Abstract
Background and Aims
Previous laboratory studies have suggested selection for root hair traits in future crop breeding to improve resource use efficiency and stress tolerance. However, data on the interplay between root hairs and open-field systems, under contrasting soils and climate conditions, are limited. As such, this study aims to experimentally elucidate some of the impacts that root hairs have on plant performance on a field scale.
Methods
A field experiment was set up in Scotland for two consecutive years, under contrasting climate conditions and different soil textures (i.e. clay loam vs. sandy loam). Five barley (Hordeum vulgare) genotypes exhibiting variation in root hair length and density were used in the study. Root hair length, density and rhizosheath weight were measured at several growth stages, as well as shoot biomass, plant water status, shoot phosphorus (P) accumulation and grain yield.
Key Results
Measurements of root hair density, length and its correlation with rhizosheath weight highlighted trait robustness in the field under variable environmental conditions, although significant variations were found between soil textures as the growing season progressed. Root hairs did not confer a notable advantage to barley under optimal conditions, but under soil water deficit root hairs enhanced plant water status and stress tolerance resulting in a less negative leaf water potential and lower leaf abscisic acid concentration, while promoting shoot P accumulation. Furthermore, the presence of root hairs did not decrease yield under optimal conditions, while root hairs enhanced yield stability under drought.
Conclusions
Selecting for beneficial root hair traits can enhance yield stability without diminishing yield potential, overcoming the breeder’s dilemma of trying to simultaneously enhance both productivity and resilience. Therefore, the maintenance or enhancement of root hairs can represent a key trait for breeding the next generation of crops for improved drought tolerance in relation to climate change.
Keywords: Agricultural sustainability, barley, drought tolerance, grain yield, Hordeum vulgare, plant water status, phosphorus, rhizosheath, root hairs, soil texture
INTRODUCTION
Root traits are critical features for more resource-efficient and stress-tolerant crop varieties (Lynch, 2007). Common targets in breeding programmes are deep roots to capture stored water and leached nitrogen, and abundant shallow roots to capture strongly bound nutrients such as phosphorus (P; White et al., 2013). However, in the context of crop breeding, the selection of genotypes with abundant root growth in low fertility conditions may be counterproductive, as resources would be allocated to the root system at the expense of shoot and reproductive output (i.e. yield; Bloom et al., 1985). Root hairs represent an attractive target for future crop breeding given their role in P uptake, relatively simple genetic control and relatively small associated metabolic cost (Bates and Lynch, 2000; Gahoonia and Nielsen, 2004; Brown et al., 2013, 2017; George et al., 2020).
Root hairs are tubular protrusions (typically 10 µm diameter) arising from epidermal cells (trichoblasts; Jungk, 2001). These specialized structures represent about 2 % of the root mass (Röhm and Werner, 1987; Clarkson, 1991), and significantly increase the interaction between the plant and the soil. Early estimates by Dittmer (1937) that a single rye plant had 14 billion root hairs that provided a potential surface area in contact with the soil of 400 m2 clearly demonstrate that they increase the nutrient- and water-absorbing root surface area, which can be up to 3-fold larger (Gahoonia et al., 1997; Dolan, 2001; Gahoonia and Nielsen, 2004; Holz et al., 2018). In addition to increasing surface area, root hairs access finer pores than the main root axis, so the volume of soil influenced by roots can increase significantly (Ruiz et al., 2020). Root hairs also assist rhizosphere development by facilitating the diffusion of root mucilage (Watt et al., 1994; Ahmadi et al., 2017), citrate and acid phosphatase (Narang et al., 2000; Gahoonia et al., 2001), and promoting microbiota diversification (Robertson-Albertyn et al., 2017). Soil that is very strongly bound to roots in the rhizosphere forms a rhizosheath, operationally defined as the weight of soil that adheres strongly to roots on excavation (George et al., 2014). Rhizosheath size is greatly affected by the properties of root hairs (Haling et al., 2010), which is important as it protects the root from drought and heat stress (Benard et al., 2016; Basirat et al., 2019), nutrient deficiencies (Brown et al., 2012), soil acidity (Haling et al., 2010) and soil strength (Haling et al., 2013, 2014). Root hairs also have a positive impact on soil carbon sequestration, with greater carbon allocation below-ground in the presence of root hairs (Holz et al., 2018). Root hairs may also be important for growth into strong soils as they provide anchorage. Bengough et al. (2016) found that, from a loose seedbed, maize primary roots with root hairs could penetrate soil that was five times stronger than that penetrated by root hairless maize mutants. Taken together, roots hairs are involved in a number of processes that enhance crop tolerance to abiotic stresses.
Root hairs show intra- and interspecific variations in length and density (i.e. root hair traits; Brown et al., 2017). In angiosperms, the average length of root hairs varies from zero (i.e. species with no root hairs) to 1.5 mm (e.g. in Hordeum vulgare; Brown et al., 2017). Root hair length is particularly important in P-deficient conditions, where they increase shoot P accumulation, biomass and yield (Gahoonia and Nielsen, 2004; Brown et al., 2012). Most studies investigating root hairs have focused on plant tolerance to P deficiency and rhizosheath formation, comparing wild types (WTs) and hairless mutants of major crops under controlled conditions (Brown et al., 2012, 2017; Delhaize et al., 2012; Kole et al., 2015), such as growth cabinets with artificial lighting and sieved soil packed to optimal density (for details, see Supplementary data Table S1). Such experiments provide controlled conditions that are ideal for contrasting root hair traits with few environmental variables, but they do not reflect conditions that plants may experience in the field, such as fluctuating water availability.
A few controlled-environment studies have explored the impact of soil water content (% of field capacity), varying it from 100 % (Brown et al., 2012), to 80 % (Brown et al., 2017), to 75 % (Brown et al., 2012), to 70 % (Bailey and Scholes, 1997; Adu et al., 2017), to 50 % (George et al., 2014) and to 30 % (Adu et al., 2017). Under the described controlled-environmental conditions, plants were generally harvested and root traits examined after a short growing period ranging from a few days (e.g. 3 d in Delhaize et al., 2015) to nearly a month (e.g. 26 d in Brown et al., 2017). The effects of soil density and soil texture on root hair development were studied on plants grown for 4 d in artificial soil mixtures with different particle fractions (Haling et al., 2014). Highly controlled environments were used to image the soil–root interface of the WT and a hairless barley mutant by high-resolution synchrotron scanning of individual roots grown in syringe barrels filled with finely sieved soil (Koebernick et al., 2017, 2019). While these laboratory studies were able to identify fundamental processes, such as genetic associations with fine-scale rhizosphere characteristics, they oversimplify complex field conditions where soil and climate variables interact.
Despite the pressing need for field-validated laboratory experiments and general improvement of agricultural production and food security, few studies, often limited to barley and rice, have tested the effect of root hairs under real field conditions (Gahoonia and Nielsen, 2004; George et al., 2014; Nestler and Wissuwa, 2016; Nestler et al., 2016). In barley, root hair length and rhizosheath size suggested consistent ranking of the tested genotypes between laboratory and field conditions (Gahoonia and Nielsen, 2004; George et al., 2014). Under field conditions, grain yields of barley genotypes with shorter root hairs were much less in P-deficient soils than for barley genotypes with longer root hairs (Gahoonia and Nielsen, 2004). This agreement between studies in the field and more controlled conditions is promising, but to date most field trials on root hairs have complemented more detailed trials in a controlled environment (e.g. growth cabinet). The field trials have measured only a few plant or soil properties, for only a single growing season. For instance, although Gahoonia and Nielsen (2004) and George et al. (2014) provided a detailed soil characterization (e.g. soil texture and pH), data on climate conditions (e.g. temperature and precipitations) and soil water status were not presented, limiting data interpretation and generalization. Indeed, soil water content affected rhizosheath weight in laboratory experiments, with rhizosheaths formed under dry conditions being larger than those formed in wet soils, with a possible function in nutrient acquisition in dry soils (Watt et al., 1994; Liu et al., 2019). Moreover, plant variables, measured in the field, were limited to yield in Gahoonia and Nielsen (2004) and to rhizosheath weight in George et al. (2014), while correlations (e.g. root length vs. rhizosheath weight) in the same studies were generally based on data gathered in a controlled environment.
In the field, plants may be subject to abrupt changes in soil moisture (e.g. see climate data in Fig. 1), with fluctuations in water availability preventing the development of stress tolerance and therefore inducing water stress. On the contrary, in laboratory experiments, plants are often subject to constant water stress or optimal conditions. To our knowledge, laboratory experiments to date on root hairs and water stress have imposed water deficit treatments (Brown et al., 2012; Adu et al., 2017) often without directly measuring plant water stress. Brown et al. (2012) showed that root hairless mutants of barley were up to 2.3-fold smaller than genotypes with root hairs under combined P and water stress. However, plant water status was not measured.
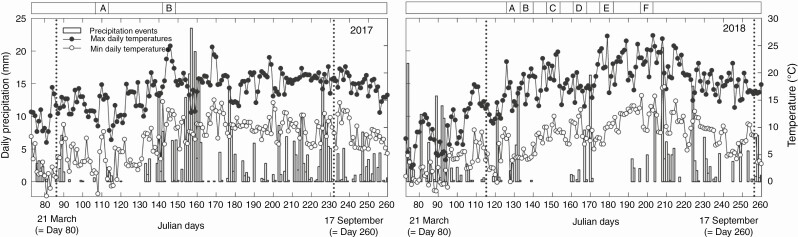
Fig. 1.
Daily precipitation and minimum and maximum daily temperatures recorded at the experimental sites between 11 March and 17 September in 2017 and 2018. Dotted lines indicate sowing (24 March in 2017 and 25 April in 2018) and harvesting (19 August in 2017 and 13 September in 2018) times for both years. Also reported are the samplings for both years: 2017 – A (Julian days 107–113: plant traits), B (Julian days 142–148: plant traits; leaf P concentration); 2018 – A (Julian days 127–133: soil P concentration), B (Julian days 134–140: plant traits; soil water content), C (Julian days 148–154: plant traits; soil P concentration; soil water content), D (Julian days 162–168: plant traits; soil P concentration; soil water content; plant water status; leaf P concentration), E (Julian days 176–182: plant traits; soil P concentration; soil water content; plant water status), F (Julian days 197–203: soil P concentration; soil water content; plant water status).
Although root hairs and the rhizosphere are assumed to play a key role in regulating plant water relations, their effect on plant water uptake has rarely been investigated. Although the hairless mutant of Arabidopsis thaliana took up less water than the WT in hydroponic culture, its ability to take up water from soil was not assessed (Tanaka et al., 2014). Under low evaporative demand (<1.5 kPa) in a controlled environment, a root hairless barley mutant (brb) and its WT had the same transpiration rate, suggesting that root hairs were redundant in regulating water uptake (Dodd and Diatloff, 2016). In contrast, enclosing shoots of these genotypes in a cuvette at higher evaporative demands demonstrated that root hairs were necessary to sustain transpiration and prevent leaf water deficits (Carminati et al., 2017). Nevertheless, to our knowledge, the role of root hairs in regulating leaf water status in field-grown plants has not been investigated.
