Abstract
BACKGROUND:
Globally, 30% of female over 50 years old have osteoporosis. This disease is one of the major causes of disability and death in the elderly. This research was aimed to determine the prevalence of primary osteoporosis and low bone density based on bone mineral density in postmenopausal women and its sociodemographic, obstetric, and life style risk factors.
MATERIALS AND METHODS:
This cross-sectional descriptive-analytical study was performed by simple random sampling on 850 postmenopausal women aged 50–65 years covered by all health centers, from August 2018 to April 2019, in Tabriz-Iran. Four hundred and forty-five eligible women underwent densitometry using dual-energy X-ray absorptiometry in the lumbar spine and femoral neck. Socio-individual, obstetric-medical, international physical activity questionnaires-short form, and anthropometric questionnaires were completed. Data analyzed using descriptive and analytical statistics including multivariate logistic regression in SPSS 21 software.
RESULTS:
The prevalence of primary osteoporosis based on lumbar vertebra T-score, femoral neck T-score, and total was 23.4%, 3.4%, and 24.5%, respectively, and the prevalence of primary osteopenia based on lumbar vertebra T-score, femur neck T-score, and total was 42%, 35.5%, and 43.6%, respectively. The present study showed that the odds of osteoporosis increased by increment of age (odds ratio [OR]: 1.18; 95% confidence interval [CI]: 1.07–1.30), but it decreased by increasing menopausal age (OR: 0.92; 95% CI: 0.85–1.01), body mass index (OR: 0.87; 95% CI: 0.78–0.97), arm circumference (OR: 0.84; 95% CI: 0.74–0.95), and education level (P = 0.028). It was higher in unmarried women (OR: 2.65; 95% CI: 0.99–7.08) and those with nonpersonal housing (OR: 4.02; 95% CI: 1.24–13.07).
CONCLUSIONS:
Given the high prevalence of primary osteoporosis and low bone mass in postmenopausal women, health education is necessary for preventing modifiable risk factors and reducing the complications of this disease.
Keywords: Bone density, postmenopause, prevalence, risk factors
Introduction
Osteoporosis is a metabolic bone disease that is associated with reduced bone mineral density (BMD) and microarchitectural deterioration in bone tissue.[1,2] Osteoporosis occurs when the process of bone resorption and bone formation lose their balance.[3] This disease is one of the major causes of disability and death in the elderly.[4] There are two types of osteoporosis. The primary type of osteoporosis occurs in postmenopausal female due to estrogen deficiency (Type I) that results in the loss of bone material or due to the normal process of aging (Type II). While in secondary osteoporosis, which accounts for at least 20% of the causes of osteoporosis, an underlying disease or deficiency or drug use especially glucocorticoids can cause osteoporosis.[5,6] The most significant decrease in BMD occurs approximately 5%/year in the 1st years after menopause, and it reaches 1%–1.5%/year in subsequent years.[7]
According to available statistics, the prevalence of osteoporosis in the world is between 4 and 40%.[8] Over 200 million people have osteoporosis in the world, which is projected to increase by 240% by 2050 in women.[9,10] The clinical consequence of osteoporosis is fracture due to bone fragility. The incidence of osteoporosis fractures varies from region to region, which may be related to population age distribution, genetic background, or lifestyle.[11] Common osteoporosis fractures are associated with significant complications and mortality and are a gateway to other serious fractures such as the pelvis.[12] Osteoporosis is often called a silent disease because the process of bone loss is so slow and gradual that it cannot be diagnosed until the first fracture occurs.[13]
Globally, 30% of female over 50 years old have osteoporosis.[14] 8%–9% of bone fractures per year are caused by osteoporosis, which results in back pain, impaired quality of life, and interference with daily activities.[15] Bone mass in female in all age groups is significantly lower than male in the same age and race.[16]
Factors associated with osteoporosis are numerous and include lifestyle and drug use (corticosteroid, anti-cancer, antacids, and thyroid hormones); diseases (hyperthyroidism, parathyroid, insulin-dependent diabetes, and kidney disease); diet (low calcium, Vitamin D, high phosphorus, protein, and coffee intake);[17,18] and other risk factors such as female gender, decreased sex hormone levels, increased age, menopause before 45 years old, low body mass index, lack of physical activity, positive family history, history of bone fractures, stress, lactation, alcohol consumption, and smoking.[19,20] The findings also show that female with five children or more have lower BMD.[21]
According to statistical indicators, the trend of elderly population growth in Iran has started. The General Census in 1996 showed that 6.62% of the Iranian population was over 60 years old. This figure reached 7.26% in the 2006 census and is projected to rise to 11.5% by 2026.[22] Osteoporosis and osteopenia are major health problems that in addition to physical problems impose significant economic costs on individuals and society. According to a meta-analysis done in 2018, the prevalence of osteoporosis in the Iranian postmenopausal female was 32% and the prevalence of low bone density was 51%.[23]
Tabriz is the center of a province and metropolitan area in Northwest Iran. However, according to the authors’ research, there is no comprehensive and new statistics available regarding the prevalence of primary osteoporosis and its risk factors from this populous city of Iran. Moreover, no any information in this regard was reported in Iranian osteoporosis meta-analysis. Because of increasing elderly population and the higher rates of osteoporosis in women especially after menopause, serious attention and planning for screening of postmenopausal osteoporosis and prevention of modifiable risk factors in this area seem necessary. The purpose of this research was to determine the prevalence of primary osteoporosis and low bone mass and its social-demographic, obstetric, and life style risk factors among postmenopausal female aged 50–65 years in Tabriz.
Materials and Methods
This cross-sectional descriptive-analytical study is a part of a megaproject approved by the Tabriz University of Medical Sciences (No: 58943) entitled “assessment of primary osteoporosis status and effect of three intervention of Curcumin, Nigella Sativa, and Curcumin-Nigella Sativa on cellular-molecular and clinical outcomes in postmenopausal women of Tabriz.” This was performed by simple random sampling on 850 postmenopausal women covered by all health centers aged 50–65 years, from August 2018 to April 2019, in Tabriz-Iran. The city of Tabriz, the center of East Azerbaijan Province, located in the northwestern part of Iran in which the population of women is 799,254 people. 163,228 of them (20.4%) in the age group of 50 years old and over.[24]
In this study, the prevalence of primary osteoporosis and low bone mass in eligible 50–65 years old women in Tabriz was calculated using the anterior-posterior (AP) lumbar spine and proximal femur neck BMD data at the Densitometry Center of Sina Hospital, Tabriz, 2018–2019 through Hologic QDR 4500W (S/N 50266) dual energy X-ray absorptiometry device.
