Abstract
Objectives:
In this study, we examined whether visual exposure to the heated tobacco product (HTP) IQOS, which was authorized for sale by the US Food and Drug Administration in 2019, acts as a cue to increase cigarette craving and smoking behavior among smokers.
Methods:
Young adult smokers (N = 105) were randomly assigned to view a video depicting use of either IQOS or bottled water. Main outcomes were changes in cigarette and e-cigarette desire and latency to smoke between the groups. We also examined participants’ attitudes about the actors using IQOS and drinking water in the videos.
Results:
Exposure to the use of IQOS acutely increased observers’ ratings of smoking urge and desire for a cigarette and an e-cigarette. The IQOS cue, compared with the water cue, also produced a marginally significant shorter latency to smoke. Participants perceived actors as less likeable and friendly when using IQOS than when drinking water.
Conclusions:
Results showed that exposure to IQOS produced smoking urge and behavior in young adult smokers, implicating IQOS use as a smoking and vaping cue. As HTPs gain popularity, product impact on passive observers should be included in their risk-benefit profile.
Keywords: heated tobacco products, electronic nicotine delivery systems (ENDS), IQOS, cue reactivity, heat-not-burn
Electronic nicotine delivery systems (ENDS) have continued to rise in both popularity and variety since competing brands were introduced to western markets in 2010.1–3 The prevalence of ENDS, namely e-cigarettes, varies by country and age group. For example, approximately 3.2% of adults in the United States (US), including 7.6% of young adults (age 18-24) and 4.2% of adults aged 25-44, report current use of e-cigarettes with rates substantially increasing globally during 2014-2018.4,5 Heated tobacco products (HTPs) are similar to ENDS, yet they differ from e-cigarettes in several important ways. First, HTPs use a battery-powered system that heats tobacco leaf to produce a nicotine-containing aerosol, whereas e-cigarettes aerosolize a liquid that may or may not include nicotine.6 Second, HTPs use heat-pressed tobacco sticks resembling short cigarettes instead of the e-liquids or pods used in vape products. Third, current generation HTPs are relatively new products compared to e-cigarettes as the HTP global market leader IQOS7 was first introduced to the market in Japan in 2014. However, similar to e-cigarettes, HTPs have seen rapid adoption and are available in over 50 countries worldwide and have been approved for sale in the US since 2019.
Beyond differences between HTPs and ENDS in product constituents and availability across certain markets, they also share similarities, particularly in their resemblance to traditional cigarette smoking in the hand-to-mouth motions involved in inhalation and exhalation behavior and their visible, exhaled aerosol that mimics combustion. This may be important as stimuli paired with smoking have been shown to serve as conditioned cues and evoke subjective and objective responses in smokers.8–10 As e-cigarette use resembles smoking, our group and others have found that exposure to vaping products not only elicits interest in ENDS products11–13 and desire to vape,14–17 but also cross-generalizes as a smoking cue and increases smoking desire14–19 similar to that produced by a cigarette cue. To our knowledge, as HTPs are relatively novel, no studies have examined whether they act as a vaping or smoking cue.
As in other countries, an accelerated proliferation of HTPs is likely in the US, particularly among young adults, as their point-of-sale, design, and marketing tactics may specifically appeal to this demographic.20 In Japan, HTP use is higher in smokers aged 20-39 than in middle-aged or older smokers.21 Currently, few people in the US have been exposed to the use of HTPs since there has not yet been widespread adoption due to its limited availability in select cities; as such, this presents a unique opportunity for examining their cue salience in young adult smokers with minimal confounding influence of prior exposure. Thus, we conducted a randomized laboratory study to examine visual exposure to the use of the HTP IQOS to determine if it affects smoking and vaping desire as well as smoking behavior among young adult smokers. We predicted that visual observation of its use, compared with a nonsmoking control cue, would elicit urge and desire for both combustible and electronic cigarettes, and produce shorter latency to smoke in a smoking lapse paradigm. We also explored whether observing IQOS use would affect participants’ ratings of product interest and curiosity and the likeability of its users.
METHODS
Design
We used a between-subjects design with participants equally randomized by a computer number generator to exposure to the use of an HTP (active cue) or bottled water (control cue). Testing took place from July 2019 to January 2020 at the Clinical Addictions Research Laboratory at the University of Chicago.
Procedure
Recruitment and screening.
Candidates were recruited via flyers and online advertisements for a study described as examining “moods, behaviors, and social interactions.” This description was chosen to mask the study purpose and minimize expectancy, as used successfully in prior studies.14–17 During a brief telephone screening, candidates were interviewed to assure they met the basic inclusion criteria: age between 18-35 years, daily smoking (5-30 cigarettes per day at least 5 days per week), no intention to quit smoking, never participated in a prior study at our laboratory, fluent in English, and no major physical or mental disorders or disabilities (eg, hearing or visual impairments) that would preclude participation. Candidates were eligible regardless of their ENDS use status. This was done to avoid attention on vaping and alternative tobacco products that might have affected responses in study sessions; all participants were assessed on ENDS history during an end of session survey.