A major gap in understanding the benefits of root hairs for crop productivity is field verification under contrasting environmental conditions. This study explored the performance of contrasting barley root hair genotypes in 2017 (a typical year) and 2018 (the driest growing season ever recorded at this site), with measurements of plant and soil properties over the growing season. We quantified the influence of root hairs on plant performance under contrasting field conditions to address the following hypotheses: (1) root hair traits are robust over time, in contrasting soil textures and climate conditions; (2) the presence of root hairs increases shoot mass and shoot P accumulation in the field; and (3) root hairs enhance plant water status and grain yield under soil moisture deficit. The hypotheses were tested in a full-scale field experiment using barley genotypes exhibiting variations in root hair length and density.
MATERIALS AND METHODS
Study site and experimental plots
The study was carried out at The James Hutton Institute, Dundee, UK (56°27'34.80''N, 3°4'21.01''W) at two locations denoted as South Bullion and North Bullion (Supplementary data Fig. S1A). The South Bullion soil is a sandy loam (60 % sand, 24 % silt and 16 % clay; 22.5 ± 1.4 g kg–1 carbon, 1.6 ± 0.3 g kg–1 nitrogen, 5.48 ± 0.07 soil pH in CaCl2) and classified as a Dystric Cambisol, whereas the North Bullion soil is a clay loam (44 % sand, 30 % silt and 26 % clay; 29.5 ± 1.2 g kg–1 carbon, 2.3 ± 0.2 g kg–1 nitrogen, 5.15 ± 0.04 soil pH in CaCl2) and classified as a Haplic Cambisol (Naveed et al., 2018). From hereon, we refer to the locations by their soil texture, i.e. sandy loam or clay loam.
Five barley (Hordeum vulgare) genotypes exhibiting variation in root hair length were used in the study. These included a WT and three mutant lines from an ethylmethane sulfonate (EMS) mutant barley population in an ‘Optic’ cultivar genetic background: no root hair (NRH), a ‘bud’ root hair (BRH, with root hair initiation but not developing any further) and a short root hair (SRH). One line of each genotype was selected from the three used by Brown et al. (2012), where further details on genotype screening are provided. Additionally, the cultivar ‘Sassy’ was included in the study as it was previously found to have abundant root hairs. Seeds were sown using a Wintersteiger Seedmatic drill at 4 cm depth in each of the sandy loam and clay loam fields, for two consecutive years, 2017 (24 March) and 2018 (25 April), following a random block design. Each treatment was replicated four times for a total of 40 experimental plots (1.5 × 6 m each). In order to prevent edge effects on the experimental plots, at both locations guard plots of 1.5 m width were planted with a standard barley cultivar (‘Concerto’; Supplementary data Fig. S1B). Nitrogen, P, potassium and sulfur (SO3) were applied using fertilizer with a 22–4–14 + 7.5 SO3 mix at 273 and 280 kg ha–1 for both soil textures in 2017 and 2018, respectively. Nitrogen fertilizer was supplied in the form of ammonium nitrate. Pesticides were added when required after sowing following the local agronomic practices for spring barley.
Weather conditions were recorded by the weather station of The James Hutton Institute. In 2017, maximum and minimum daily temperatures, from sowing to harvesting, averaged 16.9 ± 3.2 °C and 7.9 ± 3.7 °C, respectively, and in 2018 averaged 18.8 ± 3.5 °C and 8.8 ± 3.3 °C, respectively (Fig. 1). Daily precipitation differed markedly between the two experimental years, being frequent and abundant during the growing season in 2017, while only occasional precipitation events were recorded in 2018 (Fig. 1). Total precipitation from sowing to harvesting was 42 % less in 2018, measuring 323.4 mm in 2017 compared with 186.6 mm in 2018 (Supplementary data Fig. S2). This offered the possibility to investigate the importance of the presence and abundance of root hairs for plant growth and crop yield under different water availability conditions. Climate data for the area in the period 1971–2000 report an average total precipitation of 313.7 mm from April to September, so 2018 was a particularly dry growing season (Supplementary data Fig. S2).
Root traits
Root traits were measured during contrasting growing seasons and at different growing stages, from GS 11 – first leaf unfolded – (Tottman, 1987) to harvesting. In 2017 plants were harvested on two occasions, 24 (17 April) and 56 (22 May) days from sowing. During the following year, sampling was performed four times, 19 (14 May), 33 (28 May), 49 (13 June) and 61 (25 June) days from sowing. One and two whole plant samples were harvested per treatment plot in 2017 and 2018, respectively. A spade or trowel was used to ensure as much of the root mass of the plant as possible was removed. Samples were placed in a plastic bag and processed on the same day in the laboratory. Roots were gently washed with water to remove any adhering soil and patted dry, with due care being taken to minimize any potential damage to root hairs (Brown et al., 2012).
To analyse total root length, root samples were spread out in a standard Petri dish, suspended in a small amount of water and placed against a white background. An image was collected in greyscale (600 dpi) using an Epson Expression 10000XL scanner (Epson UK, London). The software WinRHIZO pro (Regent Instruments, Quebec City, Canada) was used to digitally map root samples and calculate total root length, as well as the total number of root tips and forks.
A compound light microscope (Leica MZ FLIII; Leica Microsystems, Wetzlar, Germany) at magnification ×5 was used along with a Leica DC480 camera (Leica Microsystems) to capture images of roots for root hair length and density quantification. Three images were taken per plant and ten fully elongated root hairs per image were selected for measurement. The software ImageJ (ImageJ 1.46r; NIH, Bethesda, MD, USA) was used to measure root hair length (Schneider et al., 2012). The segmented line drawing tool was used to trace along the length of root hairs, and the length in millimetres was established utilizing a conversion factor gained by measuring the known gap on a set of digital callipers at various widths. From the ten samples, the root hairs were averaged to obtain the average root hair length per sample.
Using the root images captured for root hair length analysis, root hair density was assessed. This was an observational assessment of samples. Counts of root hairs were completed on 25 sample images of roots. These samples were classified into five categories based on the approximate number of root hairs per millimetre: (1) bald, 0–7 root hairs mm–1; (2) sparse, 7–15 root hairs mm–1; (3) moderate, 15–35 root hairs mm–1; (4) thick, 35–50 root hairs mm–1; and (5) dense, ≥50 root hairs mm–1. Root and shoot mass were measured by weighing oven-dried plant material, which had been dried at 70 °C for 4 d.
An estimation of rhizosheath weight was carried out by calculating the difference between the fresh root weight including attached soil and the clean fresh root weight. Specific rhizosheath weight (mg cm–1) was determined on a per unit root length basis by dividing the rhizosheath weight (mg) by the total root length (cm) for each plant.
Soil properties
During the growing season of 2018, a range of soil properties were measured in the field. Shortly after sowing, access tubes were installed in each plot and location of the following barley genotypes: NRH, WT and ‘Sassy’. Soil volumetric water content (m3 water m–3 soil) was then measured using PR2 probes (Delta-T Devices Ltd, Cambridge, UK) on five occasions: 19 (14 May), 37 (1 June), 49 (13 June), 62 (26 June) and 84 (18 July) days from sowing. Measurements were taken at three soil depths (0.2, 0.3 and 0.4 m) using a portable HH2 moisture meter (Delta-T Devices Ltd). Surface water content (0.1 m) was measured in proximity to the tubes using ML3 sensors (Delta-T Devices Ltd). On 14 May, analogic jet-filled tensiometers (Soilmoisture Equipment Corp, USA, practical limit of –80 kPa) at 0.2 and 0.5 m depths were placed in each plot of the barley WT and its hairless mutant. The tensiometers were carefully installed with soil slurry to allow for good soil–tensiometer contact. Tensiometer readings were recorded approximately every 3–5 d.
Soil P concentration and distribution along depth were assessed in 2018 in each plot of WT, NRH and ‘Sassy’ genotypes in both locations and at five time points: 15 (10 May), 34 (29 May), 50 (11 June), 61 (25 June) and 82 (16 July) days from sowing. Soil samples were collected using a screw-auger at three depths within a 40 cm deep profile (0–13, 14–27 and 28–40 cm) for a total of four samples per treatment. Olsen-P was derived according to Olsen and Sommers (1982) and Irving and McLaughlin (1990).
Plant performance
Water status of barley NRH, WT and ‘Sassy’ in both locations was monitored by measurements of leaf water potential at 48 (12 June), 62 (26 June) and 85 (19 July) days from sowing in 2018. Pre-dawn leaf water potential (Ψ pd) was measured between 05.00 and 06.00 h (solar time). Four leaves per plot, each from a different individual (16 leaves per experimental treatment) were collected and immediately wrapped in clingfilm, stored in a refrigerated bag and transported to the laboratory. Water potential was measured using a pressure chamber (Plant Moisture System, Skye Instruments, Powys, UK). The sampling was repeated on the same day between 12.00 and 13.00 h (solar time), to estimate minimum daily leaf water potential (Ψ min). Leaf samples for abscisic acid (ABA) determination were collected from NRH, WT and ‘Sassy’ plots in both locations at 48, 62 and 85 d from sowing, freeze-dried and finely ground. Deionized water was added at a 1:50 weight ratio and an aqueous extract was obtained after incubating them in a shaker at 4 °C overnight. The extracts were analysed by a radioimmunoassay (Quarrie et al., 1988) to determine leaf ABA concentration.
Chlorophyll concentration (CHL) was assessed on the same plots and dates as leaf water potential measurements. An optical meter (CCM-200; Opti-Sciences, Hudson, USA) was used to estimate CHL in situ of four leaves per plot (16 leaves per experimental treatment). A single universal optical/absolute chlorophyll relationship derived by Parry et al. (2014) was used to relate the output from the CCM-200 to absolute CHL in µmol m–2. The photosynthetic efficiency was estimated on the same plots, dates and time as Ψ min using chlorophyll a fluorescence emission measurements performed on four leaves from separate plants per plot (16 leaves per treatment). Measurements were done with a portable fluorimeter (Pocket Pea; Hansatech, Norfolk, UK) on leaves previously darkened for 20 min, and Fv/Fm was calculated as a proxy for quantum yield of photosystem II (PSII) (Maxwell and Johnson, 2000).
To determine shoot P concentration, leaf samples (newest fully extended leaf) were sampled at 56 and 48 d from sowing, in 2017 and 2018, respectively. Diagnostic leaves were collected from all plots of the genotypes NRH, WT and ‘Sassy’ at both locations and frozen at –80 °C, freeze-dried and milled. Powdered leaf samples (50 µg) were digested for 20 min at 180 °C in 3 mL of 15.8 m HNO3 (Aristar grade, VWR International, Poole, UK), followed by oxidation for 20 min at 180 °C with 1 mL of H2O2 in closed vessels using a MARSXpress microwave oven (CEM, Buckingham, UK). Digested samples were diluted to a final volume of 50 mL with de-ionized water, and the concentrations of P in diluted digests were determined by reaction with malachite green (Irving and McLaughlin, 1990). Shoot P accumulation (mg P per shoot) was calculated as the product of shoot P concentration (mg P g–1) and total shoot biomass (g), measured at 56 and 49 d from sowing, in 2017 and 2018, respectively. Following plant senescence, harvesting was performed at 148 (19 August 19) and 141 (13 September 13) days from sowing in 2017 and 2018, respectively. Grain weight was recorded as a measurement representative of yield.