All research stages were approved by the Regional Research Ethics Committee (IR.TBZMED.REC.1397.930) and written consent was obtained from all participants. The inclusion criteria were 50–65 years old postmenopausal women, resident of Tabriz, menstruation pause for at least 12 consecutive months, ability to verbally communicate for questions, and no menopause before 40 years old. The exclusion criteria included bone disease other than osteoporosis with endorsement of endocrinologist, rheumatoid arthritis, metastatic bone disease, taking drugs affecting bone metabolism including intravenous bisphosphonate over the past 5 years, oral bisphosphonate use in the past 6 months, cumulative oral bisphosphonate use for more than 3 years or more than 1 month between 6 and 12 months prior to study, taking parathyroid hormone analogs, estrogen or corticosteroids over the past 12 months, high thyroxine hormone use, cytotoxic drugs, immunosuppressive drugs such as cyclosporines, long-term use of some types of anticonvulsant drugs (e.g., phenytoin), hereditary diseases (hemophilia, thalassemia, hemochromatosis) based on patients’ report, diseases related to internal secretory glands (Cushing's syndrome, hyperthyroidism) with endorsement of endocrinologist or endocrinology tests, gastrointestinal diseases (chronic liver diseases such as primary biliary cirrhosis, celiac, Crohn's disease, complete stomach surgery, gastric surgery), and malabsorption syndromes.
The sample size was calculated based on the study of Bayat et al.,[25] considering the prevalence of osteoporosis equal to 25.5%, α = 0.05 and d = 0.16 (study precision), using the formula for estimating a single proportion as much as 440 individuals. It was also calculated based on osteoporosis risk factors,[3] taking into account odds ratio (OR) = 2.141, α = 0.05 and β = 0.9 for the duration of menopause; OR = 3.122 and OR = 9.12, α = 0.05 and β = 0.9 for overweight and normal comparing obese body mass index (BMI); OR = 2.58, OR = 2.36, and OR = 3.144, α = 0.05, and β = 0.9 for secondary, primary and illiteracy compared to college education, equal to 125, 63, 29, 85, 101, and 67 individuals. Finally, the largest sample size (440) was considered for sampling. Samples were randomly selected from Tabriz health centers. Tabriz has 87 health centers and details of all postmenopausal women including phone number and address are available at these centers. First, the population of women aged 50–65 years was extracted out of 87 health centers in Tabriz using the Integrated Health System (SIB) and was listed individually. Of the 108,778 individuals, 850 women (about twice the sample size according to a pilot assessment) were selected by simple random sampling through www.random.org website. Eighty-four health centers were sampled from 87 centers through this random sampling. Due to the nature of sampling, all people had an equal chance of being selected. The menopausal women were then contacted by telephone, a brief description of the aims and method of the study was explained to them. If they met the inclusion criteria and were willing to participate in the study, they were asked to attend the health center at a specified time. At the meeting, the aims of the study were again explained and the exclusion criteria were assessed. If they were eligible, informed consent was obtained and questionnaires of socio-individual characteristics, obstetric-medical history, lifestyle information, anthropometric indicators, and the international physical activity questionnaires (IPAQ) were completed. Then, 10cc blood samples were taken from the participant for complete blood count with differential, calcium, phosphorus, alkaline phosphatase, thyroid stimulating hormone (TSH), creatinine, fasting blood sugar, and Vitamin D tests to differentiate primary osteoporosis from secondary osteoporosis after 10–12 h of fasting. The women were then referred to Sina Hospital's bone density department for dual energy X-ray absorptiometry (DEXA). Densitometry of lumbar spine and femoral neck was performed. All DEXA measurements were performed by a trained and experienced radiology technician through one determined densitometer. In the measurement of bone density, the criteria including T-score (standard deviations [SD] between patient bone mass and mean bone mass in young adults), Z-score (SD between patient bone mass and mean bone mass in same year and same weight), and BMD in g/cm2 are measured. According to the WHO criteria, the T-score ≥−1 is normal, between −1 and −2.5 cause osteopenia (low bone mass), and ≤−2.5 is osteoporosis, which is done by assessing lumbar spine AP, proximal femoral neck, and/or femur and forearm.[26] Blood samples were analyzed and interpreted by an expert in the Nutrition Research Laboratory and the final diagnosis of primary osteoporosis was made by an endocrinologist.
In this study, 850 women (about twice the required sample size) were selected by simple random sampling (this individual simple random sampling was consisted 84 centers from a total 87 centers) and were contacted and the inclusion criteria were assessed. Seven hundred and thirty of them met the inclusion criteria and referred to health centers. During the in-person visit, the exclusion criteria were checked and 194 people were excluded due to various reasons. Serum samples were taken for 536 patients and sent to laboratory to doing required tests for the differentiation of primary and secondary osteoporosis. Of these, 74 were excluded due to the results of tests for secondary osteoporosis and 17 due to unwillingness to continue the study. Finally, 445 patients were referred to densitometry and the necessary information was collected [Figure 1].
Figure 1.
