Abstract
Background
Guidelines recommend evaluation for electrographic seizures in neonates and children at risk, including after cardiopulmonary bypass (CPB). Although initial research using screening electroencephalograms (EEGs) in infants after CPB found a 21% seizure incidence, more recent work reports seizure incidences ranging 3–12%. Deep hypothermic cardiac arrest was associated with increased seizure risk in prior reports but is uncommon at our institution and less widely used in contemporary practice. This study seeks to establish the incidence of seizures among infants following CPB in the absence of deep hypothermic cardiac arrest and to identify additional risk factors for seizures via a prediction model.
Methods
A retrospective chart review was completed of all consecutive infants ≤ 3 months who received screening EEG following CPB at a single center within a 2-year period during 2017–2019. Clinical and laboratory data were collected from the perioperative period. A prediction model for seizure risk was fit using a random forest algorithm, and receiver operator characteristics were assessed to classify predictions. Fisher’s exact test and the logrank test were used to evaluate associations between clinical outcomes and EEG seizures.
Results
A total of 112 infants were included. Seizure incidence was 10.7%. Median time to first seizure was 28.1 h (interquartile range 18.9–32.2 h). The most important factors in predicting seizure risk from the random forest analysis included postoperative neuromuscular blockade, prematurity, delayed sternal closure, bypass time, and critical illness preoperatively. When variables captured during the EEG recording were included, abnormal postoperative neuroimaging and peak lactate were also highly predictive. Overall model accuracy was 90.2%; accounting for class imbalance, the model had excellent sensitivity and specificity (1.00 and 0.89, respectively).
Conclusions
Seizure incidence was similar to recent estimates even in the absence of deep hypothermic cardiac arrest. By employing random forest analysis, we were able to identify novel risk factors for postoperative seizure in this population and generate a robust model of seizure risk. Further work to validate our model in an external population is needed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12028-021-01313-1.
Keywords: Cardiopulmonary bypass, EEG, Seizure, Infant
Introduction
Cardiopulmonary bypass (CPB) was invented in the late 1950s and was rapidly adapted to pediatrics to facilitate surgical repair of congenital cardiac defects. Achieving adequate oxygenation was a particular challenge in early CPB; thus, cardiology teams were appropriately concerned about the neurologic sequelae of reduced cerebral oxygenation during surgery.
Early studies in the 1970s and 80s using continuous electroencephalogram (EEG) for prospective seizure detection after CPB reported 4–9.1% seizure incidence in larger trials but few or no seizures were captured in smaller reports [1–6]. Seizure incidence in subsequent populations with a spectrum of cardiac lesions and broad age distribution ranged 1.5–11.5% [7–12]. In contrast, studies restricted to infants with more complex anomalies found a higher incidence of seizure: infants with transposition of the great arteries had an incidence of 21.3% [13], and patients undergoing Norwood staged palliation for hypoplastic left heart, typically performed shortly after birth, had an incidence of 18% [14].
Seizure risk factors included deep hypothermic cardiac arrest (DHCA) [8, 9, 13], longer bypass time [13], acidosis [13], delayed sternal closure [9], younger age [8, 9], abnormal neuroimaging [7, 9], and postoperative cardiac arrest or need for extracorporeal membrane oxygenation [9]. Despite publication of these risk factors, there are no prediction models that integrate these individual risk factors to triage patients by seizure risk. The 2011 American Clinical Neurophysiology Society (ACNS) guidelines suggest 24 h of continuous EEG monitoring in infants at risk [15], including those who undergo CPB, cardiac arrest, or extracorporeal membrane oxygenation.
Our institution adopted a protocol of continuous EEG monitoring in all infants less than 3 months of age for 48 h following CPB. We recognized that intraoperative characteristics influencing seizure risk in our population might differ from those previously published given the evolution of surgical, anesthetic, and CPB techniques, together with shorter or no DHCA [8, 12]. We hypothesized that these factors would contribute to lower seizure incidence in our cohort. Our objective was to determine seizure incidence in infants following CPB at our center and develop a predictive model for postoperative seizures based on a broad spectrum of possible preoperative and perioperative risk factors.
Methods
Data Collection
We included all consecutive infants less than 3 months old undergoing continuous EEG in the 48 h following CPB, as per our institutional protocol, over the 24-month period from July 2017 through June 2019 at Lucile Packard Children’s Hospital. Prior to the start of our study period, clinicians were educated on this protocol. The postoperative admission order set used for every child returning from cardiac surgery was also adapted to include an automatic order for EEG. Although we cannot exclude the possibility that infants may have been cared for outside of our clinical protocols, our standard of care was that every infant received EEG after CPB. If a patient received two EEGs that met inclusion criteria, only the first EEG was considered. Retrospective chart review and data extraction was completed by child neurologists. A pediatric cardiologist reviewed and confirmed the type of congenital cardiac defect and surgical procedure. Data collected included postmenstrual age at birth, growth parameters, oxygen saturation and respiratory support prior to surgery, preoperative and postoperative neuroimaging findings (subcategorized by common findings or other findings), laboratory values indicative of metabolic stress, duration of bypass and cross clamp during surgery, number of code events, presence of abnormal neurologic examination findings, and family history of seizure or epilepsy. EEG data included time to EEG connection, time to first seizure, whether seizures had a clinical correlate, status epilepticus (defined as one seizure lasting > 30 min or frequent seizures occupying > 50% of a 1-h recording segment), and antiseizure medications. We analyzed surgery type directly instead of calculating a Society of Thoracic Surgeons-European Association for Cardio-Thoracic Surgery (STAT) mortality score to better assess for high-risk subtypes of surgery.