Statistical analysis
Statistical analysis was performed using GenStat 19th edition (VSN International) and SigmaPlot14 (Systat Software Inc.). Measurements that were repeated over time were analysed using restricted maximum likelihood (REML) for repeated measurements. The sampling time was included as a repeated measurement, with genotype and soil texture as fixed factors. Differences between treatments within one sampling time were determined with two-way analysis of variance (ANOVA). Factors were genotype and soil texture. Differences between genotypes within the same sampling and soil texture were established with one-way ANOVA, followed by post-hoc Tukey’s test. Significant differences in the frequency distribution of roots between categories of root hair density (bald, 0–7 root hairs mm–1; sparse, 7–15 root hairs mm–1; moderate, 15–35 root hairs mm–1; thick, 35–50 root hairs mm–1; and dense, ≥50+ root hairs mm–1) were determined for each genotype with one-way ANOVA, followed by post-hoc Tukey’s test. Soil volumetric water content and P concentration were analysed using REML, with depth as repeated measurement, genotype and soil texture as fixed factors and sampling time as random factor. In the analysis of all recorded data, plot distribution in the field was used as a block factor. Data that did not follow a normal distribution were log-transformed and checked again for normal distribution prior to ANOVA. The significance of correlations established in this study was tested by regression analyses. Results were considered statistically significant when the P-value was ≤0.05.
RESULTS
Dynamic adjustment of root traits under field conditions
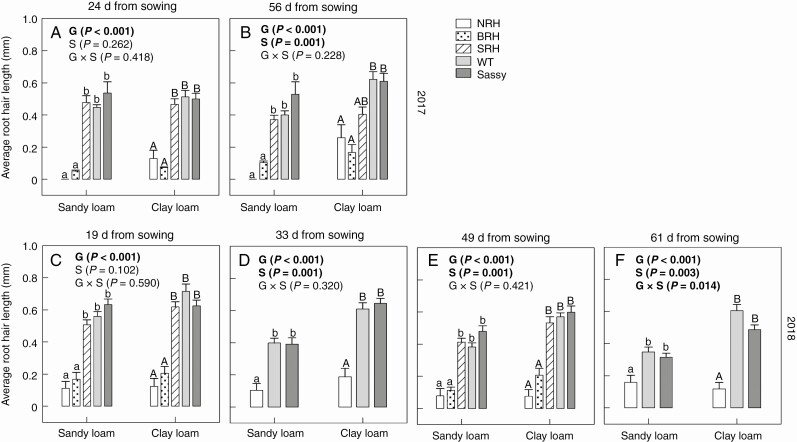
Barley genotypes differed in average root hair length and density when grown in the field. Indeed, average root hair length significantly differed (P < 0.001) between genotypes on each day that they were examined after 24 and 56 d growth in 2017 and after 19, 33, 49 and 61 d growth in 2018 (Fig. 2). Specifically, root hairs were significantly longer in the WT compared with the NRH and BRH mutants (P < 0.001) regardless of sampling day or soil texture and as plants aged for both years (Fig. 2). However, no significant differences were found between the WT, the SRH mutant and ‘Sassy’ across time or soil texture. The two experimental years showed the same trends in root hair length between genotypes; however, within the same genotype, root hairs were significantly longer (P = 0.034) for the WT grown in clay loam soil during the dry year in 2018 (0.71 mm) compared with the wet year in 2017 (0.51 mm), a few weeks after planting. Similarly, the SRH mutant grown in clay loam soil exhibited significantly longer (P = 0.015) root hairs later in the season in 2018 (0.53 mm) compared with 2017 (0.40 mm). Root hair length varied over the growing season, with root hairs growing as plants aged during the wet year and shrinking with time during the dry year. During the wet growing season of 2017, root hairs of the WT grown in clay loam were significantly longer (P = 0.006) at 56 d (0.62 mm) compared with 24 d (0.51 mm) growth. Even in BRH, that had very short root hairs, they were significantly longer (P = 0.002) at 56 d (0.10 mm) compared with 24 d (0.05 mm) growth. In contrast, the dry growing season of 2018 highlighted a decreasing trend in root hair length as plants aged, with significantly longer (P = 0.002) root hairs in the WT grown in sandy loam soil at 19 d growth (0.56 mm) compared with 33 d (0.40 mm), 49 d (0.38 mm) and 61 d (0.35 mm) growth. During the same year, a significant decrease in root hair length as plants aged was found for ‘Sassy’ grown in sandy loam (P < 0.001) and clay loam (P = 0.02). During both growing seasons, there were significant differences in average root hair length between the two soil textures, with 35 and 46 % longer root hairs in the clay loam compared with the sandy loam across all genotypes, in 2017 and 2018, respectively.
Fig. 2.
Variation in average root hair length (mm) of contrasting root hair genotypes grown in the field in sandy loam and clay loam soils for two subsequent years: 2017 (A, B) and 2018 (C–F). Two sampling campaigns were done in 2017: 24 and 56 d from sowing; four sampling campaigns were done in 2018: 19, 33, 49 and 61 d from sowing. Data are the mean of four (2017) and eight replicates (2018), with error bars representing the s.e. Differences between genotypes and soil textures were established using two-way ANOVA, P-values are reported and significant (P ≤ 0.05) parameters are in bold, with ‘G’ representing genotype, ‘S’ representing soil texture, and ‘G × S’ representing the interaction of genotype and soil texture. Identical letters indicate no significant differences as tested using one-way ANOVA followed by a post-hoc Tukey’s test. NRH represents the no root hair genotype; BRH, the bud root hair genotype; SRH, the short root hair genotype; and WT, the wild type, all in the ‘Optic’ background, together with ‘Sassy’.
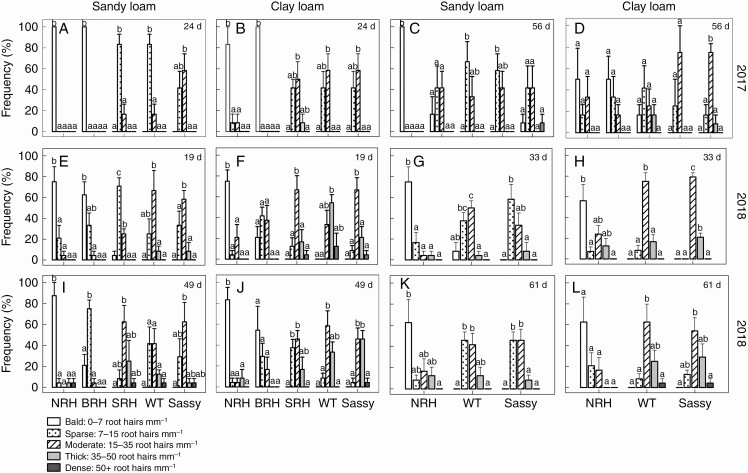
The frequency distribution of root hair density classes varied in relation to the genotype (Fig. 3). The majority of roots of the WT sampled at 24 d growth in 2017 were characterized by a sparse root hair density (7–15 root hairs mm–1) in the sandy loam field and moderate hair density (15–35 root hairs mm–1) in the clay loam. The frequency distribution of root hair density for the SRH mutant did not differ from that of the WT, while ‘Sassy’ showed a greater root hair density in the sandy loam, but not in the clay loam. In contrast, root hair density was markedly different for the NRH and BRH mutants compared with the WT, with most of the roots sampled 24 d from sowing having 0–7 root hairs mm–1 (bald) in both soil textures. Later in the season, at 56 d growth, differences in the frequency distribution of root hair density classes within genotypes were not as significant, with an increase in hair density for the BRH mutant in both soil textures and for the NRH mutant in clay loam as plants aged. The early sampling in 2018, at 19 d from sowing, highlighted differences in root hair density between the WT (15–35 root hairs mm–1) and mutants NRH (0–7 root hairs mm–1), BRH (0–7; 7–15 root hairs mm–1) and SRH (7–15 root hairs mm–1) grown in sandy loam (Fig. 3). Similarly, greater root hair density in the WT compared with its mutants was also found in clay loam soil. However, during both years, there was a trend leading to an overall greater root hair density for plants grown in clay loam compared with sandy loam soil (Fig. 3). Similarly to the previous year, as plants aged, root hair density increased for the mutants grown in sandy loam soil, going from bald to sparse for BRH and from sparse to moderate in the case of SRH.
Fig. 3.
Frequency distribution of the root hair density of contrasting root hair genotypes grown in the field in sandy loam and clay loam soils for two subsequent years: 2017 (A–D) and 2018 (E–L). Samples were classified into five categories based on the approximate number of root hairs per millimetre: bald, 0–7 root hairs mm–1; sparse, 7–15 root hairs mm–1; moderate, 15–35 root hairs mm–1; thick: 35–50 root hairs mm–1; and dense: ≥50 root hairs mm–1. Two sampling campaigns were done in 2017 at 24 and 56 d from sowing; and four sampling campaigns were done in 2018 at 19, 33, 49 and 61 d from sowing. Identical letters indicate no significant differences as tested using one-way ANOVA followed by a post-hoc Tukey’s test. NRH represents the no root hair genotype; BRH, the bud root hair genotype; SRH, the short root hair genotype; and WT, the wild type, all in the ‘Optic’ background, together with ‘Sassy’.
