Abstract
Sport fishing is an important recreational and economic activity, especially in Australia, Europe and North America, and the condition of sport fish populations is a key ecological indicator of water body condition for millions of anglers and the public. Despite its importance as an ecological indicator representing the status of sport fish populations, an index for measuring this ecosystem service has not been quantified by analyzing actual fish taxa, size and abundance data across the U.S.A. Therefore, we used game fish data collected from 1,561 stream and river sites located throughout the conterminous U.S.A. combined with specific fish species and size dollar weights to calculate site-specific recreational fishery index (RFI) scores. We then regressed those scores against 38 potential site-specific environmental predictor variables, as well as site-specific fish assemblage condition (multimetric index; MMI) scores based on entire fish assemblages, to determine the factors most associated with the RFI scores. We found weak correlations between RFI and MMI scores and weak to moderate correlations with environmental variables, which varied in importance with each of 9 ecoregions. We conclude that the RFI is a useful indicator of a stream ecosystem service, which should be of greater interest to the USA public and traditional fishery management agencies than are MMIs, which tend to be more useful for ecologists, environmentalists and environmental quality agencies.
Keywords: sport fishing, MMI, IBI, ecosystem services
Introduction
One goal of the U.S.A. Clean Water Act (USA, 1972) is fishable waters and this statute reflects the importance of fish in U.S.A. society. Sport fishing is enjoyed by approximately 12 million anglers, supports over 400,000 jobs, and generates over $63 billion in sales in the U.S.A. (USFWS, 2012; NMFS, 2015). In some rural U.S.A. counties with high-quality fisheries, fishing is the major source of income and jobs (Hughes, 2015; Woody, 2018; Colvin et al., 2019). Gamefish was the top ecosystem service item listed by Willamette Basin, Oregon, survey participants (Weber and Ringold, 2019). Southwick and Loftus (2017) estimated fish replacement prices based on fish culture costs because of the need to assess damages from fish kills to pollutant dischargers. Their price estimates varied by species and individual sizes, which in turn are affected by hatchery location, productivity and consumer demand. Clearly, fish and fishing have great value to U.S.A. anglers, citizens and economies.
As part of USEPA’s National Rivers and Streams Assessment (NRSA; USEPA, 2016b) fish assemblages and their environments are sampled across the U.S.A. at over 2000 sites every five years through use of standard methods (Hughes and Peck, 2008; USEPA, 2013a, b) as recommended by Bonar et al. (2009). Although the fish sampling method is standardized, it offers only a one-day snapshot and is not a quantitative measure of all fish species, sizes or absolute abundances at a site (Reynolds et al., 2003; Kanno et al., 2009). In addition, sampling was restricted from sites with listed species, especially when adult salmonids might have been present. Despite those limitations, the USEPA survey provides a means to make rigorous national and regional estimates of fish assemblage status and trends through the use of multimetric indicators (MMIs), as well as the natural and anthropogenic pressures and stressors limiting those assemblages (Esselman et al., 2013; USEPA, 2016b; Herlihy et al., 2019; 2020). The Esselman et al., USEPA and Herlihy et al. assessments were based on the condition of entire fish assemblages—both game and non-game species—as indicated by the MMI scores. The ecological indicators used in those assessments were designed for assessing ecological status and trends.
Recreational fishery indicators have been focused on game fishery assessments. Anglers are beneficiaries of ecosystem goods and services provided by streams (Ringold et al., 2013) and angling satisfaction is largely indicated by the size, abundance and accessibility of game fish, as well as the aesthetic appeal of the site and social factors. For example, Hunt (2006) generated a conceptual model for predicting fishing site choice based on costs, fishing quality, environmental quality, facility development, encounters with other anglers, and fishing regulations.
Hickman (2000) developed a sportfishing quality index for Tennessee Valley Authority reservoirs based largely on fish population data for specific sport species. Oliveira et al. (2009) produced a fishery quality index for all Portuguese streams and found that higher scores were correlated positively with stream size and IBI (index of biotic integrity) scores in coldwater streams, but not in warmwater streams. Melstrom et al. (2015) created a random utility maximization model for predicting the monetary benefits of recreational fishing based on game fish biomass and fishing trip information in Michigan hydrologic units. Other similar fishery quality models based on economic predictors have also been developed from angler reports (e.g., Morey et al., 1993; 2002; Jakus et al., 1998; Hunt et al., 2007), water body condition (e.g., Phaneuf, 2002; Von Haefen, 2003), or a combination of both (e.g. Jones and Lupi, 2000; Murdock, 2006) at the basin or state/province spatial extents. Ringold et al. (2013) used the combination of game fish abundance, site appeal and site access to predict the proportion of stream length having low, medium, or high levels of fishing quality for each of 12 western U.S.A. states. Esselman et al. (2015) estimated biomasses for five separate gamefish taxa across Michigan through use of 16 environmental predictor variables. Most of these models are based on environmental predictors of recreational fishery quality, the relative desirability of the fishing site, and the human benefits and costs of the angling experience. Furthermore, all but Oliveira et al. (2009) and Ringold et al. (2013) are based on data collected across relatively small spatial extents (i.e., a single state, province, basin, or lake).
Clearly, many factors, both ecological and social, affect angler satisfaction (Arlinghaus 2006). However, the Clean Water Act’s fishable goal is focused on what aquatic ecosystems alone may provide for anglers, not the multiple environmental, social, economic, and aesthetic factors that affect the angling experience. In addition, angler goals differ markedly. For example, the objective of many youthful anglers may be to simply catch a lot of fish, that of many anglers fishing for food is to provide one or more meals, whereas that of trophy anglers is to catch very large fish. Some recreational anglers may simply seek solitude in a beautiful place.
Recreational fishery indices (RFIs) provide numerical estimates of sport fishing that could be used for determining quantitative relationships between recreational fisheries and natural and anthropogenic predictor variables. Nonetheless, to date there has been no rigorous national or ecoregional estimate of recreational fisheries for U.S.A. lotic waters based on site-specific fish assemblage surveys. In addition, there has been no estimate of site-specific recreational fishery values that link fish assemblage survey results with a consistent set of weights reflecting actual fish taxa, sizes, and abundances (i.e., the costs of culturing those fish). Although Herlihy et al. (2020) modeled MMI site scores against environmental predictors across the conterminous U.S.A., RFI scores have not been rigorously linked to landscape predictor variables nationally or regionally. Therefore, our objective in this paper is to develop an RFI for conterminous U.S.A. streams and rivers based on the NRSA data together with the monetary costs of culturing individual game fish species to various sizes provided by the American Fisheries Society in Appendix A of Southwick and Loftus (2017). We used the Southwick and Loftus (2017) numbers because they offer a nationally consistent data set that is widely used for evaluating the costs of culturing the fish lost in fish kills resulting from industrial waste spills and for assessing damages in legal proceedings. The fish culturing costs are based on six regional estimates of hatchery production costs (structures, water, water treatment, electricity, prophylactic chemicals, equipment, employees, overhead) obtained by surveying private, state, tribal and federal hatchery managers. For large fish of many species, the cost per pound was extrapolated from length-weight data. The Southwick and Loftus fish culturing costs are the only nationally consistent dataset that we are aware of for weighting virtually all game species. But they underestimate the true value of fish losses and are inappropriate for species that are listed as endangered or threatened or for trophy-sized fish. Also, the Southwick and Loftus (2017) numbers do not represent aesthetic or social (nonuse) values nor the values to anglers (users) of any particular fish species. Furthermore, local values may vary from regional or national estimates and the value of an individual mass-produced sport fish derived from these weights may be markedly less than that of a less popular species that is not mass-produced. Finally, hatchery fish lack the genetic and survivability characteristics of wild fish (Christie et al. 2016; Salvanes 2017; Winans et al. 2017). The USEPA (1990) determined that most U.S.A. states used Southwick and Loftus values for determining the monetary value of fish lost in fish kills. For example, the state of Virginia (DEQ 2002) used those specific fish species values for assessing losses and the costs of their replacement following fish kills. King (2015) also used fish replacement costs for assessing salmonid losses in an Irish river. Therefore, we relate the variations in RFI scores to variations in several regional predictor variables measured at catchment and local spatial extents as recommended in Hughes et al. (2019) and compare them with the MMI results of Herlihy et al. (2020). Herlihy et al. (2020) found that MMI scores were only weakly to moderately correlated with specific environmental variables and that the most important predictor variables varied with ecoregion. Therefore, we predicted that RFI scores would also show similar weak to moderate correlations with environmental variables, as well as varying by ecoregion. The MMI scores are based on entire fish assemblages and least-disturbed reference conditions, whereas the RFI scores are based on game fish and their hatchery production costs. Therefore, we predicted weak correlations between MMI and RFI scores. It is common to have very aesthetically attractive angling sites, including high quality riparian areas, that have water quality problems (particularly toxics). On the other hand, eutrophication linked with poorer ecological conditions can be expected to produce more and bigger tolerant fish (Oliveira et al., 2009; Esselman et al., 2015), and thus produce higher RFI scores. In addition, the NRSA site attractiveness is a subjective indicator. Field crews are asked to judge the site based on what they see, hear, smell and feel, as well as how likely they would be to return to the site to recreate. Because of such antagonistic relationships between sport fish and ecological quality, as well as the subjective nature of the site attractiveness assessment, we also predicted that RFI scores would be weakly correlated with site attractiveness.
Material and methods
Study design
The NRSA field crews sampled 2,288 sites with fish assemblages during summer 2013 and 2014 across the conterminous U.S.A. through use of a probability-based design (Stevens and Olsen, 2004; Olsen and Peck, 2008; USEPA, 2016a). The NRSA selected sites from the National Hydrography Dataset (USGS 2013) representing ~1,231,000 km of lotic waters ranging from great rivers to headwater streams. The study design was spatially balanced and stratified to distribute sites as evenly as possible geographically and by ecoregion and stream size. In addition, 497 hand-picked least-disturbed reference sites were sampled. The NRSA uses nine ecoregions for reporting results (Herlihy et al., 2008; 2020). Out of all those 2,785 sampled sites (Figure 1), 1,561 produced consistent environmental data and game fish species at an average of six species per site. Site numbers ranged from as few as 88 sites in the Xeric Ecoregion to as many as 250 sites in the Northern Appalachians Ecoregion (Table 1). During each one-day sampling visit, crews collected fish assemblage data and measured chemical and physical habitat variables.
Figure 1.
