Abstract
Cyanobacterial harmful algal blooms are the most common form of harmful algal blooms in freshwater systems throughout the world. However, in situ sampling of cyanobacteria in inland lakes is limited both spatially and temporally. Satellite data has proven to be an effective tool to monitor cyanobacteria in freshwater lakes across the United States. This study uses data from the European Space Agency Envisat MEdium Resolution Imaging Spectrometer and the Sentinel-3 Ocean and Land Color Instrument to provide a national overview of the percentage of lakes experiencing a cyanobacterial bloom on a weekly basis for 2008–2011, 2017, and 2018. A total of 2321 lakes across the contiguous United States were included in the analysis. We examined four different thresholds to define when a waterbody is classified as experiencing a bloom. Across these four thresholds, we explored variability in bloom percentage with changes in seasonality and lake size. As a validation of algorithm performance, we analyzed the agreement between satellite observations and previously established ecological patterns, although data availability in the wintertime limited these comparisons on a year-round basis. Changes in cyanobacterial bloom percentage at the national scale followed the well-known temporal pattern of freshwater blooms. The percentage of lakes experiencing a bloom increased throughout the year, reached a maximum in fall, and decreased through the winter. Wintertime data, particularly in northern regions, were consistently limited due to snow and ice cover. With the exception of the Southeast and South, regional patterns mimicked patterns found at the national scale. The Southeast and South exhibited an unexpected pattern as cyanobacterial bloom percentage reached a maximum in the winter rather than the summer. Lake Jesup in Florida was used as a case study to validate this observed pattern against field observations of chlorophyll a. Results from this research establish a baseline of annual occurrence of cyanobacterial blooms in inland lakes across the United States. In addition, methods presented in this study can be tailored to fit the specific requirements of an individual system or region.
Keywords: Remote sensing, Harmful algal blooms, Cyanobacteria, Water quality, Inland waters
1. Introduction
Cyanobacterial harmful algal blooms (cyanoHABs) are the most common form of harmful algal blooms in freshwater systems (Hudnell et al., 2008). While the classification of a harmful algal bloom has regional, seasonal, and species-specific aspects, generally, the term harmful algal bloom is a colloquial way of indicating when algal growth has resulted in negative environmental or health consequences (Smayda, 2003). Human exposure occurs through skin contact, accidental ingestion, inhalation during recreational activities, or the consumption of contaminated drinking water (Chorus et al., 2000). A wide range of health risks have been associated with human exposure to cyanobacteria including skin and eye irritation, adverse effects on liver and kidney function, and flu-like symptoms including headache, nausea, diarrhea, and vomiting (World Health Organization, 2003).
CyanoHABs occur worldwide and have been documented across the United States (Loftin et al., 2016). The significance of several recent bloom events has increased national awareness of cyanoHABs. In 2010, Grand Lake St. Marys in Ohio experienced an extensive cyanobacterial bloom that killed thousands of fish and forced a “No Contact” advisory to be issued for the lake. An event in Lake Erie resulted in over half a million people being left without access to clean drinking water in the City of Toledo, Ohio in 2014 (Sonich-Mullin, 2014). Lake Okeechobee in Florida experienced a substantial bloom in 2016 that spread to the coast, prompting a state of emergency in four coastal counties (Neuhaus, 2016).
Traditionally, in situ sampling has been used to assess the status of a bloom in a specific lake. However, these efforts are often limited both spatially and temporally due to the time and cost associated with collecting data. These considerations have encouraged exploration of alternative means for assessing cyanoHAB status. Satellite data has proven to be an effective monitoring tool for inland lakes across the United States (Urquhart et al., 2017; Clark et al., 2017; Stumpf et al., 2016; Stumpf et al., 2016).
While satellite technology cannot currently be used to detect toxins (Stumpf et al., 2016), it can be used effectively to identify cyanobacterial blooms (Wynne et al., 2008) and to quantify cyanobacterial abundance (Kutser, 2009). Satellite data also has the potential to provide fairly frequent observations both temporally and spatially, although wintertime data are still limited due to snow and ice cover. Estimates of cyanobacteria concentration from satellite data offer a cost-effective approach to fill in temporal and spatial gaps when field sampling is not feasible and when cloud- and ice-free observations are possible.
Several studies have used satellite remote sensing to define the onset or quantify the duration of a cyanobacterial bloom by setting a minimum threshold based on the spatial coverage of the bloom. However, there is no consensus on what this threshold should be, and there are no recommendations regarding differences that might arise based on the threshold chosen. Zhang et al. (2012) defined the onset of cyanobacteria as the earliest date at which cyanobacterial blooms were first recorded by remote sensing. Using satellite observations from Taihu Lake in China, Hu et al. (2010) defined a significant bloom as one that covered 25% of the surface area. Qin et al. (2015) set thresholds at 10%, 40%, and 60% of the lake surface area to define three risk management levels. Most recently, Davis et al. (2019) used a threshold of 30% of the spatial area to classify a cyanobacterial bloom.
This study uses satellite remote sensing to report on the weekly percentage of lakes across the contiguous United States (CONUS) that are experiencing detectable cyanobacterial blooms. First, four different thresholds on spatial bloom area are explored for classifying whether a waterbody is experiencing a cyanobacterial bloom. Next, we qualitatively consider the agreement between satellite estimates of cyanobacterial occurrence and previously established ecological patterns. Data from the MEdium Resolution Imaging Spectrometer (MERIS) were employed from 2008 to 2011, and Sentinel-3A Ocean and Land Color Instrument (OLCI) data were used for 2017 and 2018. This study aims to address the following research questions:
How many large waterbodies nationally and regionally are impactedby cyanobacterial blooms throughout the year?
Does the satellite algorithm result in the expected seasonality relative to published ecological patterns?
Can satellite data be used to find the most appropriate spatial area threshold for classifying a waterbody as experiencing a cyanobacterial bloom?
2. Data and methods
2.1. Satellite observations
MERIS and OLCI are European Space Agency sensors onboard the Envisat and Sentinel-3A satellites, respectively. The MERIS archive includes a consistent time series over CONUS for the years 2008 through 2011. The OLCI sensor provides data for the years 2017 and 2018, and will be available to perform similar analyses in the future. The five-year data gap between the two sensors is a result of the Envisat mission ending in April 2012 due to a loss of communication with the satellite; no equivalent sensor was available until the launch of Sentinel-3A in February 2016.
Both sensors have a temporal resolution of approximately 2–3 days and a spatial resolution of 300 m at nadir, where nadir is defined as the point on the Earth surface directly below the satellite. With the inclusion of Sentinel-3B data in future analyses, temporal coverage will increase to near-daily across CONUS. Standard MERIS Level-1B data were obtained from the NASA Ocean Color website (https://oceandata.sci.gsfc.nasa.gov) and were processed as described in Urquhart and Schaeffer (2019).
A spectral shape algorithm was used to assess cyanobacteria presence, quantifying its abundance as the cyanobacteria index (CI). Originally described in Wynne et al. (2008), this spectral shape (SS) algorithm uses a baseline defined as a straight line drawn between the 665 nm and the 709 nm bands. If the fluorescence band (681) falls below the baseline between 709 nm and 665 nm, yielding a negative SS (681), cyanobacteria is likely present. In cases without cyanobacteria, the reflectance at 681 nm falls above the 709 nm and 665 nm baseline, yielding a positive SS(681).
