo. J Pharmacol Exp Ther 1996;279:1365–1378. [PubMed] [Google Scholar]
- 38. Kim YV, Di Cello F, Hillaire CS, Kim KS.. Differential Ca2+ signaling by thrombin and protease-activated receptor-1-activating peptide in human brain microvascular endothelial cells. Am J Physiol Cell Physiol 2004;286:C31–C42. [DOI] [PubMed] [Google Scholar]
- 39. McLaughlin JN, Shen L, Holinstat M, Brooks JD, Dibenedetto E, Hamm HE.. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem 2005;280:25048–25059. [DOI] [PubMed] [Google Scholar]
- 40. Bae J-S, Yang L, Manithody C, Rezaie AR.. The ligand occupancy of endothelial protein C receptor switches the protease-activated receptor 1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood 2007;110:3909–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaneider NC, Leger AJ, Agarwal A, Nguyen N, Perides G, Derian C, Covic L, Kuliopulos A. ‘ Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol 2007;8:1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Comp PC, Jacocks RM, Ferrell GL, Esmon CT.. Activation of protein C in vivo. J Clin Invest 1982;70:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Colucci M, Stassen JM, Collen D.. Influence of protein C activation on blood coagulation and fibrinolysis in squirrel monkeys. J Clin Invest 1984;74:200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hanson SR, Griffin JH, Harker LA, Kelly AB, Esmon CT, Gruber A.. Antithrombotic effects of thrombin-induced activation of endogenous protein C in primates. J Clin Invest 1993;92:2003–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taylor FB Jr, Peer GT, Lockhart MS, Ferrell G, Esmon CT.. Endothelial cell protein C receptor plays an important role in protein C activation in vivo. Blood 2001;97:1685–1688. [DOI] [PubMed] [Google Scholar]
- 46. Esmon CT. The protein C pathway. Chest 2003;124:26S–32S. [DOI] [PubMed] [Google Scholar]
- 47. Feistritzer C, Riewald M.. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood 2005;105:3178–3184. [DOI] [PubMed] [Google Scholar]
- 48. Mosnier LO, Sinha RK, Burnier L, Bouwens EA, Griffin JH.. Biased agonism of protease-activated receptor 1 by activated protein C caused by noncanonical cleavage at Arg46. Blood 2012;120:5237–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sinha RK, Wang Y, Zhao Z, Xu X, Burnier L, Gupta N, Fernández JA, Martin G, Kupriyanov S, Mosnier LO, Zlokovic BV, Griffin JH.. PAR1 biased signaling is required for activated protein C in vivo benefits in sepsis and stroke. Blood 2018;131:1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MAF, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP.. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med 2007;13:1349–1358. [DOI] [PubMed] [Google Scholar]
- 51. Wang J, Yang L, Rezaie AR, Li J.. Activated protein C protects against myocardial ischemic/reperfusion injury through AMP-activated protein kinase signaling. J Thromb Haemost 2011;9:1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Loubele STBG, Spek CA, Leenders P, van Oerle R, Aberson HL, Hamulyák K, Ferrell G, Esmon CT, Spronk HMH, ten Cate H.. Activated protein C protects against myocardial ischemia/reperfusion injury via inhibition of apoptosis and inflammation. Arterioscler Thromb Vasc Biol 2009;29:1087–1092. [DOI] [PubMed] [Google Scholar]
- 53. Borissoff JI, Otten JJT, Heeneman S, Leenders P, van Oerle R, Soehnlein O, Loubele STBG, Hamulyák K, Hackeng TM, Daemen MJAP, Degen JL, Weiler H, Esmon CT, van Ryn J, Biessen EAL, Spronk HMH, ten Cate H.. Genetic and pharmacological modifications of thrombin formation in apolipoprotein e-deficient mice determine atherosclerosis severity and atherothrombosis onset in a neutrophil-dependent manner. PLoS One 2013;8:e55784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lyden P, Pryor KE, Coffey CS, Cudkowicz M, Conwit R, Jadhav A, Sawyer RN, Claassen J, Adeoye O, Song S, Hannon P, Rost NS, Hinduja A, Torbey M, Lee J-M, Benesch C, Rippee M, Rymer M, Froehler MlT, Clarke Haley E, Johnson M, Yankey J, Magee K, Qidwai J, Levy H, Mark Haacke E, Fawaz M, Davis TP, Toga AW, Griffin JH, Zlokovic