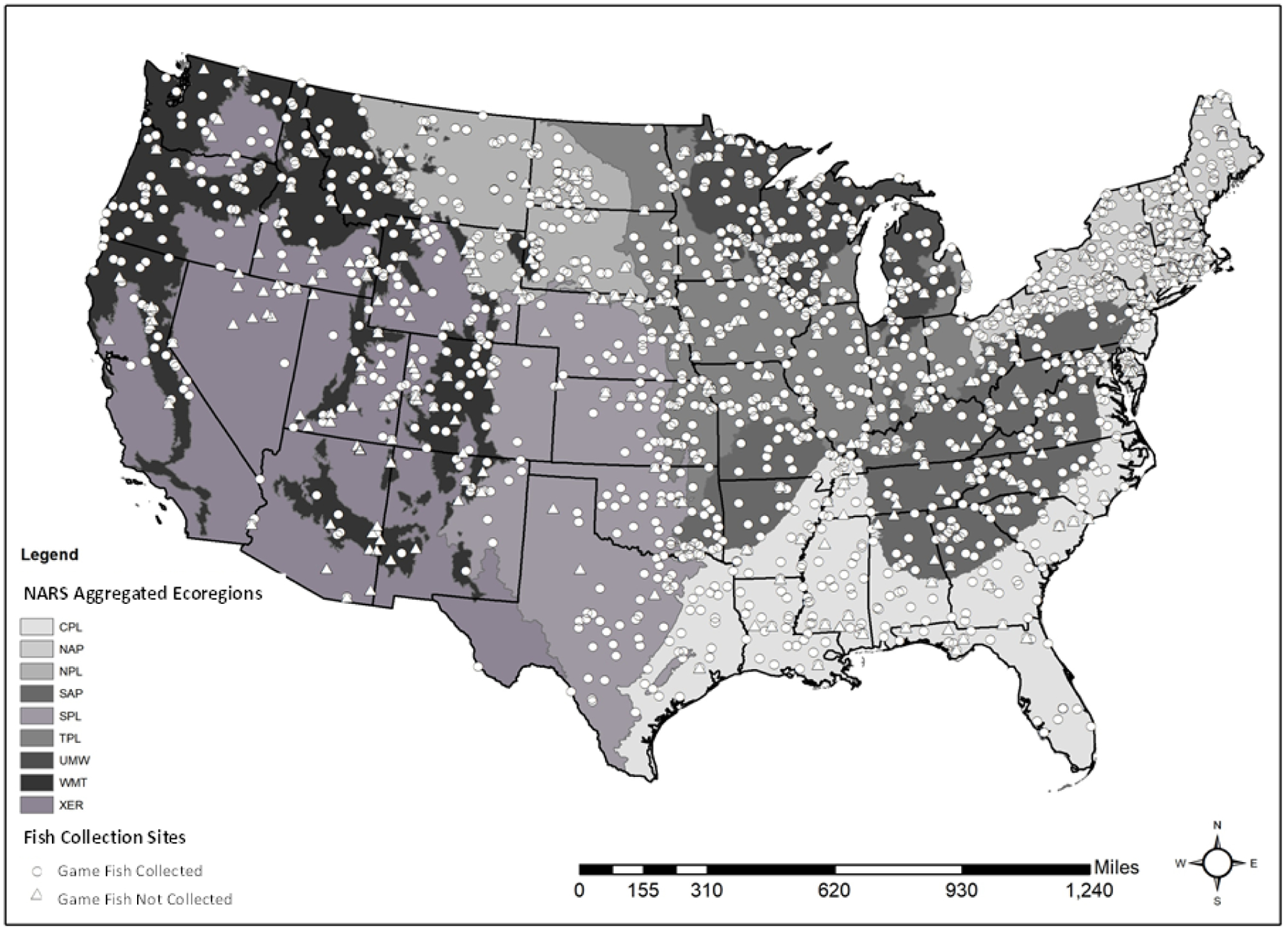
Locations of the 1,561 NRSA sites with game fish and consistent environmental predictor data by nine ecoregions. Ecoregion codes are defined in Table 1.
Table 1.
Number of NRSA sites in each ecoregion with fish RFI scores
| Ecoregion | Code | Game Fish Sites |
|---|---|---|
| Coastal Plain | CPL | 203 |
| Northern Appalachians | NAP | 250 |
| Southern Appalachians | SAP | 216 |
| Upper Midwest | UMW | 156 |
| Temperate Plains | TPL | 224 |
| Northern Plains | NPL | 132 |
| Southern Plains | SPL | 128 |
| Xeric West | XER | 88 |
| Western Mountains | WMT | 164 |
| Total | 1561 |
Fish data
Fish were collected by backpack or boat electrofishing, except 2% of the sites were seined because of high conductivity water (USEPA, 2013a, b). Site lengths were determined around the randomly chosen sample points to ensure adequate characterization of the fish assemblage at the site (Reynolds et al., 2003; Hughes and Herlihy, 2007; 2012; Hughes and Peck, 2008). In wadeable sites <13 m wide, the site length was 40 channel widths, or a minimum of 150 m. In wadeable sites >13 m wide, and boatable sites, the site length was the longer of 500 m or 20 channel widths. In the large wadeable and boatable sites, sampling continued until 500 fish were collected or a site length of 40 channel widths was sampled. Fish were identified, measured (total length) and counted at the site, then released alive unless used for subsequent analyses. Fish names were taken from Page et al. (2013).
Environmental data
We examined 39 environmental variables for associations with RFI scores (Table 2). For water quality, one grab sample was collected at in the middle of wadeable stream sites, and at the site downriver boundary in boatable rivers (USEPA, 2013a, b). Samples were shipped to the U.S. Environmental Protection Agency laboratory in Corvallis, Oregon, and to a few state laboratories. In the laboratory, pH, conductivity and turbidity were measured by meters, sulfate and chloride were measured by ion chromatography, total phosphorus and total nitrogen were measured by persulfate digestion and colorimetry, and dissolved organic carbon was measured with a carbon analyzer.
Table 2.
Variables used to predict RFI scores and their codes. Variables are ordered by class used for subsequent data interpretation.
| Variable (units) | Code | Variable (units) | Code |
|---|---|---|---|
| Water Quality | Watershed Land Use | ||
| Total Nitrogen (μg/L) | TN* | Agriculture (%) | AGR_WS |
| Total Phosphorus (μg/L) | TP* | Developed Land (%) | DEVL_WS |
| Conductivity (μS) | COND* | Wetlands (%) | WETL_WS |
| Dissolved Organic Carbon (mg/L) | DOC* | Population Density (#/km2) | POPDEN* |
| Chloride (μeq/L) | CL* | Road Density | ROADDEN* |
| Sulfate (μeq/L) | SO4* | Dam Disturbance Index | DAM* |
| Turbidity (NTU) | TURB* | Watershed Integrity Index | IWI |
| pH | PH | Climate | |
| Mean Precipitation (cm/yr) | PRECIP* | ||
| Physical Habitat Condition | Mean Runoff (cm/yr) | RUNOFF* | |
| Riparian Cover Index | RIPCOV* | Maximum Temperature (°C) | TEMPMAX |
| Natural Fish Cover (% area) | FISHCOV* | Minimum Temperature (°C) | TEMPMIN |
| Fast Water Habitat (% length) | FASTPCT | ||
| Pool Habitat (% length) | POOLPCT | Geophysical | |
| Riparian Disturbance Index | RIP_DIST | Latitude (degrees) | LAT |
| Agricultural Riparian Disturb. | RIP_AGR | Longitude (degrees) | LON |
| Non-Agricultural Riparian Disturb. | RIP_NOAG | Site Elevation (m) | ELEV* |
| Catchment Area (km2) | WSAREA* | ||
| Substrate | Mean Thalweg Depth (cm) | DEPTH* | |
| Fine Substrate (% area) | FINES | Mean Wetted Width (m) | WIDTH* |
| Sand+Fine Substrate (% area) | SANDFINE | Channel Slope (%) | SLOPE* |
| Relative Bed Stability Index | RBS | Soil Erodibility Factor | ERODE |
| Substrate size | DMM | Boatable or Wadeable | LOTIC |
Log10(x+1) transformed for data analysis, except for SLOPE (Log10(x+0.001), and DAM, RIPCOV, and FISHCOV (Log10(x+0.1).
Physical habitat conditions were determined as indicated in Hughes and Peck (2008), USEPA (2013a, b) and Kaufmann et al. (1999). Multiple measurements were taken at 11 evenly spaced transects along the site. Woody riparian vegetation, anthropogenic disturbances, fish concealment, substrate composition, and wetted and bankfull stream depth and width data were recorded at each transect on standardized field forms. Between transects, channel slope, depths and widths, habitat unit types and substrates were recorded at 11 systematic intervals. Field data were converted into metrics (Table 2) as described by Kaufmann et al. (1999, 2008).
Natural and anthropogenic landscape variables were based on site or catchment spatial extents. Latitude, longitude, elevation and site appeal data were based on the site. Catchment climate, soil, and anthropogenic pressure data are from StreamCat (Hill et al., 2016). Site appeal was scored subjectively by the field crew from 1 (low) to 5 (high) depending on the overall desirability or attractiveness of the site for recreation (USEPA, 2013a,b).
Data analyses
MMI development.
In NRSA, fish assemblage condition was assessed through use of separate multimetric indices (MMIs) for each of the nine ecoregions. MMI metrics and scoring were based on screening hundreds of potential metrics by assessing their ranges, evaluating their repeatability, adjusting for natural variation, determining their sensitivity to anthropogenic disturbance, and assessing their redundancy (Hughes et al., 1998; McCormick et al., 2001; USEPA, 2016a). Each fish MMI has 8 metrics representing each of eight classes: non-native, taxonomic composition, habitat guild, reproductive guild, migratory strategy, richness, tolerance to disturbance, and trophic guild. Metrics were adjusted for catchment area if the R2 of the metric-area relationship at reference sites was > 0.10. Each final metric was scored from 0 to 10 linearly between bottom and top reference values (USEPA, 2016a). The eight fish metric 0–10 scores (ranging from 0 to 10) were summed and multiplied by 1.25 to produce an MMI ranging from 0 to 100. Because MMI metrics, raw values, reference conditions, and metric scoring differ among ecoregions, the MMI scores are not equivalent across all ecoregions.
RFI development.