This iteration of CI was found to potentially identify other erroneous blooms including chlorophytes (Wynne et al., 2013), causing false positives. To address this issue, the CI algorithm was updated in Lunetta et al. (2015) to examine the ability to separate cyanobacteria from other blooms using a derivative that includes the 620 nm band, which is sensitive to phycocyanin, where SS(665) with λ = 665 nm, λ+ = 681 nm, and λ− = 620 nm. This condition was defined by Matthews et al. (2012) (Eqs. 3–4) to separate cyanobacteria from other blooms in African lakes. The updated CI algorithm was termed in Lunetta et al. (2015) as the CI-multi. The CI-multi uses the CI to estimate biomass, but uses the spectral shape around the 665 nm band as an exclusion criterion. Elevated phycocyanin absorption is presumed to depress the reflectance at 620 nm (e.g., Simis et al. (2005)), causing SS (665) to change from negative to positive. Accordingly, when SS (665) < 0, cyanobacteria are presumed absent, and when SS (665) > 0, cyanobacteria are presumed present.
While this algorithm has evolved since its first application in Wynne et al. (2008), hereafter, it will be referred to as CI. CI was calculated for each pixel and for each satellite overpass. Observations were then aggregated into weekly composites that preserve the maximum value for each pixel. This algorithm has been well documented across several U.S. states including Florida, Ohio, Rhode Island, Massachusetts, New Hampshire, Vermont, Connecticut, and Maine (Lunetta et al., 2015; Clark et al., 2017); Lake Erie (Stumpf et al., 2016); from 25 state health advisories in California, Oregon, New York, Idaho, New Jersey, Utah, and Vermont (Schaeffer et al., 2018); and was used by the Wyoming Department of Environmental Quality to issue eight health advisories in 2018 (e.g., in Big Sandy Reservoir (Wyoming, 2018a), Eden Reservoir (Wyoming, 2018b), and Pathfinder Reservoir (Wyoming, 2018c)).
2.2. National and regional coverage of waterbodies
Given the spatial resolution of both MERIS and OLCI (300 m at nadir), only lakes of sufficient size and shape were considered for analysis. Waterbodies used in this analysis were selected according to Urquhart and Schaeffer (2019). A total of 2,321 waterbodies across CONUS were classified as resolvable with at least one resolvable waterbody in each state, except for West Virginia and Delaware, in which all lakes were either of insufficient size or shape to meet the criteria. These 2,321 waterbodies represent only 0.61% of the 379,097 lakes, ponds, and reservoirs included in the National Hydrography Dataset Plus version 2.0 (NHD). Waterbodies considered in this analysis ranged in size from approximately 0.75 km2 to over 4,000 km2 which limits this analysis to relatively large lakes and excludes smaller waterbodies across the United States.
Satellite pixels containing cloud cover, those that fell along the landwater interface, and those that contained snow and ice were discarded as they can confound the satellite signal. Discarding data that contained snow and ice led to some data gaps during the winter months, particularly in northern latitude states. Thus, central and southern latitude states have a more complete time series than northern latitude states. Although this does limit wintertime data in several regions, we can assume that at least recreational exposure to cyanobacteria is limited when snow and ice are present in a waterbody.
To investigate the percentage of lakes experiencing a bloom at a regional level, we used the nine U.S. climate regions defined by the National Center for Environmental Information (Karl and Koss, 1984). The nine U.S. climate regions represent climatically consistent states across CONUS, and were selected for this study in an effort to group together areas that are prone to missing data due to snow and ice events during the winter months (Fig. 1).
Fig. 1.
CONUS was divided into nine climatically consistent regions (Karl and Koss, 1984) to consider the percentage of lakes experiencing a cyanobacterial bloom at a regional level. Each color indicates the states included in each climate region and gray polygons indicate all lakes considered in the analysis. The number of resolvable lakes in each region is noted under each region name. The Ohio Valley has the fewest number of resolvable lakes with 95, while the Upper Midwest has the largest number of resolvable lakes with 697.
2.3. Analyzing cyanobacterial bloom percentage
For each weekly composite, each lake was classified according to the satellite derived cyanobacteria count: either the lake was experiencing a bloom, the lake was not experiencing a bloom, or the lake did not have sufficient data for the given weekly composite. A lake was considered to not be experiencing a bloom for the given weekly composite if the given threshold was not met, but there was at least one observable pixel in the lake (i.e. all pixels within the lake were not discarded). A lake was considered to have insufficient data for the given weekly composite if all pixels within the lake were discarded. For each week across the six years of data, percentages were calculated with a constant denominator of 2321, the total number of resolvable lakes. A constant denominator was chosen for all figures except for Fig. 2. A dynamic denominator would imply resolvable lakes are representative of all lakes across the country or region; however, it is not reasonable to assume resolvable lakes in southern latitude states such as Florida or Texas are representative of lakes in northern latitude states such as Minnesota or Washington. This is particularly important during the winter months when snow and ice cover much of the northern part of the country. While a constant denominator was used in this study, a dynamic denominator might be appropriate on a regional scale in future studies.
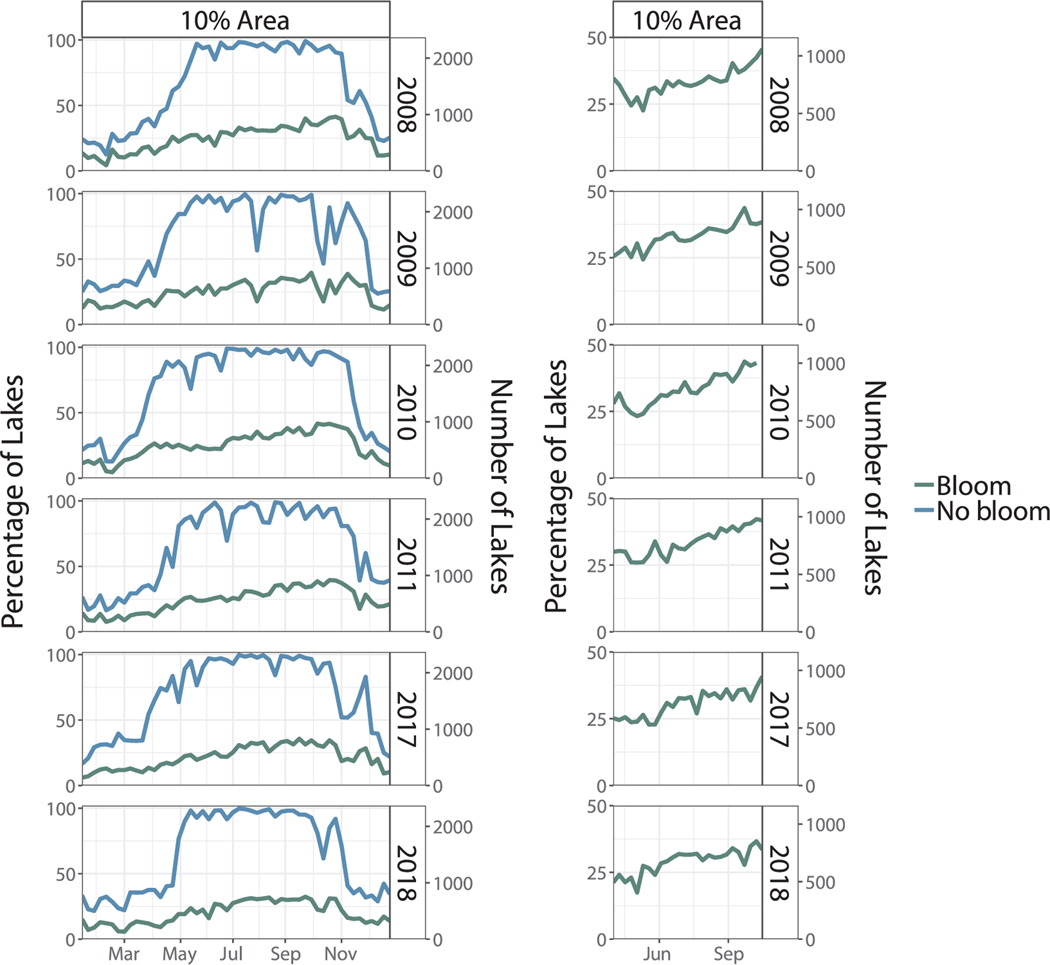
Fig. 2.