All EEGs were recorded using the 10–20 system with Nihon Kohden software. EEGs were reviewed clinically by board-certified pediatric epileptologists. EEG data were extracted from clinical reports.
All research was approved by an independent institutional review board. This study was conducted under an approved wavier of consent. Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Stanford.
Statistical Analysis
Demographics and seizure characteristics were summarized as counts and percentages for categorical variables and medians and interquartile ranges (IQR) for continuous variables.
A prediction model was derived on 88 demographic and perioperative features identified preoperatively and/or during the EEG recording period and a subgroup of 72 features that were exclusively preoperative using a conditional random forest [16]. The outcome of interest was whether a seizure occurred during EEG. Random forest models are often used on high dimensional data when the number of features exceeds the sample size. Because of the large number of features included relative to the sample size, we chose to fit the random forest model to 5,000 trees. A variable importance plot was generated to visually inspect features most important in predicting seizure risk, with features ordered by their mean decrease in classification accuracy after permuting over all trees. Because of low seizure incidence, the predicted probability threshold for classifying seizures (default of 0.5) was recalibrated to account for this imbalance by optimizing Youden’s index, which maximizes sensitivity and specificity [17]. Accuracy was reported for the continuous risk model, whereas area under the curve, sensitivity, and specificity were reported using the derived risk threshold. Features with the highest variable importance values were further examined by predicted seizure risk.
Fisher’s exact test was used to assess the association between observed seizure status and whether lactate normalized post operatively, whereas the logrank test was used to assess whether time from operation to death or transfer, death only, or transfer only (as measures of immediate clinical outcomes) significantly differed by seizure status. For time-to-event outcomes, patients who did not die or transfer were censored at their date of discharge. Median survival time and p values were reported.
The cforest function from the R package party was used to construct the random forest. The forest was grown on 5,000 trees, with five variables sampled at each split. All analyses were performed using R version 3.5.2 [18]. A p value < 0.05 was considered statistically significant.
Results
Patient Demographics
In the 24-month study period, 112 infants ≤ 3 months at time of cardiac surgery requiring CPB received continuous EEG monitoring postoperatively. On average, infants were 41 weeks post menstrual age [gestational age + chronologic age) at time of surgery, with characteristics given in Table 1. Notably, 20% of infants were critically ill on ventilators prior to surgery and 17% were on inotrope or vasopressor infusions, whereas 33% were chronically hypoxemic. Twenty-three percent of patients had abnormal preoperative neuroimaging (ultrasound, computed tomography (CT), and/or magnetic resonance imaging (MRI). One infant had a history of prior seizures and was already on an antiseizure medication initiated at an outside institution.
Table 1.
Patient demographics
| Characteristic | Patients (n = 112) |
|---|---|
| Gestational age at birth, median (min, max) | 39.0 week (27.0, 41.0) |
| Chronological age at operation, median (min, max) | 24.5 day (1.00, 119) |
| Corrected postmenstrual age at operation, median (min, max) | 41.2 week (31.3, 56.3) |
| Male sex, n (%) | 69 (61.6) |
| Discharge status, n (%) | |
| Deceased | 6 (5.4) |
| Transfer | 7 (6.3) |
| Home | 99 (88.4) |
| Genetic defect identified, n (%) | |
| No | 24 (21.4) |
| Yes | 27 (24.1) |
| Presumed but unknown | 61 (54.5) |
| Confirmed 22q11 deletion syndrome | 7 (6.2) |
| Confirmed trisomy 21 | 10 (8.9) |
| Cardiac anomaly, n (%) | |
| VSD | 27 (24.1) |
| TGA + / − VSD | 21 (18.7) |
| ToF | 14 (12.5) |
| Cardiac surgery, n (%) | |
| VSD repair | 27 (24.1) |
| ASO | 21 (18.7) |
| ToF repair | 27 (24.1) |
| Coarctation or aortic arch repair | 21 (18.7) |
| Pre-op oxygenation, n (%) | |
| > 90% on room air | 52 (46.4) |
| > 90% on supplemental O2 | 23 (20.5) |
| 80–90% on supplemental O2 | 26 (23.2) |