For each of the 1,561 sites with sufficient samples of game fish, we calculated a site-specific recreational fishery index (RFI) score:
where S = a particular game fish species; L = the number of length classes of that species; N = the number of individuals of that species for that length class and W = the dollar cost (or weight) of that length class for that species (from Southwick and Loftus, 2017). We used log10 for the RFI because initial results indicated raw RFI scores ranged from 0.12 to 3,211.8 (a few cents to thousands of dollars). The Southwick and Loftus numbers were calculated from the costs of culturing fish in hatcheries to various lengths, with increased costs accruing to larger individuals. Those values for a 25 cm individual ranged from $0.70 (drum) to $34 (sturgeon). Fish species were deemed as game fish as in FishBase (https://www.fishbase.se/search.php) and ancillary web descriptions if needed. Taxa missing from Southwick and Loftus (2017) were given dollar weights for taxa in the same fish family or as fish in a family occupying a similar trophic/habitat guild. For example, Tench Tinca tinca were weighted in the same manner as Common Carp Cyprinius carpio and Striped Mullet Mugil cephalus were weighted as if they were a sciaenid. Because the fish size classes in the NRSA database (2.5–15 cm, >15–30 cm, >30 cm) differed from those in Southwick and Loftus (2017), we used the average dollar weight of the Southwick and Loftus lengths matching the NRSA size classes. The NRSA size classes were chosen to reflect fish lengths deemed desirable to most anglers, common length requirements in state angling regulations, and what many consider a large fish. In the few cases where our fish lengths exceeded those in Southwick and Loftus (2017), we used the existing values and extrapolated linearly. Because ours is a national index, because our analytical ecoregions differ markedly from the regions in Southwick and Loftus, and because we wanted to evaluate ecosystem services in a consistent manner, we used the national average fish culturing costs of Southwick and Loftus rather than their regional costs. We note that their fish species culturing costs do not necessarily equate with angler preferences, and some mass-produced species may cost less to culture but be preferentially targeted by anglers.
Statistical analyses.
Following USEPA (2016b) and Herlihy et al. (2020), we assessed RFI scores in each of the nine ecoregions versus the MMIs and site appeal ratings through use of Pearson correlations and the 38 environmental predictor variables via multiple linear regressions. Prior to regression analyses, we checked correlations among variables to avoid multicollinearity then removed the least ecologically robust variable when correlations were ≥0.8. After performing initial regressions, we omitted one environmental variable (RBS, relative bed stability) that was not available for all sites, and then repeated the regressions. MMI scores, percentage variables, variables ranging from 0–10, pH, and logarithmic variables (substrate size, relative bed stability index) were not transformed. The other predictor variables were log transformed (Table 2). We then ran a full 37-variable stepwise linear regression for each of the nine ecoregions using SYSTAT v.13 (2009). For each ecoregion, the final model was selected based on the value for the F-test and variables were selected for each ecoregion based on variable entry and exit values of p=0.05. We checked model fit with scatter plots and the amount of variability accounted for by the models was assessed using adjusted R2. For each of the nine ecoregions, we also ran full model linear regressions by each of the predictor classes of variables and assessed variability accounted for using adjusted R2. Pearson correlations were performed using RFI, NRSA ecoregion-specific fish MMI scores, and NRSA site appeal scores for each of the nine ecoregions.
Results
The RFI scores ranged across three orders of magnitude in each of the nine ecoregions; however, median scores were lower in the Northern Plains, Western Mountains and Xeric Ecoregions (Figure 2). Median scores were highest in the Coastal Plains, Northern Appalachian Plateau, Southern Appalachians, and Upper Midwest Ecoregions; but the central tendencies of the RFI scores in all nine ecoregions indicated high proportions of sites with highly valued fisheries (RFI ~100). The 15 most commonly collected game fish occurred in 11.8% (Brook Trout Salvelinus fontinalis) of the stream length to 60% (Bluegill Lepomis macrochirus) of the river length and the weights (dollar costs) of the largest individuals collected varied from $0.72 (Common Carp Cyprinus carpio) to $33.46 (Smallmouth Bass Micropterus dolomieu) each (Appendix A). Low RFI scores in each ecoregion but the Coastal Plains were less than 1 (a few cents), whereas high RFI scores in each ecoregion were greater than 1000 ($1000) in all but the Xeric (606), Western Mountains (837), and Northern Plains (919) (Appendix B).
Figure 2.
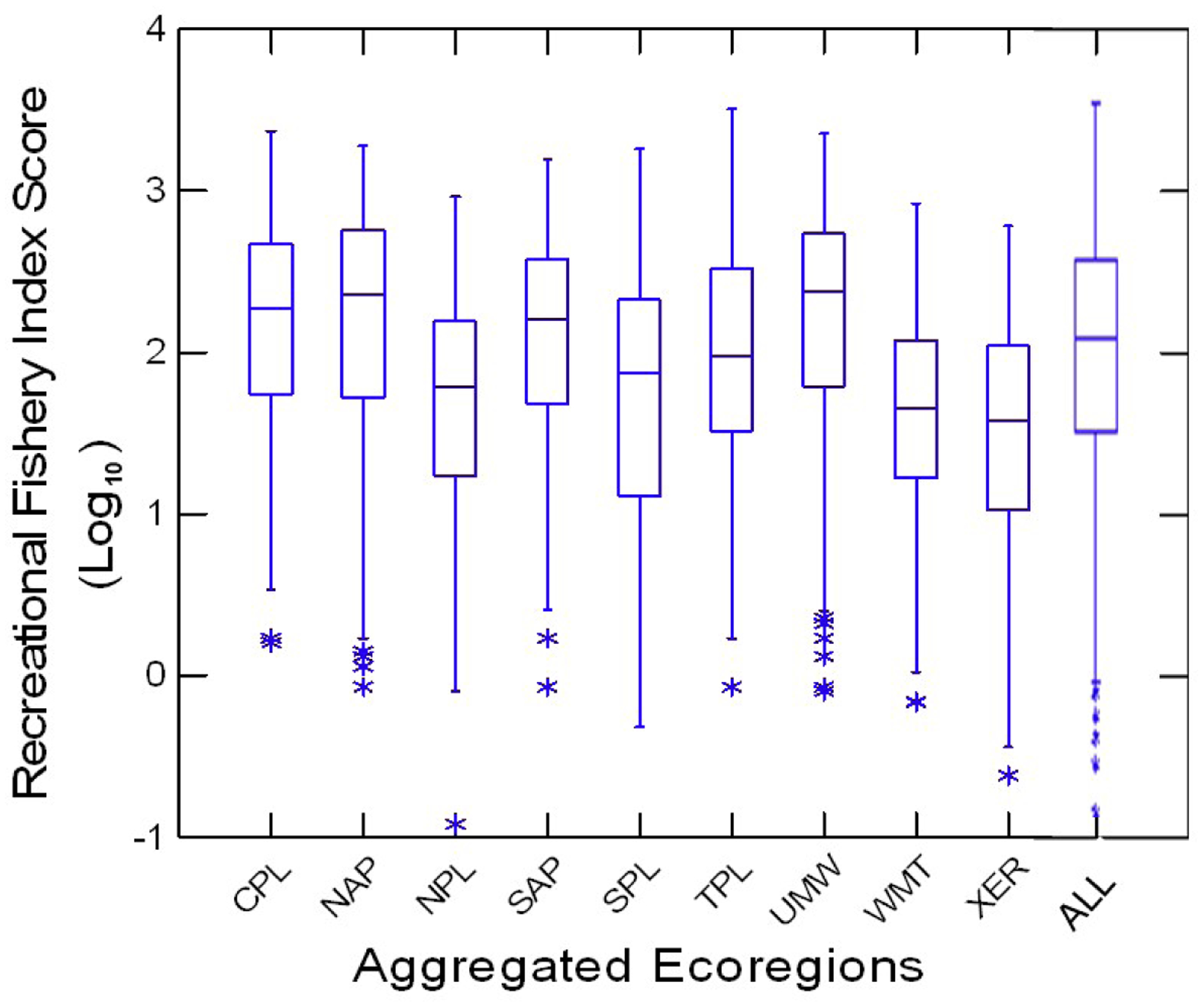
Recreational fishery index (RFI) scores by ecoregion and for the entire conterminous U.S.A. (ALL). Note that RFI scores are expressed as log10. Horizontal lines are medians and quartiles, vertical lines are ranges, and asterisks are outliers. Ecoregion codes defined in Table 1.
The regression model fits between the RFI scores and the environmental predictor variables varied widely among ecoregions (Table 3). Moderately high coefficients of determination (R2; 0.569–0.695), explaining 57–70% of the variation, were obtained for the Upper Midwest, Coastal Plains, Southern Appalachian Plateau, Temperate Plains, and Northern Appalachian Plateau ecoregions. The R2 values were low (0.214–0.454), explaining 21–45% of the variation for the Southern Plains, Northern Plains, Western Mountains, and Xeric ecoregions, as well as the whole conterminous USA, which also reflected their higher standard errors.
Table 3.
Number of ecoregion sites for each stepwise RFI multiple regression model, adjusted R2, standard error, F statistic, and degrees of freedom. Ecoregion codes are defined in Table 1.
| Ecoregion | N | R2 | SE | F | df |
|---|---|---|---|---|---|
| CPL | 198 | 0.635 | 0.406 | 69.562 | 5 |
| NAP | 250 | 0.588 | 0.465 | 60.163 | 6 |
| SAP | 205 | 0.569 | 0.450 | 54.772 | 5 |
| UMW | 152 | 0.695 | 0.406 | 35.359 | 10 |
| TPL | 212 | 0.586 | 0.449 | 23.969 | 13 |
| NPL | 132 | 0.357 | 0.574 | 9.077 | 9 |
| SPL | 125 | 0.454 | 0.579 | 18.17 | 6 |
| XER | 87 | 0.218 | 0659 | 8.976 | 3 |
| WMT | 160 | 0.214 | 0.596 | 8.194 | 6 |
| ALL | 1456 | 0.449 | 0.553 | 92.172 | 13 |
No single environmental variable or class of environmental variables were significant for predicting RFI scores across all nine ecoregions (Table 4; Table 5). Channel width was significant in six ecoregions, channel slope and percent and sand and fines in four, and fish cover, percent fast habitat types, longitude, catchment area, catchment integrity and erodibility in three ecoregions each (Table 4). The number of significant predictor variables varied from 3 to 12 in the Xeric and Temperate Plains ecoregions, respectively (Table 4). The class of geophysical variables explained >50% of the RFI variation in four ecoregions (Table 5). The RFI and MMI scores were only weakly to moderately correlated, with R2 ranging from 0.01% for the Upper Midwest and Xeric ecoregions to 36% for the Northern Appalachian Plateau (Table 5). As predicted, all RFI-MMI correlations were weakly negative except for the Northern Plains. The RFI-site appeal correlations were weakly positive in all nine ecoregions as expected, but somewhat higher than most RFI-MMI correlations (Table 5), explaining from 2.5% (Western Mountains Ecoregion) to 19.1% (Northern Appalachians). Sites in higher order streams tended to have higher RFI scores; however, those scores varied by one to three orders of magnitude by stream order (Figure 3).