The candidates who met the basic criteria were invited to an in-person screening. They were instructed to abstain from recreational drugs and alcohol for 24 hours and smoking for at least one hour prior to arriving to the visit. After the participant provided informed consent and presented a state-issued photo ID to verify their age and identity, they underwent the screening session that included explanation of study procedures and objective and subjective measures. Breath alcohol readings were required to be ≤ 0.003 mg% (Alco-Sensor III, Intoximeter, MO, USA). Expired-air carbon monoxide levels were used as a general indicator of smoking status and time since last cigarette (ie, 90% of candidates had carbon monoxide levels consistent with being a smoker (> 5 ppm) with the remainder reporting 6 or more hours since last cigarette). Surveys included the Beck Depression Inventory,22 Spielberger Trait Anxiety Inventory,23 and the Timeline Followback calendar24 for past month smoking and alcohol use. This was followed by an interview with the Structured Clinical Interview for DSM-IV research version25 including screening items for major psychiatric disorders (schizophrenia, bipolar, etc). Candidates were excluded if they had survey scores outside standard clinical thresholds for severe depression or anxiety or had a major untreated psychiatric disorder. Of the 120 candidates screened, 108 (90%) were deemed eligible and agreed to participate in the 1- to 1-1/2-hour study session that followed screening. The 12 candidates who were ineligible did not meet age (N = 2) or smoking (N = 6) criteria, or arrived to screening with a positive breath alcohol reading (N = 4). The latter were given a car service ride home to assure safety. Three participants were outliers on primary outcomes and were excluded from analyses (see “Data Analysis” for details).
Experimental session.
The session began with the participant completing baseline surveys on the computer. Then the participant was instructed to view and focus on a video approximately 5 minutes long as they would be asked later to answer questions about its general content. Depending on randomization, the video included content depicting use of either the active HTP cue or the control cue of bottled water drinking (for details, see “Cues” section). Before the video began, the research assistant left the room to assure they were blind to study condition. After the video ended, the participant completed a repeated set of surveys. This was followed by a short rest break and a wash-out task (the digit symbol substitution task).26 The participant then completed a final set of surveys approximately 20 minutes after the onset of the video. These surveys concluded with an open-ended item asking the participant: “In your opinion, what was the purpose of the study?” Responses to this item indicated that our masking was successful as only 15% (16/105) correctly deduced the study’s purpose.
After this cue phase, the final 50 minutes of the session included the smoking latency portion of the Smoking Lapse Paradigm.27,28 The research assistant presented the participant with a cigarette of their preferred brand, a lighter, an ashtray, and a buzzer. The participant was informed they could choose to smoke at any time else receive $0.20 for every 5 minutes of refraining from smoking. No other activities were allowed, ie, no reading or use of smartphone/watches and there was no clock in the room. This procedure has been used successfully in prior studies of young adult smokers.16,17,19,29 After this portion, the participant completed questionnaires on their history of ENDS use; these were not administered earlier to avoid focus on ENDS and reduce expectancy. Once all measures were completed, the participant was paid $100 for participation. A full debriefing was provided to all participants after study enrollment was completed.
Cues.
The videos were presented on a 60-centimeter (cm) computer monitor with the participant seated approximately a half meter from the monitor. The HTP cue was a white IQOS device, 9 cm long by 1.2 cm wide, and attached to a 4.5 cm long white HEET stick inserted inside the holder. The brand logo of 3 vertical lines on the HEET sticks was not visible to the viewer as there were no product close-ups. This device was consistent with what was approved and available in the US at the time we conducted the study. The control cue was a standard 16.9 oz. clear bottle of water with a label but with brand name obscured. Bottled water was chosen as the control cue as it is a consummatory behavior with hand-to mouth motions but neutral in terms of associations to smoking.9,14,16,19
The videos were created by a pre-professional videographer and edited with Adobe Premiere Pro. The acting, cinematography, and editing were identical for both videos such that the only difference between them was the product used by the actors (Figures 1 and 2). Scenes were filmed on the same day with one cue scene filmed immediately followed by filming of the other cue. The same 3 racially diverse, young adult actors were included in both videos, ie, one Asian woman, one White woman, and one Black man -- all were current smokers and vapers with prior acting experience. Background noise and voice sounds were edited out of the scenes and the videos were overlaid with an identical ambient soundtrack using freely available instrumental music30 as used in our prior study.15
Figure 1.

Example of Visual Stimulus as Cue – Water Cue Excerpt
Figure 2.

Example of Visual Stimulus as Cue – IQOS Cue Excerpt
Note.
The top image is an excerpt from the water video (a) and the bottom image is from the IQOS video (b). This was the 14th scene at 2:33 of the 5-minute video. The actual video was displayed in full color.
The videos included 29 9-10 second duration scenes including single-person or 2- or 3-person interactions (76%, 17%, and 7%, respectively; Table S1). Two-thirds of the video depicted outdoor environments with the actors sitting or talking in parks or in front of residences, walking and conversing on sidewalks, driving, or viewing park or lakefront scenery (Table S1). The other one-third of scenes were shot in indoor settings resembling an apartment living room or home office. Of the 29 scenes, 27 included hand-to-mouth movements of product use while 2 scenes showed the product held stationary in hand (Table S2). One single product (ie, one bottled water or one HTP) was displayed in each scene (see Supplement for more details; videos available upon request).
Measures
Primary dependent measures were the total score on the 10-item Brief Questionnaire of Smoking Urges (BQSU)31 and scores on 100mm visual analog scale (VAS) items scored from 0 (not at all) to 100 (most ever) on desire for a regular cigarette, an e-cigarette (separate items for a mod/vape pen and a JUUL), and water. Additional visual analog scale (VAS) items, eg, desire for conversation, salty foods, etc were imbedded in the surveys to mask the study purpose. The primary variable from the smoking behavior phase was median latency to smoke (in minutes). As in prior research,17,28,29,32 values for persons who did not choose to smoke (N = 39/108, 36%) were coded as 50 minutes and median values were examined as the data demonstrated a bimodal distribution.