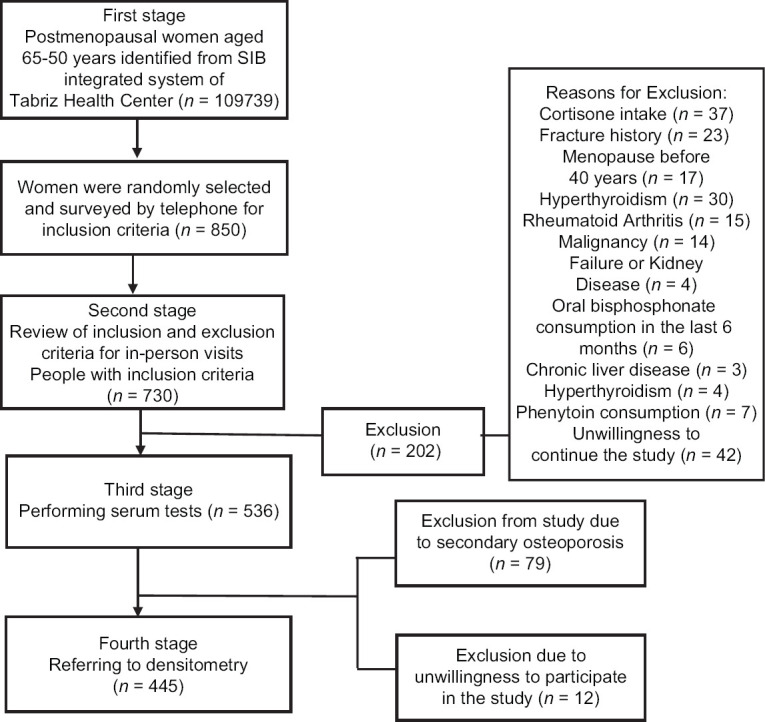
Flowchart of the study subjects selection
The required data were collected using sociodemographic characteristics questionnaires (age, menopausal age, marital status, occupation, family income, education, residential type, drug use history, tobacco, alcohol, and supplement use history, sun exposure, using or not using sunscreen, daily exercise and type of exercise, and family fracture history), anthropometric indicators (height, weight, BMI, waist circumference, hip circumference, arm circumference, and waist-to-hip ratio), obstetric-medical (number of pregnancies and delivery, type of delivery, history of lactation and lactation duration, history of oral and injectable contraceptive use, and duration of use), and the IPAQ short form. Investigating the eligibility of individuals for the study was done from the checklist for study direction criteria along with laboratory tests record sheet and recording of bone densitometry response using DEXA method. The weight of women was measured without wearing shoes and minimal cloths using a lever scale of 0.1 kg precision (Seca, Germany). The correct performance of the scales was evaluated every morning using a 500 g control weight. Height was measured in standing position, straight without shoes and hats, while the head, back, hips, and heels were perfectly tangent to the caliber wall with 0.1 cm accuracy and the woman was facing upward. BMI was obtained from the following formula:
BMI = weight (kg)/(height [m])2
To measure the arm circumference, the hand was fully relaxed next to the body, and mid-upper circumference of the left arm, was measured with a tape measure. Waist-to-hip ratio was obtained by dividing the waist in the thinnest area, by the hip in the largest circumference.[27] In this study, physical activity was assessed by IPAQ questionnaire. The questionnaire, developed in Geneva in 1998, is a tool for measuring physical activity. Its validity and reliability have been confirmed in 12 countries. This questionnaire has been used in various studies in Iran and its validity and reliability have been confirmed.[28] The questionnaire was developed on two scales of long (31 questions) and short (7 questions). In this study, the short form of the questionnaire consisted of 7 questions (2 questions on vigorous physical activity, 2 questions on moderate physical activity, 2 questions on light physical activity, and 1 question on sitting). The questions are about the times during which the person has been active during the past 7 days. The total energy value of the activities during the last 7 days was calculated according to the IPAQ guidelines. Physical activity minutes per week were calculated based on the total amount of physical activity in MET (metabolic equivalent of task)-minutes/week units last week.[27]
Content and face validity of the questionnaire were used by 10 experts to determine the validity of sociodemographic and obstetric-medical questionnaires. In this study, each questionnaire was administered by a trained interviewer who had MSc or BSC degree in one of the medical science fields. Blood samples were collected by a qualified laboratory technician who was not involved in administering the questionnaires. To determine the reliability of the laboratory tests, the first 10 samples were sent to the laboratory with two different names, and then the correlation coefficient between the results of the experiments was calculated and its value was calculated using intraclass correlation coefficient (95% CI) = 0.94 (0.92–0.96).
Data analysis
After data collection, data were analyzed by SPSS/Ver 23 (IBM SPSS Statistics, IBM Corporation, Chicago, IL). Descriptive statistics including frequency and percentage were used to determine the prevalence of primary osteoporosis and low bone density. Normality of quantitative data was investigated using Skewness and Kurtosis. Analytical statistics including Chi-square, independent t-test, Mann–Whitney, one-way analysis of variance, Kruskal–Wallis, Fisher's exact test were used to determine the relationship of primary osteoporosis with individual-social, obstetric-medical, lifestyle, and anthropometric risk factors. Univariate and multivariate logistic regression was used to determine the association of primary osteoporosis with mentioned risk factors. In order to predict primary osteoporosis based on the mentioned risk factors, variables with P < 0.1 in the bivariate tests were entered into multivariate logistic regression model using backward strategy. The Hosmer–Lemeshow test was applied for goodness of fit for logistic regression model. P < 0.05 was considered as the significant level.
Results
The demographic and clinical characteristics of the participants are shown in Table 1. Results showed that there was a statistically significant difference between age, menopausal age, education level, and housing status between the three groups with primary osteoporosis, low bone density, and normal subjects (P < 0.05). The median (p25–p75) of physical activity in the osteoporosis, osteopenia, and normal density groups was 346.6 (346.6–826.5), 462.0 (346.5–1275.5), and 396 (346.5–884.0), respectively (P = 0.488) that all were in low level. The frequency of primary osteoporosis, osteopenia, and normal bone density in the age group of 50–55 years was 13.7%, 41.7%, 44.6%, respectively; in the age group of 56–60 years were 29.4%, 44.4%, 6 26.6%, and in the age group of 61–65 years were 35.6%, 50%, 18.4%, respectively.
Table 1.