Table 4.
Variables predicting RFI scores. Numeric values are the stepwise regression coefficients, variable units and transforms are listed in Table 2, -- indicates the variable was not selected in the regression. All variables significant at p≤0.05. Ecoregion codes are defined in Table 1.
| Variable | CPL | NAP | SAP | UMW | TPL | NPL | SPL | XER | WMT | |
|---|---|---|---|---|---|---|---|---|---|---|
| Water Quality | ||||||||||
| TP | -- | −0.2199 | -- | -- | -- | -- | -- | -- | -- | |
| TN | -- | -- | -- | -- | -- | -- | -- | -- | 0.287 | |
| COND | -- | --- | -- | -- | -- | −0.434 | -- | -- | 0.948 | |
| DOC | -- | -- | -- | −0.732 | -- | -- | -- | -- | -- | |
| CL | -- | -- | -- | −0.255 | -- | -- | -- | -- | -- | |
| SO4 | -- | -- | -- | -- | -- | -- | −0.224 | -- | ||
| TURB | -- | -- | -- | −0.329 | -- | −0.177 | -- | -- | -- | |
| PH | -- | -- | -- | -- | 0.308 | -- | -- | -- | −0.508 | |
| Physical Habitat | ||||||||||
| FISHCOV | 0.125 | -- | -- | -- | −0.205 | 0.298 | -- | -- | -- | |
| FASTPCT | -- | −0.005 | -- | 0.004 | −0.009 | -- | -- | -- | -- | |
| POOLPCT | -- | -- | 0.003 | -- | -- | -- | -- | -- | -- | |
| RIP_AGR | -- | -- | -- | -- | -- | -- | 0.179 | -- | -- | |
| Substrate | ||||||||||
| FINES | -- | -- | -- | -- | -- | -- | -- | -- | −0.007 | |
| SANDFINE | -- | -- | −0.003 | -- | −0.004 | −0.011 | -- | −0.009 | -- | |
| RBS | -- | -- | -- | -- | -- | −0.305 | -- | -- | -- | |
| Catchment Land Use | ||||||||||
| AGR_WS | -- | -- | -- | -- | -- | −0.017 | 0.013 | -- | -- | |
| DEVL_WS | -- | -- | -- | -- | -- | −0.158 | -- | −0.142 | -- | |
| ROAD_DEN | -- | -- | −0.785 | -- | −1.021 | -- | -- | -- | -- | |
| DAM | -- | -- | -- | −0.382 | -- | -- | -- | -- | -- | |
| IWI | -- | -- | -- | −0.476 | −3.087 | -- | 0.757 | -- | -- | |
| Climate | ||||||||||
| PRECIP | -- | -- | -- | -- | 1.228 | -- | 2.281 | -- | -- | |
| RUNOFF | -- | −1.406 | -- | -- | -- | --- | -- | -- | -- | |
| TEMPMIN | 0.116 | 0.044 | -- | -- | -- | -- | -- | -- | -- | |
| Geophysical | ||||||||||
| LAT | −0.949 | -- | -- | -- | -- | -- | -- | -- | -- | |
| LON | 0.822 | -- | -- | 0.032 | 0.040 | -- | -- | -- | -- | |
| ELEV | -- | -- | -- | -- | 0.885 | -- | -- | -- | -- | |
| WSAREA | 0.448 | -- | 0.291 | 0.321 | -- | - | -- | -- | -- | |
| DEPTH | -- | −0.288 | - | -- | -- | -- | -- | -- | -- | |
| WIDTH | -- | 0.598 | -- | 0.502 | −0.484 | 0.721 | 1.273 | -- | 0.308 | |
| SLOPE | -- | -- | −0.227 | -- | −0.204 | −0.297 | -- | −0.465 | -- | |
| ERODE | -- | - | - | 1.343 | −2.691 | -- | -- | -- | −2.766 | |
| Model Intercept | −1.910 | 6.548 | 0.728 | 4.442 | −0.112 | 2.659 | −5.960 | 1.717 | 3.267 |
Table 5.
RFI multiple regression R2 for models based only on the variables within each predictor class by ecoregion and correlations (r) by ecoregion for the RFI versus the NRSA fish ecoregion-specific MMI scores. Bold signifies >50% variation explained. Ecoregion codes are defined in Table 1.
| CPL | NAP | SAP | UMW | TPL | SPL | NPL | WMT | XER | |
|---|---|---|---|---|---|---|---|---|---|
| Water Quality | 0.183 | 0.238 | 0.291 | 0.385 | 0.259 | 0.249 | 0.194 | 0.110 | 0.097 |
| Physical Habitat | 0.193 | 0.350 | 0.192 | 0.192 | 0.212 | 0.137 | 0.075 | 0.025 | 0.053 |
| Substrate | 0.107 | 0.103 | 0.113 | 0.161 | 0.060 | 0.115 | 0.074 | 0.036 | 0.124 |
| Land Use | 0.231 | 0.324 | 0.270 | 0.355 | 0.343 | 0.174 | 0.165 | 0.083 | 0.145 |
| Geophysical | 0.616 | 0.553 | 0.551 | 0.579 | 0.479 | 0.426 | 0.302 | 0.136 | 0.222 |
| Climate | 0.093 | 0.039 | 0.045 | 0.057 | 0.119 | 0.186 | 0.175 | 0.042 | 0.101 |
| Correlations (r) MMI X RFI | −0.03 | −0.60 | −0.46 | −0.01 | −0.17 | −0.09 | +0.49 | −0.15 | −0.01 |
| Site Appeal X RFI | 0.305 | 0.441 | 0.217 | 0.394 | 0.317 | 0.185 | 0.267 | 0.159 | 0.309 |
Figure 3.
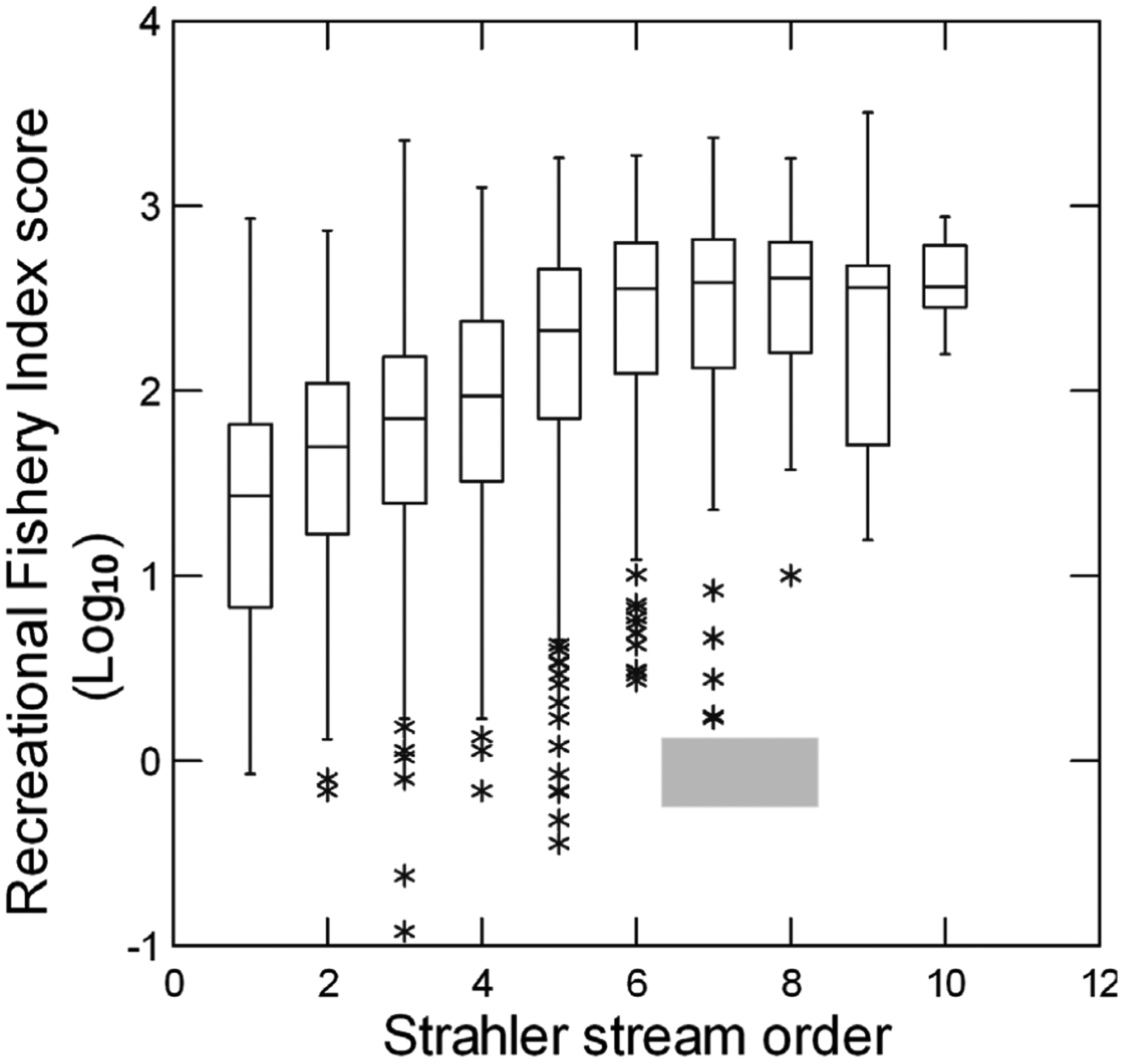
Recreational fishery index (RFI) scores as a function of Strahler stream order. Note that the RFI scores are expressed as log10 and that zero-order channels are mostly misclassified side channels of larger rivers. Horizontal lines are medians and quartiles, vertical lines are ranges, and asterisks are outliers.