Time series indicating the national percentage (left axis) and number (right axis) of lakes experiencing a cyanobacterial bloom on a weekly basis for the years 2008 through 2011, 2017 and 2018 based on a spatial area threshold of 10%. The lefthand figure shows a stacked (cumulative) time series indicating lakes experiencing a bloom in green and not experiencing a bloom in blue. The righthand figure shows a time series of just the months of May through October during which snow and ice extent are limited, increasing the number of observable lakes. The righthand figure also uses a dynamic denominator in which the denominator reflects the number of observable lakes each week rather than a constant denominator of 2321.
2.4. Defining a cyanobacterial bloom based on spatial bloom area
In this study, four different area thresholds were tested, with any pixels above the minimum detection limit counting toward bloom area. The minimum detection limit for cyanobacteria is currently estimated to be at or above a CI value of 1. For reference, this corresponds to the World Health Organization (WHO) low-risk threshold at which there is a relatively low probability of adverse health effects in recreational waters (World Health Organization, 2003). Given the localized characterization at which an algal bloom becomes harmful, this study reports solely on the presence of cyanobacteria and not whether the observed blooms had a negative impact on human or environmental health.
The most sensitive area threshold used in this study requires that at least one pixel in the waterbody be above the detection limit for the entire lake to be classified as experiencing a bloom. Thresholds on the percentage of pixels indicating a bloom rather than the number of pixels indicating a bloom were also tested. Thresholds of 10%, 20%, and 30% were used; if the percentage of pixels indicating a bloom out of all valid pixels was at or above the given threshold, the lake was classified as experiencing a bloom.
Currently, there is no consensus regarding the minimum spatial area a bloom must cover for a waterbody to be characterized as experiencing a bloom; However, several recent studies have defined the onset of a bloom or quantified the duration of a bloom using satellite remote sensing. These studies required that some portion of a waterbody be classified as a bloom before classifying the entire waterbody as a bloom. The most sensitive threshold found in the literature defined the onset of cyanobacteria as the earliest date on which cyanobacteria was first recorded by remote sensing (Zhang et al., 2012). This classification method is similar to using a single point sample to indicate a bloom is present. Thresholds on percent of lake surface area have also been used (Hu et al., 2010; Davis et al., 2019; Qin et al., 2015).
Herein we outlined a method to calculate weekly bloom percentage; therefore, while four discrete area thresholds were tested, the threshold used can be adjusted to meet the needs of a particular system or study. To understand if some thresholds might be more appropriate for a specific analysis, the effects of both seasonality and lake size were analyzed using 2018 data. To quantitatively compare results across seasons and lake sizes, the mean absolute deviation (MAD) was calculated by comparing bloom percentage for each subset to bloom percentage for all lakes. This allows for an investigation into whether a certain threshold under- or overclassifies bloom percentage when compared to all waterbodies. MAD is calculated similarly to Mean Absolute Error. For each subset of data (i.e. either seasons or lake sizes), the sum of the deviation between observations within each subset and the total population is divided by the number of observations within each subset. Higher values indicate less agreement with the total population.
To evaluate the effect of seasonality on each threshold, a time series was generated to display the difference in bloom percentage between each threshold on a weekly basis. This time series was then normalized to the number of lakes with valid data for each week. In other words, for a given week, the difference in bloom percentage using a 1 pixel threshold and bloom percentage using a 10% area threshold was normalized by the number of valid lakes for that weekly composite. This was repeated to compare bloom percentage between 10% and 20% and between 20% and 30%. This allows for an investigation into whether the spread between thresholds is affected by time of year. When quantifying MAD for seasonality, meteorological seasons were considered with winter as December through February, spring as March through May, summer as June through August, and fall as September through November.
To evaluate the effect of lake size on each threshold, lakes were divided into small, medium, and large lakes. Small lakes were those with fewer than 11 pixels. This cutoff was chosen since this is the smallest number at which if a lake meets the most sensitive threshold (1 pixel), then it automatically meets at least one other threshold. For example, if a lake has 7 pixels and 1 pixel indicates a cyanobacterial bloom, then this lake meets both the 1 pixel and the 10% area thresholds. However, if a lake has 11 pixels and 1 pixel indicates a cyanobacterial bloom, then this lake only meets the 1 pixel threshold. Medium lakes were classified as those with less than 50 pixels, which was chosen to keep the sample size of the three categories similar. All analyses were conducted in R Version 3.4.0 (R Core Team, 2017).
3. Results and discussion
3.1. National cyanobacterial bloom percentage
Based on the four spatial area thresholds considered in this study, a threshold of 10% was shown to reduce variability in the results, which will be further discussed in Section 3.3. Therefore, both national and regional results will be shown based on this threshold. The national pattern of bloom percentage was fairly consistent across the six years of data. Fig. 2 shows two time series representing results at the CONUS scale based on a 10% threshold. The lefthand figure shows a stacked (cumulative) time series indicating the percentage of lakes experiencing a bloom and the percentage of lakes not experiencing a bloom, as a portion of the total 2,321 lakes investigated. Results based on thresholds of 1 pixel, 20%, and 30% are shown in Fig. 9. The righthand figure shows a subset of the year, May through October, which represents the months in which the majority of the country had fairly complete spatial coverage due to reduced snow and ice extent. This time series shows just bloom percentage (i.e. the percentage of lakes without a bloom is not displayed). Bloom percentage displayed here was calculated by dividing the number of lakes experiencing a bloom by the number of observable lakes for a given week (a dynamic denominator), as opposed to the aforementioned method of dividing by the total number of lakes (a static denominator). Including this time series along with the full annual time series highlights the increase in cyanobacterial abundance seen throughout the growing season.
Fig. 9.
Stacked time series indicating the national percentage (left axis) and number (right axis) of lakes experiencing a cyanobacterial bloom (green) and not experiencing a cyanobacterial bloom (blue). Results are shown for each week for the years 2008 through 2011, 2017 and 2018 based on spatial area thresholds of 1 pixel, 20%, and 30%.
The minimum bloom percentage across the six years of data ranged from 6% to 15% per year, while the maximum ranged from 63% to 73%. The lowest minimum and lowest maximum both occurred in 2018, while the highest minimum and highest maximum both occurred in 2009. The percentage of lakes with no bloom reached a maximum early in the season and with relative consistency from year to year, ranging from 59% in 2009 to 66% in 2011 and occurring almost always in early July.
While summer and fall have fairly comprehensive temporal coverage, large gaps in observable data persist throughout the winter. Approximately 70% of lakes had no valid data from December through mid-March. A sharp increase in data availability typically occurred in March to April, corresponding to decreased snow and ice cover in northern latitude states. Data availability sharply declined again in November and December. There were a few instances in the summer when data availability momentarily declined which can be attributed to widespread cloud cover resulting in large data gaps across CONUS.
At the CONUS scale, the shapes of seasonal periodicity were as expected, with initiation of blooms around April, a maximum between September and November, and senescence of cyanobacteria occurring in November and December. Qualitatively, this offers some support that the satellite data agrees with previously published ecological studies describing the phenology of cyanobacteria in inland lakes. Most studies to date have been limited to either a smaller spatial or temporal subset, such as a single waterbody or a single season. Of the few year-round datasets that do exist, most report cyanobacterial bloom occurrence is highest during late-summer with minimum occurrence during winter months. Using chlorophyll a as a proxy for cyanobacteria, Lake Taihu, China indicated maximum levels from 1992–2012 in late summer and early fall with lower values observed in winter and spring (Xu et al., 2017). Using data collected from 2001–2016 in the Cheney Reservoir Watershed in Kansas, Graham et al. (2017) found cyanobacterial abundances to reach a maximum in late summer and early fall. At several sites along the Sodus Bay in Lake Ontario, the highest concentrations of cyanobacteria pigment occurred toward the end of the summer in 2011 and 2012 (Lugliè et al., 2017).