| < 80% on supplemental O2 | 11 (9.8) |
| Critical patient when pre-op, n (%) | |
| No | 83 (74.1) |
| Sustained ventricular dysrhythmia | 1 (0.9) |
| Mechanically ventilated | 22 (19.6) |
| Inotropic support | 19 (17.0) |
| Pre-op imaging abnormalities, n (%) | |
| No | 68 (60.7) |
| Yes | 26 (23.2) |
| Not performed | 18 (16.1) |
| Preop imaging abnormalities n (% of all patients imaged) | |
| IPH | 8 (7.1) |
| IVH | 5 (4.5) |
| Ventriculomegaly | 4 (3.6) |
| Postop imaging abnormalities, n (%) | |
| No | 29 (25.9) |
| Yes | 25 (22.3) |
| Not performed | 58 (51.8) |
| Postop imaging abnormalities, n (% of all patients imaged) | |
| SDH | 5 (4.5) |
| IPH | 6 (5.4) |
| IVH | 4 (3.6) |
| Ventriculomegaly | 5 (4.5) |
| Stroke/encephalomalacia | 5 (4.5) |
ASO arterial switch operation for transposition of the great arteries, IPH intraparenchymal hemorrhage, IVH intraventricular hemorrhage, max maximum, min minimum, O2 oxygen, pre-op preoperative, SDHsubdural hemorrhage, TGA transposition of the great arteries, ToF tetralogy of Fallot, VSD ventricular septal defect
EEG and Seizures
Median time from end of CPB to EEG connection was 7.8 h (IQR 4.6–18.8 h; Fig. 1). This time delay includes the completion of surgery and transport time to the cardiovascular intensive care unite (CVICU). Twelve infants out of 112 developed seizures during the course of EEG recording, yielding a seizure incidence of 10.7% (Table 2). Median time from the end of CPB to the first seizure was 28.1 h (IQR 18.9–32.2 h, full range 6–52.3 h). One infant had seizures detected immediately after EEG was initiated. Only two infants underwent DHCA; neither developed seizures. Of the 12 infants with seizures, 5 of 12 (42%) had status epilepticus. Only 2 of 12 infants experienced clinical seizures; both of them also had seizures without clinical correlate. Two patients had brief, isolated seizures without recurrence and were not started on medication. The remaining ten patients were loaded with 1–4 antiseizure medications and achieved seizure control on phenobarbital and/or levetiracetam.
Fig. 1.
Latency to EEG and first seizure. Duration of time in hours from end of CPB to EEG connection (dark gray bar), and then first seizure (light gray bar) is depicted along the x-axis for all 12 patients with seizures. Median time from bypass to EEG connection in all 112 patients was 7.8 h, indicated by the vertical dashed line. CPB cardiopulmonary bypass, EEG electroencephalogram
Table 2.
Seizure statistics
| Variable | Result |
|---|---|
| Time from CPB to EEG, median (IQR) (h) | 7.8 (4.6–18.8) |
| Seizures observed on EEG, n (% of seizures) | 12 (10.7) |
| Status epilepticus | 5 (42) |
| Subclinical seizures | 12 (100) |
| Clinical seizures | 2 (16.7) |
| Time from CPB to first seizure, median (IQR) (h) | 28.1 (18.9–32.2) |
| Discharged home on ASM, n (%) | 10 (83.3) |
ASM antiseizure medication, CPB cardiopulmonary bypass, EEG electroencephalogram, IQR interquartile range
Predicting Seizure Risk
The random forest model predicting seizure risk demonstrated excellent model fit, with an overall accuracy rate of 90.2% (95% confidence interval 83.1–95%). Predicted probabilities of seizure risk were classified into a yes/no dichotomy based on the recalibrated probability threshold of 0.15. Using this threshold, all 12 infants who seized were correctly classified (sensitivity of 1.00 and specificity of 0.89), with an area under the curve of 0.96 (95% confidence interval 0.927–0.995; Table 3).
Table 3.
Sensitivity and specificity table for seizure prediction model
| Seized | Predicted to seize | |
|---|---|---|
| No | Yes | |
| No | 89 | 11 |
| Yes | 0 | 12 |
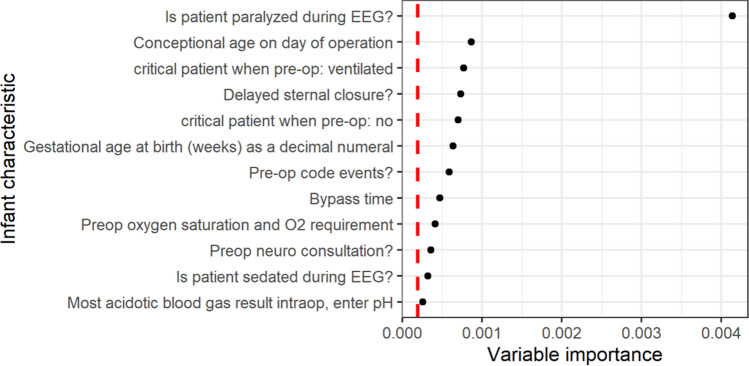
A subset of the variable importance plot derived from the random forest model is presented in Fig. 2 (full plot in Supplemental Figure 1A). The 12 variables that were identified as most important in predicting seizures were postoperative neuromuscular blockade, prematurity and corrected gestational age at surgery, delayed sternal closure, preoperative mechanical ventilation or critical preoperative status, preoperative oxygen requirement, preoperative code events, bypass duration, preoperative neurology consultation, and peak acidosis intraoperatively. When we consider variables collected during the EEG recording period as well, postoperative neuroimaging abnormalities and peak postoperative lactate were also informative in predicting seizures (Supplemental Figure 1B).