Secondary measures included VAS items that were based on previous studies on vaping advertisements,11,33,34 and likability of a target individual.35 These items included rating liking of the actors in the video (“The people in the video appeared to be likable.” and “The people in the video appeared to be friendly.”) and curiosity about (“I am curious about the product in this video.”) and interest (“I would like to use the product in this video.”) in using the product. The participant also rated overall liking of the video (“Did you like this video?” and “Would you like to watch more of this video?”).
Data Analysis
Primary data were examined first for normality. There were 3 outliers identified (3% of sample; 2 in the IQOS group, one in the water group) who had urge or desire ratings > 2 SD from the mean, and thus, were excluded from analysis. This resulted in a final sample size of 105 participants (N = 52 IQOS group, N = 53 water group). As in our prior studies, ratings for each measure were summarized as change scores, ie, ratings at each time point minus the baseline score.
We compared demographic and smoking characteristics between groups using t-tests and chi-square tests, as appropriate. We analyzed outcomes using Generalized Estimating Equations (GEE) with an exchangeable correlation matrix and identity link function. Baseline measures were included as a covariate in all models as values that are very high or low at baseline can impact outcomes. We examined statistically significant effects by postestimation contrasts with Bonferroni corrections for multiple comparisons. We used Mood’s median test to examine group differences in smoking latency.36 We ran separate GEE models to examine cue responses by ENDS use status (naïve, lifetime, and past month use). We repeated all analyses with relevant covariates, including age, sex,37 cigarettes smoked per day, and ENDS use status.
RESULTS
Sample Characteristics
The average age of the sample was 28.6 ± 4.4 (SD) years (51% female), and the racial background was diverse (45% white, 36% black, 19% other). The groups did not differ on most sociodemographic characteristics with the exception of age (Table 1); results did not change when age was included as a covariate. Participants averaged smoking 9.9 ± 5.0 cigarettes per day with a mean FTCD of 3.6 ± 2.1, indicating moderate nicotine dependence, and 73% preferred mentholated cigarettes. Lifetime use of ENDS was common (80% of the sample) with over one-third (34%) reporting past month use, and prior use of IQOS was rare, with only 6 participants (5.7%) reporting ever use of this product. The groups did not differ on any smoking or ENDS use characteristics.
Table 1.
Group Characteristics
| Water (N = 53) |
IQOS (N = 52) |
p | |
|---|---|---|---|
| Demographics and background | |||
| Age (years) | 29.5 (0.6) | 27.7 (0.6) | .03 |
| Education (years) | 13.5 (0.2) | 13.2 (0.2) | .27 |
| Sexb (Percent male) | 26 (49.1%) | 25 (48.1%) | .99 |
| Race | .47 | ||
| White | 27 (51%) | 20 (39%) | - |
| Black | 16 (30%) | 22 (42%) | - |
| Other | 10 (19%) | 10 (19%) | - |
| Sexual Orientation | |||
| Percent Heterosexual/Straight | 37 (70%) | 44 (81.5%) | .28 |
| Beck Depression Inventory | 8.8 (1.0) | 11.1 (1.1) | .14 |
| Spielberger Trait Anxiety (t-score) | 59.7 (0.7) | 60.0 (0.6) | .75 |
| Alcohol drinks/week | 13.1 (2.2) | 9.5 (1.6) | .19 |
| Smoking patterns and use | |||
| Days smoked (of past 28 days) | 27.7 (0.2) | 27.9 (0.1) | .42 |
| Cigarettes smoked per smoking day | 9.7 (0.7) | 10.1 (0.7) | .71 |
| FTCD (nicotine dependency, 0-10) | 3.4 (0.3) | 3.9 (0.3) | .23 |
| Hours since last reported cigarettea | 4.3 (0.7) | 4.7 (0.6) | .34 |
| CO (ppm)a | 14.8 (1.4) | 15.2 (1.4) | .89 |
| Previous ENDS use | .47 | ||
| Naïve ENDS user (never used ENDS) | 10 (19%) | 11 (21%) | - |
| Lifetime ENDS user (no past month use) | 23 (43%) | 25 (48%) | - |
| Current ENDS user (past month use) | 20 (38%) | 16 (31%) | - |
| Other tobacco use in the past year: | |||
| Hookah | 9 (17%) | 16 (31%) | .10 |
| Cigars | 17 (32%) | 15 (29%) | .63 |
| Other (Smokeless, Pipe, Cloves, etc) | 8 (15%) | 7 (13%) | .81 |
| Baseline Urge and Desire Ratings | |||
| BQSUc | 38.8 (1.8) | 40.6 (1.7) | .46 |
| Regular Cigarette desired | 51.1 (3.4) | 48.8 (3.0) | .61 |
| Vape Pen/Mod desired | 12.3 (3.0) | 10.3 (2.4) | .62 |
| JUUL desired | 9.7 (2.8) | 10.1 (2.7) | .90 |
Note.
Values are Mean (SEM) or N (%), as indicated. Variables were compared between groups with Student’s t-test or chi-square as appropriate.
CO and hours since last reported cigarette values are medians due to non-normal distribution, and compared via Mann-Whitney U test.
Participant’s biological sex.
Total score range:10-70.
Range: 0-100. CO = carbon monoxide; ENDS = electronic nicotine delivery systems; FTCD = Fagerström Test for Cigarette Dependence; BQSU = Brief Questionnaire of Smoking Urges.