BV, the NeuroNEXT Clinical Trials Network NN104 Investigators. Final results of the RHAPSODY trial: a multi-center, phase 2 trial using a continual reassessment method to determine the safety and tolerability of 3K3A-APC, a recombinant variant of human activated protein C, in combination with tissue plasminogen activator, mechanical thrombectomy or both in moderate to severe acute ischemic stroke. Ann Neurol 2019;85:125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Feistritzer C, Lenta R, Riewald M.. Protease-activated receptors-1 and-2 can mediate endothelial barrier protection: role in factor Xa signaling. J Thromb Haemost 2005;3:2798–2805. [DOI] [PubMed] [Google Scholar]
- 56. Bae J-S, Yang L, Rezaie AR.. Factor X/Xa elicits protective signaling responses in endothelial cells directly via PAR-2 and indirectly via endothelial protein C receptor-dependent recruitment of PAR-1. J Biol Chem 2010;285:34803–34812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rana S, Yang L, Hassanian SM, Rezaie AR.. Determinants of the specificity of protease-activated receptors 1 and 2 signaling by factor Xa and thrombin. J Cell Biochem 2012;113:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Manithody C, Yang L, Rezaie AR.. Identification of exosite residues of factor Xa involved in recognition of PAR-2 on endothelial cells. Biochemistry 2012;51:2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stavenuiter F, Mosnier LO.. Noncanonical PAR3 activation by factor Xa identifies a novel pathway for Tie2 activation and stabilization of vascular integrity. Blood 2014;124:3480–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Levi M, Ten Cate H.. Disseminated intravascular coagulation. N Engl J Med 1999;341:586–592. [DOI] [PubMed] [Google Scholar]
- 61. Laszik Z, Mitro A, Taylor FB Jr, Ferrell G, Esmon CT.. Human protein C receptor is present primarily on endothelium of large blood vessels: implications for the control of the protein C pathway. Circulation 1997;96:3633–3640. [DOI] [PubMed] [Google Scholar]
- 62. Gu JM, Katsuura Y, Ferrell GL, Grammas P, Esmon CT.. Endotoxin and thrombin elevate rodent endothelial cell protein C receptor mRNA levels and increase receptor shedding in vivo. Blood 2000;95:1687–1693. [PubMed] [Google Scholar]
- 63. Faust SN, Levin M, Harrison OB, Goldin RD, Lockhart MS, Kondaveeti S, Laszik Z, Esmon CT, Heyderman RS.. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N Engl J Med 2001;345:408–416. [DOI] [PubMed] [Google Scholar]
- 64. Li W, Zheng X, Gu J, Hunter J, Ferrell GL, Lupu F, Esmon NL, Esmon CT.. Overexpressing endothelial cell protein C receptor alters the hemostatic balance and protects mice from endotoxin. J Thromb Haemost 2005;3:1351–1359. [DOI] [PubMed] [Google Scholar]
- 65. Sundaram J, Keshava S, Gopalakrishnan R, Esmon CT, Pendurthi UR, Rao LVM.. Factor VIIa binding to endothelial cell protein C receptor protects vascular barrier integrity in vivo. J Thromb Haemost 2014;12:690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Clark CA, Vatsyayan R, Hedner U, Esmon CT, Pendurthi UR, Rao LVM.. Endothelial cell protein C receptor-mediated redistribution and tissue-level accumulation of factor VIIa. J Thromb Haemost 2012;10:2383–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kondreddy V, Wang J, Keshava S, Esmon CT, Rao LVM, Pendurthi UR.. Factor VIIa induces anti-inflammatory signaling via EPCR and PAR1. Blood 2018;131:2379–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. von DrygalskiA, Furlan-Freguia C, Ruf W, Griffin JH, Mosnier LO.. Organ-specific protection against lipopolysaccharide-induced vascular leak is dependent on the endothelial protein C receptor. Arterioscler Thromb Vasc Biol 2013;33:769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bouwens EAM, Stavenuiter F, Mosnier LO.. Cell painting with an engineered EPCR to augment the protein C system. Thromb Haemost 2015;114:1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Loghmani H, Conway EM.. Exploring traditional and nontraditional roles for thrombomodulin. Blood 2018;132:148–158. [DOI] [PubMed] [Google Scholar]