Fig. 2.
Subset of top seizure risk factors extracted from the random forest model, excluding variables captured during EEG recording. Variable importance plot of the most important variables to predict seizure risk. Magnitude of variable importance increases along the x-axis. Red dashed line represents the absolute value of the lowest scoring important variable, with values to the right of the line indicating variables more important to the outcome. EEG electroencephalogram, O2 oxygen, pre-op preoperative
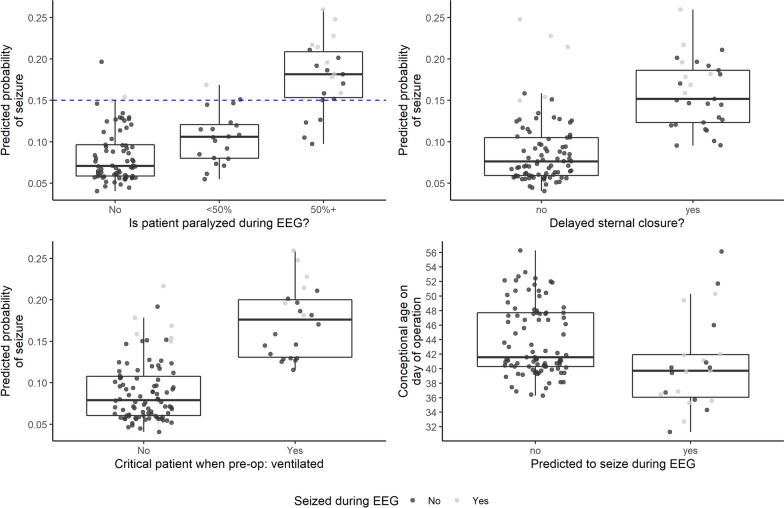
Further examination of these features identified that patients with postoperative neuromuscular blockade, surgically delayed sternal closure, critical illness preoperatively, and younger age at time of surgery have higher seizure risk (Fig. 3). If we include immediately postoperative variables obtained during EEG recording, any type of abnormal neuroimaging and higher peak lactate in the 24 h during and after surgery also contributed to seizure risk (Supplemental Figure 2).
Fig. 3.
Seizure risk factors. a–d Box plots of four most important variables in predicting seizure risk. Risk categories are on x-axis (a–c), and continuous risk variables are on y-axis (d); predicted probability of seizure is on the other axis. Patients with seizure are gray marks and patients without seizure are black marks. Threshold of significance for seizure incidence was calculated as 0.15, indicated as blue dashed line (a). Probability of seizure increased in patients with neuromuscular blockade (a), delayed sternal closure (b), preoperative mechanical ventilator dependence (c), and younger age at time of surgery (d), corrected for prematurity. EEG electroencephalogram, pre-op preoperative
Seizures and Clinical Outcomes
Presence or absence of postoperative seizures was not associated with whether lactate normalized within the 48 h after the operation (normalized in 93% patients without seizures versus 75% with seizures, Fisher’s exact test p = 0.074). Time from operation to death/transfer, death only, or transfer only were not significantly different among infants who did or did not seize (Supplemental Table 1).
Discussion
We present a large contemporary cohort of infants monitored after CPB and developed a novel predictive model based on seizure risk factors. Seizure incidence was 10.7% (12 of 112 patients), comparable with recent published findings [7–11], despite minimal DHCA at our institution (2 of 112 patients). The incidence in our cohort, which includes a spectrum of cardiac defects, is lower than was reported in studies of subpopulations considered to be at the very highest risk, such as infants with single ventricle physiology or transposition of the great arteries [13, 14].
The median time until first seizure was 28.1 h, nearly three times longer than the median time to EEG initiation (7.8 h), indicating most seizures were likely captured on EEG despite short delays in EEG initiation attributable to surgery completion, patient transport, and stabilization. Moreover, the full range of time to first seizure was 6–52 h, delineating a window of highest yield for EEG monitoring that is concordant with current ACNS guidelines [15]. In the largest prior study using EEG after infant heart surgery, the range of time to first seizure was 10–36 h [7]. A systematic review estimated that approximately 90% of seizures in children with recent cardiac arrest or cardiac surgery are detected within 24 h [19]. The majority of seizures were electrographic only, reaffirming guidelines for continuous EEG as opposed to clinical observation or intermittent EEG monitoring for accurate diagnosis.