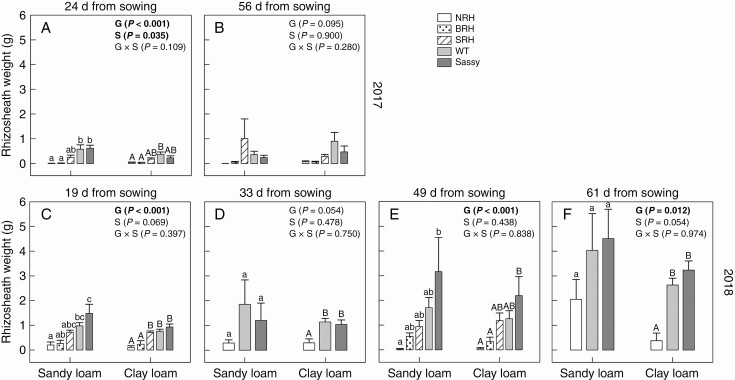
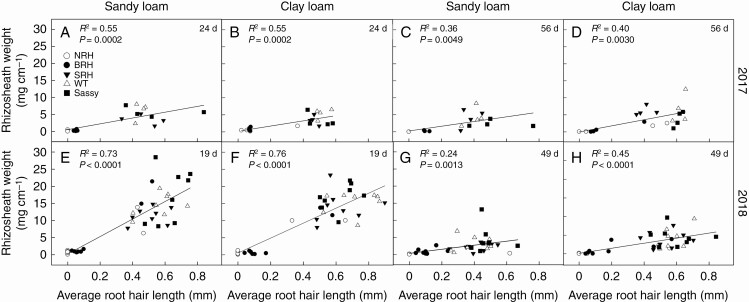
Soil bound to the root hairs was reflected in rhizosheath weight, which varied significantly (P < 0.001) between genotypes from the early sampling in 2017 and from all four samplings in 2018 (Fig. 4). Rhizosheath weight was significantly greater (P < 0.001) for the WT (0.58 and 0.37 g in sandy and clay loam, respectively) compared with NRH (0.01 and 0.03 g in sandy and clay loam, respectively) and BRH mutants (0.01 and 0.04 g in sandy and clay loam, respectively) at 24 d growth in 2017. The same sampling highlighted smaller rhizosheath weights for the WT, SRH and ‘Sassy’ in the clay loam compared with the sandy loam soil, with an average decrease of 36, 30 and 64 %, respectively. However, no significant differences were found in rhizosheath weight between genotypes, soil textures or their interaction at 56 d growth. During the dry season of 2018, rhizosheath weight for plants grown in sandy loam was significantly greater (P < 0.001) for the WT (0.98 g) and ‘Sassy’ (1.48 g) compared with NRH (0.20 g; Fig. 4), at 19 d growth. In the clay loam, significant differences (P < 0.001) were found between the WT (0.76 g) and both NRH (0.10 g) and BRH (0.24 g) mutants. At 33 and 61 d from sowing, rhizosheath weight was significantly greater (P = 0.005; P < 0.001) for WT and ‘Sassy’ compared with NRH only in clay loam, while no differences were found for plants grown in sandy loam (Fig. 4). During the last sampling of 2018, smaller rhizosheath weights were recorded for plants grown in clay loam compared with those in sandy loam, with an average reduction of 82, 35 and 28 % for NRH, the WT and ‘Sassy’, respectively. The two experimental years resulted in significant (P < 0.001) differences in the rhizosheath weight measured early in plant establishment (24 and 19 d growth in 2017 and 2018, respectively). Indeed, rhizosheath weight was greater for all genotypes in 2018, with significant differences for SRH (P = 0.027) in sandy loam, and the WT (P < 0.001), SRH (P = 0.007) and ‘Sassy’ (P = 0.018) in clay loam. There was a positive relationship between average root hair length and specific rhizosheath weight for all sampling dates and soil textures; however, the relationship yielded an R2 > 0.70 and P < 0.0001 only following the first sampling during the dry season of 2018 (Fig. 5).
Fig. 4.
Variation in average rhizosheath weight (g) of contrasting root hair genotypes grown in the field in sandy loam and clay loam soils for two subsequent years: 2017 (A, B) and 2018 (C–F). Two sampling campaigns were done in 2017 at 24 and 56 d from sowing; four sampling campaigns were done in 2018 at 19, 33, 49 and 61 d from sowing. Data are the mean of four (2017) and eight replicates (2018), with error bars representing the s.e. Differences between genotypes and soil textures were established using two-way ANOVA, P-values are reported and significant (P ≤ 0.05) parameters are in bold, with ‘G’ representing genotype, ‘S’ representing soil texture and ‘G × S’ representing the interaction of genotype and soil texture. Identical letters indicate no significant differences as tested using one-way ANOVA followed by a post-hoc Tukey’s test. NRH represents the no root hair genotype; BRH, the bud root hair genotype; SRH, the short root hair genotype; and WT, the wild type, all in the ‘Optic’ background, together with ‘Sassy’.
Fig. 5.
Relationship between average root hair length (mm) and specific rhizosheath weight (mg cm–1 root) for contrasting root hair genotypes grown in the field in sandy loam and clay loam soils for two subsequent years: 2017 (A–D) and 2018 (E–H). Two sampling campaigns were done in 2017 (24 and 56 d from sowing) and 2018 (19 and 49 d from sowing). The coefficient of determination R2 and corresponding P-values are reported upon fitting with equation y = y0 + a × x.
Other measurements taken after 24 and 56 d growth in 2017 and 19, 33, 49 and 61 d growth in 2018 are summarized in Table 1 and Supplementary data Table S2. In 2017, dry weights of shoots were greater (P = 0.022) in the WT (253 ± 25 g) and ‘Sassy’ (288 ± 49 g) genotypes grown in sandy loam soil compared with NRH (112 ± 18 g), with intermediate values recorded for the other two genotypes (Table 1). No significant differences were found in shoot dry weight between genotypes grown in the clay loam; however, this was generally less (–30 %; P = 0.006) compared with plants grown in sandy loam. In 2018, significant differences (P = 0.003) in shoot dry weight were recorded between genotypes (Table 1). At 49 d growth, shoot biomass was significantly different between ‘Sassy’ (1211 ± 31 g) and NRH (509 ± 57 g) in sandy loam (P = 0.006), while in the clay loam differences (P = 0.004) were also found between the WT (792 ± 80 g) and NRH (308 ± 43 g), with intermediate values (but not significant differences) found for the other mutants. Overall shoot biomass was generally smaller in the clay loam (–29 %; P = 0.010) compared with the sandy loam. It should be noted that overall, the shoot biomass in 2018 at 61 d from sowing was approx. 10-fold that recorded in 2017 at 56 d from sowing (Table 1) as a result of the respective sowing dates. Indeed, seeds were sown a month later (i.e. 25 April) in 2018 compared with 2017 (i.e. 24 March), resulting in a different growth rate, with 56 d from sowing in 2017 corresponding to 22 May, while 61 d from sowing in 2018 corresponded to 25 June. Total root lengths did not differ significantly between genotypes in 2017; however, these were significantly different between soil textures (P = 0.045), being generally shorter in clay loam compared with the sandy loam (–45 %; Table 1). Although there was a significant effect of time on root length from 24 to 56 d from sowing in 2017 (P = 0.014), some plants did not exhibit longer roots at 56 d (i.e. NRH and SRH in clay loam; the WT and ‘Sassy’ in sandy loam soil; Table 1), which may be the result of weather conditions (i.e. low temperatures and scarce precipitation) between the two sampling times (Fig. 1). In 2018, total root lengths were shorter in the clay loam (–36 % overall; P < 0.001) and varied between genotypes (P < 0.001) only in the clay loam, with NRH displaying 37 and 48 % shorter total root lengths than ‘Sassy’ at 19 and 61 d growth, respectively (Table 1). The total number of root tips and forks did not differ significantly between genotypes in 2017 (Supplementary data Table S2), while the number of tips was generally less in the clay loam compared with the sandy loam in 2017 (–62 %; P = 0.018) and 2018 (–36 %; P < 0.001). During the latter year, the number of root tips and forks was significantly less in NRH (–56 and –57 %, respectively; P < 0.001) compared with the WT for plants grown in clay loam at 19 and 33 d growth.
Table 1.
Total root length and shoot dry mass of three root hair mutants (NRH = no root hair; BRH = bud root hair; SRH = short root hair) and the wild type (WT) in the ‘Optic’ background and in ‘Sassy’
| Days from sowing | Soil | Genotype | Total root length 2017 (cm) | Shoot dry mass 2017 (mg) | Total root length 2018 (cm) | Shoot dry mass 2018 (mg) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 (2017) and 19 (2018) | Sandy loam | NRH | 71.5 ± 8.6a | – | 60.5 ± 4.7a | 24.3 ± 1.5a | ||||
| BRH | 79.3 ± 14.5a | – | 66.6 ± 6.5a | 25.9 ± 2.2a | ||||||
| SRH | 66.9 ± 7.7a | – | 62.2 ± 3.4a | 22.0 ± 2.6a | ||||||
| WT | 98.7 ± 11.8a | – | 66.1 ± 4.0a | 26.0 ± 1.5a | ||||||
| ‘Sassy’ | 106.5 ± 8.7a | – | 78.3 ± 6.4a | 28.9 ± 1.0a | ||||||
| Clay loam | NRH | 60.7 ± 5.9a | – | 35.9 ± 3.6a | 13.3 ± 1.9a | |||||
| BRH | 50.5 ± 2.9a | – | 51.7 ± 4.0ab | 18.8 ± 2.2a | ||||||
| SRH | 49.4 ± 3.9a | – | 51.9 ± 1.6ab | 22.0 ± 2.6a | ||||||
| WT | 68.3 ± 7.3a | – | 49.1 ± 1.8ab | 18.3 ± 1.6a | ||||||
| ‘Sassy’ | 62.1 ± 3.7a | – | 56.9 ± 4.3b | 20.4 ± 1.3a | ||||||
| 33 (2018) | Sandy loam | NRH | – | – | 347.1 ± 43.5a | 182.0 ± 15.8a | ||||
| BRH | – | – | – | – | ||||||
| SRH | – | – | – | – | ||||||
| WT | – | – | 393.5 ± 51.7a | 216.3 ± 41.0a | ||||||
| ‘Sassy’ | – | – | 374.0 ± 57.4a | 213.9 ± 18.6a | ||||||
| Clay loam | NRH | – | – | 173.2 ± 22.2a | 95.5 ± 36.0a | |||||
| BRH | – | – | – | – | ||||||
| SRH | – | – | – | – | ||||||
| WT | – | – | 277.0 ± 21.8a | 152.5 ± 40.1a | ||||||
| ‘Sassy’ | – | – | 256.2 ± 27.5a | 157.3 ± 31.5a | ||||||
| 49 (2018) | Sandy loam | NRH | – | – | 439.2 ± 51.0a | 509.4 ± 56.6a | ||||
| BRH | – | – | 716.8 ± 40.9a | 919.4 ± 169.3ab | ||||||
| SRH | – | – | 428.0 ± 43.7a | 972.1 ± 125.4ab | ||||||
| WT | – | – | 567.6 ± 46.3a | 802.3 ± 88.3ab | ||||||
| ‘Sassy’ | – | – | 665.6 ± 58.6a | 1210.9 ± 31.1b | ||||||
| Clay loam | NRH | – | – | 265.6 ± 23.2ab | 308.4 ± 43.2a | |||||
| BRH | – | – | 185.8 ± 17.0a | 560.0 ± 171.0ab | ||||||
| SRH | – | – | 266.5 ± 18.3ab | 706.6 ± 226.2ab | ||||||
| WT | – | – | 307.9 ± 24.5ab | 791.5 ± 79.9b | ||||||
| ‘Sassy’ | – | – | 418.0 ± 35.7b | 826.3 ± 82.8b | ||||||
| 56 (2017) and 61 (2018) | Sandy loam | NRH | 280.3 ± 106.9a | 111.5 ± 18.0a | 398.4 ± 50.3a | 1857.1 ± 779.6a | ||||
| BRH | 263.4 ± 73.1a | 182.8 ± 25.1ab | – | – | ||||||
| SRH | 175.8 ± 66.4a | 176.0 ± 36.4ab | – | – | ||||||
| WT | 95.6 ± 19.8a | 252.5 ± 25.2b | 663.6 ± 99.0a | 1579.6 ± 300.1a | ||||||
| ‘Sassy’ | 99.1 ± 21.3a | 287.8 ± 48.5b | 735.8 ± 103.8a | 2088.3 ± 267.1a | ||||||
| Clay loam | NRH | 58.7 ± 2.1a | 123.0 ± 15.9a | 269.4 ± 33.8a | 1223.9 ± 122.6a | |||||
| BRH | 116.2 ± 22.9a | 111.8 ± 12.8a | – | – | ||||||
| SRH | 47.7 ± 7.6a | 150.8 ± 27.8a | – | – | ||||||
| WT | 128.9 ± 5.9a | 127.3 ± 9.6a | 396.0 ± 34.4ab | 1761.3 ± 76.7a | ||||||
| ‘Sassy’ | 103.9 ± 17.7a | 178.8 ± 67.7a | 514.2 ± 23.8b | 1863.6 ± 204.3a | ||||||
| REML | F | P | F | P | F | P | F | P | ||
| Genotype | 0.32 | 0.862 | 3.42 | 0.022 | 7.22 | <0.001 | 4.33 | 0.003 | ||
| Soil texture | 4.44 | 0.045 | 8.98 | 0.006 | 59.48 | <0.001 | 6.97 | 0.010 | ||
| Time | 6.84 | 0.014 | – | – | 64.61 | <0.001 | 189.96 | <0.001 | ||
| Genotype × Soil texture | 0.60 | 0.663 | 1.44 | 0.249 | 1.45 | 0.225 | 0.99 | 0.416 | ||
| Genotype × Time | 0.70 | 0.598 | – | – | 1.43 | 0.187 | 1.75 | 0.09 | ||
| Soil texture × Time | 1.70 | 0.202 | – | – | 5.67 | 0.001 | 1.26 | 0.291 | ||
| Genotype × Soil texture × Time | 1.11 | 0.370 | – | – | 0.69 | 0.701 | 0.75 | 0.644 |
Plants were grown in 2017 and 2018 at two locations with different soil textures: sandy loam and clay loam. Two sampling campaigns were done in 2017 at 24 and 56 d from sowing; four sampling campaigns were done in 2018 at 19, 33, 49 and 61 d from sowing. Data are the mean of four (2017) and eight replicates (2018), with differences between genotypes, soil textures and time of sampling established using REML for repeated measurements from which the F- and P-value data are derived. Significant parameters (P ≤ 0.05) are in bold. Identical superscript letters indicate no significant differences between genotypes as tested using one-way ANOVA followed by a post-hoc Tukey’s test. It should be noted that overall, the shoot biomass in 2018 (61 d from sowing) was approx. 10-fold of that recorded in 2017 (56 d from sowing) as a result of the respective sowing dates.