- 71. Morser J. Thrombomodulin links coagulation to inflammation and immunity. Curr Drug Targets 2012;13:421–431. [DOI] [PubMed] [Google Scholar]
- 72. Martin FA, Murphy RP, Cummins PM.. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am J Physiol Heart Circ Physiol 2013;304:H1585–H1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yamakawa K, Aihara M, Ogura H, Yuhara H, Hamasaki T, Shimazu T.. Recombinant human soluble thrombomodulin in severe sepsis: a systematic review and meta-analysis. J Thromb Haemost 2015;13:508–519. [DOI] [PubMed] [Google Scholar]
- 74. Zhang C, Wang H, Yang H, Tong Z.. Recombinant human soluble thrombomodulin and short-term mortality of infection patients with DIC: a meta-analysis. Am J Emerg Med 2016;34:1876–1882. [DOI] [PubMed] [Google Scholar]
- 75. Ireland H, Konstantoulas CJ, Cooper JA, Hawe E, Humphries SE, Mather H, Goodall AH, Hogwood J, Juhan-Vague I, Yudkin JS, di Minno G, Margaglione M, Hamsten A, Miller GJ, Bauer KA, Kim YT, Stearns-Kurosawa DJ, Kurosawa S.. EPCR Ser219Gly: elevated sEPCR, prothrombin F1 + 2, risk for coronary heart disease, and increased sEPCR shedding in vitro. Atherosclerosis 2005;183:283–292. [DOI] [PubMed] [Google Scholar]
- 76. Sesin CA, Yin X, Esmon CT, Buyon JP, Clancy RM.. Shedding of endothelial protein C receptor contributes to vasculopathy and renal injury in lupus: in vivo and in vitro evidence. Kidney Int 2005;68:110–120. [DOI] [PubMed] [Google Scholar]
- 77. Menschikowski M, Hagelgans A, Eisenhofer G, Siegert G.. Regulation of endothelial protein C receptor shedding by cytokines is mediated through differential activation of MAP kinase signaling pathways. Exp Cell Res 2009;315:2673–2682. [DOI] [PubMed] [Google Scholar]
- 78. Ku S-K, Lee W, Yoo H, Han C-K, Bae J-S.. Inhibitory effects of epi-sesamin on endothelial protein C receptor shedding in vitro and in vivo. Inflamm Res 2013;62:895–902. [DOI] [PubMed] [Google Scholar]
- 79. Ku S-K, Kim JA, Bae J-S.. Piperlonguminine downregulates endothelial protein C receptor shedding in vitro and in vivo. Inflammation 2014;37:435–442. [DOI] [PubMed] [Google Scholar]
- 80. Ku S-K, Yang E-J, Song K-S, Bae J-S.. Rosmarinic acid down-regulates endothelial protein C receptor shedding in vitro and in vivo. Food Chem Toxicol 2013;59:311–315. [DOI] [PubMed] [Google Scholar]
- 81. Ku S-K, Lee I-C, Han M-S, Bae J-S.. Inhibitory effects of rutin on the endothelial protein C receptor shedding in vitro and in vivo. Inflammation 2014;37:1424–1431. [DOI] [PubMed] [Google Scholar]
- 82. Ishii H, Uchiyama H, Kazama M.. Soluble thrombomodulin antigen in conditioned medium is increased by damage of endothelial cells. Thromb Haemost 1991;65:618–623. [PubMed] [Google Scholar]
- 83. Boffa MC, Karochkine M, Bérard M.. Plasma thrombomodulin as a marker of endothelium damage. Nouv Rev Fr Hematol 1991;33:529–530. [PubMed] [Google Scholar]
- 84. Esmon CT, Fukudome K, Mather T, Bode W, Regan LM, Stearns-Kurosawa DJ, Kurosawa S.. Inflammation, sepsis, and coagulation. Haematologica 1999;84:254–259. [PubMed] [Google Scholar]
- 85. Wu KK, Matijevic-Aleksic N.. Thrombomodulin: a linker of coagulation and fibrinolysis and predictor of risk of arterial thrombosis. Ann Med 2000;32(Suppl. 1):73–77. [PubMed] [Google Scholar]
- 86. Reinhart K, Bayer O, Brunkhorst F, Meisner M.. Markers of endothelial damage in organ dysfunction and sepsis. Crit Care Med 2002;30:S302–S312. [DOI] [PubMed] [Google Scholar]
- 87. Laszik ZG, Zhou XJ, Ferrell GL, Silva FG, Esmon CT.. Down-regulation of endothelial expression of endothelial cell protein C receptor and thrombomodulin in coronary atherosclerosis. Am J Pathol 2001;159:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sommeijer DW, Beganovic A, Schalkwijk CG, Ploegmakers H, van der Loos CM, van Aken BE, ten Cate H, van der Wal AC.. More fibrosis and thrombotic complications but similar expression patterns of markers for coagulation and inflammation in symptomatic plaques from DM2 patients. J Histochem Cytochem 2004;52:1141–1149. [DOI] [PubMed] [Google Scholar]