Sociodemographic characteristics of postmenopausal women by bone density
| Variable | Osteoporosis (n=109), n (%) | Osteopenia (n=194), n (%) | Normal (n=142), n (%) | P | P** |
|---|---|---|---|---|---|
| Age©, mean (SD) | 58.2 (3.8) | 57.1 (3.9) | 55.4 (3.8) | <0.001€ | <0.001* |
| Age group | |||||
| 50-55 | 23 (21.5) | 70 (36.8) | 75 (54.3) | <0.001¥ | <0.001¥ |
| 56-60 | 53 (49.5) | 80 (42.1) | 47 (34.1) | ||
| 61-65 | 31 (29.0) | 40 (21.1) | 16 (11.6) | ||
| Menopause age© | 47.7 (6.0) | 48.2 (5.2) | 49.1 (3.9) | 0.031€ | 0.012* |
| Marital status | |||||
| Single, widow, divorced | 28 (25.7) | 35 (18.0) | 17 (12.0) | 0.063¥ | 0.069¥ |
| Married | 81 (74.3) | 152 (82.0) | 125 (88.0) | ||
| Education | |||||
| Illiterate | 36 (33.0) | 60 (40) | 25 (17.6) | 0.002ǂ | 0.069ǂ |
| Primary school | 44 (40.4) | 44 (22.7) | 44 (31.0) | ||
| Secondary school | 11 (10.1) | 28 (14.4) | 23 (16.2) | ||
| High school/diploma | 13 (11.9) | 45 (15.2) | 34 (23.9) | ||
| Academic | 5 (4.6) | 17 (8.7) | 16 (11.3) | ||
| Job | |||||
| Housewife | 100 (91.7) | 159 (82.4) | 122 (85.9) | 0.082¥ | 1.000ƍ |
| Employed | 9 (8.3) | 34 (7.6) | 20 (14.1) | ||
| Family income | |||||
| Enough | 20 (18.5) | 34 (17.7) | 35 (24.6) | 0.065ǂ | 0.050ƍ |
| Somewhat enough | 70 (64.8) | 132 (68.8) | 93 (65.5) | ||
| Insufficient | 18 (16.7) | 26 (13.5) | 14 (9.9) | ||
| Housing status | |||||
| Personal | 82 (80.4) | 188 (97.0) | 132 (94.3) | <0.001¥ | 0.478¥ |
| Nonpersonal | 20 (19.6) | 6 (3.0) | 9 (6.7) | ||
| Smoking status | |||||
| No history of use | 103 (95.4) | 188 (97.4) | 136 (96.5) | 0.434¥ | 0.730¥ |
| Current or previous use | 5 (4.6) | 5 (3.6) | 5 (3.5) | ||
| Smoker in roommate | |||||
| Yes | 26 (24.5) | 44 (24.0) | 34 (25.0) | 0.981¥ | 0.904ǂ |
| Hookah consumption | |||||
| No history of use | 107 (98.2) | 189 (97.4) | 140 (98.6) | 0.744¥ | 0.411ǂ |
| Current use | 2 (1.8) | 5 (2.6) | 2 (1.4) | ||
| Alcohol consumption | |||||
| No history of use | 109 (100) | 193 (99.5) | 142 (100) | ||
| Current use | 0 (0.0) | 1 (0.5) | 0 (0.0) | ||
| Exposure to sunlight | |||||
| Yes | 48 (44.0) | 97 (50.5) | 61 (43.0) | 0.310¥ | 0.328ǂ |
| Supplement use (yes) | 65 (59.6) | 103 (54.2) | 70 (49.3) | 0.310¥ | 0.328ǂ |
| Supplement type | |||||
| Vitamin D | 15 (24.3) | 38 (35.8) | 31 (34.4) | 0.577¥ | 0.683¥ |
| Calcium | 12 (19.4) | 18 (17.0) | 16 (17.8) | ||
| Vitamin D and calcium | 17 (27.4) | 31 (29.3) | 25 (27.8) | ||
| Other supplements | 18 (29.0) | 19 (17.9) | 18 (20.0) | ||
| Exercise (yes) | 64 (58.7) | 118 (61.5) | 90 (63.4) | 0.753¥ | 0.602ǂ |
| Total physical activity (MET-min.week)©, median (p25-p75) | 346.6 (346.6-826.5) | 462.0 (346.5-1275.5) | 396.0 (346.5-884) | 0.488€ | 0.587* |
| Sunscreen use (yes) | 24 (22.0) | 66 (34.2) | 40 (28.2) | 0.078¥ | 0.738ǂ |
| Fracture history in close relatives (yes) | 15 (13.9) | 23 (12.0) | 0 (7.1) | 0.194¥ | 0.100ǂ |
©Variables indicated by this symbol have been reported by mean (SD), physical activity <600: low; physical activity between 600-3000: moderate; physical activity >3000: high. P: Difference between osteoporosis, osteopenia and normal groups, **P: Difference between osteoporosis and osteopenia groups with normal, *Independent t-test, ƍExact Fisher’s test, One-way ANOVA,¥ Chi-square, ǂLinear by linear Chi-square. SD=Standard deviation
The obstetric profile is shown in Table 2. Results showed that there was a statistically significant difference between primary osteoporosis, low bone density, and normal subjects in terms of number of pregnancies, number of children, and lactation duration (P < 0.05). The mean (SD) number of pregnancies in patients with primary osteoporosis, osteopenia, and normal bone density were 4.9 (2.3), 4.1 (2.2), and 3.8 (1.7). The mean (SD) number of children in patients with primary osteoporosis, osteopenia, normal bone density was 4 (1.8), 3.5 (1.9), 3 (1.3), respectively. The mean duration of lactation in the three groups was 68.7 (31), 59.7 (27), and 54.5 (17.2) months, respectively.
Table 2.
Obstetric characteristic of postmenopausal women by bone density
| Variable | Osteoporosis (n=109), n (%) | Osteopenia (n=194), n (%) | Normal (n=142), n (%) | P | P** |
|---|---|---|---|---|---|
| Gravida | |||||
| 0-2 | 14 (13.3) | 46 (25.1) | 30 (22.2) | 0.005ǂ | 0.015ǂ |
| 3-4 | 40 (38.1) | 69 (38.0) | 68 (50.4) | ||
| ≥5 | 51 (48.6) | 68 (37.2) | 37 (27.4) | ||
| Mean (SD) | 4.9 (2.3) | 4.1 (2.2) | 3.8 (1.7) | <0.001* | <0.001* |
| Parity | |||||
| 1-2 | 24 (22.9) | 61 (33.9) | 56 (41.5) | 0.001ǂ | 0.001ǂ |
| 3-4 | 50 (47.6) | 73 (40.6) | 64 (47.4) | ||
| ≥5 | 31 (29.5) | 46 (25.6) | 15 (11.1) | ||
| Mean (SD) | 4.0 (1.8) | 3.5 (1.9) | 3.0 (1.3) | <0.001* | >0.001* |
| Type of delivery | |||||
| Vaginal | 79 (75.2) | 126 (69.4) | 101 (74.8) | 0.290ǂ | 0.306ǂ |
| Cesarean | 7 (6.7) | 24 (13.3) | 19 (14.1) | ||
| Both | 19 (18.1) | 30 (16.7) | 15 (11.1) | ||
| History of lactation (yes) | 101 (96.2) | 165 (90.2) | 128 (94.8) | 0.097ǂ | 0.414ƍ |
| Breastfeeding duration (months), mean (SD) | 68.7 (31.0) | 59.7 (27.0) | 54.5 (17.2) | 0.033* | 0.026* |
| History of oral contraceptive use (yes) | 45 (42.9) | 73 (40.3) | 70 (52.6) | 0.086ǂ | 0.035ƍ |
| History of injectable contraceptive use (yes) | 7 (6.7) | 12 (6.6) | 6 (4.6) | 0.722ǂ | 0.509ƍ |
P: Difference between osteoporosis, osteopenia and normal groups, **P: Difference between osteoporosis and osteopenia groups with normal, *Independent t-test, ƍExact Fisher’s test, €One-way ANOVA, ¥Chi-square. SD=Standard deviation
Table 3 shows the anthropometric characteristics of the subjects in the three study groups. There was a significant difference between the three groups in terms of mean weight, height, BMI, waist circumference, hip circumference, and arm circumference (P < 0.001).