Effects of root hairs on soil water and phosphorus
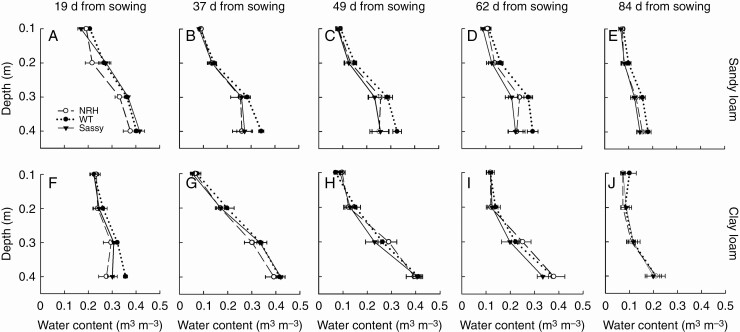
Soil water content was measured in 2018 at five time points, from 19 to 84 d from sowing (Fig. 6). At 19 d from sowing, this averaged 0.19 and 0.23 m3 m–3 in surface soil (10 cm) for sandy and clay loam, respectively, while at 40 cm depth this in turn averaged 0.40 and 0.31 m3 m–3. It dried substantially by 37 d plant growth, dropping to 0.09 and 0.06 m3 m–3 in sandy loam and clay loam, respectively, and was maintained below 0.12 m3 m–3 for the rest of the growing season in both soil textures, with no significant differences between these. In deeper soil (40 cm), significant differences (P < 0.001) were found between soil textures, with the clay loam exhibiting greater water content throughout the season (Fig. 6). There was no significant effect of the genotype on soil water content.
Fig. 6.
Soil water content measured in the field at five time points (19, 37, 49, 62 and 84 d from sowing) in 2018. Measurements are for sandy loam (A–E) and clay loam (F–J) soils, and in each experimental plot of the barley wild type (WT; ‘Optic’) and its hairless mutant (NRH), together with ‘Sassy’.
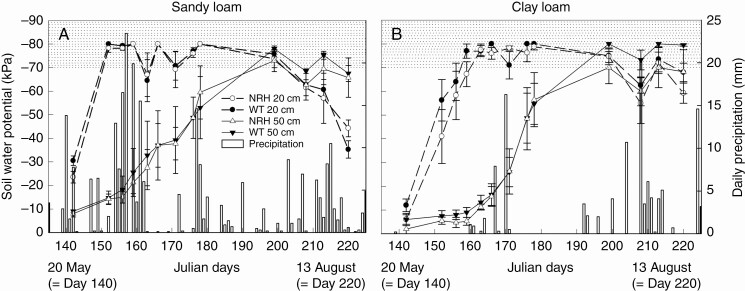
Soil water potential was measured to investigate plant water availability during the growing season (Fig. 7). Soil water potential at 20 cm depth decreased down to the tensiometer’s limit (less than –70 kPa) 37 d after sowing (1 June) in the sandy loam field, and 7 d later in the clay loam field. Soil water potential at 50 cm depth became less than –70 kPa by 84 d after sowing (18 July) in both soil textures (Fig. 7). There were no significant differences in soil water potential between the WT and its hairless mutant in both soil textures and at either soil depth.
Fig. 7.
Soil water potential at 20 and 50 cm depth measured from 22 May to 8 August in 2018. Measurements are for sandy loam (A) and clay loam (B) soils and experimental plots planted with the barley wild type (WT; ‘Optic’) and its hairless mutant (NRH). Data are the mean of four replicates, with error bars representing the s.e. The dotted area represents the tensiometers limit (less than –70 kPa). Also presented is the daily precipitation recorded at the experimental sites between 15 May and 13 August in 2017 and 2018.
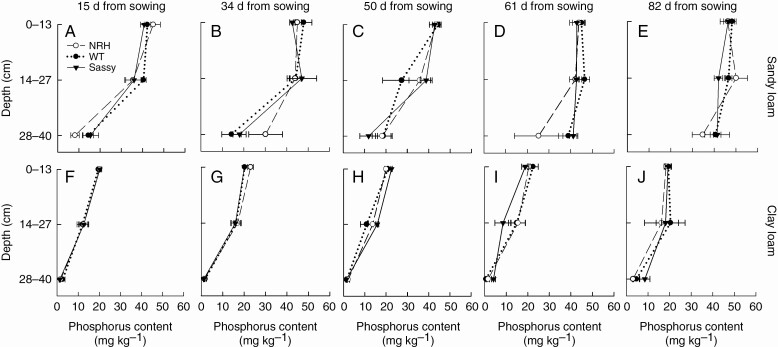
Olsen P differed significantly (P < 0.001) between soil textures and sampling depths (P < 0.001), such that the clay loam had an average 45, 64 and 90 % decrease in soil P content compared with the sandy loam in the top (0–13 cm), middle (14–27 cm) and bottom soil sampling depths (28–40 cm), respectively (Fig. 8). Specifically, during the growing season, in the sandy loam field, Olsen P averaged 44.4, 41.2 and 24.8 mg P kg–1 in the top, middle and bottom soil depths, respectively. In the clay loam field, Olsen P averaged 20.4, 14.7 and 2.5 mg P kg–1 in the top, middle and bottom soil section, respectively. In the sandy loam, compared with clay loam, there was a greater variability in soil P content in relation to the barley genotype. However, no significant difference was found in soil P content between samples collected from plots planted with different genotypes.
Fig. 8.
Soil phosphorus content measured in the field at five time points (15, 34, 50, 61 and 82 d from sowing) in 2018. Measurements are for sandy loam (A–E) and clay loam (F–J) soils and experimental plots planted with the barley wild type (WT; ‘Optic’) and its hairless mutant (NRH), together with ‘Sassy’. Data are the mean of four replicates, with error bars representing the s.e.
Effects of root hairs on plant performance under drought
Measurements of plant water status taken at 48, 62 and 85 d growth in 2018 are summarized in Table 2. In June 2018, similar values of Ψ pd and Ψ min were found between soil treatments and genotypes, averaging –0.43 and –1.26 MPa, respectively. During the following month, soil water availability decreased even in deep soil, as shown by measurements of soil water content (Fig. 6) and water potential (Fig. 7), leading to more negative leaf water potentials (Table 2). Indeed, in July 2018, Ψ min decreased below the permanent wilting point (–1.5 MPa), while limited soil water availability was confirmed by measurements of Ψ pd, which averaged –0.85 MPa in clay loam and –1.57 MPa in sandy loam. In these conditions, significant differences in Ψ min (P < 0.001) were found between soil treatments, with substantially lower values for sandy loam (Ψ min = –2.02 MPa) than clay loam (Ψ min = –1.60 MPa). More interestingly, Ψ min differed significantly (P = 0.021) between NRH (–1.76 MPa) and the WT (–1.43 MPa) grown in clay loam, with the mutant exhibiting greater water stress. However, no significant differences were found in sandy loam soil despite data showing the same trend. As expected, leaf ABA concentration increased with decreasing water availability during the growing season (Table 2). In July 2018, at the peak of water stress, leaf ABA concentration was significantly (P = 0.023) greater for NRH (394 ng g–1) than for the WT (250 ng g–1) grown in clay loam soil, in agreement with leaf water potential measurements. When plants were grown in sandy loam soil, we observed the same trend between genotypes in ABA concentrations, but no significant differences. The same sampling highlighted a soil treatment effect, with plants grown in sandy loam having larger ABA concentrations (+140%; P < 0.001) than those grown in clay loam.
Table 2.