Discussion
The median ecoregion RFI scores (Figure 2) were not consistently explained by any of the individual predictor variables that we examined (Table 4). Herlihy et al. (2020) also reported that the importance of various factors for predicting MMI scores varied by ecoregion and that local factors tended to be more important than catchment factors for predicting index scores. Similar results were reported for midwestern rivers (Wang et al., 2003; Esselman et al., 2015), French rivers (Marzin et al., 2012), and Brazilian streams in the Cerrado, Atlantic Forest and Amazon biomes (Macedo et al., 2014; Terra et al., 2015; Leal et al., 2018). However, RFI scores in all nine ecoregions were most strongly explained by the class of natural geophysical variables occurring in, and differing among, those ecoregions (Table 5). Fausch et al. (1984), Hughes et al. (1987) and Rohm et al. (1987) also reported that fish assemblage composition and species richness varied with ecoregion for midwestern U.S.A., Oregon and Arkansas rivers, respectively. But McCormick et al. (2000), Van Sickle and Hughes (2000) and Herlihy et al. (2019) found that the spatial patterns they recognized for Mid-Atlantic, Oregon and U.S.A. streams were associated most with geographic proximity rather than geographic classifications such as ecoregions, basins, hydrologic units or political units. Clearly, we cannot predict RFI scores accurately from environmental factors and geographical classifications.
There was a tendency for lower RFI scores in several ecoregions with increased levels of fines and sand and fines, decreased bed stability and water quality, increased catchment erodibility, and intensified land uses (dams, agriculture, development, roads, human population) (Table 4). Herlihy et al. (2020) also found that several of those same variables were associated with lower MMI scores. Such relationships have been reflected at basin levels of resolution. For example, in Oregon’s Willamette River basin, an estimated 46% of the stream and river miles in the basin were classified as most disturbed (poor condition), with agricultural land use associated with 62% of the most impaired miles (but representing only 30% of the miles in the basin; Mulvey et al. 2009). As in the Willamette Valley, agriculture was closely linked with negative biological effects on streams or lakes in the upper Mississippi River basin (Deweber et al. 2019), Tennessee-Mississippi basins (Perkin et al. 2019), Kansas River basin (Bruckerhoff and Gido 2019), and Northern Forests, Eastern Temperate Forests and Great Plains ecoregions (Jacobson et al. 2019). Presumably, this is because agriculture is the most widely distributed land use in the nation, there are strong gradients in agriculture across river basins and ecoregions, and its diffuse pollutants (excess sediments and nutrients) are weakly regulated or controlled.
Larger streams and rivers tended to have higher RFI scores (Figure 3). Similarly, Fausch et al. (1984) demonstrated that fish species richness increased with stream order and catchment area in midwestern U.S.A. rivers. McGarvey and Hughes (2008) and McGarvey and Ward (2008) reported that the number of fish species increased with increased discharge in Oregon and Alabama rivers, respectively. Hitt and Angermeier (2008) found increasing numbers of game fish individuals and species with increased proximity to large Mid-Atlantic Highlands rivers and Oberdorff and Hughes (1992) reported increased catch per unit effort with increased river size in the Seine basin, France. Hughes and Gammon (1987), Gammon (1976) and Lyons et al. (2001) included biomass collected per unit effort in the MMIs that they developed for large Oregon and midwestern rivers because of the substantial adult size differences that they found for some fish species in rivers versus those found in smaller streams. Hughes et al. (2020) reported that pristine Alaskan river sites supported more and larger game fish species and individuals than did the river tributary sites. In summary and as expected, larger water bodies tend to yield higher RFI scores because such waters support more and larger game fish, as well as more game fish species.
Although all NRSA MMIs include a non-native species metric, the RFI-MMI correlations were weakly (-0.01) to moderately (-0.60) negative for all ecoregions but the Northern Plains (Table 5). We believe this occurred for four major reasons. 1) MMIs assess all fish species, including many highly sensitive or rare non-game prey species—not only game species. Hughes and Herlihy (2012) described how such species are often replaced in rivers by more tolerant, common and piscivorous game fish species. 2) MMIs are developed and scored by using minimally or least-disturbed reference conditions, whereas the environmental conditions required for supporting recreational fisheries span a broad range of ecological conditions. Hughes and Gammon (1987) and Davies and Jackson (2006) described how sensitive species are replaced by more tolerant and common game fish species as the levels of anthropogenic disturbance increase. 3) RFIs include non-native game fish species, many of which are deliberately introduced, actively sought by anglers, and negatively affect native fish assemblages and the MMIs used to assess them. For example, Oliveira et al. (2009) reported a positive correlation between their coldwater fishery quality index that included no non-native fish and a coldwater MMI, but a negative correlation between their warmwater fishery quality index that included three non-native game fish (Common Carp, Largemouth Bass Micropterus salmoides, Pumpkinseed Lepomis gibbosus) and warmwater MMIs. Lomnicky et al. (2007) estimated that three non-native salmonid game-fish species (Brook Trout Salvelinus fontinalis, Brown Trout Salmo trutta, Rainbow Trout Oncorhynchus mykiss) occupied 17%, 16% and 14%, respectively, of the stream length assessed in the western USA, and Whittier et al. (2007) included number of non-native species as a negative metric in their western U.S.A. fish MMIs. 4) RFI scores tended to be higher in larger rivers. Although larger rivers tend to support larger game fish, they also tend to be more disturbed (Hughes and Gammon, 1987; Lyons et al., 2001; Mebane et al., 2003; Rinne et al., 2005). Nonetheless, Dietermann et al. (2019) reported that sites with high catch per unit effort of game fish tended to have high MMI scores in rivers of the Eastern Temperate Forest ecoregion of Minnesota, although many sites with high MMI scores had relatively low abundances of game fish. We believe that the moderately positive RFI-MMI correlation in the Northern Plains is a result of two factors. 1) A preponderance of large rivers and relatively few small streams produced many large-bodied native game species in the Northern Plains (Appendix B). 2) In addition, unlike the other ecoregions, the Northern Plains RFI score was strongly associated with its IWI (index of watershed integrity) score (Table 4), which is moderately correlated with higher MMI scores as well (r = 0.30). Nonetheless, RFIs and MMIs often assess markedly different aspects of riverine fish assemblages, just as indicators of ecological integrity markedly differ from indicators of ecosystem services to humans (Hughes 2019).
The weak RFI-site appeal correlations (Table 5) were predicted, indicating subjective evaluations of site attractiveness is poorly related to RFI scores. We presume this occurs because production of some moderately tolerant game fish species tends to increase with moderate levels of disturbance, as reported by Oliveira et al. (2009) for Largemouth Bass and Pumpkinseed as well as by Esselman et al. (2015) for Smallmouth Bass (Micropterus dolomieu) and Walleye (Sander vitreus). Also, our RFI is based on the fish collected at a site and their costs of culturing—not the attractiveness of a site. However, this is contrary to the assumptions modeled by Ringold et al. (2013) who estimated higher recreational fishery condition in western U.S.A. streams would be associated with higher site appeal.
It is important to indicate what our RFI signifies. First, the species weights are simply the variable costs of producing different sizes of each game fish in hatcheries. The RFI score is a function of those weights times the lengths and numbers of game fish species collected. The numbers and sizes of some game fish are sometimes related to MMI scores and environmental conditions; at other times they are not (Oliveira et al. 2009). Likewise, the scores are not the total economic or subjective value of the recreational fishing experience at a site or in the nation or one of its ecoregions. Those values are calculated by other means, typically by travel cost estimates or willingness to pay studies (Ward and Loomis 1986; Wilson and Carpenter 1999; Bockstael and McConnell 2007; Mendelsohn and Olmstead 2009). In addition, in a national survey, Arlinghaus (2006) found that non-catch attributes of fishing were major motivators for German anglers, despite catch-expectation being the major driver for angler satisfaction. Those studies are important and useful, but generally only provide insights for specific areas and are not directly tied to the production, distribution, size, quality or abundance of fish at a site or in a region.
Conclusions
Because our RFI assessed markedly different aspects of fish assemblages than MMI scores determined from the same site samples, we believe that it would be a useful indicator to add to the USEPA’s National Rivers and Streams Assessments. RFI scores should be of greater interest to traditional state and federal fishery management agencies than are MMI scores because of the RFI focus on game fish variables versus entire fish assemblages that tend to be of greater interest to state and federal environmental quality agencies. Similarly, RFI scores should be of greater interest to the angling public versus ecologists and environmentalists that are more concerned with indicators like MMIs that assess overall ecological condition. There is also a need to determine what should constitute good, fair, or poor RFI scores, such as those used in MMIs, to aid in score interpretation by the public.
Acknowledgments
We thank Karen Blocksom, Alan Herlihy, Marc Weber and Ryan Hill for providing us with the fish and environmental data bases and USEPA contracts C000620098 to RTI International and EP-D-16-021 to CSS for funding. We are also grateful for the many state, federal, and contractor personnel involved with collecting and managing the NRSA data. We appreciate the helpful reviews of earlier manuscripts by Alan Herlihy, Andrew Loftus, James Markwiese, Michael Nye and two journal reviewers. This manuscript was subjected to Agency review prior to submission for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
Appendix A.
The 15 most common game fish species found in the National Rivers and Streams Assessment (NRSA). Numbers are percents of total target wadeable stream length (1,438,000 km) or total target boatable river length (123,300 km).
| Fish Taxa | Boatable Length (%) | Wadeable Length (%) | Species size class weight range ($US) |
|---|---|---|---|
| Bluegill Lepomis macrochirus | 60.0 | 35.4 | 0.85–4.00 |
| Common Carp Cyprinus carpio | 49.5 | 0.12–0.72 | |
| Largemouth Bass Micropterus salmoides | 48.2 | 21.2 | 1.41–16.14 |
| Channel Catfish Ictalurus punctatus | 46.8 | - | 0.68–4.71 |
| Smallmouth Bass Micropterus dolomieu | 36.5 | - | 2.92–33.46 |
| Freshwater Drum Aplodinotus grunniens | 34.9 | - | 0.23–1.51 |
| Longear Sunfish Lepomis megalotis | 33.0 | 16.7 | 0.85–4.00 |
| Spotted Bass Micropterus punctulatus | 30.3 | - | 0.85–4.00 |
| Green Sunfish Lepomis cyanellus | 28.6 | 44.5 | 0.85–4.00 |
| White Sucker Catostomus commersonii | 28.3 | 27.8 | 1.14–15.88 |
| Golden Redhorse Moxostoma erythrurum | 24.2 | - | 1.14–15.88 |
| Rock Bass Ambloplites rupestris | 24.1 | - | 0.85–4.00 |
| Flathead Catfish Pylodictis olivaris | 22.1 | - | 0.68–15.25 |
| Yellow Bullhead Ameiurus natalis | - | 23.8 | 0.80–2.90 |
| Brook Trout Salvelinus fontinalis | - | 11.8 | 1.05–10.14 |
Appendix B.