We developed a highly sensitive and specific prediction model of seizure risk that identified 14 key risk factors for postoperative seizures in our population. Importantly, 12 of these variables are known even before the EEG recording period begins, and the other 2 can be ascertained during the recording period. The principle classes of risk include preoperative and postoperative illness severity and confirmed prior findings of risk associated with longer bypass time [13], acidosis [13], delayed sternal closure [9], younger age [8, 9], and abnormal neuroimaging [7, 9]. Preoperative code events, prematurity, mechanical ventilation, and oxygen requirement can indicate preoperative illness, whereas neuromuscular blockade, delayed sternal closure, increased bypass time, and higher lactate peak reflect intraoperative and postoperative instability. Clinically, delayed sternal closure and prolonged postoperative neuromuscular blockade are techniques to improve cardiac output and ventilation in patients with thoracic or myocardial edema, poor lung compliance, or prolonged low cardiac output state. Moreover, neuromuscular blockade can mask the motor manifestation of seizures and lead to a delay in clinical detection. Abnormal neuroimaging can indicate postoperative complications, such as infarct or hemorrhage, that may acutely trigger seizures [20]. Thus, we hypothesize that many of the most important risk factors are indicative of an overall critical condition that predisposes to brain injury and thus seizures.
Although not all these factors can be preemptively modified, many can be optimized, screened, and treated to improve neurologic outcomes. Under current guidelines, all infants undergoing CPB should be monitored with EEG regardless of the number of risk factors [15]. Identifying risk factors may help stratify patients if EEG resources are limited, identify a priori that patients’ EEGs or clinical status should be scrutinized most closely, and inform discussions with families.
In our study, intermediate outcomes of lactate trend and discharge status were not significantly associated with development of seizures. Given the small number of patients with seizures, our sample is likely too limited to detect these effects in the face of variability in clinical status, length of stay, and patient demographics. All patients in this study are offered developmental surveillance through our High-Risk Infant Follow-Up clinic, which may permit analysis of longer-term outcomes, such as development, neurologic examination, and rates of epilepsy in future studies.
Several features distinguish our study from prior evaluations of EEG monitoring after CPB. First, because very few of our patients underwent DHCA, this type of anesthesia was not a seizure risk factor in our population. This allowed us to determine alternative factors that might have been masked in previous populations. Moreover, we employed a data-driven prediction algorithm to identify important risk factors for postoperative seizures, thereby uncovering new seizure risk associations, including critical illness preoperatively, increased number of code events, elevated perioperative lactate, postoperative neuromuscular blockade, and abnormal postoperative neuroimaging. We also confirmed previously reported risk factors, including prematurity, increased duration of bypass, and delayed sternal closure. Although there are models that attempt to preemptively identify children at high risk of seizures, most postoperative infants automatically fall into the high-risk category, and thus the models do not discriminate well in this population [21–23]. Similarly, adult clinical prediction models for risk of seizures in the CVICU setting are based on clinical factors not experienced by infants [24]. There is therefore a need for a clinically based risk assessment in cardiac infants.
Our study’s first limitation is that it represents a single center and lacks a replication group for newly discovered predictive factors. Next, even though EEG was initiated rapidly, we may have missed some early seizures in the hours before EEG began. We were also unable to predict what EEG background features indicate low risk of seizure to risk stratify patients prospectively.
Our sample was moderately sized and larger than many prior reports but remained underpowered to query several specific types of risk due to the low incidence of seizures. We were therefore unable to determine whether genetic syndromes, individual cardiac malformations, or particular neuroimaging findings were associated with altered seizure risk. Of note, we did not divide cardiac lesions into cyanotic and acyanotic types as we analyzed each individual type of lesion. Patients with cyanotic lesions are considered to have higher risk of neurologic injury and hypoxia, thus it is possible that seizure risk factors may be different in a cohort restricted to cyanotic heart disease [14]. Many larger patient cohorts excluded children with genetic syndromes, potentially limiting the applicability of the results in light of the substantial prevalence of genetic syndromes in children with congenital cardiac disease. Although the classification threshold derived from the random forest model yielded high specificity and sensitivity, this threshold may not generalize to external cohorts and therefore should be further validated. We hope that combining data sets could yield larger numbers of patients and thus power to analyze these rare but clinically relevant potential risk factors.
As a predictive model, multicollinearity in our dataset does not affect the random forest model accuracy. Hypothetically, interpretation of the data could lead to incorrect conclusions that one feature is a strong predictor, whereas others in a similar category are not, when in fact they are similar in terms of their association to the outcome. However, Genuer et al. [25] found that although the magnitude of importance values was affected by collinearity, the order of importance variables remained the same with truly important variables to the outcomes still able to be differentiated from noise. Therefore, we are not concerned about multicollinearity affecting the identification of the most predictive variables in our dataset.
In our model, postoperative neuroimaging abnormalities were predictive of seizure risk. However, postoperative imaging was commonly obtained after seizures were captured to evaluate for new neurologic injury, so this relationship may be confounded by indication. Conversely, there was no relationship between preoperative neuroimaging abnormalities and seizure. A reasonable explanation is that acquired perioperative neurologic injury is a risk factor for seizures, though a causative relationship cannot be proven with our current data set.
Finally, we did not collect or analyze additional immediate, intermediate, or long-term outcomes with clinical relevance such as duration of intubation or vasoactive support, discharge neurological examination, developmental outcomes, or development of epilepsy later in life as these were not consistently available on chart review.