Pre-dawn and minimum leaf water potential, leaf abscisic acid concentration (ABA), chlorophyll concentration (CHL) and Fv/Fm of the barley wild type (WT; ‘Optic’) and its hairless mutant (NRH) as well as ‘Sassy’, grown in the field in 2018 at two locations with different soil textures: sandy loam and clay loam
| Days from sowing | Soil | Genotype | Pre-dawn water potential (MPa) | Minimum water potential (MPa) | ABA (ng g–1 DW) | CHL (µmol m-2) | Fv/Fm | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 48 | Sandy loam | NRH | –0.52 ± 0.03a | –1.05 ± 0.09a | 193.21 ± 30.00a | 371.79 ± 3.76ab | 0.80 ± 0.00a | |||||
| WT | –0.40 ± 0.05a | –1.03 ± 0.08a | 193.84 ± 19.31a | 408.60 ± 15.61a | 0.81 ± 0.00b | |||||||
| ‘Sassy’ | –0.54 ± 0.11a | –1.20 ± 0.17a | 192.51 ± 19.54a | 358.96 ± 11.64b | 0.80 ± 0.00a | |||||||
| Clay loam | NRH | –0.66 ± 0.05a | –1.16 ± 0.07a | 161.46 ± 29.07a | 406.16 ± 10.99a | 0.76 ± 0.01a | ||||||
| WT | –0.60 ± 0.02a | –1.09 ± 0.01a | 158.43 ± 21.78a | 450.91 ± 21.52a | 0.69 ± 0.03a | |||||||
| ‘Sassy’ | –0.49 ± 0.09a | –1.13 ± 0.04a | 119.11 ± 14.68a | 403.94 ± 12.90a | 0.74 ± 0.01a | |||||||
| 62 | Sandy loam | NRH | –0.45 ± 0.09a | –1.40 ± 0.07a | 163.56 ± 19.31a | 428.21 ± 32.93ab | 0.64 ± 0.05a | |||||
| WT | –0.31 ± 0.02a | –1.32 ± 0.08a | 117.70 ± 20.69a | 481.27 ± 19.48a | 0.66 ± 0.04a | |||||||
| ‘Sassy’ | –0.29 ± 0.07a | –1.40 ± 0.06a | 248.74 ± 58.77a | 398.53 ± 26.76b | 0.68 ± 0.06a | |||||||
| Clay loam | NRH | –0.41 ± 0.11a | –1.50 ± 0.09a | 176.25 ± 31.28a | 524.88 ± 21.41a | 0.66 ± 0.02a | ||||||
| WT | –0.26 ± 0.06a | –1.47 ± 0.05a | 144.47 ± 16.31a | 557.25 ± 27.92a | 0.65 ± 0.06a | |||||||
| ‘Sassy’ | –0.27 ± 0.01a | –1.39 ± 0.12a | 199.44 ± 39.65a | 519.43 ± 13.05a | 0.64 ± 0.04a | |||||||
| 85 | Sandy loam | NRH | –1.53 ± 0.04a | –2.13 ± 0.08a | 958.84 ± 173.13a | 390.32 ± 12.54a | 0.78 ± 0.01a | |||||
| WT | –1.64 ± 0.10a | –1.92 ± 0.15a | 569.97 ± 85.47a | 403.53 ± 20.16a | 0.76 ± 0.01a | |||||||
| ‘Sassy’ | –1.65 ± 0.05a | –1.99 ± 0.19a | 822.59 ± 114.03a | 409.49 ± 28.76a | 0.75 ± 0.00a | |||||||
| Clay loam | NRH | –0.91 ± 0.17a | –1.76 ± 0.09a | 393.82 ± 41.55a | 505.94 ± 62.52ab | 0.68 ± 0.04a | ||||||
| WT | –0.77 ± 0.12a | –1.43 ± 0.11b | 250.40 ± 39.92b | 611.89 ± 37.18a | 0.76 ± 0.02a | |||||||
| ‘Sassy’ | –0.88 ± 0.03a | –1.51 ± 0.08ab | 333.13 ± 44.00ab | 450.79 ± 18.51b | 0.70 ± 0.04a | |||||||
| REML | F | P | F | P | F | P | F | P | F | P | ||
| Genotype | 1.22 | 0.068 | 2.21 | 0.029 | 2.09 | 0.152 | 10.60 | <0.001 | 0.02 | 0.981 | ||
| Soil texture | 26.59 | <0.001 | 5.65 | 0.003 | 20.68 | <0.001 | 56.63 | <0.001 | 13.72 | <0.001 | ||
| Time | 311.83 | <0.001 | 70.04 | <0.001 | 80.19 | <0.001 | 17.33 | <0.001 | 17.56 | <0.001 | ||
| Genotype × Soil texture | 0.49 | 0.299 | 0.71 | 0.192 | 0.73 | 0.493 | 1.03 | 0.281 | 0.09 | 0.918 | ||
| Genotype × Time | 1.21 | 0.170 | 1.19 | 0.096 | 2.57 | 0.044 | 0.18 | 0.892 | 0.55 | 0.527 | ||
| Soil texture × Time | 73.86 | <0.001 | 12.78 | <0.001 | 8.27 | <0.001 | 3.91 | 0.003 | 1.65 | 0.101 | ||
| Genotype × Soil texture × Time | 1.50 | 0.070 | 0.14 | 0.959 | 0.85 | 0.545 | 2.36 | 0.007 | 1.17 | 0.174 |
Samples were taken 48, 62 and 85 d from sowing. Data are the mean of 16 replicates, with differences between genotypes, soil textures and time of sampling established using REML for repeated measurements from which the F- and P-value data are derived. Significant parameters (P ≤ 0.05) are in bold. Identical superscript letters indicate no significant differences between genotypes as tested using one-way ANOVA followed by a post-hoc Tukey’s test.
Table 2 reports significant differences between genotypes and soil textures in terms of photosynthetic efficiency. The WT grown in sandy loam at 48 d from sowing had a significantly (P = 0.01) greater Fv/Fm compared with its hairless mutant; however, for both genotypes, values were in the optimum range (0.79–0.84; Maxwell and Johnson, 2000). Progressively smaller values were recorded during the following samplings. Overall, plants grown in sandy loam displayed greater values of Fv/Fm, indicating better photosynthetic efficiency, compared with those grown in clay loam at 48 d (P < 0.001) and 85 d (P = 0.014) growth. The CHL showed marked differences between plants grown in different soil textures as this was significantly greater in the clay loam (+21 %; P < 0.001) compared with the sandy loam. No significant differences in terms of CHL were found between the WT and its hairless mutant, while it varied significantly (P = 0.001) between the WT (‘Optic’) and the other elite cultivar ‘Sassy’.
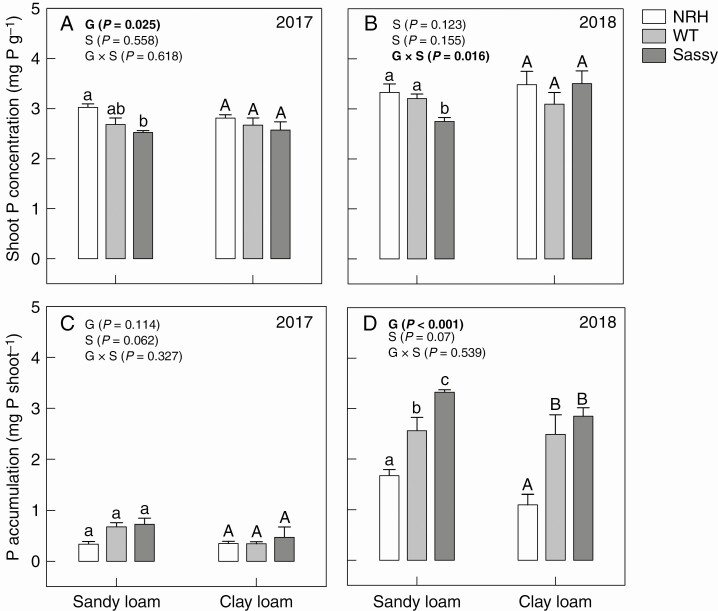
Significant differences were found between genotypes with respect to shoot P concentrations in sandy loam soil in both 2017 (P = 0.033) and 2018 (P < 0.001), with NRH having concentrations that were 20 and 21 % greater than those of Sassy in 2017 and 2018, respectively (Fig. 9). Calculations using shoot P concentrations and total shoot biomass to produce shoot P accumulation data resulted in significant differences (P < 0.001) between genotypes only in 2018 (Fig. 9). It should be noted that overall shoot P accumulation in 2017 was approx. 5-fold less than in 2018 (Fig. 9C, D) as a result of differences in shoot biomass (Table 1). Under water deficit conditions in sandy loam soil (Fig. 9D), there was a 53 % increase (P < 0.001) in average shoot P accumulation by the WT (2.56 mg P per shoot) compared with NRH (1.67 mg P per shoot). In clay loam soil, shoot P accumulation in the WT (2.49 mg P per shoot) was over twice that in NRH (1.10 mg P per shoot).
Fig. 9.
Variation in shoot P concentration (mg P g–1; A, B) and shoot P accumulation (mg P per shoot; C, D) of contrasting root hair genotypes grown in the field in sandy loam and clay loam soils for two subsequent years: 2017 and 2018. Data are the mean of four (2017) and eight replicates (2018), with error bars representing the s.e. Differences between genotypes and soil textures were established using two-way ANOVA, P-values are reported and significant (P ≤ 0.05) parameters are in bold, with ‘G’ representing genotype, ‘S’ representing soil texture and ‘G × S’ representing the interaction of genotype and soil texture. Identical letters indicate no significant differences as tested using one-way ANOVA followed by a post-hoc Tukey’s test. NRH represents the no root hair genotype and WT the wild type (‘Optic’), together with ‘Sassy’.
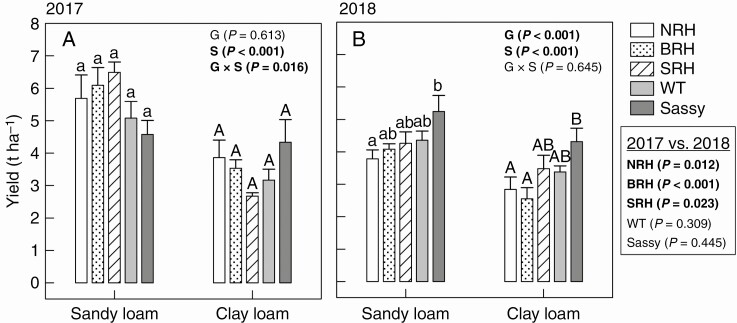
Grain weights obtained from harvesting in August 2017 and September 2018 were used as a measure of crop yield (Fig. 10). This measurement produced significant differences (P < 0.001) between the soil treatments in both experimental years. Plants grown in sandy loam generally produced greater yield than those grown in clay loam (+66 % in 2017; +33 % in 2018). However, plants grown in clay loam exhibited a smaller yield drop between years (i.e. 6 % in clay loam vs. 22 % in sandy loam). Yield responses to soil types and years (i.e. water availability) varied greatly between genotypes. While the yield of NRH significantly (P = 0.012) decreased from 2017 to 2018 in both clay (–26 %) and sandy (–33 %) loam soils, no significant differences were found between years in the yield of the WT. No significant differences were found between genotypes in 2017, while during the following year we recorded better yield (P < 0.001) for ‘Sassy’ (5.25 and 4.31 t ha–1 in sandy (+39 %) and clay loam (+55 %), respectively) compared with the hairless genotype NRH (3.79 and 2.84 t ha–1 in sandy and clay loam, respectively) in both soil textures.
Fig. 10.