3.2. Regional cyanobacterial bloom percentage
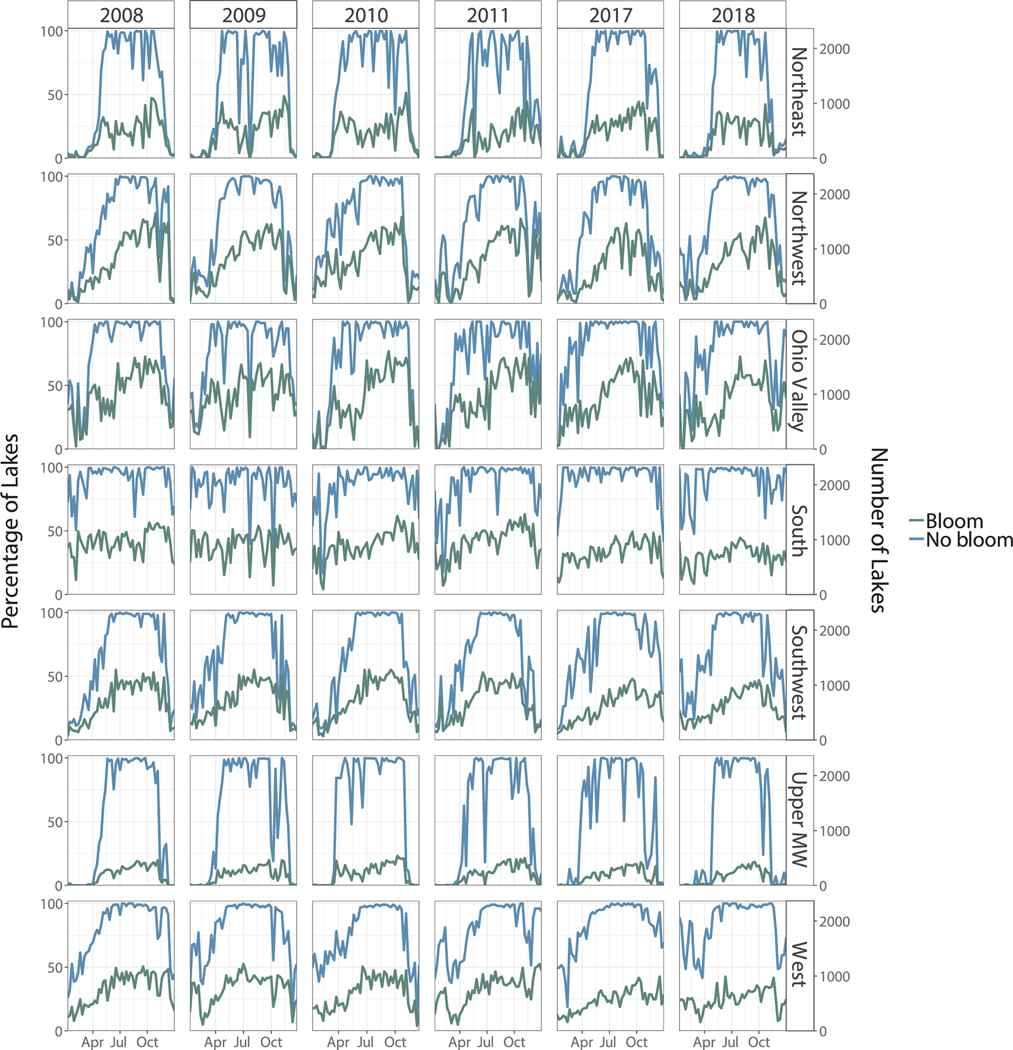
To account for states more prone to snow and ice cover during winter, a regional bloom percentage assessment was performed where states with similar climates were grouped together. A stacked time series was generated for the percentage of lakes with a bloom and the percentage of lakes without a bloom for each of the nine climate regions based on a threshold of 10% (Fig. 3, Fig. 8).
Fig. 3.
Stacked time series indicating regional percentage (left axis) and number (right axis) of lakes experiencing a bloom in green and not experiencing a bloom in blue for the years 2008 to 2011, 2017, and 2018. Results are shown for the Northwest Rockies & Plains (NWR&P) and the Southeast based on a spatial area threshold of 10%.
Fig. 8.
Stacked time series indicating the regional percentage (left axis) and number (right axis) of lakes experiencing a bloom and percentage of lakes not experiencing a bloom for each weekly composite from 2008 through 2011, 2017, and 2018. Results are based on a 10% minimum surface area threshold for a waterbody to be classified as experiencing a bloom. Climate regions include Northeast, Northwest, Ohio Valley, South, Southwest, Upper Midwest, and West. Results for the Northwestern Rockies & Plains and the Southeast are shown in Fig. 3.
Regardless of notable differences in the timing and duration, every region reached a maximum bloom percentage of approximately 75%. Northern regions such as the Ohio Valley and Northwest Rockies and Plains reached this maximum only briefly, while the Southeast and the West had several weeks throughout the year in which at least 75% of lakes contained cyanobacteria. Bloom percentage in the South and the Southeast was persistently high with only a few weeks dropping below the 50% line across the entire time series.
Despite differences in cyanobacterial bloom percentage across each climate region, there is fairly strong temporal agreement within each climate region. With only a few exceptions, the timing of maximums and minimums as well as their relative magnitude is fairly consistent from year-to-year in each climate region, suggesting temporal stability across the years of data.
The Northwest Rockies and Plains and the Southeast were selected for Fig. 3 since each illustrates a distinct seasonal pattern. Results from the Northwest Rockies and Plains highlight difficulties in collecting year-round data in colder regions. During the winter months, the percentage of lakes with valid data was at or near 0%, indicating that we cannot fully evaluate annual time series in this region. During snow- and ice-free months, results were consistent with those observed across CONUS.
Unlike the higher latitude regions of the United States, the Southeast has a nearly complete annual time series. There were only a few notable missing data peaks in the summertime due to widespread cloud cover as confirmed with aerial imagery in both June 2009 and June 2011. Given the near-continuous time series in the Southeast, a unique pattern was observed compared to the other eight climate regions: the maximum bloom percentage actually occurred in the winter as opposed to summer. The percentage of lakes experiencing a bloom hovered at nearly 90% at the beginning of the year before gradually decreasing, reaching a minimum in late summer, and increasing again through the end of the year. The percentage of lakes without a bloom reached its highest value in late summer before decreasing through the winter. To a lesser degree, the South also showed this pattern. This finding will be further discussed in Section 3.4.
3.3. The effect of threshold choice on bloom percentage
When comparing national cyanobacterial occurrence across different thresholds, there are noticeable shifts in the magnitude of lakes with a bloom, but the overall pattern remains consistent (Fig. 9). Bloom percentage between the four thresholds is nearly identical during the winter months when observable data is at a minimum. Results begin to separate during the spring, but differences between each threshold remain approximately stable throughout the growing season. Their deviation begins to close again during the fall and into the winter.
While all thresholds displayed the same pattern of cyanobacterial blooms throughout the year, the reported number of lakes experiencing cyanobacterial blooms at the regional and national scale can differ drastically between the most sensitive (a minimum of 1 pixel) and least sensitive (a minimum of 30% surface area) thresholds. We believe the choice of threshold should be up to those making management decisions; however, we aimed to explore how seasonality or lake size might affect bloom percentage under different threshold requirements.