Table 3.
Anthropometric characteristics of postmenopausal women by bone density
| Variable | Osteoporosis (n=109), n (%) | Osteopenia (n=194), n (%) | Normal (n=142), n (%) | P | P** |
|---|---|---|---|---|---|
| Weight | 67.4 (9.8) | 72 (10.2) | 78.2 (12.7) | <0.001€ | <0.001* |
| BMI (kg/m2)* | 28.3 (3.7) | 29.6 (4.1) | 31 (4.9) | <0.001€ | <0.001* |
| Normal | 15 (14.0) | 23 (11.9) | 10 (7.1) | <0.001ǂ | <0.001ǂ |
| Overweight | 58 (54.2) | 86 (44.3) | 39 (27.7) | ||
| Obese | 34 (31.8) | 85 (43.8) | 92 (65.2) | ||
| Waist circumference | 93.5 (9.3) | 94.6 (10.0) | 97.6 ( 9.8 ) | <0.001€ | <0.001* |
| Hip circumference | 105.4 (7.9) | 109.3 (9.2) | 112.4 (11.7) | 0.002€ | <0.001* |
| Waist to hip ratio | 0.88 (0.7) | 0.86 (0.7) | 0.87 (0.7) | 0.306€ | 0.785* |
| Arm circumference | 31 (2.9) | 32.5 (3.30) | 34 (3.9) | <0.001€ | <0.001* |
*Independent t-test, ƍExact Fisher’s test, €One-way ANOVA, ¥Chi-square, ǂLinear by linear Chi-square, P=Difference between osteoporosis, osteopenia and normal groups, **P=Difference between osteoporosis and osteopenia groups with normal. BMI=Body mass index
The Frequency of primary osteoporosis and low bone density in postmenopausal women based on T-score index in lumbar spine was more than femoral neck. So that, 23.4% (95% CI: 19.5%–27.3%) of women had primary osteoporosis based on lumbar vertebrae and 3.4% (95% CI: 1.7%–5.1%) women had primary osteoporosis in femoral neck. The prevalence of osteopenia based on lumbar spine T-score was 42% (95% CI: 37.4%–46.6%) and femoral neck T-score 35.5% (95% CI: 31.1%–39.9%). Overall, of the 445 women in the study, 109 (24.5% [95% CI: 21.1%–27.9%]) had primary osteoporosis, 194 (43.6% [95% CI: 39%–48.2%]) had osteopenia, and 142 (31.9% [95% CI: 27.6%–36.2%]) were normal [Figure 2].
Figure 2.
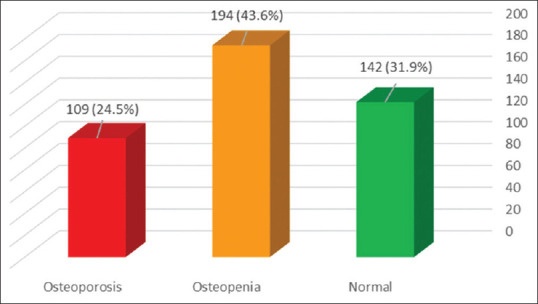
Prevalence of osteoporosis, osteopenia and normal bone density among postmenopausal women in Tabriz
Multiple logistic regression model indicated that age, menopausal age, BMI, arm circumference, education level, and housing status were significant risk factors for primary osteoporosis in postmenopausal 50–65-year-old women. Primary osteoporosis increased with increasing age (OR = 1.18, 95% CI: 1.07–1.30; P < 0.001) and decreased with increasing menopausal age (OR = 0.92, 95% CI: 0.85–1.01; P = 0.071), BMI (OR = 0.87, 95% CI: 0.78–0.97; P = 0.010), and arm circumference (OR = 0.84, 95% CI: 0.74–0.95); P = 0.005). The risk of primary osteoporosis decreased with increasing education (P = 0.028), but in high school/diploma, the risk of primary osteoporosis was lower than college education. In addition, the odds of developing primary osteoporosis were four times higher in individuals with nonpersonal (rented/relatives home) in comparison to personal housing (OR = 4.02: 95% CI: 1.24–13.07; P = 0.021). In nonmarried (single/widowed/divorced) women, its risk was 2.5 times more than married individuals (OR = 2.65 95% CI: 0.99–7.08; P = 0.051) [Table 4].
Table 4.
Predictors of osteoporosis based on multivariate logistic regression (comparison of osteoporosis with normal group)
| Variable | Adjusted odds ratio (95% confidence interval) | P* |
|---|---|---|
| Age | 1.18 (1.07-1.30) | <0.001 |
| Menopause age | 0.92 (0.85-1.01) | 0.071 |
| Body mass index | 0.87 (0.78-0.97) | 0.010 |
| Arm circumference | 0.84 (0.74-0.95) | 0.005 |
| Education level (academic:reference) | 0.028 | |
| Illiterate | 3.73 (0.71-19.52) | 0.119 |
| Primary school | 3.23 (0.66-15.76) | 0.147 |
| Secondary school | 2.42 (0.41-14.22) | 0.327 |
| High school/diploma | 0.74 (0.13-4.14) | 0.730 |
| Housing status (personal: reference) | 4.02 (1.24-13.07) | 0.021 |
| Marital status (married: reference) | 2.65 (0.99-7.08) | 0.051 |
Multivariate logistic regression test: Hosmer and Lemeshow P=0.13, χ2=12.97, df=8. All variables in this table remained as predictors in the last stage of backward strategy.