- 89. Yoshii Y, Okada Y, Sasaki S, Mori H, Oida K, Ishii H.. Expression of thrombomodulin in human aortic smooth muscle cells with special reference to atherosclerotic lesion types and age differences. Med Electron Microsc 2003;36:165–172. [DOI] [PubMed] [Google Scholar]
- 90. Lentz SR, Fernández J, Griffin JH, Piegors DJ, Erger RA, Malinow MR, Heistad DD.. Impaired anticoagulant response to infusion of thrombin in atherosclerotic monkeys associated with acquired defects in the protein C system. Arterioscler Thromb Vasc Biol 1999;19:1744–1750. [DOI] [PubMed] [Google Scholar]
- 91. Wilk G, Osmenda G, Matusik P, Nowakowski D, Jasiewicz-Honkisz B, Ignacak A, Cześnikiewicz-Guzik M, Guzik TJ.. Endothelial function assessment in atherosclerosis: comparison of brachial artery flow-mediated vasodilation and peripheral arterial tonometry. Pol Arch Med Wewn 2013;123:443–452. [DOI] [PubMed] [Google Scholar]
- 92. Monteiro JP, Bennett M, Rodor J, Caudrillier A, Ulitsky I, Baker AH.. Endothelial function and dysfunction in the cardiovascular system: the long non-coding road. Cardiovasc Res 2019;115:1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lacolley P, Regnault V, Avolio AP.. Smooth muscle cell and arterial aging: basic and clinical aspects. Cardiovasc Res 2018;114:513–528. [DOI] [PubMed] [Google Scholar]
- 94. Guzik TJ, Sadowski J, Kapelak B, Jopek A, Rudzinski P, Pillai R, Korbut R, Channon KM.. Systemic regulation of vascular NAD(P)H oxidase activity and nox isoform expression in human arteries and veins. Arterioscler Thromb Vasc Biol 2004;24:1614–1620. [DOI] [PubMed] [Google Scholar]
- 95. Djordjevic T, Pogrebniak A, BelAiba RS, Bonello S, Wotzlaw C, Acker H, Hess J, Görlach A.. The expression of the NADPH oxidase subunit p22phox is regulated by a redox-sensitive pathway in endothelial cells. Free Radic Biol Med 2005;38:616–630. [DOI] [PubMed] [Google Scholar]
- 96. BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, Görlach A.. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med 2007;42:446–459. [DOI] [PubMed] [Google Scholar]
- 97. Guzik TJ, Chen W, Gongora MC, Guzik B, Lob HE, Mangalat D, Hoch N, Dikalov S, Rudzinski P, Kapelak B, Sadowski J, Harrison DG.. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J Am Coll Cardiol 2008;52:1803–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Takahashi K, Omae T, Ono S, Kamiya T, Tanner A, Yoshida A.. Thrombin-induced responses via protease-activated receptor 1 blocked by the endothelium on isolated porcine retinal arterioles. Curr Eye Res 2018;43:1374–1382. [DOI] [PubMed] [Google Scholar]
- 99. Brailoiu E, Shipsky MM, Yan G, Abood ME, Brailoiu GC.. Mechanisms of modulation of brain microvascular endothelial cells function by thrombin. Brain Res 2017;1657:167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kitasato L, Yamaoka-Tojo M, Hashikata T, Ishii S, Kameda R, Shimohama T, Tojo T, Ako J.. Factor Xa in mouse fibroblasts may induce fibrosis more than thrombin. Int Heart J 2014;55:357–361. [DOI] [PubMed] [Google Scholar]
- 101. Jobi K, Rauch BH, Dangwal S, Freidel K, Doller A, Eberhardt W, Fischer JW, Schrör K, Rosenkranz AC.. Redox regulation of human protease-activated receptor-2 by activated factor X. Free Radic Biol Med 2011;51:1758–1764. [DOI] [PubMed] [Google Scholar]
- 102. Rahadian A, Fukuda D, Salim HM, Yagi S, Kusunose K, Yamada H, Soeki T, Shimabukuro M, Sata M.. Thrombin inhibition by dabigatran attenuates endothelial dysfunction in diabetic mice. Vascul Pharmacol 2020;124:106632. [DOI] [PubMed] [Google Scholar]
- 103. Kossmann S, Lagrange J, Jäckel S, Jurk K, Ehlken M, Schönfelder T, Weihert Y, Knorr M, Brandt M, Xia N, Li H, Daiber A, Oelze M, Reinhardt C, Lackner K, Gruber A, Monia B, Karbach SH, Walter U, Ruggeri ZM, Renné T, Ruf W, Münzel T, Wenzel P.. Platelet-localized FXI promotes a vascular coagulation-inflammatory circuit in arterial hypertension. Sci Transl Med 2017;9:eaah4923. [DOI] [PubMed] [Google Scholar]
- 104. Clément M, Mallat Z.. Unravelling immune cell complexity in atherosclerosis. Cardiovasc Res 2018;114:1306–1307. [DOI] [PubMed] [Google Scholar]