To consider the effect of seasonality, we used weekly data from 2018 and compared the normalized and non-normalized differences in bloom percentage between each of the four thresholds (Fig. 4). The difference between bloom percentage based on a 1 pixel threshold and a 10% surface area threshold was highly variable over time. When normalized by the number of observable lakes per week, the difference between 10% and 20% and the difference between 20% and 30% was relatively consistent throughout the year, indicating that the time of year does not cause large deviations in observed bloom percentage using these thresholds. This result suggests the 1 pixel threshold might produce more sporadic bloom percentage results than a threshold based on spatial coverage. MAD was calculated to compare bloom percentage for each season to annual bloom percentage (Table 1). Within each threshold, MAD was fairly consistent across seasons, but, of the four thresholds considered, a 1 pixel threshold had the highest MAD.
Fig. 4.
The difference in bloom percentage for 2018 between each of the four thresholds, both non-normalized (left) and normalized by the number of observable lakes for each week (right).
Table 1.
Mean absolute deviation (MAD) comparing bloom percentage for each season to annual bloom percentage for each of the four spatial area thresholds (1 pixel, 10%, 20%, and 30%). Spring, summer, fall, and winter were defined by meteorological seasons. A larger MAD indicates increased variability for a particular season compared to all seasons.
| Season | |||||
|---|---|---|---|---|---|
| Threshold | Spring | Summer | Fall | Winter | Total |
| 1 Pixel | 22.54 | 25.21 | 22.80 | 23.30 | 93.84 |
| 10% Area | 19.58 | 23.26 | 21.04 | 20.05 | 83.93 |
| 20% Area | 17.47 | 21.37 | 19.25 | 17.79 | 75.89 |
| 30% Area | 15.11 | 19.12 | 17.44 | 15.47 | 67.14 |
We also tested whether different sized lakes were under- or overclassified at each threshold. Lakes were categorized into small, medium, and large lakes based on the number of potential satellite pixels contained within the lake (Fig. 5). At the 1 pixel threshold, large lakes tend to be overclassified and small lakes tend to be underclassified relative to all lakes. Large lakes are likely overclassified due to the extreme sensitivity of the 1 pixel threshold relative to others. This also makes the 1 pixel threshold more susceptible to false positive bloom classifications based on rare erroneous pixels. At 10%, there is very little variation indicating lake size does not seem to cause deviation at this threshold. At the 20% and 30% surface area thresholds, large lakes tend to be underclassified, although only slightly. This is likely because a much larger bloom would be required for these lakes to indicate that a bloom is present. MAD confirmed these findings with a much larger value found for the 1 pixel threshold compared to all lakes. A 30% threshold had the lowest MAD across all lake sizes, although results for 10%, 20%, and 30% were similar (Table 2). Based on the analyses of seasonality and lake size, it seems that a threshold on spatial area, rather than just a single pixel, is most appropriate to avoid potentially erroneous bloom percentage results.
Fig. 5.
Bloom percentage for 2018 for each spatial area threshold (1 pixel, 10%, 20%, and 30%) and for different lake sizes. Lakes were divided into small, medium, and large lakes based on the number of valid pixels within the waterbody.
Table 2.
Mean absolute deviation (MAD) comparing bloom percentage for each lake size to all lakes for each of the four spatial area thresholds (1 pixel, 10%, 20%, and 30%). Small lakes were those with less than 11 pixels, medium lakes were those with less than 50 pixels, and large lakes were those with 50 or more pixels. A larger MAD indicates increased variability for a given lake size compared to all lakes.
| Lake Size | ||||
|---|---|---|---|---|
| Threshold | Small | Medium | Large | Total |
| 1 Pixel | 10.16 | 2.35 | 15.83 | 28.34 |
| 10% Area | 1.56 | 2.17 | 3.88 | 7.61 |
| 20% Area | 1.91 | 1.52 | 4.07 | 7.50 |
| 30% Area | 2.09 | 1.248 | 3.73 | 7.05 |
3.4. A case study at Lake Jesup in Florida
Regional bloom percentage was consistent with results at the CONUS scale, indicating agreement with previously published ecological patterns for all regions except the Southeast and South. These regions showed a pattern contrary to what was expected in which bloom percentage reached a maximum in the winter months, with the effect in the Southeast being more prominent than the South. To investigate the observed anomaly at a finer spatial scale, we tested our approach on Lake Jesup in Florida using monthly averages of weekly maximum CI values for each pixel in a given month (Fig. 6). In 2018, Lake Jesup began the year with relatively low levels of cyanobacteria, but an extensive bloom began in February and persisted until June with the highest cyanobacterial values occurring in the month of March. This bloom dissipated when we would typically expect to see maximum cyanobacterial occurrence (in July through September), with the lowest values occurring in August. Another bloom began in October lasting through the end of the year. A detailed investigation of several lakes across the Southeast region revealed that this pattern is not unique to Lake Jesup.
Fig. 6.
2018 monthly composite CI values for Lake Jesup in Florida (28.7302°N, 81.2023°W) computed as the average of the weekly maximum CI values for each pixel in a given month. The data for Lake Jesup shows an anomalous pattern where CI reaches its highest values in the spring and winter months rather than the summer months.
To verify the phenology suggested by satellite data, monthly field observations of chlorophyll a were obtained for three sites in Lake Jesup during the months of February through November (Fig. 7). Satellite observations were converted from CI to chlorophyll a estimates using a conversion from Tomlinson et al. (2016) which established a relationship between the two parameters using data from inland waterbodies across the state of Florida. Although there is a slight offset in the data, with satellite data consistently indicating lower values than field data, the observed patterns are well-supported between the two datasets. Low values of chlorophyll a are observed in February followed by a strong increase that reaches a maximum in spring. The lowest values occur in July and August, depending on the site, before increasing again throughout the fall and early winter. This supports the phenology observed through satellite data in this system, suggesting the bloom season may not be as well-understood as previously thought, particularly in subtropical climates where there is little seasonality.
Fig. 7.
(A) Location of monthly chlorophyll a measurements used to validate monthly satellite imagery at Lake Jesup in Florida. (B) Monthly match-ups for field observations (dashed lines) and satellite observations (solid lines) of chlorophyll a at three point locations in Lake Jesup.
3.5. Exploring cold-season blooms across the Southeast
Although most studies are focused on the growing season, cold-weather cyanobacterial blooms have been noted throughout the literature. Cyanobacteria have been documented under ice (Bertilsson et al., 2013), in high-latitude freshwater systems (Vincent et al., 2012), and in Antarctic, Arctic, and alpine systems (Zakhia et al., 2008). Binding et al. (2012) used satellite remote sensing to observe winter blooms in Lake Erie in February 2011. Over an 11-year period from 1999–2010, Babanazarova et al. (2013) found cyanobacterial blooms throughout the winter months in Lake Nero in Western Russia. In January 2017, a winter cyanobacterial bloom was studied at a lake in Western Poland (Wejnerowski et al., 2018).
The occurrence of winter blooms in the southeast could be explained by either environmental conditions or artifacts in the satellite data. While several studies have found a minimum temperature threshold below which cyanobacterial growth is inhibited (Reynolds, 2006; Carey et al., 2012), little research has been done to investigate if a maximum temperature threshold exists. Paerl (2014) found that cyanobacteria growth diminished once reaching a water temperature of about 35 °C. It is possible that water temperatures in some lakes in the Southeast region are too warm during the summer for optimal cyanobacterial growth meaning their biomass peaks in colder months when water temperatures are lower.