Study Outcomes
For the main study measures, the IQOS (vs water) cue produced significant increases in BQSU smoking urge and desire for a cigarette, a mod/vape pen, and a JUUL (group, all ps < .05; Figures 3–4 and Table S3). For those exposed to IQOS, within-subject comparisons between desire for cigarettes, a mod/vape pen, and JUUL were not statistically significant (p = .096). As expected, the water cue (vs IQOS) elicited a greater desire for water (group, p = .04). In the smoking behavior task, 65.7% of the total sample chose to smoke during the task, with 71.2% and 60.4% in the IQOS and water groups, respectively, choosing to smoke. The IQOS cue group had a marginally significant shorter latency to smoke than the water cue group [median latency: 19.2 vs 32.9 minutes; χ2(1,105) = 3.44, p = .06; Figure 5]. The most commonly endorsed reasons for choosing to smoke were “I wanted to smoke” (94% agreement) and “I was bored” (82% agreement; Table S4). All aforementioned results did not change with the inclusion of covariates.
Figure 3.
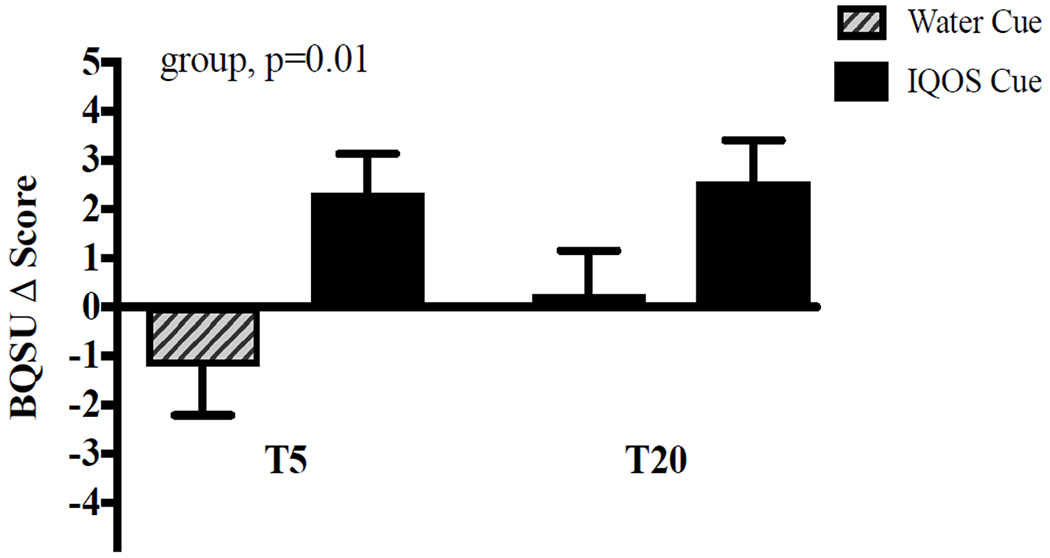
Smoking Urge
Note.
Data are mean ± SEM. Subjective ratings of smoking urge represented as change scores from the baseline rating. Ratings were total scores on the 10-item Brief Questionnaire of Smoking Urges (BQSU). T represents time after exposure to the cue, with T5 = 5 minutes after cue exposure and T20 = 20 minutes after cue exposure.
Figure 4.
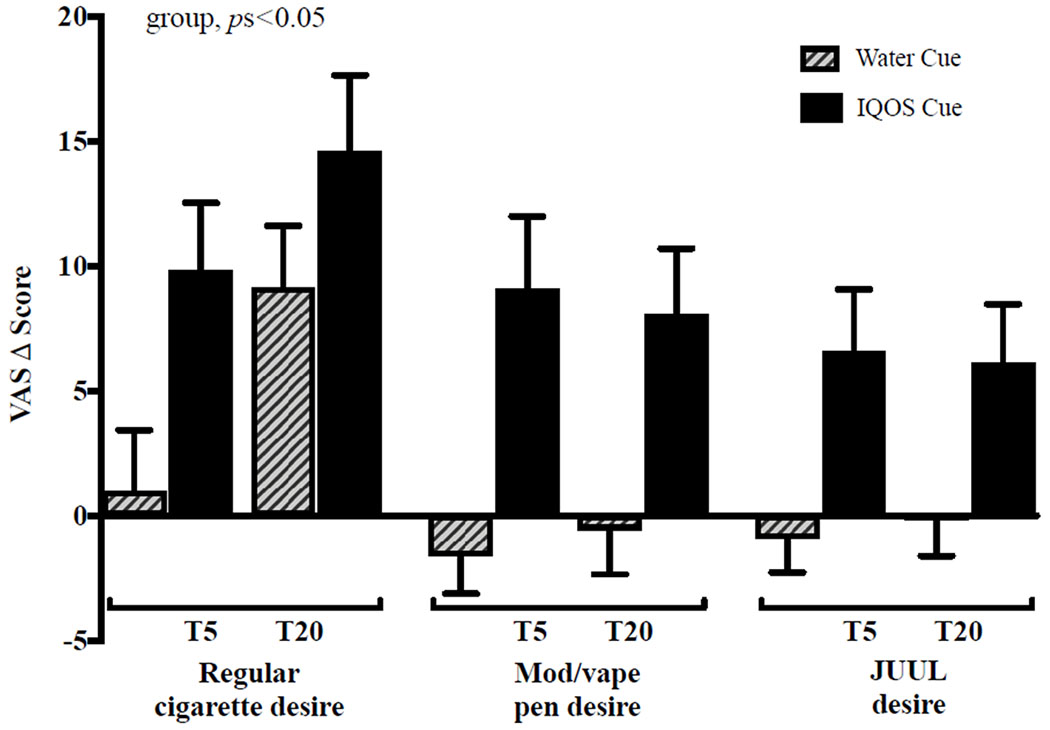
Desire for Cigarette, Mod/vape Pen and JUUL
Note.