- 105. Abdellatif M, Zirlik A.. Immunometabolism: a key target to improve microcirculation in ageing. Cardiovasc Res 2020;116:e48–e50. [DOI] [PubMed] [Google Scholar]
- 106. Caligiuri G, Norata GD.. Fuel for thought: immunometabolism is a paradigm shift in understanding immunity in cardiovascular disease. Cardiovasc Res 2019;115:1383–1384. [DOI] [PubMed] [Google Scholar]
- 107. Minnema MC, Ten Cate H, Hack CE.. The role of factor XI in coagulation: a matter of revision. Semin Thromb Hemost 1999;25:419–428. [DOI] [PubMed] [Google Scholar]
- 108. Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost 2016;14:28–39. [DOI] [PubMed] [Google Scholar]
- 109. van Montfoort ML, Meijers JCM.. Recent insights into the role of the contact pathway in thrombo-inflammatory disorders. Hematol Am Soc Hematol Educ Program 2014;2014:60–65. [DOI] [PubMed] [Google Scholar]
- 110. Undas A, Ariëns RAS.. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol 2011;31:e88–e99. [DOI] [PubMed] [Google Scholar]
- 111. Ariëns RAS. Novel mechanisms that regulate clot structure/function. Thromb Res 2016;141:S25–S27. [DOI] [PubMed] [Google Scholar]
- 112. Asada Y, Yamashita A, Sato Y, Hatakeyama K.. Thrombus formation and propagation in the onset of cardiovascular events. J Atheroscler Thromb 2018;25:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Spronk H, Padro T, Siland J, Prochaska J, Winters J, van der Wal A, Posthuma J, Lowe G, d’Alessandro E, Wenzel P, Coenen D, Reitsma P, Ruf W, van Gorp R, Koenen R, Vajen T, Alshaikh N, Wolberg A, Macrae F, Asquith N, Heemskerk J, Heinzmann A, Moorlag M, Mackman N, van der Meijden P, Meijers J, Heestermans M, Renné T, Dólleman S, Chayouâ W, Ariëns R, Baaten C, Nagy M, Kuliopulos A, Posma J, Harrison P, Vries M, Crijns H, Dudink E, Buller H, Henskens Y, Själander A, Zwaveling S, Erküner O, Eikelboom J, Gulpen A, Peeters F, Douxfils J, Olie R, Baglin T, Leader A, Schotten U, Scaf B, van Beusekom H, Mosnier L, van der Vorm L, Declerck P, Visser M, Dippel D, Strijbis VJ, Pertiwi K, ten Cate-Hoek A, ten Cate H.. Atherothrombosis and thromboembolism: position paper from the second Maastricht Consensus Conference on Thrombosis. Thromb Haemost 2018;118:229–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Maier RV, Ulevitch RJ.. The induction of a unique procoagulant activity in rabbit hepatic macrophages by bacterial lipopolysaccharides. J Immunol 1981;127:1596–1600. [PubMed] [Google Scholar]
- 115. Chapman HA Jr, Allen CL, Stone OL, Fair DS.. Human alveolar macrophages synthesize factor VII in vitro. Possible role in interstitial lung disease. J Clin Invest 1985;75:2030–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Østerud B, Bjørklid E.. Sources of tissue factor. Semin Thromb Hemost 2006;32:011–023. [DOI] [PubMed] [Google Scholar]
- 117. Pejler G, Lunderius C, Tomasini-Johansson B.. Macrophages synthesize factor X and secrete factor X/Xa-containing prothrombinase activity into the surrounding medium. Thromb Haemost 2000;84:429–435. [PubMed] [Google Scholar]
- 118. Grover SP, Mackman N.. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol 2018;38:709–725. [DOI] [PubMed] [Google Scholar]
- 119. D’Alessandro E, Posma JJN, Spronk HMH, ten Cate H.. Tissue factor (:Factor VIIa) in the heart and vasculature: more than an envelope. Thromb Res 2018;168:130–137. [DOI] [PubMed] [Google Scholar]
- 120. Wilcox JN, Smith KM, Schwartz SM, Gordon D.. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A 1989;86:2839–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Grover SP, Mackman N.. Tissue factor in atherosclerosis and atherothrombosis. Atherosclerosis 2020;307:80–86. [DOI] [PubMed] [Google Scholar]
- 122. Kopp CW, HöLzenbein T, Steiner S, Marculescu R, Bergmeister H, Seidinger D, Mosberger I, Kaun C, Cejna M, Horvat R, Wojta J, Maurer G, Binder BR, Breuss JM, Ecker RC, de Martin R, Minar E.. Inhibition of restenosis by tissue factor pathway inhibitor: in vivo and in vitro evidence for suppressed monocyte chemoattraction and reduced gelatinolytic activity. Blood 2004;103:1653–1661. [DOI] [PubMed] [Google Scholar]