Ecoregions with high and low RFI site scores and number of species collected per size class.
| Ecoregion | No. per size class | |||||||
|---|---|---|---|---|---|---|---|---|
| Site (RFI High) | Site (RFI Low) | Species (Common Name) | <15 | 15–30 | 31–45 | >45 | ||
| XER | ORR9–0907 (606.62) | Common Carp | 0 | 0 | 0 | 1 | ||
| Channel Catfish | 0 | 0 | 8 | 6 | ||||
| Rainbow Trout | 1 | 1 | 0 | 0 | ||||
| Smallmouth Bass | 43 | 42 | 0 | 0 | ||||
| NVR9–0910 (0.24) | Common Carp | 2 | 0 | 0 | 0 | |||
| WMT | MTRO-01064 (837.17) | Rainbow Trout | 5 | 29 | 7 | 0 | ||
| Brown Trout | 0 | 0 | 2 | 1 | ||||
| Bull Trout | 0 | 0 | 1 | 1 | ||||
| Mountain Whitefish | 18 | 90 | 28 | 0 | ||||
| Westslope Cutthroat Trout | 0 | 1 | 1 | 0 | ||||
| ORRF-0108 (0.68) | Chinook Salmon | 1 | 0 | 0 | 0 | |||
| UMV | MNLS-1091 (2256.28) | Green Sunfish | 6 | 0 | 0 | 0 | ||
| Rainbow Trout | 0 | 2 | 0 | 0 | ||||
| Brook Trout | 26 | 230 | 29 | 0 | ||||
| Brown Trout | 0 | 0 | 0 | 1 | ||||
| Largemouth Bass | 0 | 1 | 1 | 0 | ||||
| White Sucker | 127 | 65 | 145 | 0 | ||||
| WIS9–0926 (0.8) | Yellow Bullhead | 1 | 0 | 0 | 0 | |||
| TPL | SDR9–0913 (3211.8) | RIiver Carpsucker | 3 | 3 | 42 | 4 | ||
| Common Carp | 0 | 0 | 4 | 5 | ||||
| Freshwater Drum | 0 | 2 | 1 | 1 | ||||
| Goldeye | 0 | 6 | 22 | 0 | ||||
| Channel Catfish | 0 | 3 | 1 | 0 | ||||
| Flathead Catfish | 0 | 0 | 0 | 1 | ||||
| Shortnose Gar | 0 | 0 | 0 | 8 | ||||
| Longnose Gar | 0 | 0 | 0 | 15 | ||||
| Sauger | 0 | 0 | 5 | 0 | ||||
| Walleye | 0 | 1 | 1 | 3 | ||||
| Smallmouth Bass | 0 | 1 | 0 | 0 | ||||
| Shovelnose Sturgeon | 0 | 0 | 0 | 26 | ||||
| ILS9–0922 (0.85) | Green Sunfish | 1 | 0 | 0 | 0 | |||
| SPL | OKRM-1006 (1811.6) | River Carpsucker | 6 | 9 | 3 | 2 | ||
| Smallmouth Buffalo | 0 | 0 | 3 | 8 | ||||
| Freshwater Drum | 12 | 3 | 2 | 0 | ||||
| White Bass | 18 | 6 | 1 | 0 | ||||
| Green Sunfish | 1 | 0 | 0 | 0 | ||||
| Longear Sunfish | 32 | 0 | 0 | 0 | ||||
| Channel Catfish | 3 | 6 | 6 | 6 | ||||
| Flathead Catfish | 0 | 0 | 0 | 2 | ||||
| Blue Catfish | 0 | 0 | 0 | 2 | ||||
| White Crappie | 1 | 0 | 0 | 0 | ||||
| Largemouth Bass | 1 | 0 | 0 | 0 | ||||
| Longnose Gar | 1 | 0 | 0 | 44 | ||||
| Striped Bass | 3 | 0 | 0 | 0 | ||||
| Golden Redhorse | 0 | 0 | 0 | 1 | ||||
| Shovelnose Sturgeon | 0 | 0 | 1 | 7 | ||||
| Orangespotted Sunfish | 3 | 0 | 0 | 0 | ||||
| Bluegill | 5 | 0 | 0 | 0 | ||||
| NER9–0911 (0.48) | Common Carp | 4 | 0 | 0 | 0 | |||
| SAP | OHR9–0905 (1569.10) | River Carpsucker | 0 | 2 | 5 | 0 | ||
| Common Carp | 0 | 0 | 1 | 21 | ||||
| Smallmouth Buffalo | 2 | 2 | 7 | 8 | ||||
| Freshwater Drum | 3 | 41 | 36 | 5 | ||||
| White Bass | 1 | 3 | 2 | 0 | ||||
| Green Sunfish | 1 | 0 | 0 | 0 | ||||
| Hybrid Lepomis | 1 | 0 | 0 | 0 | ||||
| Longear Sunfish | 1 | 0 | 0 | 0 | ||||
| Channel Catfish | 0 | 5 | 14 | 23 | ||||
| Flathead Catfish | 0 | 3 | 1 | 0 | ||||
| Black Crappie | 0 | 2 | 0 | 0 | ||||
| Wiper | 2 | 7 | 2 | 0 | ||||
| Largemouth Bass | 0 | 1 | 1 | 0 | ||||
| Longnose Gar | 0 | 0 | 4 | 38 | ||||
| Sauger | 0 | 5 | 3 | 1 | ||||
| Golden Redhorse | 0 | 1 | 4 | 0 | ||||
| River Redhorse | 0 | 0 | 1 | 0 | ||||
| Silver Redhorse | 0 | 0 | 4 | 2 | ||||
| Smallmouth Bass | 0 | 0 | 1 | 0 | ||||
| Black Buffalo | 0 | 0 | 0 | 2 | ||||
| Orangespotted Sunfish | 1 | 0 | 0 | 0 | ||||
| Spotted Bass | 4 | 2 | 7 | 0 | ||||
| Bluegill | 0 | 2 | 0 | 0 | ||||
| ALSS-1065 (0.85) | Green Sunfish | 1 | 0 | 0 | 0 | |||
| NPL | MTRM-1010 (919.27) | Smallmouth Buffalo | 0 | 0 | 2 | 0 | ||
| Freshwater Drum | 0 | 11 | 3 | 1 | ||||
| Goldeye | 33 | 7 | 1 | 0 | ||||
| White Bass | 0 | 0 | 1 | 1 | ||||
| Channel Catfish | 0 | 3 | 4 | 0 | ||||
| Sauger | 2 | 4 | 15 | 0 | ||||
| Walleye | 0 | 4 | 9 | 2 | ||||
| Shovelnose Sturgeon | 0 | 0 | 0 | 4 | ||||
| Burbot | 0 | 1 | 0 | 0 | ||||
| NELS-1083 (0.12) | Common Carp | 1 | 0 | 0 | 0 | |||
| NAP | NYS9–0930 (1897.87) | Common Carp | 0 | 0 | 3 | 15 | ||
| White Perch | 26 | 13 | 2 | 0 | ||||
| Yellow Bullhead | 0 | 2 | 0 | 0 | ||||
| Black Crappie | 2 | 1 | 0 | 0 | ||||
| White Crappie | 0 | 1 | 0 | 0 | ||||
| Yellow Perch | 0 | 17 | 0 | 0 | ||||
| Largemouth Bass | 10 | 20 | 13 | 1 | ||||
| Longnose Gar | 0 | 0 | 2 | 0 | ||||
| White Sucker | 0 | 1 | 2 | 0 | ||||
| Muskellunge | 0 | 0 | 0 | 1 | ||||
| Smallmouth Bass | 3 | 6 | 0 | 1 | ||||
| Brown Bullhead | 0 | 3 | 0 | 0 | ||||
| Pumpkinseed | 36 | 134 | 0 | 0 | ||||
| Rock Bass | 2 | 1 | 0 | 0 | ||||
| Bluegill | 15 | 174 | 0 | 0 | ||||
| NYS9–0931 (0.85) | Pumpkinseed | 1 | 0 | 0 | 0 | |||
| CPL | ARR9–0903 (2341.95) | River Carpsucker | 0 | 0 | 6 | 10 | ||
| Common Carp | 0 | 0 | 8 | 45 | ||||
| Smallmouth Buffalo | 0 | 3 | 9 | 6 | ||||
| Freshwater Drum | 8 | 15 | 5 | 1 | ||||
| White Bass | 0 | 0 | 2 | 1 | ||||
| Green Sunfish | 2 | 0 | 0 | 0 | ||||
| Longear Sunfish | 8 | 0 | 0 | 0 | ||||
| Channel Catfish | 4 | 1 | 2 | 2 | ||||
| Flathead Catfish | 4 | 3 | 3 | 6 | ||||
| Blue Catfish | 0 | 1 | 6 | 39 | ||||
| White Crappie | 1 | 3 | 0 | 0 | ||||
| Largemouth Bass | 0 | 0 | 1 | 0 | ||||
| Shortnose Gar | 0 | 0 | 1 | 20 | ||||
| Spotted Gar | 0 | 0 | 1 | 51 | ||||
| Longnose Gar | 0 | 0 | 0 | 10 | ||||
| Black Buffalo | 0 | 0 | 0 | 4 | ||||
| Orangespotted Sunfish | 1 | 0 | 0 | 0 | ||||
| Spotted Bass | 3 | 0 | 0 | 0 | ||||
| Warmouth | 2 | 0 | 0 | 0 | ||||
| Bluegill | 1 | 0 | 0 | 0 | ||||
| TXSS-1243 (1.6) | Yellow Bullhead | 2 | 0 | 0 | 0 | |||
Literature Cited
- Arlinghaus R 2006. On the apparently striking disconnect between motivation and satisfaction in recreational fishing: the case of catch orientation of German anglers. North American Journal of Fisheries Management 26:592–605. [Google Scholar]
- Bockstael NE, and McConnell KE, editors. 2007. Environmental and resource valuation with revealed preferences: a theoretical guide to empirical models. Springer, New York, New York. [Google Scholar]
- Bonar S, Hubert W, and Willis D, editors. 2009. Standard methods for sampling North American freshwater fishes. American Fisheries Society, Bethesda, Maryland, USA. [Google Scholar]
- Bruckerhoff LA, and Gido KB. 2019. Assessing site-selection strategies for modeling the influence of landscape factors on stream fish assemblages. Pages 159–177 in Hughes RM, Infante DM, Wang L, Chen K, and Terra BF, editors. Advances in understanding landscape influences on freshwater habitats and biological assemblages. Symposium 90. American Fisheries Society, Bethesda, Maryland, USA. [Google Scholar]
- Christie MR, Marine ML, Fox SE, French RA, and Blouin MS. 2016. A single generation of domestication heritably alters the expression of hundreds of genes. Nature Communications 7: 10676. 10.1038/ncomms10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin SAR, Sullivan SMP, Shirey PD, Colvin RW, Winemiller KO, Hughes RM, Fausch KD, Infante DM, Olden JD, Bestgen KR, Danehy RJ, and Eby L. 2019. Headwater streams and wetlands are critical for sustaining fish, fisheries, and ecosystem services. Fisheries 2:73–91. [Google Scholar]
- Davies SB, and Jackson SK. 2006. The biological condition gradient: a descriptive model for interpreting change in aquatic ecosystems. Ecological Applications 16:1251–1266. [DOI] [PubMed] [Google Scholar]
- DEQ (Virginia Department of Environmental Quality). 2002. Fish kill investigation guidance manual, second edition. Water Quality Standards and Biological Monitoring Programs. Richmond, Virginia, USA. [Google Scholar]
- Deweber JT, Sleezer L, and Frimpong EA. 2019. A new regionalization framework to quantify how physiography mediates the effect of land use on stream fishes. Pages 321–350 in Hughes RM, Infante DM, Wang L, Chen K, and Terra BF, editors. Advances in understanding landscape influences on freshwater habitats and biological assemblages. Symposium 90. American Fisheries Society, Bethesda, Maryland, USA. [Google Scholar]
- Dieterman DJ, Hoxmeier RJH, and Krumm EJ. 2019. Association between biotic integrity and sport fish populations in upper Midwest, USA rivers, with emphasis on smallmouth bass. Environmental Management 63:732–746. [DOI] [PubMed] [Google Scholar]
- Esselman PC, Infante DM, Wang L, Cooper AR, Wieferich D, Tsang Y-P, Thornbrugh DJ, and Taylor WW. 2013. Regional fish community indicators of landscape disturbance to catchments of the conterminous United States. Ecological Indicators 26:163–173. [Google Scholar]
- Esselman PC, Stevenson RJ, Lupi F, Riseng CM, and Wiley MJ. 2015. Landscape prediction and mapping of game fish biomass, an ecosystem service of Michigan rivers. North American Journal of Fisheries Management 35:302–320. [Google Scholar]