Data are mean ± SEM and represent change scores from the baseline rating on 100mm visual analog scales scored from 0 (not at all) to 100 (most ever) for ratings of desire for a regular cigarette, mod/vape pen, and JUUL. T represents time after exposure to the cue, with T5 = 5 minutes after the initiation of cue exposure and T20 = 20 minutes after initiation of cue exposure. There was a significant group effect for each variable, all ps < .05.
Figure 5.
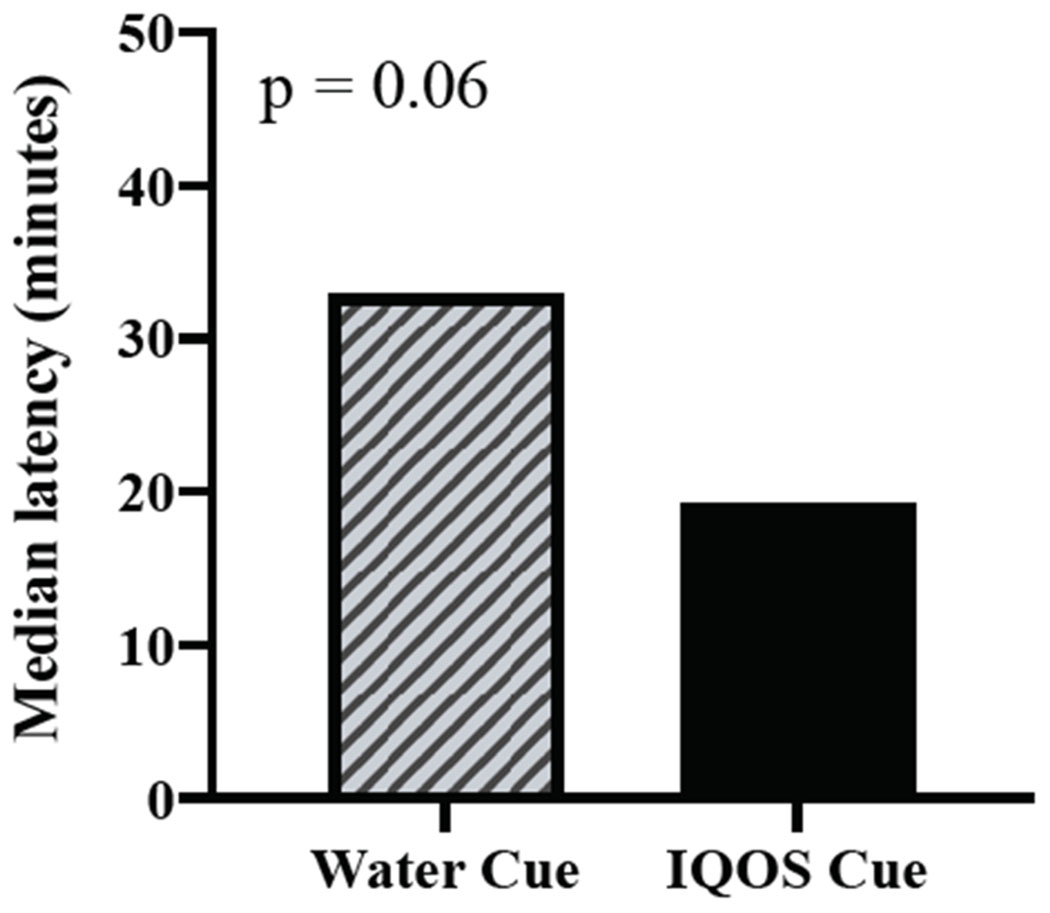
Latency to Smoke in Choice Phase
Note.
Data are median latency. During the 50-minute Smoking Lapse Paradigm, the participant was informed they could choose to smoke at any time else receive $0.20 for every 5 minutes of refraining from smoking. All participants who did not choose to smoke were counted as the duration of the task, ie 50 minutes. There was a trend for a significant group effect, p = .06.
Despite the videos being identical except for the product used, participants who viewed the IQOS (vs water) cue rated the actors as significantly less likable (p = .003) and friendly (p = .04) and the product as significantly less desirable (p = .002). There were no differences between groups on ratings of product curiosity, overall liking of the video, or wanting to watch more. Table 2 shows the full details.
Table 2.
Video Perception Mean Ratings
| Response item | Water (N = 53) |
IQOS (N = 52) |
p |
|---|---|---|---|
| Actor Perception | |||
| The people in the video appeared to be likable. | 74.5 (2.5) | 60.4 (3.8) | .003 |
| The people in the video appeared to be friendly | 75.9 (2.7) | 66.8 (3.3) | .04 |
| Product Interest | |||
| I would like to use the product in this video. | 55.0 (4.3) | 34.2 (5.0) | .002 |
| I am curious about the product in this video. | 38.0 (4.5) | 43.7 (5.2) | .40 |
| Video Liking | |||
| Did you like this video? | 47.5 (3.9) | 40.3 (4.0) | .20 |
| Would you like to watch more of this video? | 29.1 (4.1) | 27.3 (4.0) | .75 |
Note.
Values are Mean (SD). Each item was rated on a 100mm visual analog scale ranging from 0 (not at all) to 100 (most ever). Group differences on mean ratings were analyzed via Student’s t-test.
Responses to the cues by ENDS use status (past month, lifetime, and naïve users) revealed that, as expected, past month ENDS users, relative to lifetime and naïve users, reported higher baseline desire for both a mod/vape pen (baseline desire 21.6 compared with 5.6 and 1.5 for lifetime and naïve users, respectively) and a JUUL (baseline desire 20.9 compared with 3.1 and 0.8 for lifetime and naïve users, respectively). The IQOS cue (vs water) produced significantly higher increases in desire for a mod/vape pen [mean change scores of +10.5 and +14.2 in past month and lifetime users, respectively, compared with +0.7 in naïve users; group x ENDS status, Wald χ2(5) = 14.92, p < .05]. ENDS use status did not significantly affect smoking urge, cigarette desire, or desire for a JUUL (all ps > .05).