Postoperative seizures have been implicated in worse neurodevelopmental outcomes in many but not all cohorts. In the Boston cohort, where postoperative seizures went untreated, patients who seized had worse motor performance at 1 year old, lower IQ, more neurologic examination abnormalities at 4 years, and worse psychosocial outcomes as adolescents [26–28]. Gaynor et al. [29] also found reduced executive function and social skills at 4 years, though IQ was equivalent. In the small Norwood recipient study, seizures were associated with increased immediate mortality [14]. In contrast, there are a few reports that postoperative seizures or EEG background abnormalities did not or only minimally affected developmental outcomes [30, 31]. Overall, the clinical approach at most institutions, including ours, is to maximize neurodevelopmental outcomes by detecting and treating seizures rapidly in hopes of preventing adverse neurodevelopmental sequelae.
This study provides additional data that continuous EEG monitoring after CPB in infants identifies postoperative seizures in a significant proportion of infants. Based on the range of time to first seizure in our population, the 48 h of monitoring recommended by current guidelines are likely sufficient to capture the majority of seizures following CPB in infants. We identified novel seizure risk factors that may help institutions predict the highest risk patients if there is a need to prioritize which infants receive EEG or other services in the setting of limited resources. These risk factors warrant study in older infants and children after CPB, as well as replication in an independent cohort, prior to clinical use.
Conclusions
This study demonstrates that seizure incidence remains significant in infants who undergo CPB even without DHCA, which was previously identified as a risk factor for seizures. Postbypass infants merit continuous EEG monitoring for 48 h for seizure detection. We report several novel risk factors for seizures and confirm additional previously reported factors, enabling practitioners to identify and risk stratify patients accurately. The highest risk infants according to our study experienced preoperative oxygen or ventilatory requirements, more code events, neurology consultation, and younger age; intraoperatively, they had worse acidosis, longer bypass times, and delayed sternal closure; and in the immediate postoperative period, during EEG recording, they were under neuromuscular blockade, had a higher peak lactate, and had new abnormalities on brain imaging.
Supplementary Information
Below is the link to the electronic supplementary material.
Complete variable importance plot of all variables included in the random forest analysis. Magnitude of variable importance increases along the x-axis. Red dashed line represents the absolute value of the lowest scoring important variable, with values to the right of the line indicating variables more important to the outcome. A) Complete plot with exclusively immediate postoperative variables. B) Complete plot with variables that may occur during EEG recording. EEG, electroencephalogram (TIF 653 kb)
Seizure risk factors, including those collected during EEG recording. A–F, Box plots of six most important variables in predicting seizure risk. Risk categories are on x-axis (A–D), and continuous risk variables are on y-axis (E–F); predicted probability of seizure is on the other axis. Patients with seizure are orange marks and patients without seizure are blue marks. Threshold of significance for seizure incidence was calculated as 0.15, indicated by the blue dashed line (A). Probability of seizure increased in patients with neuromuscular blockade (A), postoperative neuroimaging abnormalities (B), delayed sternal closure (c), preoperative mechanical ventilator dependence (D), higher postoperative lactate level (E), and younger age at time of surgery (F), corrected for prematurity. EEG, electroencephalogram (TIF 192 kb)
Time to event outcomes analysis for patients with and without seizures. Abbreviations: CI 95% confidence interval; N/A indicates the median or upper limit of survival could not be calculated since >50 or 95% of the patients survived. P-value calculated from the logrank test (PDF 30 kb)
Acknowledgements
We are appreciative of support from our neurodiagnostic technologists, led by Betty Cobb, and the CVICU and neurology teams who cared for the patients. Rajani Kaimal contributed to interim statistical analysis not represented in this article.
Author Contributions
RJL and CJW designed the study and wrote the manuscript with input from all authors. RJL, EWM, MI, KRR, and AGSK collected the data. RJL and NP analyzed the data. Authorship requirements have been met, and the final manuscript was approved by all authors.
Source of support
The Maternal and Child Health Research Institute at Stanford provided support for statisticians. Research information technology (IT) grant support via Stanford Clinical and Translational Science Awards (CTSA) award number UL1 TR001085 from National Institutes of Health/National Center for Research Resources (NIH/NCRR) supported use of REDCap database.
Conflict of interest
Dr. Wusthoff reports personal fees from Persyst, unrelated to the submitted work. Drs. Levy, Mayne, Iqbal, Ryan, Sandoval Karamian, and Ms. Purington have nothing to disclose.
Ethical approval/informed consent
We confirm adherence to ethical guidelines and received ethical approvals from our institutional review board; we received a waiver of consent from patients and families because of the retrospective nature of the research.