According to the results of the analysis, the mean (SD) TSH test was 1.79 (1.5), 1.7 (1.21) 1.64 (1.21) mU/L, Vitamin 25 (OH) Vitamin D was 42 (19.5), 44 (23.2), 47 (26.3) ng/mL, Alkaline phosphatase was 191 (57), 186 (60.3), 157.8 (48) IU/L, FBS was 87.3 (22), 92 (24.5), 88 (23) mg/dL, creatinine was 0.93 (0.14), 0.93 (0.17), 0.95 (0.15) mg/dL, phosphorus was 3.4 (0.61), 3.4 (0.61), 3.31 (0.57) mg/dL, calcium was 9.3 (0.64), 9.3 (0.64), 9.2 (0.68) mg/dL in the three groups of primary osteoporosis, osteopenia and normal in the, in, in test, and in test. According to the above statistics, there was no significant relationship between the results of the tests and the bone density.
Discussion
In the present study, the prevalence of primary osteoporosis and demographic, obstetric, life style, and anthropometric risk factors were studied in postmenopausal women aged 50–65 years old in Tabriz.
Prevalence
The prevalence of primary osteoporosis was evaluated based on the two lumbar spine and femoral neck areas. The results of this study showed that the rate of primary osteoporosis was 23.4% in the lumbar region, 3.4% in femur neck area, and 24.5% in total.
The prevalence of osteoporosis for women over 50 years was reported in 2010 as 9% in the United Kingdom, 15% in France and Germany, 16% in the United States, and 38% in Japan while this statistics for men was 1% in the United Kingdom, 4% in Japan, 3% in Canada, and 8% in France.[29] The prevalence of osteoporosis in the United States in the population over 50 years was 10.3%.[7] Comparison of the mentioned studies with our results shows that the prevalence of osteoporosis in Iran is significantly higher than in the European and American countries. Despite ample sunshine, the Middle East and Africa register the highest rates of rickets worldwide. Low levels of Vitamin D are prevalent throughout the region.[30]
Based on the results of a meta-analysis conducted in Iranian postmenopausal women, the prevalence of osteoporosis in 2018 was estimated as much as 32% and the prevalence of low bone density was as much as 51% (21). The rate of osteoporosis in postmenopausal women in our study (24.5%) was lower. The lower rate may be related to the study on specific age range of 50–65 in the present study. In the meta-analysis, postmenopausal women in all age groups were examined. It is noteworthy that the risk of osteoporosis increases with aging.[31]
The results of another meta-analysis conducted in 2011 on 9657 Iranian women with an average age of 55.8 indicated that the prevalence of osteoporosis was 18.9 ranged from 6.5 to 43%.[21] The mentioned study has been conducted on women of all age groups. Hence, the low prevalence in the meta-analysis may be contributed to age difference.
In the meta-analysis of Irani et al., 19% of postmenopausal women had osteoporosis and 40% of them had osteopenia based on lumbar spine densitometry.[32] In line with our study, this research has been conducted on postmenopausal women. Although it has determined the prevalence of osteoporosis in all age groups after menopause, the rate of osteoporosis and osteopenia was less than our results. This difference may be attributed to the increasing rate of osteoporosis over time and also to the higher rate of osteoporosis in the northwestern region of the country. The subgroup analysis of the mentioned meta-analysis also illustrated that the prevalence of osteoporosis and osteopenia in the northern regions of the country was significantly higher than that in the southern regions.[32] Moreover, the comparison of the two above meta-analysis in Iran[21,32] indicates a significant increase in the prevalence of postmenopausal osteoporosis.
According to a study in Tehran-Iran, the prevalence of osteoporosis in women 40–60 years was 15.8% in the lumbar spine and 2.9% in the femur neck.[33]
Risk factors
Based on the results of the present study, variables such as age, menopausal age, BMI, arm circumference, education level, housing status, and marital status were significant risk factors for osteoporosis in postmenopausal women aged 50–65 years old so that developing of osteoporosis increased as much as 18% with 1 year increase of age and decreased by 1 year increment in menopausal age as much as 8%. The development of osteoporosis decreased as much as 13% and 16%, respectively, with one unit increase in BMI and arm circumference. The risk of developing osteoporosis decreased with increasing education, but the risk of developing osteoporosis in high school/diploma was lower than college level. Moreover, the chance of developing osteoporosis in individuals with nonpersonal housing (rented/relatives’ homes) were four times higher than those with personal homes It was 2.5 times higher in unmarried people (single/widowed/divorced) than married people. The median of physical activity was in low level in the all groups.
A review of the literature shows that bone density decreases with age, which can increase the risk of fractures. The risk of fracture doubles with every 10% reduction in BMD.[34] Low bone mass and bone tissue weakness are associated with increased bone fragility and fracture risk.[35] As age increases, the bone mass decreases and the bones become porous. Due to the altered structural quality, the risk of fracture in the bones is increased. One of the reasons is the imbalance between new bone formation and old bone resorption that results in bone loss and structural quality. Furthermore, decreasing the sexual hormones also have a greater effect on the process of reducing bone density.[36] Various studies have shown that there is a significant relationship between marital status and menopausal age with the prevalence of osteoporosis, which may be due to the effects of estrogen on menopausal age,[37] which are consistent with the present study.
Several studies have shown that one of the potent protective factors against osteoporosis has been obesity.[38] Similar results were found in the present research, so that the risk of osteoporosis was decreased in people with high BMI. The precise mechanism of this effect is not clear, but it is generally accepted that high BMI imposes a high mechanical burden on the bone and so as a result bone mass is increased to accommodate this additional burden. In addition, adipocytes are an important source of estrogen in postmenopausal women. Estrogen is also known to inhibit osteoclasts. Increased adipose tissue in postmenopausal women appears to suppress osteoclasts and increase bone mass due to increased estrogen production.[39]
Consistent with the present research, several studies have shown that increased arm circumference and forearm have a significant relationship with bone mass, so that increased arm circumference is associated with increased bone mass and bone formation composition.[40]
Various studies have shown that people with lower levels of education are more likely to develop osteoporosis, and previous findings have shown a relationship between lower education and the risk of osteoporosis.[41] Education levels may affect BMD, which may be explained by the knowledge of more educated people about the prevention of osteoporosis.[42] In highly educated individuals, the prevalence of osteoporosis may be low due to higher activity levels and the consumption of healthy foods containing high nutrients specially calcium and appropriate diets.[43]
As a main strong point of present study, we determined the prevalence and risk factors of primary osteoporosis in early postmenopausal period for the first time. The screening of osteoporosis and low bone mass in early postmenopausal period can better help us in timely prevention and treatment of primary osteoporosis to promote healthy aging.