Variation in yield (t ha–1), calculated from grain weight, of contrasting root hair genotypes grown in the field in sandy loam and clay loam soils for two subsequent years: 2017 (A) and 2018 (B). Data are the mean of four replicates, with error bars representing the s.e. Differences between genotypes and soil textures were established using two-way ANOVA, P-values are reported and significant (P ≤ 0.05) parameters are in bold, with ‘G’ representing genotype, ‘S’ representing soil texture and ‘G × S’ representing the interaction of genotype and soil texture. Identical letters indicate no significant differences as tested using one-way ANOVA followed by a post-hoc Tukey’s test. Cross comparisons between experimental years were established using two-way ANOVA, P-values are reported in the box, with significant (P ≤ 0.05) parameters in bold. NRH represents the no root hair genotype; BRH, the bud root hair genotype; SRH, the short root hair genotype; and WT, the wild type all in the ‘Optic’ background, together with ‘Sassy’.
DISCUSSION
While root hairs did not confer a notable advantage to barley under optimal conditions (i.e. adequate water availability in 2017), under drought (i.e. the 2018 growing season) root hairs enhanced plant water status, P accumulation and yield (Table 2; Figs 9 and 10). Importantly, the presence of root hairs did not decrease yield under optimal conditions, suggesting that selecting for beneficial root hair traits can enhance yield stability without diminishing yield potential, overcoming the breeder’s dilemma of trying to simultaneously enhance both productivity and resilience. It is therefore important to understand the physiological mechanisms by which root hairs can enhance yields under sub-optimal conditions. To our knowledge, the present findings provide the first evidence of the effect of root hairs upon drought in open field conditions (i.e. a real agricultural system). Therefore, along with the well-recognized role for P uptake, maintenance or enhancement of root hairs can represent a key trait for breeding the next generation of crops for improved drought tolerance in relation to climate change.
Dynamic adjustment of root traits under field conditions
Root hair length in the field differed notably between clay and sandy loam soils during both growing seasons. Root hair length was significantly longer (+35 % in 2017 and +46 % in 2018) in clay loam considering all genotypes, in contrast to data from Haling et al. (2014), who found shorter root hairs in soil with smaller particles. While that study grew barley genotypes in pots filled with artificial mixtures of different sand fractions, our plants were grown in natural soils with different textures in the field, where soil texture, structure (e.g. aggregates and macropores), hydrology and climate might all affect root hair growth. Average root hair length (0–0.13 mm for NRH and 0.45–0.71 mm for the WT; Fig. 2), recorded during the first sampling in 2017 and 2018 (i.e. 24 and 19 d from sowing), was only slightly shorter than that in Brown et al. (2012), where the same genotypes were grown in a different sandy soil in a controlled environment for 7 d. However, root hairs grew longer in 2017 (e.g. in the WT from 0.51 mm at 24 d from sowing to 0.62 mm at 56 d from sowing), while root hair length decreased progressively in 2018 (e.g. in the WT from 0.56 mm at 19 d from sowing to 0.35 mm at 61 d from sowing; Fig. 2). We measured root hair length from older, field-grown, plants compared with previous laboratory studies (Brown et al., 2012; Haling et al., 2014; Delhaize et al., 2015), which may have missed the plastic adjustment of root hair length upon changing environmental conditions. Similarly, root hair density showed remarkable changes during both growing seasons, overshadowing initial differences between genotypes (Fig. 3). For instance, root hair density in BRH increased from 0–7 root hairs mm–1 (24 d after sowing in 2017) to 7–15 and 15–35 root hairs mm–1 for most roots (56 d after sowing; Fig. 3).
Root hair length was positively correlated with rhizosheath weight during both growing seasons (Fig. 5), as in controlled environments (Brown et al., 2012, 2017; Delhaize et al., 2012; George et al., 2014; Adu et al., 2017). This correlation is based on field data and highlights trait robustness upon variable environmental conditions, although the strength (i.e. coefficient of determination) of the correlation changed between growing seasons (2017 vs. 2018), as well as during the same growing season (i.e. the correlation weakened as plants aged; Fig. 5). During the wet summer of 2017, the slope of the correlation did not change as plants aged, but it decreased notably during the dry growing season of 2018 as both root hair length (Fig. 2) and rhizosheath weight (Figs 4 and 5) changed. Both root hair traits and mucilage traits determine rhizosheath weight (Akhtar et al., 2018; Galloway et al., 2018), and their relative importance may explain the shift in the relationship between root hair length and rhizosheath weight. Differences in the soil moisture dynamics (e.g. drying/re-wetting) down the soil profile and during the growing season may have also impacted rhizosheath weight, as shown by Watt et al. (1994). A similar decrease in rhizosheath weight during the growing season was reported by George et al. (2014) for the WT and SRH grown in a sandy loam field. A diminished rhizosheath during the exceptionally dry summer of 2018 seems to contradict previous controlled-environment studies (Haling et al., 2014; Liu et al., 2019), where soil drying enhanced rhizosheath mass. The dynamic change in the relationship between root hair length and rhizosheath observed here can explain the reported variability in the strength of this relationship between laboratory-based studies.
Root hairs improve plant water status under drought
The summer of 2018 ranked amongst the hottest and driest June–July period in Scottish records dating back to 1910 (Parry, 2018), providing an ideal opportunity to investigate the eco-physiological responses of the tested genotypes under drought conditions. Early in the growing season, Ψ pd did not differ significantly between genotypes and soil types. This absence of severe water stress agreed with the less negative water potentials found in deeper soil (Fig. 7), as plants tend to establish equilibrium overnight with wetter zones (e.g. deeper soil layer in Figs 6 and 7) of bulk soil. By mid-July, soil water potential (less than –70 kPa) decreased even in the deeper soil (50 cm depth; Fig. 7), and Ψ pd dropped to –0.85 and –1.57 MPa in clay and sandy loam, respectively, consistent with drier soil at depth in the sandy loam field than in the clay loam field (Fig. 6). The Ψ min highlighted a consistent trend, with significant differences between soils in mid-July (Table 2; 85 d from sowing). Under water stress conditions (Fig. 7; Table 2), Ψ min measurements also highlighted consistent genotypic rankings for both soil types, with the presence of root hairs significantly enhancing plant water status in clay loam soil. This novel field evidence is consistent with controlled-environment conditions where leaf water potential of the hairless mutant decreased more rapidly at high transpiration rates than the that of the WT (Carminati et al., 2017). Indeed, root hairs facilitate the uptake of water by substantially reducing the decline in water potential at the interface between root and soil in rapidly transpiring plants, given the greater water carrying capacity of root hairs and the smaller tortuosity of the water path with respect to unsaturated soil (Segal et al., 2008; Carminati et al., 2017). Thus, decreased Ψ min of the root hairless mutant was consistent with its higher leaf ABA concentrations, suggesting a greater degree of stomatal closure and hence possibly more limited photosynthesis.
Influence of root hairs on grain yield stability under drought
The remarkably different climate conditions between growing seasons (Fig. 1; Supplementary data Fig. S2) offered the opportunity to test the overall field performance of barley genotypes differing in root hair abundance. Grain yield decreased by an average of 14 % in 2018 compared with the previous year, as did total cereal yields in Scotland (i.e. –9 %), including spring barley (i.e. –6 %, from 5.9 t ha–1 in 2017 to 5.5 t ha–1 in 2018; The Scottish Government 2017, 2018), as a result of poor weather conditions. Grain yield and shoot biomass differed significantly between the clay and sandy loam fields, with an average decrease in clay loam by 37 % in 2017 and 24 % in 2018 (Table 1; Fig. 10). More interestingly, yield responses to soil types and years (i.e. water availability) varied greatly between genotypes. While yield of NRH decreased significantly from 2017 to 2018 in both soils, yield of WT plants grown in clay loam soil increased by 7 % over the same period. When barley was grown in clay loam soil, the presence of root hairs significantly affected plant water status (23 % drop in Ψ min and 58 % increase in ABA for NRH compared with the WT; Table 2) and P accumulation (+126 % for the WT compared with NRH), maintaining a stable grain yield during exceptional climate conditions such as the drought in 2018. Although Scotland is generally considered a wet country, a large interannual variability of precipitation is predicted for the next decades (Brown et al., 2008; ASC, 2016) which may cause drought stress in crops unless more resilient genotypes are developed (e.g. selection of new crops based on root traits). We may expect root hairs to contribute to drought tolerance in other crops too, but further investigation is needed as root hair traits vary greatly between species (Brown et al., 2017) and there is a lack of field investigations looking at their role under water deficit conditions.
The role of root hairs in P accumulation has been associated with barley yields in the field (Gahoonia and Nielsen, 2004), but this response varies with environmental conditions. While root hairs may have a negligible effect on plant performance (Gahoonia and Nielsen, 2004) or even represent a cost (Brown et al., 2012; George et al., 2014) when P and water availability are optimal, root hairs could have a key role in maintaining yield stability if P and water are limiting. We found that the presence and abundance of root hairs is critical for stress tolerance, supporting laboratory data by Brown et al. (2012) on the biomass accumulation of barley genotypes upon combined P and water deficiency. Our data demonstrate that all genotypes achieved adequate P nutrition (Fig. 9) under both soil conditions, regardless of the prevailing weather conditions that year, due to the relatively large content of available P in the surface of both soils. Field studies comparing the impact of drought conditions on root hair genotypes in P-limited soils are needed to explore this more fully.
In summary, root hair traits were important in real agricultural conditions to maintain plant water status and P accumulation when soil water availability was limiting, with potential implications for maintaining a stable grain yield under extreme precipitation patterns (e.g. prolonged summer drought). However, the genotypic differences in root hair length and abundance as well as plant performance varied in relation to plant age and soil texture, which need to be considered in future work assessing the role of root traits. Furthermore, the effects of root hairs on soil physical and hydrological properties in the field should be evaluated in relation to their potential benefits for both crops and soils.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: location of two experimental fields and schematic representation of the experimental lots. Figure S2: monthly precipitation from March to September. Table S1: list of examples of studies investigating root hairs that were conducted under controlled environmental conditions. Table S2: total number of root tips and forks and root diameter of three root hair mutants and the wild type all in the ‘Optic’ background, together with ‘Sassy’.
ACKNOWLEDGEMENTS
We thank Mr Richard Keith, Mr Christopher Warden and the field staff of The James Hutton Institute for setting up, managing and maintaining the field trials.
FUNDING
Aberdeen University and James Hutton staff were funded by BBSRC BB/J00868/1 and Dundee University staff by BBSRC BB/L025825/1. Contributions by Southampton University were funded by BBSRC SARISA BB/L025620/1. The James Hutton Institute receives financial support from the Rural & Environment Science & Analytical Services Division of the Scottish Government, and T.R. is also funded by EPSRC EP/M020355/1, ERC 646809DIMR, BBSRC SARIC BB/P004180/1 and NERC NE/L00237/1. I.C.D. is grateful for the support of a Newton Advanced Fellowship (NA160430).