DISCUSSION
In this study, we examined the cue salience of FfTPs to determine whether the novel product IQOS generalizes as a cue for smoking and vaping in a group of young adult daily smokers. Visual imagery depicting the use of IQOS, but not water, resulted in statistically significant increases in smoking urge and desire for a cigarette, a mod/vape pen, and JUUL. Further, exposure to visual imagery of IQOS use (vs water) produced shorter smoking latencies versus receiving a monetary reinforcer. On the other hand, visual imagery depicting the use of water (vs IQOS) increased ratings of desire for water, further confirming the validity of the exposures to induce craving for the cue depicted in each video. These findings suggest that the HTP IQOS, similar to first-to-fourth generation ENDS, elicits both smoking and vaping craving in young adult smokers.
The findings show that HTPs, similar to ENDS, cross-generalize as cues for smoking. Whereas HTPs are marketed as products that can help smokers abstain from cigarette smoking, there may be some population-wide trade-offs in the adoption of this product. Although manufacturers market HTP products as viable options in the harm reduction continuum for smokers,7 the features and use of these products closely resemble smoking38 which may play a role in their cue salience. Importantly, in our US sample, most participants had not yet been exposed to IQOS use, but they still had statistically significant increases in smoking urge, desire, and behavior after exposure to its use, implicating the cross-generalization of this product even among young smokers not yet familiar with the product. A small minority of 6 participants (5.7% of sample) endorsed having used an HTP. Visual aids were provided for all device types, but there was no interview conducted to ensure participants were not confusing an HTP with a vape pen or other ENDS device. Because IQOS was available in 39 countries and 2 US cities (but not Chicago) at the time of data collection, it is possible they were exposed to the product via one of these routes. Despite differences between HTPs and ENDS, the magnitude of craving responses to the HTP cue was similar to that shown to be elicited by ENDS16,17 and traditional cigarettes,14,19 and smoking latency was comparable to that observed in prior work with a variety of ENDS cues.17,19
Interestingly, despite the fact that the same actors appeared in each video, they were rated as less likeable and less friendly when they used IQOS than when they drank water. Although survey data suggest growing awareness and interest in using IQOS among youth39 our study showed that, when observing direct use of the product, user judgments seem to arise, curiously among young adults who smoke. Furthermore, participants exposed to IQOS reported less interest in using the product shown despite reporting cigarette craving. We speculate that our study participants’ negative judgements of IQOS users related to the effects on craving in a situation where an immediate opportunity to smoke or vape to assuage those urges was not available, thereby creating a negative mental state that affected impressions of the actors and of HTP use in general. Another possibility is that participants formed a negative attribution of the product used to resolve the cognitive dissonance40 of experiencing conflicting desire to use while at the same time being aware of the possible product harms and lung injury.41,42 Whereas further research is needed, it is certainly the case that the cue salience of novel tobacco products is not without some emotional responses in young adult smokers who may be receiving conflicting messages about non-combustible tobacco products from a variety of sources ranging from health authorities to social media influencers.
The strengths of this study include the use of a randomized design with cue exposure in a controlled laboratory setting and standardized cues allowing for a range of real-life exposures between groups to be identical besides the product shown. Despite these strengths in design and ecological validity, there are some limitations to note. First, the sample consisted of young adult daily smokers in a between-subjects design. Our sample was split somewhat evenly between those aged 18-29 (53%) and 30-35 (47%), encompassing a relatively wide age range for a young adult sample. Young adults were chosen because they report the highest rate of ENDS use and at the age range most likely to be exposed to ENDS in their social environments ie, parties, social media, school, etc.5,43 It is unknown if the findings would generalize to older or former smokers, nondaily smokers, or to sole e-cigarette users, or whether a cross-over design would have produced different results. Future studies could investigate differences in cue reactivity among different product users (ENDS-only users, dual users, former users, etc). Second, inherent in video cue exposure is the lack of sensory aspects one would encounter if the product were to be used in their presence. However, the magnitude of the increases in smoking desire were similar to that found in our prior research with direct confederate-delivered product use cues.16,17,19
Finally, the videos were created for the purpose of this study and were not actual video portrayals that smokers regularly encounter, ie, as in television or Internet advertisements. However, advertisements were not possible with IQOS as video advertising for tobacco products varies substantially by country due to differences in regulations; in the US, the Food and Drug Administration tightly regulates the depiction of tobacco product use in advertising. The advantage of our study’s videos were that they to portrayed IQOS use across diverse racial/ethnic actors, from a variety of angles, and settings mimicking real-life exposures rather than using advertisements that showcase product use to increase sales.
IMPLICATIONS FOR TOBACCO REGULATION
The present investigation is the first to show that exposure to the use of the novel HTP IQOS increases smoking and vaping craving as well as smoking behavior in young adult daily smokers. Thus, it appears that HTPs, similar to a variety of e-cigarettes, generalize as a cue for smoking. Despite these findings, those in the IQOS group rated the actors in the video as less likeable, friendly, and the device itself as less desirable. Although it is currently unclear how HTPs such as IQOS will fare in the variable regulatory climates across countries, this study is timely for our understanding of how their proliferation might impact susceptible groups such as young adult smokers who are targeted as the next generation of regular tobacco users, whether for combustible or newer alternative products. These observer effects should be weighed against the possible mitigation of health risks in adults smokers who are able to stop using combustible cigarettes by switching to use of HTPs, although notably, long-term benefits of HTPs have not been determined. However, our findings raise further consideration of where and how IQOS and other HTPs are marketed and designed. As novel tobacco products are continuously developed, it is possible that reducing their resemblance to traditional cigarettes could help tip the risk/benefit ratio to enhance public health and consider effects for society at large.