Another possible explanation is the timing of the summer rainy season in Florida. The large influx of freshwater from rain events and tropical systems could dilute the water in these lakes, resulting in lower detectable levels of cyanobacteria. Berger et al. (2008) found that dilution of a cyanobacterial bloom can be used as a mitigation technique. Additionally, increased precipitation leads to less water stratification; well-mixed environments may lead to underrepresentation of cyanobacteria as the bloom is distributed throughout the water column (Reynolds, 2006), and the satellite cannot always detect blooms that are deeper in the water column.
Additionally, light availability has been found to affect the buoyancy of cyanobacteria, as these cells prefer conditions without excessive amounts of direct sunlight (Mur et al., 1999). Cyanobacteria can adjust their buoyancy (Reynolds et al., 1987), and it may be possible that under direct summer sunlight, they move lower in the water column and are not as readily detected by the sensor, leading to a reduced CI. Perhaps the increased direct solar radiation in southern latitude states during the summer months, likely in combination with warmer water temperatures, is causing cyanobacteria to shift toward more optimal conditions lower in the water column.
Field observations at Lake Jesup are an indicator that the satellite data, in at least one case, seems to be accurately reflecting in situ conditions. However, there are still several limitations that could create an artifact in the data in other waterbodies, leading us to believe that winter bloom events are more prevalent than they actually are. Of the 2,321 NHD lakes considered in this study, 297 of them are controlled by at least one dam (U.S. Army Corps of Engineers, 2016). Fluctuating water levels in these lakes could expose more pixels along the edge of the lake, or, particularly in optically shallow water, could cause bottom reflectance or benthic ecosystems to be misinterpreted as cyanobacteria.
3.6. Limitations
While this study offers a unique analysis that includes weekly observations of large inland waterbodies across CONUS, there are several limitations that should be acknowledged. Only waterbodies of sufficient size and shape were considered in order to accommodate the spatial resolution of the satellite data. As explained in Section 2.2, pixels along the land–water interface were removed which prevents consideration of the shoreline where blooms often occur. Finer resolution sensors such as those on Landsat-8 or Sentinel-2 could potentially provide more complete data across additional waterbodies in the future. Moreover, regions affected by snow and ice in the winter cannot provide year-round datasets. Thus, the observed patterns discussed here could change if a more continuous dataset were available. The snow and ice mask used here is likely more conservative than is needed; however, it is the best approach available at this time to avoid artificial winter peaks caused by signal contamination. Another satellite limitation is the inability of the sensor to detect lower portions of the water column, particularly in high attenuation waters. Cyanobacteria in well-mixed systems could appear to be less concentrated than cyanobacteria that has become stratified at the top of the water column.
4. Conclusion
This study provides a validation of algorithm performance through comparing annual changes with previously established ecological patterns on cyanobacteria population dynamics. Here, we also demonstrate a metric to quantify the percentage of lakes experiencing cyanobacterial blooms for each week from 2008–2011, 2017, and 2018. Using satellite data, we presented results for the percentage of lakes with a bloom and the percentage of lakes without a bloom for each weekly composite at both a national and regional level. Our analysis focused on relatively large lakes across the United States. At this time, there is no evidence that these findings can be extrapolated to smaller lakes, although finer spatial resolution data would offer insight into this limitation. The CONUS bloom season exhibited phenology that is well-supported in the literature, with cyanobacterial blooms increasing gradually throughout the growing season before reaching a maximum in late-summer or early-autumn. Wintertime data was persistently limited due to snow and ice cover, particularly in northern latitude regions. Contrary to the pattern seen at the CONUS scale, the percentage of lakes experiencing a bloom in the Southeast reached its highest values during winter. A small case study on Lake Jesup in Florida illustrated this phenomenon on a monthly timescale, and field data validated these findings for chlorophyll a. Several possible explanations were addressed, including environmental conditions and satellite artifacts, but further research is needed.
Results presented throughout the manuscript were largely based on a spatial area threshold of 10% for a waterbody to be classified as experiencing a bloom. A threshold of 1 pixel was found to potentially be more prone to error when analyzing both seasonal differences and the effect of lake size. Therefore, a threshold based on spatial coverage of a bloom is suggested, although of the three percentages considered, all appeared to perform fairly equally. Methods presented in this study can be used to monitor annual patterns of cyanobacterial presence in inland lakes across CONUS. Additionally, as finer spatial resolution data become available, this study can be expanded to include waterbodies not considered in this study due to spatial resolution limitations.
The primary objective of this study was to develop a methodology for monitoring inland cyanobacterial blooms across the United States on a weekly basis. Several studies have summarized large-scale drivers of cyanobacteria. Eutrophication is commonly cited as a primary driver of cyanobacterial blooms (Huisman et al., 2018; Pick, 2016; Paerl et al., 2011). A review article by Huisman et al., 2018 also credited increases in cyanobacterial occurrence to rising CO2 concentrations and global warming. In addition to eutrophication and climate change, Pick, 2016 also identified food web alterations as a compounding factor contributing to a rise in algal blooms across Canada. Although outside the scope of this study, future research should focus on drivers of these cyanobacterial blooms, particularly on a regional basis to better understand the phenology presented here.
Acknowledgments
This work was supported by the NASA Ocean Biology and Biogeochemistry Program/Applied Sciences Program (proposal 14-SMDUNSOL14-0001) and by U.S. EPA, NOAA, U.S. Geological Survey Toxic Substances Hydrology Program, and Oak Ridge Institute for Science and Technology (ORISE). This article has been reviewed by the National Exposure Research Laboratory and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use by the U.S. Government. The views expressed in this article are those of the authors and do not necessarily reflect the views or policies of the U.S. EPA. Portions of this analysis were included as a conference proceeding appearing originally as ”A method for quantifying the number of US lakes with cyanobacterial harmful algal blooms using satellite remote sensing” in Proceedings Volume 10767, Remote Sensing and Modeling of Ecosystems for Sustainability XV. This conference proceeding was published in 2018 with the following authors: M. M. Coffer, Blake A. Schaeffer, Erin A. Urquhart, John A. Darling, and Wilson B. Salls. Field observations at Lake Jesup were obtained from Florida LAKEWATCH (http://lakewatch.ifas.ufl.edu/), School of Forest Resources and Conservation, Institute of Food and Agricultural Sciences, University of Florida.
Footnotes
Appendix A. Bloom percentage for each climate region
The following figure is included to offer a more detailed representation of bloom percentage for each climate region. Fig. 8 illustrates the percentage of lakes with a bloom (green) and the percentage of lakes without a bloom (blue) for each of the remaining seven climate regions that were not included in Fig. 3.
Appendix B. Bloom percentage with different spatial area thresholds
While the majority of results throughout the manuscript are based on a 10% minimum surface area threshold, three additional spatial area thresholds were also considered. A 10% minimum surface area threshold was chosen because variability across both lake size and seasons was minimized as discussed in Section 3.3. Fig. 8 shows the percentage of lakes with a bloom (green) and the percentage of lakes without a bloom (blue) for all of CONUS based on a 1 pixel minimum threshold, a 20% surface area minimum threshold, and a 30% surface area minimum threshold. While there are obvious differences in the magnitude of lakes being classified as a bloom, overall patterns remain fairly consistent across these three thresholds and the results shown in Fig. 2. Results based on a 20% and 30% threshold were nearly identical likely because a relatively large bloom would have to be present to meet both of these thresholds.