- 123. Hsieh JY, Smith TD, Meli VS, Tran TN, Botvinick EL, Liu WF.. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomater 2017;47:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Davalos D, Akassoglou K.. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol 2012;34:43–62. [DOI] [PubMed] [Google Scholar]
- 125. Hara T, Phuong PT, Fukuda D, Yamaguchi K, Murata C, Nishimoto S, Yagi S, Kusunose K, Yamada H, Soeki T, Wakatsuki T, Imoto I, Shimabukuro M, Sata M.. Protease-activated receptor-2 plays a critical role in vascular inflammation and atherosclerosis in apolipoprotein E-deficient mice. Circulation 2018;138:1706–1719. [DOI] [PubMed] [Google Scholar]
- 126. Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, Bottazzi B, Doni A, Vincenzo B, Pasqualini F, Vago L, Nebuloni M, Mantovani A, Sica A.. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood 2006;107:2112–2122. [DOI] [PubMed] [Google Scholar]
- 127. Schaffner F, Yokota N, Carneiro-Lobo T, Kitano M, Schaffer M, Anderson GM, Mueller BM, Esmon CT, Ruf W.. Endothelial protein C receptor function in murine and human breast cancer development. PLoS One 2013;8:e61071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Graf C, Wilgenbus P, Pagel S, Pott J, Marini F, Reyda S, Kitano M, Macher-Göppinger S, Weiler H, Ruf W.. Myeloid cell-synthesized coagulation factor X dampens antitumor immunity. Sci Immunol 2019;4:eaaw8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Nystedt S, Emilsson K, Wahlestedt C, Sundelin J.. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A 1994;91:9208–9212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Elmariah SB, Reddy VB, Lerner EA.. Cathepsin S signals via PAR2 and generates a novel tethered ligand receptor agonist. PLoS One 2014;9:e99702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Compton SJ, Renaux B, Wijesuriya SJ, Hollenberg MD.. Glycosylation and the activation of proteinase-activated receptor 2 (PAR(2)) by human mast cell tryptase. Br J Pharmacol 2001;134:705–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Jones SM, Mann A, Conrad K, Saum K, Hall DE, McKinney LM, Robbins N, Thompson J, Peairs AD, Camerer E, Rayner KJ, Tranter M, Mackman N, Owens AP. III. PAR2 (protease-activated receptor 2) deficiency attenuates atherosclerosis in mice. Arterioscler Thromb Vasc Biol 2018;38:1271–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bentzon JF, Otsuka F, Virmani R, Falk E.. Mechanisms of plaque formation and rupture. Circ Res 2014;114:1852–1866. [DOI] [PubMed] [Google Scholar]
- 134. Quillard T, Franck G, Mawson T, Folco E, Libby P.. Mechanisms of erosion of atherosclerotic plaques. Curr Opin Lipidol 2017;28:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Partida RA, Libby P, Crea F, Jang I-K.. Plaque erosion: a new in vivo diagnosis and a potential major shift in the management of patients with acute coronary syndromes. Eur Heart J 2018;39:2070–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hoogen IJ van den, Gianni U, Al Hussein Alawamlh O, Wijeratne R, Jinnouchi H, Finn A, Earls JP, Virmani R, Lin FY.. What atherosclerosis findings can CT see in sudden coronary death: plaque rupture versus plaque erosion. J Cardiovasc Comput Tomogr 2019. [DOI] [PubMed] [Google Scholar]
- 137. Gaul DS, Weber J, van Tits LJ, Sluka S, Pasterk L, Reiner MF, Calatayud N, Lohmann C, Klingenberg R, Pahla J, Vdovenko D, Tanner FC, Camici GG, Eriksson U, Auwerx J, Mach F, Windecker S, Rodondi N, Lüscher TF, Winnik S, Matter CM.. Loss of Sirt3 accelerates arterial thrombosis by increasing formation of neutrophil extracellular traps and plasma tissue factor activity. Cardiovasc Res 2018;114:1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ketelhuth DFJ, Lutgens E, Bäck M, Binder CJ, Van den Bossche J, Daniel C, Dumitriu IE, Hoefer I, Libby P, O’Neill L, Weber C, Evans PC.. Immunometabolism and atherosclerosis: perspectives and clinical significance: a position paper from the Working Group on Atherosclerosis and Vascular Biology of the European Society of Cardiology. Cardiovasc Res 2019;115:1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Souilhol C, Harmsen MC, Evans PC, Krenning G.. Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc Res 2018;114:565–577. [DOI] [PubMed] [Google Scholar]