Supplementary Material
Acknowledgements
Appreciation is extended to Dingcai Cao for assistance with statistical analysis and to Martin Awano for video production. This research was supported by NIDA (R01-DA044210). Salary support for EIB was provided by National Institutes of Health (T32-DA043469).
Footnotes
Human Subjects Approval Statement
This study was approved by the University of Chicago Institutional Review Board.
Conflict of Interest Disclosure Statement
No conflicts to disclose
Copyright of Tobacco Regulatory Science is the property of Tobacco Regulatory Science Group and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder’s express written permission. However, users may print, download, or email articles for individual use.
Contributor Information
Emma I. Brett, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, Chicago, IL, United States.
Krista Miloslavich, Psychology Department, University of Illinois at Chicago, Chicago, IL, United States.
Ashley Vena, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, Chicago, IL, United States.
Nathan Didier, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, Chicago, IL, United States.
Andrea C. King, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, Chicago, IL, United States.
References
- 1.Bao W, Liu B, Du Y, et al. Electronic cigarette use among young, middle-aged, and older adults in the United States in 2017 and 2018. JAMA Intern Med. 2020;180(2):313–314. doi: 10.1001/jamainternmed.2019.4957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yong H-H, Borland R, Balmford J, et al. Trends in e-cigarette awareness, trial, and use under the different regulatory environments of Australia and the United Kingdom. Nicotine Tob Res. 2015;17(10):1203–1211. doi: 10.1093/ntr/ntu231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Regan AK, Promoff G, Dube SR, Arrazola R. Electronic nicotine delivery systems: adult use and awareness of the “e-cigarette” in the USA. Tob Control. 2013;22(1):19–23. [DOI] [PubMed] [Google Scholar]
- 4.Laverty AA, Filippidis FT, Vardavas CI. Patterns, trends and determinants of e-cigarette use in 28 European Union member states 2014—2017. Prev Med. 2018;116:13–18. doi: 10.1016/j.ypmed.2018.08.028 [DOI] [PubMed] [Google Scholar]
- 5.Dai H, Leventhal AM. Prevalence of e-cigarette use among adults in the United States, 2014–2018. JAMA. 2019;322(18):1824–1827. doi: 10.1001/jama.2019.15331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeVito EE, Krishnan-Sarin S. E-cigarettes: impact of e-liquid components and device characteristics on nicotine exposure. Curr Neuropharmacol. 2018;16(4):438–459. do i: 10.2174/1570159X15666171016164430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillip Morris International. IQOS. https://www.pmi.com/smoke-free-products/iqos-our-tobacco-heating-system. Accessed May 20, 2020.
- 8.Carpenter MJ, Saladin ME, LaRowe SD, et al. Craving, cue reactivity, and stimulus control among early-stage young smokers: effects of smoking intensity and gender. Nicotine Tob Res. 2014;16(2):208–215. doi: 10.1093/ntr/ntt147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drobes DJ, Tiffany ST. Induction of smoking urge through imaginal and in vivo procedures: physiological and self-report manifestations. J Abnorm Psychol. 1997;106(1):15–25. doi: 10.1037/0021-843X.106.1.15 [DOI] [PubMed] [Google Scholar]
- 10.Erblich J, Bovbjerg DH. In vivo versus imaginal smoking cue exposures: is seeing believing? Exp Clin Psychopharmacol. 2004;12(3):208–215. doi: 10.1037/1064-1297.12.3.208 [DOI] [PubMed] [Google Scholar]
- 11.Kim AE, Lee YO, Shafer P, et al. Adult smokers’ receptivity to a television advert for electronic nicotine delivery systems. Tob Control. 2015;24(2):132–135. doi: 10.1136/tobaccocontrol-2013-051130 [DOI] [PubMed] [Google Scholar]
- 12.Lochbuehler K, Wileyto EP, Tang KZ, et al. Do current and former cigarette smokers have an attentional bias for e-cigarette cues? J Psychopharmacol (Oxf). 2018;32(3):316–323. doi: 10.1177/0269881117728418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pepper JK, Emery SL, Ribisl KM, et al. Effects of advertisements on smokers’ interest in trying e-cigarettes: the roles of product comparison and visual cues. Tob Control. 2014;23:iii31–iii36. doi: 10.1136/tobaccocontrol-2014-051718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King AC, Smith LJ, McNamara PJ, et al. Passive exposure to electronic cigarette (e-cigarette) use increases desire for combustible and e-cigarettes in young adult smokers. Tob Control. 2015;24(5):501–504. doi: 10.1136/tobaccocontrol-2014-051563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King AC, Smith LJ, Fridberg DJ, et al. Exposure to electronic nicotine delivery systems (ENDS) visual imagery increases smoking urge and desire. Psychol Addict Behav. 2016;30(1):106–112. doi: 10.1037/adb0000123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vena A, Howe M, Cao D, King A. The role of E-liquid vegetable glycerin and exhaled aerosol on cue reactivity to tank-based electronic nicotine delivery systems (ENDS). Psychopharmacology (Berl). 2019;236(7):2083–2092. doi: 10.1007/s00213-019-05202-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vena A, Miloslavich K, Cao D, King A. Cue salience of the use of an electronic nicotine delivery system (ENDS) device marketed to women. Addict Behav. 2020;100:106116. doi: 10.1016/j.addbeh.2019.106116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maloney EK, Cappella JN. Does vaping in e-cigarette advertisements affect tobacco smoking urge, intentions, and perceptions in daily, intermittent, and former smokers? Health Commun. 2016;31(1):129–138. doi: 10.1080/10410236.2014.993496 [DOI] [PubMed] [Google Scholar]