References
- Babanazarova O, Sidelev S, Schischeleva S, 2013. The structure of winter phytoplankton in Lake Nero, Russia, a hypertrophic lake dominated by Planktothrix-like Cyanobacteria. Aquatic Biosyst. 9, 18 http://aquaticbiosystems.biomedcentral.com/articles/10.1186/2046-9063-9-18,10.1186/2046-9063-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger P, Brooks J, Evens T, Gobler C, Graham J, Berger P., Brooks J, Evens T, Graham J, Hyde J, Karner DO, Shea D, Paul K, Paerl V, Piehler H, Rosen M, Santelmann B, Tester M, Westrick P, Cyanobacterial J, 2008. Cyanobacterial Harmful Algal Blooms: Chapter 9: Causes, Prevention, and Mitigation Workgroup Report http://digitalcommons.unl.edu/usepapapers/33.
- Bertilsson S, Burgin A, Carey CC, Fey SB, Grossart HP, Grubisic LM, Jones ID, Kirillin G, Lennon JT, Shade A, Smyth RL, 2013. The under-ice microbiome of seasonally frozen lakes. Limnol. Oceanogr 58. 10.4319/lo.2013.58.6.1998.. URL: https://aslopubs.onlinelibrary.wiley.com/doi/pdf/10.4319/lo.2013.58.6.1998. [DOI] [Google Scholar]
- Binding CE, Greenberg TA, Bukata RP, Smith DE, Twiss MR, 2012. The MERIS MCI and its potential for satellite detection of winter diatom blooms on partially icecovered Lake Erie. J. Plankton Res 34, 569–573. 10.1093/plankt/fbs021. DOI: 10.1093/plankt/fbs021. [DOI] [Google Scholar]
- Carey CC, Ibelings BW, Hoffmann EP, Hamilton DP, Brookes JD, 2012. Ecophysiological adaptations that favour freshwater cyanobacteria in a changing climate. Water Res. 46, 1394–1407. 10.1016/J.WATRES.2011.12.016. [DOI] [PubMed] [Google Scholar]
- Chorus I, Falconer IR, Salas HJ, Bartram J, 2000. Health risks caused by freshwater Cyanobacteria in recreational waters. J. Toxicol. Environ. Health, Part B 3, 323–347. 10.1080/109374000436364. [DOI] [PubMed] [Google Scholar]
- Clark JM, Schaeffer BA, Darling JA, Urquhart EA, Johnston JM, Ignatius AR, Myer MH, Loftin KA, Werdell PJ, Stumpf RP, 2017. Satellite monitoring of cyanobacterial harmful algal bloom frequency in recreational waters and drinking water sources. Ecol. Ind 80, 84–95. 10.1016/j.ecolind.2017.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TW, Stumpf R, Bullerjahn GS, McKay RML, Chaffin JD, Bridgeman TB, Winslow C, 2019. Science meets policy: a framework for determining impairment designation criteria for large waterbodies affected by cyanobacterial harmful algal blooms. Harmful Algae 81, 59–64. 10.1016/J.HAL.2018.11.016..URL: https://www.sciencedirect.com/science/article/pii/S1568988318301860. [DOI] [PubMed] [Google Scholar]
- Graham JL, Foster GM, Kramer AR, Survey USG, 2017. Twenty years of water-quality studies in the Cheney Reservoir Watershed, Kansas, 1996–2016. Technical Report. Reston, VA. 10.3133/fs20173019.http://pubs.er.usgs.gov/publication/fs20173019. [DOI] [Google Scholar]
- Hu C, Lee Z, Ma R, Yu K, Li D, Shang S, 2010. Moderate Resolution Imaging Spectroradiometer (MODIS) observations of cyanobacteria blooms in Taihu Lake, China. J. Geophys. Res.: Oceans 115. 10.1029/2009JC005511. [DOI] [Google Scholar]
- Hudnell HK, Dortch Q, Zenick H, 2008. An Overview of the Interagency, International Symposium on Cyanobacterial Harmful Algal Blooms (ISOC-HAB): Advancing the Scientific Understanding of Freshwater Harmful Algal Blooms. In: Hudnell HK (Ed.), Springer; New York, New York, NY, pp. 1–16 URL: 10.1007/978-0-387-75865-7_1,10.1007/978-0-387-75865-7_1. [DOI] [PubMed] [Google Scholar]
- Huisman J, Codd GA, Paerl HW, Ibelings BW, Verspagen JMH, Visser PM, 2018. Cyanobacterial blooms. Nat. Rev. Microbiol 16, 471–483. 10.1038/s41579-018-0040-1. [DOI] [PubMed] [Google Scholar]
- Karl T, Koss WJ, 1984. Regional and National Monthly, Seasonal, and Annual Temperature Weighted by Area, 1895–1983. [Google Scholar]
- Kutser T, 2009. Passive optical remote sensing of cyanobacteria and other intense phytoplankton blooms in coastal and inland waters. Int. J. Remote Sens 30, 4401–4425. 10.1080/01431160802562305. [DOI] [Google Scholar]
- Loftin KA, Graham JL, Hilborn ED, Lehmann SC, Meyer MT, Dietze JE, Griffith CB, 2016. Cyanotoxins in inland lakes of the United States: Occurrence and potential recreational health risks in the EPA National Lakes Assessment 2007. Harmful Algae 56, pp. 77–90. doi: 10.1016/j.hal.2016.04.001.http://www.sciencedirect.com/science/article/pii/S1568988315300883. [DOI] [PubMed] [Google Scholar]
- Lugliè A, Giacobbe MG, Riccardi E, Bruno M, Pigozzi S, Mariani MA, Satta CT, Stacca D, Bazzoni AM, Caddeo T, Farina P, Padedda BM, Pulina S, Sechi N, Milandri A, 2017. Paralytic shellfish toxins and cyanotoxins in the mediterranean: new data from sardinia and sicily (Italy). Microorganisms 10.3390/microorganisms5040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunetta RS, Schaeffer BA, Stumpf RP, Keith D, Jacobs SA, Murphy MS, 2015. Evaluation of cyanobacteria cell count detection derived from MERIS imagery across the eastern USA. Remote Sens. Environ 157, 24–34. 10.1016/j.rse.2014.06.008.. URL: http://www.sciencedirect.com/science/article/pii/S0034425714002211. [DOI] [Google Scholar]
- Matthews MW, Bernard S, Robertson L, 2012. An algorithm for detecting trophic status (chlorophyll-a), cyanobacterial-dominance, surface scums and floating vegetation in inland and coastal waters. Remote Sens. Environ 124, 637–652. 10.1016/j.rse.2012.05.032.. URL: http://www.sciencedirect.com/science/article/pii/S0034425712002350. [DOI] [Google Scholar]
- Mur LR, Skulberg OM, Utkilen H, 1999. Cyanobacteria in the environment. In: Chorus I, Bartram J (Eds.), Toxic Cyanobacteria in Water: A guide to their public health consequences, monitoring and management. E & FN Spon, London: chapter 2. [Google Scholar]
- Neuhaus L, 2016. Reeking, Oozing Algae Closes South Florida Beaches.https://www.nytimes.com/2016/07/02/us/reeking-oozing-algae-closes-south-florida-beaches.html?_r=2.