- Fausch KD, Karr JR, and Yant PR. 1984. Regional application of an index of biotic integrity based on stream fish communities. Transactions of the American Fisheries Society 113:39–55. [Google Scholar]
- Gammon JR 1976. The fish populations of the middle 340 km of the Wabash River. Technical Report 86. Purdue University Water Resource Research Center. West Lafayette, Indiana, USA. [Google Scholar]
- Herlihy AT, Paulsen SG, Van Sickle J, Stoddard JL, Hawkins CP, and Yuan LL. 2008. Striving for consistency in a national assessment: the challenges of applying a reference condition approach at a continental scale. Journal of the North American Benthological Society 27:860–877. [Google Scholar]
- Herlihy AT, Sifneos JC, Hughes RM, Peck DV, and Mitchell RM. 2019. Lotic fish assemblage clusters across the conterminous USA and their associations with local- and catchment-scale landscape variables. Pages 385–408 in Hughes RM, Infante DM, Wang L, Chen K, and Terra BF, editors. Advances in understanding landscape influences on freshwater habitats and biological assemblages. Symposium 90. American Fisheries Society, Bethesda, Maryland, USA. [Google Scholar]
- Herlihy AT, Sifneos JC, Hughes RM, Peck DV, and Mitchell RM. 2020. The relation of lotic fish and benthic macroinvertebrate condition indices to environmental factors across the conterminous USA. Ecological Indicators 112(May):105958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman GD 2000. Sport fishing index (SFI): a method to quantify sport fishing quality. Environmental Science and Policy 3:S117–S125. [Google Scholar]
- Hill RA, Weber MA, Leibowitz SG, Olsen AR, and Thornbrugh DJ. 2016. The stream catchment (StreamCat) dataset: a database of watershed metrics for the conterminous United States. Journal of the American Water Resources Association 52:120–128. [Google Scholar]
- Hitt NP, and Angermeier PL. 2008. Evidence for fish dispersal from spatial analysis of stream network topology. Journal of the North American Benthological Society 27:304–320. [Google Scholar]
- Hughes RM 2015. Recreational fisheries in the USA: economics, management strategies, and ecological threats. Fisheries Science 81:1–9. [Google Scholar]
- Hughes RM 2019. Ecological integrity: conceptual foundations and applications. in Wohl E, editor. Oxford bibliographies in environmental science. Oxford University Press, New York, New York, USA. http://www.oxfordbibliographies.com/view/document/obo-9780199363445/obo-9780199363445-0113.xml?rskey=Mfte5h&result=21 [Google Scholar]
- Hughes RM, and Gammon JR. 1987. Longitudinal changes in fish assemblages and water quality in the Willamette River, Oregon. Transactions of the North American Fisheries Society 116:196–209. [Google Scholar]
- Hughes RM, and Herlihy AT. 2007. Electrofishing distance needed to estimate consistent index of biotic integrity (IBI) scores in raftable Oregon rivers. Transactions of the American Fisheries Society 136:135–141. [Google Scholar]
- Hughes RM, and Herlihy AT. 2012. Patterns in catch per unit effort of native prey fish and alien piscivorous fish in 7 Pacific Northwest USA rivers. Fisheries 37:201–211. [Google Scholar]
- Hughes RM, and Peck DV, V. D, 2008. Acquiring data for large aquatic resource surveys: the art of compromise among science, logistics, and reality. Journal of the North American Benthological Society 27:837–859. [Google Scholar]
- Hughes RM, Rexstad E, and Bond CE. 1987. The relationship of aquatic ecoregions, river basins and physiographic provinces to the ichthyogeographic regions of Oregon. Copeia 1987:423–432. [Google Scholar]
- Hughes RM, Kaufmann PR, Herlihy AT, Kincaid TM, Reynolds L, and Larsen DP. 1998. A process for developing and evaluating indices of fish assemblage integrity. Canadian Journal of Fisheries and Aquatic Sciences 55:1618–1631. [Google Scholar]
- Hughes RM, Infante DM, Wang L, Chen K, and Terra BF, editors. 2019. Advances in understanding landscape influences on freshwater habitats and biological assemblages. Symposium 90. American Fisheries Society, Bethesda, Maryland, USA. [Google Scholar]
- Hughes RM, Boxall G, Herlihy AT, Adams J, and Young DB. 2020. A complete fisheries inventory of the Chulitna River Basin, Lake Clark National Park and Preserve, Alaska: example of a minimally disturbed basin. Transactions of the North American Fisheries Society 149:14–26. [Google Scholar]
- Hunt LE 2006. Recreational fishing site choice models: insights and future opportunities. Human Dimensions of Wildlife 10:153–172. [Google Scholar]
- Hunt LE, Boxall PE, and Boots B. 2007. Accommodating complex substitution patterns in a random utility model of recreational fishing. Marine Resource Economics 22:155–172. [Google Scholar]
- Jacobson PC, Hansen GJA, Olmanson LG, Wehrly KE, Hein CL, and Johnson LB. 2019. Loss of coldwater fish habitat in glaciated lakes of the midwestern United States after a century of land use and climate change. Pages 141–157 in Hughes RM, Infante DM, Wang L, Chen K, and Terra BF, editors. Advances in understanding landscape influences on freshwater habitats and biological assemblages. Symposium 90. American Fisheries Society, Bethesda, Maryland, USA.. [Google Scholar]
- Jakus PM, Dadakas D, and Fly JM. 1998. Fishing consumption advisories: incorporating angler‐specific knowledge, habits, and catch rates in a site choice model. American Journal of Agricultural Economics 80:1019–1024. [Google Scholar]
- Jones CA, and Lupi F. 2000. The effect of modeling substitute activities on recreational benefit estimates. Marine Resource Economics 14:257–274. [Google Scholar]
- Kanno Y, Vokoun JC, Dauwalter DC, Hughes RM, Herlihy AT, Maret TR, and Patton TM. 2009. Influence of rare species on electrofishing distance–species richness relationships at stream sites. Transactions of the American Fisheries Society 138:1240–1251. [Google Scholar]
- Kaufmann P, Levine P, Robison E, Seeliger C, and Peck D. 1999. Quantifying physical habitat in wadeable streams. EPA/620/R-99/003. US Environmental Protection Agency, Washington, DC., USA [Google Scholar]
- Kaufmann PR, Faustini JM, Larsen DP, and Shirazi MA. 2008. A roughness-corrected index of relative bed stability for regional stream surveys. Geomorphology 99:150–170. [Google Scholar]
- King JJ 2015. Ecology and economics of fish kills: mortality and recovery of brown trout (Salmo trutta L.) and Atlantic Salmon (Salmo salar L.) in an Irish river. Biology and Environment: Proceedings of the Royal Irish Academy 115:157–170. [Google Scholar]
- Leal CG, Barlow J, Gardner T, Hughes RM, Leitão RP, MacNally R, Kaufmann P, Ferraz SFB, Zuanon J, de Paula FR, Ferreira J, Thomson JR, Lennox GD, Dary EP, Röpke CP, and Pompeu PS. 2018. Is environmental legislation conserving tropical stream faunas? A large-scale assessment of local, riparian and catchment-scale influences on Amazonian fish. Journal of Applied Ecology 55:1312–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomnicky GA, Whittier TR, Hughes RM, and Peck DV. 2007. Distribution of nonnative aquatic vertebrates in western U.S. streams and rivers. North American Journal of Fisheries Management 27:1082–1093. [Google Scholar]
- Lyons J, Piette RR, and Niermeyer KW. 2001. Development, validation, and application of a fish-based index of biotic integrity for Wisconsin’s large warmwater rivers. Transactions of the American Fisheries Society 130:1077–1094. [Google Scholar]
- Macedo DR, Hughes RM, Ligeiro R, Ferreira WR, Castro M, Junqueira NT, Silva DRO, Firmiano KR, Kaufmann PR, Pompeu PS, and Callisto M. 2014. The relative influence of multiple spatial scale environmental predictors on fish and macroinvertebrate assemblage richness in cerrado ecoregion streams, Brazil. Landscape Ecology 29:1001–1016. [Google Scholar]
- Marzin A, Verdonschot PFM, and Pont D. 2012. The relative influence of catchment, riparian corridor, and reach-scale anthropogenic pressures on fish and macroinvertebrate assemblages in French rivers. Hydrobiologia 704:375–388. [Google Scholar]
- McCormick FH, Peck DV, and Larsen DP. 2000. Comparison of geographic classification schemes for mid-Atlantic stream fish assemblages. Journal of the North American Benthological Society 19:385–404, [Google Scholar]
- McCormick FH, Hughes RM, Kaufmann PR, Herlihy AT, Peck DV. 2001. Development of an index of biotic integrity for the Mid-Atlantic Highlands Region. Transactions of the American Fisheries Society 130:857–877. [Google Scholar]
- McGarvey DJ, and Hughes RM. 2008. Longitudinal zonation of Pacific Northwest (U.S.A.) fish assemblages and the species-discharge relationship. Copeia 2008:311–321. [Google Scholar]
- McGarvey DJ, and Ward GM. 2008. Scale dependence in the species-discharge relationship for fishes of the southeastern U.S.A. Freshwater Biology 53:2206–2219. [Google Scholar]