- 19.King AC, Smith LJ, McNamara PJ, Cao D. Second generation electronic nicotine delivery system vape pen exposure generalizes as a smoking cue. Nicotine Tob Res. 2018;20(2):246–252. doi: 10.1093/ntr/ntw327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKelvey K, Baiocchi M, Halpern-Felsher B. PMI’s heated tobacco products marketing claims of reduced risk and reduced exposure may entice youth to try and continue using these products. Tob Control. 2020;29(e1):e18–e24. doi: 10.1136/tobaccocontrol-2019-055318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sutanto E, Smith DM, Miller C, et al. Use of heated tobacco products within indoor spaces: findings from the 2018 ITC Japan Survey. Int J Environ Res Public Health. 2019;16(23):4862. doi: 10.3390/ijerph16234862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961. ;4(6) :561–571. doi: 10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- 23.Spielberger CD, Lushene RE, Gorsuch RL. STAI Manual for the State-trait Anxiety Inventory (“Self-evaluation Questionnaire”). White Bear Lake, MN: Consulting Psychologists Press; 1970. [Google Scholar]
- 24.Rueger SY, Trela CJ, Palmeri M, King AC. Self-administered web-based Timeline Followback procedure for drinking and smoking behaviors in young adults. J Stud Alcohol Drugs. 2012;73(5):829–833. doi: 10.15288/jsad.2012.73.829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. New York, NY: Biometrics Research; 1996. [Google Scholar]
- 26.Wechsler D Manual for the Wechsler Adult Intelligence Scale. Oxford, UK: Psychological Corp; 1955:vi, 110. [Google Scholar]
- 27.McKee SA, Weinberger AH, Shi J, et al. Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine Tob Res. 2012;14(11):1362–1371. doi: 10.1093/ntr/nts090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol. 2009;14(1):99–107. doi: 10.1111/j.1369-1600.2008.00135.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang RD, Leventhal AM. Sex differences in negative affect and lapse behavior during acute tobacco abstinence: a laboratory study. Exp Clin Psychopharmacol. 2013;21(4):269–276. doi: 10.1037/a0033429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zabriskie C Free Music Archive: Air Hockey Saloon. https://www.bing.com/videos/search?q=Free+Music+Archive%3a+Air+Hockey+Saloon&docid=608040174203109939&mid=D430AD210847112B5E7BD430AD210847112B5E7B&view=detail&FORM=VIRE. Published August 23, 2015. Accessed December 19, 2020.
- 31.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051 [DOI] [PubMed] [Google Scholar]
- 32.Kahler CW, Metrik J, Spillane NS, et al. Acute effects of low and high dose alcohol on smoking lapse behavior in a laboratory analogue task. Psychopharmacology (Berl). 2014;231 (24) :4649–4657. doi: 10.1007/s00213-014-3613-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicksic NE, Harrell MB, Pérez A, et al. Recall of e-cigarette advertisements and adolescent e-cigarette use. Tob Regul Sci. 2017;3(2):210–221. doi: 10.18001/TRS.3.2.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Margolis KA, Donaldson EA, Portnoy DB, et al. E-cigarette openness, curiosity, harm perceptions and advertising exposure among U.S. middle and high school students. Prev Med. 2018;112:119–125. doi: 10.1016/j.ypmed.2018.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reysen S Construction of a new scale: the Reysen Likability Scale. Soc Behav Personal Int J. 2005;33(2):201–208. doi: 10.2224/sbp.2005.33.2.201 [DOI] [Google Scholar]
- 36.Mood AM. On the asymptotic efficiency of certain nonparametric two-sample tests. Ann Math Stat. 1954;25(3):514–522. doi: 10.1214/aoms/1177728719 [DOI] [Google Scholar]
- 37.Heishman SJ, Lee DC, Taylor RC, Singleton EG. Prolonged duration of craving, mood, and autonomic responses elicited by cues and imagery in smokers: effects of tobacco deprivation and sex. Exp Clin Psychopharmacol. 2010;18(3):245–256. doi: 10.1037/a0019401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis B, Williams M, Talbot P. iQOS: Evidence of pyrolysis and release of a toxicant from plastic. Tob Control. 2019;28(1):34–41. doi: 10.1136/tobaccocontrol-2017-054104 [DOI] [PubMed] [Google Scholar]
- 39.Czoli CD, White CM, Reid JL, et al. Awareness and interest in IQOS heated tobacco products among youth in Canada, England and the USA. Tob Control. 2020;29(1):89–95. doi: 10.1136/tobaccocontrol-2018-054654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Festinger L Cognitive dissonance. Sci Am. 1962;207:93–102. doi: 10.1038/scientificamerican1062-93 [DOI] [PubMed] [Google Scholar]
- 41.Pericot-Valverde I, Gaalema DE, Priest JS, Higgins ST. E-cigarette awareness, perceived harmfulness, and ever use among U.S. adults. Prev Med. 2017;104:92–99. doi: 10.1016/j.ypmed.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.King BA, Jones CM, Baldwin GT, Briss PA. The EVA-LI and youth vaping epidemics — implications for public health. N Engl J Med. 2020;382(8):689–691. doi: 10.1056/NEJMp1916171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barrington-Trimis JL, Urman R, Leventhal AM, et al. E-cigarettes, cigarettes, and the prevalence of adolescent tobacco use. Pediatrics. 2016;138(2):e20153983. doi: 10.1542/peds.2015-3983 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