LITERATURE CITED
- Adu MO, Asare PA, Yawson DO, et al. . 2017. Quantifying variations in rhizosheath and root system phenotypes of landraces and improved varieties of juvenile maize. Rhizosphere 3: 29–39. [Google Scholar]
- Ahmadi K, Zarebanadkouki M, Ahmed MA, et al. . 2017. Rhizosphere engineering: innovative improvement of root environment. Rhizosphere 3: 176–184. [Google Scholar]
- Akhtar J, Galloway AF, Nikolopoulos G, Field KJ, Knox P. 2018. A quantitative method for the high throughput screening for the soil adhesion properties of plant and microbial polysaccharides and exudates. Plant and Soil 428: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASC . 2016. UK Climate Change Risk Assessment 2017 Evidence Report. Summary for Scotland. London: Committee on Climate Change. [Google Scholar]
- Bailey C, Scholes M. 1997. Rhizosheath occurrence in South African grasses. South African Journal of Botany 63: 484–490. [Google Scholar]
- Basirat M, Mousavi SM, Abbaszadeh S, Ebrahimi M, Zarebanadkouki M. 2019. The rhizosheath: a potential root trait helping plants to tolerate drought stress. Plant and Soil 445: 565–575. [Google Scholar]
- Bates TR, Lynch JP. 2000. The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. American Journal of Botany 87: 964–970. [PubMed] [Google Scholar]
- Benard P, Kroener E, Vontobel P, Kaestner A, Carminati A. 2016. Water percolation through the root–soil interface. Advances in Water Resources 95: 190–198. [Google Scholar]
- Bengough AG, Loades K, McKenzie BM. 2016. Root hairs aid soil penetration by anchoring the root surface to pore walls. Journal of Experimental Botany 67: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Chapin FS, Mooney HA. 1985. Resource limitation in plants – an economic analogy. Annual Review of Ecology and Systematics 16: 363–392. [Google Scholar]
- Brown I, Towers W, Rivington M. 2008. Influence of climate change on agricultural land-use potential: adapting and updating the land capability system for Scotland. Climate Research 37: 43–57. [Google Scholar]
- Brown LK, George TS, Thompson JA, et al. . 2012. What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley (Hordeum vulgare)? Annals of Botany 110: 319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LK, George TS, Dupuy LX, White PJ. 2013. A conceptual model of root hair ideotypes for future agricultural environments: what combination of traits should be targeted to cope with limited P availability? Annals of Botany 112: 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LK, George TS, Neugebauer K, White PJ. 2017. The rhizosheath – a potential trait for future agricultural sustainability occurs in orders throughout the angiosperms. Plant and Soil 418: 115–128. [Google Scholar]
- Carminati A, Passioura JB, Zarebanadkouki M, et al. . 2017. Root hairs enable high transpiration rates in drying soils. New Phytologist 216: 771–781. [DOI] [PubMed] [Google Scholar]
- Clarkson DT. 1991. Root structure and sites of ion uptake. In: Eshel A, Beeckaman T, eds. Plant roots: the hidden half. Taylor & Francis, 417–453. [Google Scholar]
- Delhaize E, James RA, Ryan PR. 2012. Aluminium tolerance of root hairs underlies genotypic differences in rhizosheath size of wheat (Triticum aestivum) grown on acid soil. New Phytologist 195: 609–619. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Rathjen TM, Cavanagh CR. 2015. The genetics of rhizosheath size in a multiparent mapping population of wheat. Journal of Experimental Botany 66: 4527–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer HJ. 1937. A quantitative study of the roots and root hairs of a winter rye plant (Secale cereale). American Journal of Botany 24: 417–420. [Google Scholar]
- Dodd IC, Diatloff E. 2016. Enhanced root growth of the brb (bald root barley) mutant in drying soil allows similar shoot physiological responses to soil water deficit as wild-type plants. Functional Plant Biology 43: 199–206. [DOI] [PubMed] [Google Scholar]
- Dolan L. 2001. The role of ethylene in root hair growth in Arabidopsis. Journal of Plant Nutrition and Soil Science 164: 141–145. [Google Scholar]
- Gahoonia ST, Nielsen NE. 2004. Root traits as tools for creating phosphorus efficient crop varieties. Plant and Soil 260: 47–57. [Google Scholar]
- Gahoonia TS, Care D, Nielsen NE. 1997. Root hairs and phosphorus acquisition of wheat and barley cultivars. Plant and Soil 191: 181–188. [Google Scholar]
- Gahoonia TS, Nielsen NE, Joshi PA, Jahoor A. 2001. A root hairless barley mutant for elucidating genetic of root hairs and phosphorus uptake. Plant and Soil 235: 211–219. [Google Scholar]
- Galloway AF, Pedersen MJ, Merry B, et al. . 2018. Xyloglucan is released by plants and promotes soil particle aggregation. New Phytologist 217: 1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TS, Brown LK, Ramsay L, et al. . 2014. Understanding the genetic control and physiological traits associated with rhizosheath production by barley (Hordeum vulgare). New Phytologist 203: 195–205. [DOI] [PubMed] [Google Scholar]
- George TS, Brown LK, Bengough AG. 2020. Advances in understanding plant root hairs in relation to nutrient acquisition and crop root function. In: Gregory PJ, ed. Understanding and improving crop root function. Burleigh Dodds Science Publishing Ltd. [Google Scholar]
- Haling RE, Richardson AE, Culvenor RA, Lambers H, Simpson RJ. 2010. Root morphology, root-hair development and rhizosheath formation on perennial grass seedlings is influenced by soil acidity. Plant and Soil 335: 457–468. [Google Scholar]
- Haling RE, Brown LK, Bengough AG, et al. . 2013. Root hairs improve root penetration, root–soil contact, and phosphorus acquisition in soils of different strength. Journal of Experimental Botany 64: 3711–3721. [DOI] [PubMed] [Google Scholar]
- Haling RE, Brown LK, Bengough AG, et al. . 2014. Root hair length and rhizosheath mass depend on soil porosity, strength and water content in barley genotypes. Planta 239: 643–651. [DOI] [PubMed] [Google Scholar]
- Holz M, Zarebanadkouki M, Kuzyakov Y, Pausch J, Carminati A. 2018. Root hairs increase rhizosphere extension and carbon input to soil. Annals of Botany 121: 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving GCJ, McLaughlin MJ. 1990. A rapid and simple field test for phosphorus in Olsen and Bray No. 1 extracts of soil. Communications in Soil Science and Plant Analysis 21: 2245–2255. [Google Scholar]
- Jungk A. 2001. Root hairs and the acquisition of plant nutrients from soil. Journal of Plant Nutrition and Soil Science 164: 121–129. [Google Scholar]
- Koebernick N, Daly KR, Keyes SD, et al. . 2017. High-resolution synchrotron imaging shows that root hairs influence rhizosphere soil structure formation. New Phytologist 216: 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebernick N, Daly KR, Keyes SD, et al. . 2019. Imaging microstructure of the barley rhizosphere: particle packing and root hair influences. New Phytologist 221: 1878–1889. [DOI] [PubMed] [Google Scholar]
- Kole C, Muthamilarasan M, Henry R, et al. . 2015. Application of genomics-assisted breeding for generation of climate resilient crops: progress and prospects. Frontiers in Plant Science 6: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TY, Ye N, Song T, et al. . 2019. Rhizosheath formation and involvement in foxtail millet (Setaria italica) root growth under drought stress. Journal of Integrative Plant Biology 61: 449–462. [DOI] [PubMed] [Google Scholar]
- Lynch JP. 2007. Roots of the second green revolution. Australian Journal of Botany 55: 493–512. [Google Scholar]
- Maxwell K, Johnson GN. 2000. Chlorophyll fluorescence—a practical guide. Journal of Experimental Botany 51: 659–668. [DOI] [PubMed] [Google Scholar]
- Narang RA, Bruene A, Altmann T. 2000. Analysis of phosphate acquisition efficiency in different Arabidopsis accessions. Plant Physiology 124: 1786–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveed M, Brown LK, Raffan AC, et al. . 2018. Rhizosphere-scale quantification of hydraulic and mechanical properties of soil impacted by root and seed exudates. Vadose Zone Journal 17: 1–12. [Google Scholar]
- Nestler J, Wissuwa M. 2016. Superior root hair formation confers root efficiency in some, but not all, rice genotypes upon P deficiency. Frontiers in Plant Science 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler J, Keyes SD, Wissuwa M. 2016. Root hair formation in rice (Oryza sativa L.) differs between root types and is altered in artificial growth conditions. Journal of Experimental Botany 67: 3699–3708. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Sommers LE. 1982. Phosphorus. In: Page AL, Miller RH, Keeney DR, eds. Methods of soil analysis. Madison, WI: American Society of Agronomy, 403–427. [Google Scholar]
- Parry C, Blonquist JM Jr, Bugbee B. 2014. In situ measurement of leaf chlorophyll concentration: analysis of the optical/absolute relationship. Plant, Cell & Environment 37: 2508–2520. [DOI] [PubMed] [Google Scholar]
- Parry S. 2018. UK Hydrological Status Update – December 2018.https://www.ceh.ac.uk/news-and-media/blogs/uk-hydrological-status-update-december-2018. (17 June 2020).
- Quarrie SA, Whitford PN, Appleford NE, et al. . 1988. A monoclonal antibody to (S)-abscisic acid: its characterisation and use in a radioimmunoassay for measuring abscisic acid in crude extracts of cereal and lupin leaves. Planta 173: 330–339. [DOI] [PubMed] [Google Scholar]
- Robertson-Albertyn S, Alegria Terrazas R, Balbirnie K, et al. . 2017. Root hair mutations displace the barley rhizosphere microbiota. Frontiers in Plant Science 8: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhm M, Werner D. 1987. Isolation of root hairs from seedlings of Pisum sativum. Identification of root hair specific proteins by in situ labeling. Physiologia Plantarum 69: 129–136. [Google Scholar]
- Ruiz S, Koebernick N, Duncan S, et al. . 2020. Significance of root hairs at the field scale – modelling root water and phosphorus uptake under different field conditions. Plant and Soil 447: 281–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal E, Kushnir T, Mualem Y, Shani U. 2008. Water uptake and hydraulics of the root hair rhizosphere. Vadose Zone Journal 7: 1027–1034. [Google Scholar]
- Tanaka N, Kato M, Tomioka R, et al. . 2014. Characteristics of a root hair-less line of Arabidopsis thaliana under physiological stresses. Journal of Experimental Botany 65: 1497–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Scottish Government. 2017. Final estimates of the cereal and oilseed rape harvest 2017. Edinburgh. [Google Scholar]
- The Scottish Government. 2018. Cereal and oilseed rape harvest 2018 final estimates. Edinburgh. [Google Scholar]
- Tottman DR. 1987. The decimal code for the growth stages of cereals, with illustrations. Annals of Applied Biology 110: 441–454. [Google Scholar]
- Watt M, McCully ME, Canny MJ. 1994. Formation and stabilization of rhizosheaths of Zea mays L. (effect of soil water content). Plant Physiology 106: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, George TS, Gregory PJ, Bengough AG, Hallett PD, McKenzie BM. 2013. Matching roots to their environment. Annals of Botany 112: 207–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.