- Mebane CA, Maret TR, and Hughes RM. 2003. An index of biological integrity (IBI) for Pacific Northwest rivers. Transactions of the American Fisheries Society 132:239–261. [Google Scholar]
- Melstrom RT, Lupi F, Esselman PC, and Stevenson RJ. 2015. Valuing recreational fishing quality at rivers and streams. Water Resources Research 51:140–150. [Google Scholar]
- Mendelsohn R, and Olmstead S. 2009. The economic valuation of environmental amenities and disamenities: methods and applications. Annual Review of Environment and Resources 34:325–347. [Google Scholar]
- Morey ER, Rowe RD, and Watson M. 1993. A repeated nested-logit model of Atlantic salmon fishing. American Journal of Agricultural Economics 75:578–592. [Google Scholar]
- Morey ER, Breffle WS, Rowe RD, and Waldman DM. 2002. Estimating recreational trout fishing damages in Montana’s Clark Fork River basin: summary of a natural resource damage assessment. Journal of Environmental Management 66:159–170. [DOI] [PubMed] [Google Scholar]
- Mulvey M, Leferink R, and Borisenko A. 2009. Willamette basin rivers and streams assessment. Oregon Department of Environmental Quality. Hillsboro, Oregon, USA. [Google Scholar]
- Murdock J 2006. Handling unobserved site characteristics in random utility models of recreation demand. Journal of Environmental Economics and Management 51:1–25. [Google Scholar]
- NMFS (National Marine Fisheries Service). 2015. Fisheries economics of the United States, 2015. Government Printing Office, Washington, DC, USA. [Google Scholar]
- Oberdorff T, and Hughes RM. 1992. Modification of an index of biotic integrity based on fish assemblages to characterize rivers of the Seine Basin, France. Hydrobiologia 228:117–130. [Google Scholar]
- Oliveira JM, Hughes RM, Ferreira MT, Teixeira A, Morgado P, Cortes RM, and Bochechas JH. 2009. A preliminary fishery quality index for Portuguese streams. North American Journal of Fisheries Management 29: 1466–1478. [Google Scholar]
- Olsen AR, Peck DV. 2008. Survey design and extent estimates for the Wadeable Streams Assessment. Journal of the North American Benthological Society 27:822–836. [Google Scholar]
- Page LM, Espinosa-Pérez H, Findley LT, Gilbert CR, Lea RN, Mandrak NE, Mayden RL, Nelson JS. 2013. Common and scientific names of fishes from the United States, Canada and Mexico. Special Publication 34. American Fisheries Society, Bethesda, Maryland, USA. [Google Scholar]
- Perkin JS, Wellemeyer JC, and Fore JD. 2019. Multiscale fish assemblage distribution models to guide riverscape conservation planning. Pages 409–440 in Hughes RM, Infante DM, Wang L, Chen K, and Terra BF, editors. Advances in understanding landscape influences on freshwater habitats and biological assemblages. Symposium 90. American Fisheries Society, Bethesda, Maryland, USA. [Google Scholar]
- Phaneuf DJ 2002. A random utility model for total maximum daily loads: estimating the benefits of watershed‐based ambient water quality improvements. Water Resources Research 38(11): 1254, doi: 10.1029/2001WR000959. [DOI] [Google Scholar]
- Reynolds L, Herlihy AT, Kaufmann PR, Gregory SV, and Hughes RM. 2003. Electrofishing effort requirements for assessing species richness and biotic integrity in western Oregon streams. North American Journal of Fisheries Management 23:450–461. [Google Scholar]
- Ringold PL, Boyd J, Landers D, and Weber M. 2013. What data should we collect? A framework for identifying indicators of ecosystem contributions to human well-being. Frontiers in Ecology and the Environment 11:98–105. [Google Scholar]
- Rinne JN, Hughes RM, and Calamusso B, editors. 2005. Historical changes in large river fish assemblages of the Americas. Symposium 45. American Fisheries Society, Bethesda, Maryland, USA. [Google Scholar]
- Rohm CJ, Giese JW, and Bennett CC. 1987. Evaluation of an aquatic ecoregion classification of streams in Arkansas. Journal of Freshwater Ecology 4:127–140. [Google Scholar]
- Salvanes AGV 2017. Are antipredator behaviours of hatchery Salmo salar juveniles similar to wild juveniles? Journal of Fish Biology 90: 1785–1796. [DOI] [PubMed] [Google Scholar]
- Southwick RI, and Loftus AJ. 2017. Investigation and monetary values of fish and freshwater mollusk kills. American Fisheries Society Special Publication 35. Bethesda, Maryland, USA. [Google Scholar]
- Stevens DL, and Olsen AR. 2004. Spatially balanced sampling of natural resources. Journal of the American Statistical Association 99:262–278. [Google Scholar]
- SYSTAT ver. 13. 2009. Systat Software, Inc. Available at: www.systatsoftware.com. [Google Scholar]
- Terra BDF, Hughes RM, and Araujo FG. 2015. Fish assemblages in Atlantic Forest streams: the relative influence of local and catchment environments on taxonomic and functional species. Ecology of Freshwater Fish 25: 527–544. [Google Scholar]
- USA (United States of America). 1972. Federal water pollution control act amendments of 1972. 33 U.S.C. 1251 et seq. https://www.epa.gov/sites/production/files/2017-08/documents/federal-water-pollution-control-act-508full.pdf.
- USEPA (United States Environmental Protection Agency). 1990. Preliminary identification of approaches used in valuating natural resources. Office of Solid Waste. Washington, DC, USA. [Google Scholar]
- USEPA (United States Environmental Protection Agency). 2013a. National rivers and streams assessment 2013/14: field operations manual -- wadeable. EPA 841/B-12/009b, Office of Water and Office of Environmental Information, Washington, DC, USA. [Google Scholar]
- USEPA (United States Environmental Protection Agency). 2013b. National rivers and streams assessment 2013/14: field operations manual --non-wadeable. EPA 841/B-12/009a, Office of Water and Office of Environmental Information, Washington, DC, USA. [Google Scholar]
- USEPA (United States Environmental Protection Agency). 2016a. National rivers and streams assessment 2008–2009 technical report. EPA 841/R-16/008, Office of Water and Office of Research and Development, Washington, DC, USA. [Google Scholar]
- USEPA (U.S. Environmental Protection Agency). 2016b. National rivers and streams assessment 2008–2009: a collaborative survey. EPA/841/R-16/007. Office of Water and Office of Research and Development. Washington, DC, USA. [Google Scholar]
- USFWS (U.S. Fish and Wildlife Service). 2012. 2011 National survey of fishing, hunting, and wildlife-associated recreation. U.S. Department of the Interior, Washington, DC, USA [Google Scholar]
- USGS (United States Geological Survey). 2013. National hydrography geodatabase. https://viewer.nationalmap.gov/viewer/nhd.html?p=nhd.
- Van Sickle J, and Hughes RM. 2000. Classification strengths of ecoregions, basins and geographic clusters for aquatic vertebrates in Oregon. Journal of the North American Benthological Society 19:370–384. [Google Scholar]
- Von Haefen RH 2003. Incorporating observed choice into the construction of welfare measures from random utility models. Journal of Environmental Economics and Management 45:145–165. [Google Scholar]
- Wang L, Lyons J, Rasmussen P, Seelbach P, Simon T, Wiley M, Kanehl P, Baker E, Niemela S, and Stewart PM. 2003. Watershed, reach, and riparian influences on stream fish assemblages in the Northern Lakes and Forest Ecoregion, U.S.A. Canadian Journal of Fisheries and Aquatic Sciences 60:491–505. [Google Scholar]
- Ward FA, and Loomis JB. 1986. The travel cost demand model as an environmental policy assessment tool: a review of literature. Western Journal of Agricultural Economics 11:164–178. [Google Scholar]
- Weber MA, and Ringold PL. 2019. River metrics by the public, for the public. PLoS ONE 14(5): e0214986. 10.1371/journal.pone.0214986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittier TR, Hughes RM, Stoddard JL, Lomnicky GA, Peck DV, and Herlihy AT. 2007. A structured approach to developing indices of biotic integrity: three examples from western USA streams and rivers. Transactions of the American Fisheries Society 136:718–735. [Google Scholar]
- Wilson MA, and Carpenter SR. 1999. Economic valuation of freshwater ecosystem services in the Unites States: 1971 to 1997. Ecological Applications 9:772–783. [Google Scholar]
- Winans GA, Baker J, McHenry M, Ward L, and Myers J. 2017. Genetic characterization of Oncorhynchus mykiss prior to dam removal with implications for recolonization of the Elwha River watershed, Washington. Transactions of the American Fisheries Society 146: 160–172. [Google Scholar]
- Woody CA 2018. Bristol Bay Alaska: natural resources of the aquatic and terrestrial ecosystems. J. Ross Publishing, Plantation, Florida, USA. [Google Scholar]