Although in this study, the selection of postmenopausal women aged 50–65 years was done by simple randomization in the whole city of Tabriz, 54 people were excluded due to unwillingness to participate in the study during the study. Given that these individuals may be different in terms of socio-economic class, this reduces the generalizability of the study. It is recommended that future studies compare the demographics and lifestyle of these individuals with those participating in the study as much as possible. Intake of calcium and Vitamin D supplements as well as exposure to direct sunlight in the participants were evaluated and compared in this study, but food intake containing these substances was not investigated and compared. Hence, it is suggested that this be evaluated in future studies. Also in this study, all the participants’ answers to the questions were considered as correct answers.
Conclusions
According to this research, one quarter of postmenopausal women aged 50–65 years old have primary osteoporosis and about half of women have low BMD, which is a warning for the complications of osteoporosis that need to be planned for screening, prevention, control, and training to enhance lifestyle. Given the increasing trend of aging in Iran and the higher prevalence of osteoporosis in northern cities such as Tabriz, the extent of this problem seems to go even further with time. Therefore, consideration of the predicting risk factors of osteoporosis and removing and amendment of modifiable factors and paying special attention to those with irreversible risk factors can play an important role in preventing or reducing this epidemic.
Financial support and sponsorship
This study has been funded by Tabriz University of Medical Sciences (grant number: 60916). The funder does monitor study progress, but does not have a role in the conduct of the study and did not contribute to the preparation of this manuscript. The study protocol has undergone peer review of the funding institute.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The present paper was extracted from a master's thesis in Midwifery. It would be grateful for us to express our appreciation to the Deputy of Research and Technology in Tabriz University of Medical Sciences for financial support (No: 60916), Faculty of Nursing and Midwifery, Deputy of Health, Sina Hospital Densitometry Department, and all women participating in the project.
References
- 1.Liu ZY, Yang Y, Wen CY, Rong LM. Serum osteocalcin and testosterone concentrations in adult males with or without primary osteoporosis: A meta-analysis. Biomed Res Int. 2017;2017:9892048. doi: 10.1155/2017/9892048. doi: 10.1155/2017/9892048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jahromi VK, Ghashghaei SB, Sharifi N. Improvement of osteoporosis-related behaviors in female students based on trans theoretical model. J Educ Health Promot. 2020;9:221. doi: 10.4103/jehp.jehp_213_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jin YX, Wu P, Mao YF, Wang B, Zhang JF, Chen WL, Liu Z, Shi XL. Chinese herbal medicine for osteoporosis: A meta-analysis of randomized controlled trials. Journal of Clinical Densitometry. 2017;20:516–25. doi: 10.1016/j.jocd.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Tian L, Yang R, Wei L, Liu J, Yang Y, Shao F, et al. Prevalence of osteoporosis and related lifestyle and metabolic factors of postmenopausal women and elderly men: A cross-sectional study in Gansu province, Northwestern of China. Medicine (Baltimore) 2017;96:e8294. doi: 10.1097/MD.0000000000008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, et al. UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12:43. doi: 10.1007/s11657-017-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawla J, Sharma N, Arora D, Arora M, Shukla L. Bone densitometry status and its associated factors in peri and post menopausal females: A cross sectional study from a tertiary care centre in India. Taiwan J Obstet Gynecol. 2018;57:100–5. doi: 10.1016/j.tjog.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 7.Cui W, Mager J. Transcriptional regulation and genes involved in first lineage specification during preimplantation development. Adv Anat Embryol Cell Biol. 2018;229:31–46. doi: 10.1007/978-3-319-63187-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pajouhi M, Maghbooli Z, Hejri SM, Keshtkar A, Saberi M, Larijani B. Bone mineral density in 10 to75 year-old Iranian healthy women: Population base study. Iran J Public Health. 2004;33(Suppl 1):57–63. [Google Scholar]
- 10.Paknahad Z, Mohammadifard N, Bonakdar Z, Hasanzadeh A. Nutritional status and its relationship with bone mass density in postmenopausal women admitted in osteodensitometry center, Isfahan-Iran. J Educ Health Promot. 2014;3:48. doi: 10.4103/2277-9531.131937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tohidi M, Dabbaghmanesh MH, Fattahi MR, Ranjbar Omrani G. Prevalence of osteoporosis in rural men of Fars based on both local and WHO reference data. Iran J Endocrinol Metab. 2010;12:393–400. [Google Scholar]
- 12.Ensrud KE. Epidemiology of fracture risk with advancing age. J Gerontol A Biol Sci Med Sci. 2013;68:1236–42. doi: 10.1093/gerona/glt092. [DOI] [PubMed] [Google Scholar]
- 13.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Mineral Res. 2014;29:2520–6. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dionyssiotis Y, Paspati I, Trovas G, Galanos A, Lyritis GP. Association of physical exercise and calcium intake with bone mass measured by quantitative ultrasound. BMC Women's Health. 2010;10:12. doi: 10.1186/1472-6874-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang HC, Hsieh CF, Lin YC, Tantoh DM, Ko PC, Kung YY, et al. Does coffee drinking have beneficial effects on bone health of Taiwanese adults? A longitudinal study. BMC Public Health. 2018;18:1273. doi: 10.1186/s12889-018-6168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naseh L, Shaikhy R, Rafiei H. General self-efficacy and associated factors among elderly residents of nursing home. J Holistic Nurs Midwifery. 2016;26:90–7. [Google Scholar]