Footnotes
This article is related to the Invite Commentary available at https://link.springer.com/article/10.1007/s12028-021-01314-0.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Venugopal P, Olszowka J, Wagner H, Vlad P, Lambert E, Subramanian S. Early correction of congenital heart disease with surface-induced deep hypothermia and circulatory arrest. J Thorac Cardiovasc Surg. 1973;66(3):375–386. doi: 10.1016/S0022-5223(19)39795-8. [DOI] [PubMed] [Google Scholar]
- 2.Brunberg JA, Doty DB, Reilly EL. Choreoathetosis in infants following cardiac surgery with deep hypothermia and circulatory arrest. J Pediatr. 1974;84(2):232–235. doi: 10.1016/S0022-3476(74)80607-4. [DOI] [PubMed] [Google Scholar]
- 3.Brunberg JA, Reilly EL, Doty DB. Central nervous system consequences in infants of cardiac surgery using deep hypothermia and circulatory arrest. Circulation. 1974;50(2 suppl):II60–II68. [PubMed] [Google Scholar]
- 4.Clarkson PM, MacArthur BA, Barratt-Boyes BG, Whitlock RM, Neutze JM. Developmental progress after cardiac surgery in infancy using hypothermia and circulatory arrest. Circulation. 1980;62(4):855–861. doi: 10.1161/01.CIR.62.4.855. [DOI] [PubMed] [Google Scholar]
- 5.Ehyai A, Fenichel GM, Bender HW. Incidence and prognosis of seizures in infants after cardiac surgery with profound hypothermia and circulatory arrest. JAMA. 1984;252(22):3165–3167. doi: 10.1001/jama.1984.03350220071035. [DOI] [PubMed] [Google Scholar]
- 6.du Plessis AJ, Jonas RA, Wypij D, Hickey PR, Riviello J, Wessel DL, et al. Perioperative effects of alpha-stat versus pH-stat strategies for deep hypothermic cardiopulmonary bypass in infants. J Thorac Cardiovasc Surg. 1997;114(6):991–1000. doi: 10.1016/S0022-5223(97)70013-8. [DOI] [PubMed] [Google Scholar]
- 7.Clancy RR, Sharif U, Ichord R, Spray TL, Nicolson S, Tabbutt S, et al. Electrographic neonatal seizures after infant heart surgery. Epilepsia. 2005;46(1):84–90. doi: 10.1111/j.0013-9580.2005.22504.x. [DOI] [PubMed] [Google Scholar]
- 8.Gaynor JW, Nicolson SC, Jarvik GP, Wernovsky G, Montenegro LM, Burnham NB, et al. Increasing duration of deep hypothermic circulatory arrest is associated with an increased incidence of postoperative electroencephalographic seizures. J Thorac Cardiovasc Surg. 2005;130(5):1278–1286. doi: 10.1016/j.jtcvs.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naim MY, Gaynor JW, Chen J, Nicolson SC, Fuller S, Spray TL, et al. Subclinical seizures identified by postoperative electroencephalographic monitoring are common after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2015;150(1):169–78. doi: 10.1016/j.jtcvs.2015.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitt B, Finckh B, Christen S, Lykkesfeldt J, Schmid ER, Bauersfeld U, et al. Electroencephalographic changes after pediatric cardiac surgery with cardiopulmonary bypass: is slow wave activity unfavorable? Pediatr Res. 2005;58(4):771–778. doi: 10.1203/01.PDR.0000180554.16652.4E. [DOI] [PubMed] [Google Scholar]
- 11.Chock VY, Reddy VM, Bernstein D, Madan A. Neurologic events in neonates treated surgically for congenital heart disease. J Perinatol. 2006;26(4):237–242. doi: 10.1038/sj.jp.7211459. [DOI] [PubMed] [Google Scholar]
- 12.Andropoulos DB, Mizrahi EM, Hrachovy RA, Stayer SA, Stark AR, Heinle JS, et al. Electroencephalographic seizures after neonatal cardiac surgery with high-flow cardiopulmonary bypass. Anesth Analg. 2010;110(6):1680–1685. doi: 10.1213/ANE.0b013e3181dd5a58. [DOI] [PubMed] [Google Scholar]
- 13.Helmers SL, Wypij D, Constantinou JE, Newburger JW, Hickey PR, Carrazana EJ, et al. Perioperative electroencephalographic seizures in infants undergoing repair of complex congenital cardiac defects. Electroencephalogr Clin Neurophysiol. 1997;102(1):27–36. doi: 10.1016/S0013-4694(96)95079-8. [DOI] [PubMed] [Google Scholar]
- 14.Gunn JK, Beca J, Penny DJ, Horton SB, d’Udekem YA, Brizard CP, et al. Amplitude-integrated electroencephalography and brain injury in infants undergoing Norwood-type operations. Ann Thorac Surg. 2012;93(1):170–176. doi: 10.1016/j.athoracsur.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Shellhaas RA, Chang T, Tsuchida T, Scher MS, Riviello JJ, Abend NS, et al. The American Clinical Neurophysiology Society’s guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28(6):611–617. doi: 10.1097/WNP.0b013e31823e96d7. [DOI] [PubMed] [Google Scholar]
- 16.Strobl C, Boulesteix A-L, Kneib T, Augustin T, Zeileis A. Conditional variable importance for random forests. BMC Bioinform. 2008;9:307. doi: 10.1186/1471-2105-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obuchowski NA. Receiver operating characteristic curves and their use in radiology. Radiology. 2003;229(1):3–8. doi: 10.1148/radiol.2291010898. [DOI] [PubMed] [Google Scholar]