- 140. van Tuijl J, Joosten LAB, Netea MG, Bekkering S, Riksen NP.. Immunometabolism orchestrates training of innate immunity in atherosclerosis. Cardiovasc Res 2019;115:1416–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 142. Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie M-A, Dubé M-P, Rhainds D, Provencher M, Blondeau L, Orfanos A, L’Allier PL, Guertin M-C, Roubille F.. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 143. Quillard T, Araújo HA, Franck G, Shvartz E, Sukhova G, Libby P.. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J 2015;36:1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Franck G, Mawson TL, Folco EJ, Molinaro R, Ruvkun V, Engelbertsen D, Liu X, Tesmenitsky Y, Shvartz E, Sukhova GK, Michel J-B, Nicoletti A, Lichtman A, Wagner D, Croce KJ, Libby P.. Roles of PAD4 and NETosis in experimental atherosclerosis and arterial injury: implications for superficial erosion. Circ Res 2018;123:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Folco EJ, Mawson TL, Vromman A, Bernardes-Souza B, Franck G, Persson O, Nakamura M, Newton G, Luscinskas FW, Libby P.. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1α and cathepsin G. Arterioscler Thromb Vasc Biol 2018;38:1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Narula N, Dannenberg AJ, Olin JW, Bhatt DL, Johnson KW, Nadkarni G, Min J, Torii S, Poojary P, Anand SS, Bax JJ, Yusuf S, Virmani R, Narula J.. Pathology of peripheral artery disease in patients with critical limb ischemia. J Am Coll Cardiol 2018;72:2152–2163. [DOI] [PubMed] [Google Scholar]
- 147. Kaplovitch E, Anand SS.. The evolving treatment of peripheral arterial disease: preventing ischaemic events in the post-COMPASS era. Cardiovasc Res 2019;115:e121–e124. [DOI] [PubMed] [Google Scholar]
- 148. Posma JJ, Grover SP, Hisada Y, Owens AP III, Antoniak S, Spronk HM, Mackman N.. Roles of coagulation proteases and PARs (Protease-Activated Receptors) in mouse models of inflammatory diseases. Arterioscler Thromb Vasc Biol 2019;39:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Puy C, Tucker EI, Matafonov A, Cheng Q, Zientek KD, Gailani D, Gruber A, McCarty OJT.. Activated factor XI increases the procoagulant activity of the extrinsic pathway by inactivating tissue factor pathway inhibitor. Blood 2015;125:1488–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Puy C, Tucker EI, Ivanov IS, Gailani D, Smith SA, Morrissey JH, Gruber A, McCarty OJT.. Platelet-derived short-chain polyphosphates enhance the inactivation of tissue factor pathway inhibitor by activated coagulation factor XI. PLoS One 2016;11:e0165172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Xiao Q, Danton MJ, Witte DP, Kowala MC, Valentine MT, Degen JL.. Fibrinogen deficiency is compatible with the development of atherosclerosis in mice. J Clin Invest 1998;101:1184–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lou XJ, Boonmark NW, Horrigan FT, Degen JL, Lawn RM.. Fibrinogen deficiency reduces vascular accumulation of apolipoprotein(a) and development of atherosclerosis in apolipoprotein(a) transgenic mice. Proc Natl Acad Sci U S A 1998;95:12591–12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Seehaus S, Shahzad K, Kashif M, Vinnikov IA, Schiller M, Wang H, Madhusudhan T, Eckstein V, Bierhaus A, Bea F, Blessing E, Weiler H, Frommhold D, Nawroth PP, Isermann B.. Hypercoagulability inhibits monocyte transendothelial migration through protease-activated receptor-1-, phospholipase-Cbeta-, phosphoinositide 3-kinase-, and nitric oxide-dependent signaling in monocytes and promotes plaque stability. Circulation 2009;120:774–784. [DOI] [PubMed] [Google Scholar]
- 154. Hara T, Fukuda D, Tanaka K, Higashikuni Y, Hirata Y, Nishimoto S, Yagi S, Yamada H, Soeki T, Wakatsuki T, Shimabukuro M, Sata M.. Rivaroxaban, a novel oral anticoagulant, attenuates atherosclerotic plaque progression and destabilization in ApoE-deficient mice. Atherosclerosis 2015;242:639–646. [DOI] [PubMed] [Google Scholar]