- 17.Harooni J, Hassanzadeh A, Mostafavi F. Influencing factors on health promoting behavior among the elderly living in the community. J Educ Health Promot. 2014;3:40. doi: 10.4103/2277-9531.131921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabiei L, Mostafavi F, Masoudi R, Hassanzadeh A. Effects of family-centered interventions on empowerment of the elderly. J Educ Health Promot. 2013;2:24. doi: 10.4103/2277-9531.112700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanhope M, Lancaster J. Community & public health nursing. 6th ed. St. Louis: Mosby; 2004. [Google Scholar]
- 20.Kular J, Tickner J, Chim SM, Xu J. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem. 2012;45:863–73. doi: 10.1016/j.clinbiochem.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Bagheri P, Haghdoost A, Dortaj rabari E, Halimi L, Vafaei Z, Farhangnya M, et al. Ultra analysis of prevalence of osteoporosis in Iranian women “a systematic review and meta-analysis. Iran J Endocrinol Metabolism. 2011;13:315–42. [Google Scholar]
- 22.Statistical Year Book of Iran Publication of Statistical Center. Statistical Center of Iran; 2007. [Google Scholar]
- 23.Hemmati F, Sarokhani D, Sayehmiri K, Motadayen M. Prevalence of osteoporosis in postmenopausal women in Iran: A systematic review and meta-analysis. Iran J Obstetrics Gynecol Infertility. 2018;21:90–102. [Google Scholar]
- 24.Statistical Center of Iran. The Results of the General Census of Population and Housing. 2016. [Last accessed on 2020 Oct 03]. Available from: https://www.amar.org.ir/
- 25.Bayat N, Haji AZ, Ali SG, Ebadi A, Hosseini M, Lalouei A. Frequency of Osteoporosis and Osteopenia in Post-Menopausal Military Family's Women. 2008 [Google Scholar]
- 26.WHO Study Group. Technical Report Series 843. Geneva: World Health Organization; 1994. Assessment of Fracture Risk and its Application to Screening for Postmenopausal Osteoporosis. [PubMed] [Google Scholar]
- 27.Hazavehei SM, Asadi Z, Hassanzadeh A, Shekarchizadeh P. Comparing the effect of two methods of presenting physical education Π course on the attitudes and practices of female Students towards regular physical activity in Isfahan University of Medical Sciences. Iran J Med Edu. 2008;8:121–31. [Google Scholar]
- 28.Committee IR. Guidelines for data processing and analysis of the international physical activity questionnaire (IPAQ)-short and long forms. Retrieved Sept. 2005;17:2008. [Google Scholar]
- 29.Wade SW, Strader C, Fitzpatrick LA, Anthony MS, O’Malley CD. Estimating prevalence of osteoporosis: Examples from industrialized countries. Arch Osteoporos. 2014;9:182. doi: 10.1007/s11657-014-0182-3. [DOI] [PubMed] [Google Scholar]
- 30.El-Hajj Fuleihan G, Adib G, Nauroy L. Switzerland: International Osteoporosis Foundation; 2011. The middle east & Africa regional audit, epidemiology, costs & burden of osteoporosis in 2011; pp. 102011–105000. [Google Scholar]
- 31.Chen PH, Lin MS, Huang TJ, Chen MY. Prevalence of and factors associated with adopting bone health promoting behaviours among people with osteoporosis in Taiwan: A crosssectional study. BMJ Open. 2017;7:e01598030. doi: 10.1136/bmjopen-2017-015980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irani AD, Poorolajal J, Khalilian A, Esmailnasab N, Cheraghi Z. Prevalence of osteoporosis in Iran: A meta-analysis. J Res Med Sci. 2013;18:759–66. [PMC free article] [PubMed] [Google Scholar]
- 33.Jamshidian TM, Kalantari N, Kamali Z, Houshyarrad A, Azadbakht L, Smaeilzadeh A, et al. Osteoporosis risk factors in Tehran women aged 40-60 years. IJEM. 2004;6(2):139–45. [Google Scholar]
- 34.Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17(Suppl 6):S164–9. [PubMed] [Google Scholar]
- 35.Morin SN, Lix LM, Leslie WD. The importance of previous fracture site on osteoporosis diagnosis and incident fractures in women. J Bone Miner Res. 2014;29:1675–80. doi: 10.1002/jbmr.2204. [DOI] [PubMed] [Google Scholar]
- 36.Bazrafshan HR, Qorbani M, Shadpour Rashti H, Aghaei M, Safari R. Prevalence of osteoporosis and its association with demographic characterirstics – Gorgan, Iran. J Hormozgan Uni Med Sci. 2011;15:56–62. [Google Scholar]
- 37.Ajamzibod H. PhD dissertation, MSc. thesis. Tehran: Tehran University of Medical Sciences; 2011. The study of relationship between life style and quality of life among the west Tehran elderly. [Google Scholar]
- 38.Lloyd JT, Alley DE, Hawkes WG, Hochberg MC, Waldstein SR, Orwig DL. Body mass index is positively associated with bone mineral density in US older adults. Arch Osteoporos. 2014;9:175. doi: 10.1007/s11657-014-0175-2. [DOI] [PubMed] [Google Scholar]
- 39.Kameda T, Mano H, Yuasa T, Mori Y, Miyazawa K, Shiokawa M, et al. Estrogen inhibits bone resorption by directly inducing apoptosis of the bone-resorbing osteoclasts. J Exp Med. 1997;186:489–95. doi: 10.1084/jem.186.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naeem ST, Hussain R, Raheem A, Siddiqui I, Ghani F, Khan AH. Bone turnover markers for osteoporosis status assessment at baseline in postmenopausal Pakistani females. J Coll Physicians Surg Pak. 2016;26:408–12. [PubMed] [Google Scholar]
- 41.Liu ZM, Wong CK, Wong SY, Leung J, Tse LA, Chan R, et al. A healthier lifestyle pattern for cardiovascular risk reduction is associated with better bone mass in Southern Chinese elderly men and women. Medicine (Baltimore) 2015;94:e1283. doi: 10.1097/MD.0000000000001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu CX, Zhang XZ, Zhang K, Tang Z. A cross-sectional study for estimation of associations between education level and osteoporosis in a Chinese men sample. BMC Musculoskelet Disord. 2015;16:382. doi: 10.1186/s12891-015-0839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasnah H, Amin I, Suzana S. Bone health status and lipid profile among post-menopausal malay women in Cheras, Kuala Lumpur. Malays J Nutr. 2012;18:161–71. [PubMed] [Google Scholar]