- Paerl HW, 2014. Mitigating harmful cyanobacterial blooms in a human- and climatically-impacted world. Life 4, 988–1012. 10.3390/life4040988.. URL: http://www.mdpi.com/2075-1729/4/4/988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paerl HW, Hall NS, Calandrino ES, 2011. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ 409, 1739–1745. 10.1016/j.scitotenv.2011.02.001.. URL: http://www.sciencedirect.com/science/article/pii/S0048969711001197. [DOI] [PubMed] [Google Scholar]
- Pick FR, 2016. Blooming algae: a Canadian perspective on the rise of toxic cyanobacteria. Can. J. Fish. Aquat. Sci 73, 1149–1158. 10.1139/cjfas-2015-0470. [DOI] [Google Scholar]
- Qin B, Li W, Zhu G, Zhang Y, Wu T, Gao G, 2015. Cyanobacterial bloom management through integrated monitoring and forecasting in large shallow eutrophic Lake Taihu (China). J. Hazard. Mater 287, 356–363. 10.1016/j.jhazmat.2015.01.047.. URL: http://www.sciencedirect.com/science/article/pii/S0304389415000588. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2017. R: A Language and Environment for. Statistical Computing. URL: http://www.R-project.org/. [Google Scholar]
- Reynolds CS, 2006. Ecology of phytoplankton. Ecology, biodiversity, and conservation. Cambridge University Press, Cambridge. URL: https://catalog.lib.ncsu.edu/record/NCSU1912943. [Google Scholar]
- Reynolds CS, Oliver RL, Walsby AE, 1987. Cyanobacterial dominance: the role of buoyancy regulation in dynamic lake environments. NZ J. Mar. Freshwat. Res 21, 379–390. 10.1080/00288330.1987.9516234. [DOI] [Google Scholar]
- Schaeffer BA, Bailey SW, Conmy RN, Galvin M, Ignatius AR, Johnston JM, Keith DJ, Lunetta RS, Parmar R, Stumpf RP, Urquhart EA, Werdell PJ, Wolfe K, 2018. Mobile device application for monitoring cyanobacteria harmful algal blooms using Sentinel-3 satellite Ocean and Land Colour Instruments. Environ. Model. Software 109, 93–103. 10.1016/j.envsoft.2018.08.015.. URL: http://www.sciencedirect.com/science/article/pii/S1364815218302482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simis SGH, Peters SWM, Gons HJ, 2005. Remote sensing of the cyanobacterial pigment phycocyanin in turbid inland water. Limnol. Oceanogr 50, 237–245. 10.4319/lo.2005.50.1.0237. [DOI] [Google Scholar]
- Smayda TJ, 2003. What is a bloom? A commentary. Limnol. Oceanogr 42, 1132–1136. 10.4319/lo.1997.42.5_part_2.1132. [DOI] [Google Scholar]
- Sonich-Mullin C, 2014. EPA Science In Action: Keeping an Eye on. Harmful Algal Blooms. [Google Scholar]
- Stumpf RP, Davis TW, Wynne TT, Graham JL, Loftin KA, Johengen TH, Gossiaux D, Palladino D, Burtner A, 2016. Challenges for mapping cyanotoxin patterns from remote sensing of cyanobacteria. Harmful Algae 54, 160–173. 10.1016/j.hal.2016.01.005.. URL: http://www.sciencedirect.com/science/article/pii/S1568988315301839. [DOI] [PubMed] [Google Scholar]
- Stumpf RP, Johnson LT, Wynne TT, Baker DB, 2016. Forecasting annual cyanobacterial bloom biomass to inform management decisions in Lake Erie. J. Great Lakes Res 42, 1174–1183. 10.1016/j.jglr.2016.08.006.. URL: http://www.sciencedirect.com/science/article/pii/S0380133016301484. [DOI] [Google Scholar]
- Tomlinson MC, Stumpf RP, Wynne TT, Dupuy D, Burks R, Hendrickson J, Fulton III RS, 2016. Relating chlorophyll from cyanobacteria-dominated inland waters to a MERIS bloom index. Remote Sens. Lett 7, 141–149. 10.1080/2150704X.2015.1117155. [DOI] [Google Scholar]
- Urquhart EA, Schaeffer BA, 2019. Envisat MERIS and Sentinel-3 OLCI satellite lake biophysical water quality flag dataset for the contiguous United States. Data in Brief, 104826. 10.1016/j.dib.2019.104826.http://www.sciencedirect.com/science/article/pii/S2352340919311813. [DOI] [PMC free article] [PubMed]
- Urquhart EA, Schaeffer BA, Stumpf RP, Loftin KA, Werdell PJ, 2017. A method for examining temporal changes in cyanobacterial harmful algal bloom spatial extent using satellite remote sensing. Harmful Algae 67, 144–152. 10.1016/J.HAL.2017.06.001.. UR: https://www.sciencedirect.com/science/article/pii/S1568988317300288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Army Corps of Engineers, 2016. National Inventory of Dams. [Google Scholar]
- Vincent WF, Quesada A, 2012. Cyanobacteria in High Latitude Lakes, Rivers and Seas. In: Whitton BA (Ed.), Ecology of Cyanobacteria II: Their Diversity in Space and Time. Springer, Netherlands, Dordrecht, pp. 371–385. 10.1007/978-94-007-3855-3_13. [DOI] [Google Scholar]
- Wejnerowski U, Rzymski P, Kokociński M, Meriluoto J, 2018. The structure and toxicity of winter cyanobacterial bloom in a eutrophic lake of the temperate zone. Ecotoxicology 27, 752–760. 10.1007/s10646-018-1957-x.. URL: http://link.springer.com/10.1007/s10646-018-1957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2003. Algae and Cyanobacteria in Fresh Water, in: Guidelines for safe recreational water environments. chapter 8, pp. 136–158. [Google Scholar]
- Wynne TT, Stumpf RP, Tomlinson MC, Fahnenstiel GL, Dyble J, Schwab DJ, Joshi SJ, 2013. Evolution of a cyanobacterial bloom forecast system in western Lake Erie: development and initial evaluation. J. Great Lakes Res 39, 90–99. 10.1016/j.jglr.2012.10.003.. URL: http://www.sciencedirect.com/science/article/pii/S0380133012002110. [DOI] [Google Scholar]
- Wynne TT, Stumpf RP, Tomlinson MC, Warner RA, Tester PA, Dyble J, Fahnenstiel GL, 2008. Relating spectral shape to cyanobacterial blooms in the Laurentian Great Lakes. Int. J. Remote Sens 29, 3665–3672. 10.1080/01431160802007640. [DOI] [Google Scholar]
- Wyoming DEQ, 2018. Big Sandy Reservoir Harmful Cyanobacterial Investigation 2018. Technical Report. Wyoming Department of Environmental Quality.http://deq.wyoming.gov/media/attachments/Water. [Google Scholar]
- Wyoming DEQ, 2018. Eden Reservoir Harmful Cyanobacterial Investigation 2018. Technical Report. Wyoming Department of Environmental Quality.http://deq.wyoming.gov/media/attachments/Water. [Google Scholar]
- Wyoming DEQ, 2018. Pathfinder Reservoir Harmful Cyanobacterial Bloom Investigation 2018. Technical Report. Wyoming Department of Environmental Quality.http://deq.wyoming.gov/media/attachments/Water. [Google Scholar]
- Xu H, Paerl HW, Zhu G, Qin B, Hall NS, Zhu M, 2017. Long-term nutrient trends and harmful cyanobacterial bloom potential in hypertrophic Lake Taihu, China. Hydrobiologia 787, 229–242. 10.1007/s10750-016-2967-4. [DOI] [Google Scholar]
- Zakhia F, Jungblut AD, Taton A, Vincent WF, Wilmotte A, 2008. Cyanobacteria in Cold Ecosystems. In: Margesin R, Schinner F, Marx JC, Gerday C (Eds.), Psychrophiles: from Biodiversity to Biotechnology. Springer, Berlin, Heidelberg, Berlin, Heidelberg, pp. 121–135. 10.1007/978-3-540-74335-4_8. [DOI] [Google Scholar]
- Zhang M, Duan H, Shi X, Yu Y, Kong F, 2012. Contributions of meteorology to the phenology of cyanobacterial blooms: Implications for future climate change. Water Res. 46, 442–452. 10.1016/j.watres.2011.11.013.. URL: http://www.sciencedirect.com/science/article/pii/S0043135411006853. [DOI] [PubMed] [Google Scholar]