- 155. Zhou Q, Bea F, Preusch M, Wang H, Isermann B, Shahzad K, Katus HA, Blessing E.. Evaluation of plaque stability of advanced atherosclerotic lesions in apo E-deficient mice after treatment with the oral factor Xa inhibitor rivaroxaban. Mediators Inflamm 2011;2011:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Posthuma JJ, Posma JJN, van Oerle R, Leenders P, van Gorp RH, Jaminon AMG, Mackman N, Heitmeier S, Schurgers LJ, Ten Cate H, Spronk HMH.. Targeting coagulation factor Xa promotes regression of advanced atherosclerosis in apolipoprotein-E deficient mice. Sci Rep 2019;9:3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Plank F, Beyer C, Friedrich G, Stühlinger M, Hintringer F, Dichtl W, Wildauer M, Feuchtner G.. Influence of vitamin K antagonists and direct oral anticoagulation on coronary artery disease: a CTA analysis. Int J Cardiol 2018;260:11–15. [DOI] [PubMed] [Google Scholar]
- 158. Gurbel PA, Fox KAA, Tantry US, Ten Cate H, Weitz JI.. Combination antiplatelet and oral anticoagulant therapy in patients with coronary and peripheral artery disease. Circulation 2019;139:2170–2185. [DOI] [PubMed] [Google Scholar]
- 159. Andreotti F, Testa L, Biondi-Zoccai GGL, Crea F.. Aspirin plus warfarin compared to aspirin alone after acute coronary syndromes: an updated and comprehensive meta-analysis of 25,307 patients. Eur Heart J 2006;27:519–526. [DOI] [PubMed] [Google Scholar]
- 160. Hirsh J, Eikelboom JW, Chan NC.. Fifty years of research on antithrombotic therapy: Achievements and disappointments. Eur J Intern Med 2019;70:1–7. [DOI] [PubMed] [Google Scholar]
- 161. Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P, Bhatt DL, Goodman S, Verheugt FW, Flather M, Huber K, Liaw D, Husted SE, Lopez-Sendon J, De Caterina R, Jansky P, Darius H, Vinereanu D, Cornel JH, Cools F, Atar D, Leiva-Pons JL, Keltai M, Ogawa H, Pais P, Parkhomenko A, Ruzyllo W, Diaz R, White H, Ruda M, Geraldes M, Lawrence J, Harrington RA, Wallentin L.. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med 2011;365:699–708. [DOI] [PubMed] [Google Scholar]
- 162. Mega JL, Braunwald E, Wiviott SD, Bassand J-P, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KAA, Goto S, Murphy SA, Plotnikov AN, Schneider D, Sun X, Verheugt FWA, Gibson CM, ATLAS ACS 2–TIMI 51 Investigators. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med 2012;366:9–19. [DOI] [PubMed] [Google Scholar]
- 163. Bosch J, Eikelboom JW, Connolly SJ, Bruns NC, Lanius V, Yuan F, Misselwitz F, Chen E, Diaz R, Alings M, Lonn EM, Widimsky P, Hori M, Avezum A, Piegas LS, Bhatt DL, Branch KRH, Probstfield JL, Liang Y, Liu L, Zhu J, Maggioni AP, Lopez-Jaramillo P, O’Donnell M, Fox KAA, Kakkar A, Parkhomenko AN, Ertl G, Störk S, Keltai K, Keltai M, Ryden L, Dagenais GR, Pogosova N, Dans AL, Lanas F, Commerford PJ, Torp-Pedersen C, Guzik TJ, Verhamme PB, Vinereanu D, Kim J-H, Ha J-W, Tonkin AM, Varigos JD, Lewis BS, Felix C, Yusoff K, Steg PG, Aboyans V, Metsarinne KP, Anand SS, Hart RG, Lamy A, Moayyedi P, Leong DlP, Sharma M, Yusuf S.. Rationale, design and baseline characteristics of participants in the cardiovascular outcomes for people using anticoagulation strategies (COMPASS) trial. Can J Cardiol 2017;33:1027–1035. [DOI] [PubMed] [Google Scholar]
- 164. Cairns JA, Eikelboom JW, Shestakovska O, Yusuf S, DeMets D.. Monitoring emerging data from the COMPASS trial of an antithrombotic agent. J Am Coll Cardiol 2019;73:2769–2772. [DOI] [PubMed] [Google Scholar]
- 165. Steffel J, Eikelboom JW, Anand SS, Shestakovska O, Yusuf S, Fox KAA.. The COMPASS trial: net clinical benefit of low-dose rivaroxaban plus aspirin as compared with aspirin in patients with chronic vascular disease. Circulation 2020;142:40–48. [DOI] [PubMed] [Google Scholar]
- 166. Anand SS, Eikelboom JW, Dyal L, Bosch J, Neumann C, Widimsky P, Avezum AA, Probstfield J, Cook Bruns N, Fox KAA, Bhatt DL, Connolly SJ, Yusuf S, COMPASS Trial Investigators. Rivaroxaban plus aspirin versus aspirin in relation to vascular risk in the COMPASS trial. J Am Coll Cardiol 2019;73:3271–3280. [DOI] [PubMed] [Google Scholar]