- 18.Team RC . A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 19.Abend NS, Dlugos DJ, Clancy RR. A review of long-term EEG monitoring in critically ill children with hypoxic-ischemic encephalopathy, congenital heart disease, ECMO, and stroke. J Clin Neurophysiol. 2013;30(2):134–142. doi: 10.1097/WNP.0b013e3182872af9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doria JW, Forgacs PB. Incidence, implications, and management of seizures following ischemic and hemorrhagic stroke. Curr Neurol Neurosci Rep. 2019;19(7):37. doi: 10.1007/s11910-019-0957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang A, Arndt DH, Berg RA, Carpenter JL, Chapman KE, Dlugos DJ, et al. Development and validation of a seizure prediction model in critically ill children. Seizure. 2015;25:104–111. doi: 10.1016/j.seizure.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sansevere AJ, Kapur K, Peters JM, Fernández IS, Loddenkemper T, Soul JS. Seizure prediction models in the neonatal intensive care unit. J Clin Neurophysiol. 2019;36(3):186–194. doi: 10.1097/WNP.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fung FW, Parikh DS, Jacobwitz M, Vala L, Donnelly M, Wang Z, et al. Validation of a model to predict electroencephalographic seizures in critically ill children. Epilepsia. 2020;61(12):2754–2762. doi: 10.1111/epi.16724. [DOI] [PubMed] [Google Scholar]
- 24.Laccheo I, Sonmezturk H, Bhatt AB, Tomycz L, Shi Y, Ringel M, et al. Non-convulsive status epilepticus and non-convulsive seizures in neurological ICU patients. Neurocrit Care. 2015;22(2):202–211. doi: 10.1007/s12028-014-0070-0. [DOI] [PubMed] [Google Scholar]
- 25.Genuer R, Poggi J-M, Tuleau-Malot C. Variable selection using random forests. Pattern Recognit Lett. 2010;31(14):2225–36. doi: 10.1016/j.patrec.2010.03.014. [DOI] [Google Scholar]
- 26.Bellinger DC, Jonas RA, Rappaport LA, Wypij D, Wernovsky G, Kuban KC, et al. Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med. 1995;332(9):549–555. doi: 10.1056/NEJM199503023320901. [DOI] [PubMed] [Google Scholar]
- 27.Bellinger DC, Wypij D, Kuban KC, Rappaport LA, Hickey PR, Wernovsky G, et al. Developmental and neurological status of children at 4 years of age after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. Circulation. 1999;100(5):526–532. doi: 10.1161/01.CIR.100.5.526. [DOI] [PubMed] [Google Scholar]
- 28.Bellinger DC, Wypij D, Rivkin MJ, DeMaso DR, Robertson RL, Dunbar-Masterson C, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124(12):1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaynor JW, Jarvik GP, Gerdes M, Kim DS, Rajagopalan R, Bernbaum J, et al. Postoperative electroencephalographic seizures are associated with deficits in executive function and social behaviors at 4 years of age following cardiac surgery in infancy. J Thorac Cardiovasc Surg. 2013;146(1):132–137. doi: 10.1016/j.jtcvs.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller G, Rodichok LD, Baylen BG, Myers JL. EEG changes during open heart surgery on infants aged 6 months or less: relationship to early neurologic morbidity. Pediatr Neurol. 1994;10(2):124–130. doi: 10.1016/0887-8994(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 31.Gaynor JW, Jarvik GP, Bernbaum J, Gerdes M, Wernovsky G, Burnham NB, et al. The relationship of postoperative electrographic seizures to neurodevelopmental outcome at 1 year of age after neonatal and infant cardiac surgery. J Thorac Cardiovasc Surg. 2006;131(1):181–189. doi: 10.1016/j.jtcvs.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Complete variable importance plot of all variables included in the random forest analysis. Magnitude of variable importance increases along the x-axis. Red dashed line represents the absolute value of the lowest scoring important variable, with values to the right of the line indicating variables more important to the outcome. A) Complete plot with exclusively immediate postoperative variables. B) Complete plot with variables that may occur during EEG recording. EEG, electroencephalogram (TIF 653 kb)
Seizure risk factors, including those collected during EEG recording. A–F, Box plots of six most important variables in predicting seizure risk. Risk categories are on x-axis (A–D), and continuous risk variables are on y-axis (E–F); predicted probability of seizure is on the other axis. Patients with seizure are orange marks and patients without seizure are blue marks. Threshold of significance for seizure incidence was calculated as 0.15, indicated by the blue dashed line (A). Probability of seizure increased in patients with neuromuscular blockade (A), postoperative neuroimaging abnormalities (B), delayed sternal closure (c), preoperative mechanical ventilator dependence (D), higher postoperative lactate level (E), and younger age at time of surgery (F), corrected for prematurity. EEG, electroencephalogram (TIF 192 kb)
Time to event outcomes analysis for patients with and without seizures. Abbreviations: CI 95% confidence interval; N/A indicates the median or upper limit of survival could not be calculated since >50 or 95% of the patients survived. P-value calculated from the logrank test (PDF 30 kb)