Abstract
Aims
Visceral adipose tissue (AT) promotes inflammation and may be associated with disease progression in heart failure with preserved ejection fraction (HFpEF). We characterized regional AT distribution in HFpEF patients and controls and analysed associations with co‐morbidities and exercise tolerance.
Methods and results
Magnetic resonance imaging was performed to quantify epicardial, liver, abdominal, and thigh skeletal muscle AT. We assessed New York Heart Association (NYHA) class, 6 min walk distance, and global well‐being score. Multivariable linear regression models adjusting for body surface area were used. We studied 55 HFpEF patients (41 women, mean age 67 ± 11 years) and 33 controls (21 women, mean age 57 ± 10 years). Epicardial AT (median [interquartile range] 4.6 [2.0] vs. 3.2 [1.4] mm, P < 0.001), thigh intermuscular fat (11.0 [11.5] vs. 5.0 [2.7] cm2, P < 0.001) and liver fat fraction (6.4% [6.1] vs. 4.1% [5.5], P = 0.001) were higher in HFpEF patients than controls. Women with HFpEF had higher abdominal and thigh subcutaneous AT than men. Greater thigh intermuscular fat was associated with higher blood pressure (β [SE] 0.73 [0.17], P < 0.001) and diabetes (odds ratio [95% confidence interval] 1.2 [1.0–1.5], P = 0.03). Greater thigh intramuscular fat was associated with both worse NYHA class (β [SE] 2.7 [1.0], P = 0.01) and shorter 6 min walk distance (β [SE] −4.1 [1.9], P = 0.03), and greater epicardial AT (β [SE] −0.2 [0.1], P < 0.001) and liver fat fraction (β [SE] −0.4 [0.2], P = 0.04) were associated with lower global well‐being score.
Conclusions
Heart failure with preserved ejection fraction patients have increased epicardial, liver, and skeletal muscle fat compared with controls out of proportion to their increased body size, and adiposity was associated with worse NYHA class and exercise tolerance in HFpEF. These results provide the basis for further investigation into the effect of interventions to reduce regional AT distribution in relation to HFpEF symptoms and pathophysiology.
Keywords: Heart failure with preserved ejection fraction, Magnetic resonance imaging, Adipose tissue, Obesity, Risk factors, Exercise tolerance
Introducton
Heart failure with preserved ejection fraction (HFpEF) is a rapidly growing form of heart failure for which we currently lack effective therapies. 1 HFpEF is associated with multiple co‐morbid conditions, including obesity, which is an independent risk factor for mortality. 2 Exercise intolerance is a hallmark of HFpEF, leading to reduced quality of life, increased heart failure (HF) hospitalizations, and an enormous societal and economic toll. 3 The pathophysiology of exercise intolerance in HFpEF remains an active area of investigation, and prior studies suggest that non‐cardiac factors, including obesity, contribute significantly to exercise intolerance in HFpEF. 4
In patients with obesity, lipids accumulate in non‐adipose tissues such as skeletal muscle and the liver when the fat storage capacity of subcutaneous adipose tissue has been overwhelmed. 5 Excess adiposity is associated with systemic inflammation, atherogenesis, and adverse metabolic changes, which can lead to cardiovascular and peripheral (skeletal muscle) dysfunction that may contribute to the pathophysiology of exercise intolerance in HFpEF. 6 It is therefore plausible that ectopic adipose tissue may contribute to exercise intolerance and co‐morbidities in HFpEF; however, the extent to which they contribute to HFpEF pathophysiology is not well understood.
Prior studies have reported that increased total body adiposity contributes to the impaired peak VO2 in HFpEF patients with obesity. 3 , 7 Moreover, recent studies also suggest that apart from total quantity of adipose tissue, regional adipose tissue distribution in the skeletal muscle, abdomen, and epicardial regions may be an important determinant of impaired cardiac function and reduced exercise tolerance in HFpEF. 4 , 8 , 9 Additionally, studies evaluating liver adiposity have shown that increased liver fat content is associated with the development of atrial fibrillation and increased mortality in HFpEF; however, the role of liver adiposity in exercise tolerance in HFpEF is not well understood. 10 Lastly, clinical studies have shown worse diastolic reserve and exercise haemodynamics in women compared with those in men with HFpEF. 11 However, the pathophysiologic differences contributing to sex differences in HFpEF, such as regional adipose tissue distribution, are not well characterized.
Therefore, the aim of this study was four‐fold: (i) to characterize regional adipose distribution in patients with HFpEF compared with controls; (ii) to evaluate sex differences in adipose tissue content and distribution, (iii) to assess the relationship between HFpEF risk factors and regional adipose tissue distribution, and (iv) to examine the associations between adipose tissue depots and measures of functional capacity and exercise intolerance.
Methods
Study population
HFpEF patients were enrolled prospectively between September 2016 and November 2019 from the HFpEF clinics at Johns Hopkins University and Northwestern University (NU) (Supporting Information, Figure S1 ). Participants with HFpEF were included if they met the following criteria: age >21 years, left ventricular ejection fraction (LVEF) ≥45% within the preceding 6 months, and symptoms of HF [New York Heart Association (NYHA) Class II–IV] at the time of enrolment. A diagnosis of HFpEF was made based on the presence of HF as defined by the Framingham criteria for HF 12 with LVEF ≥45% and at least two of the following: (i) structural heart disease as evidenced by left ventricular (LV) hypertrophy or left atrial enlargement, (ii) N terminal pro brain natriuretic peptide ≥100 pg/mL, or (iii) elevated pulmonary capillary wedge pressures on hemodynamic assessment (≥15 mmHg at rest or ≥25 mmHg with exercise). Patients were excluded if they had any prior echocardiogram with EF < 40%, were currently pregnant or nursing, or had a history of infiltrative or restrictive cardiomyopathy, history of hypertrophic cardiomyopathy, active myocarditis, constrictive pericarditis, congenital heart disease, isolated pulmonary arterial hypertension, significant valvular regurgitation or stenosis, systolic blood pressure <100 mmHg, or need for intravenous inotropic medication or mechanical circulatory support. Control participants (n = 17) with no history of HF and no symptoms of HF were enrolled from the NU adult general cardiology clinic. Additional control participants (n = 16) were recruited from outpatient internal medicine clinics at the Johns Hopkins Hospital. The study was approved by the institutional review boards of both Johns Hopkins University and NU. All participants provided written informed consent.
Demographic and clinical data
At the initial study visit, detailed demographic and medical history were collected using a standardized questionnaire, and physical examination was performed. Body mass index (BMI) was calculated as weight (kg)/height (m)2; obesity was defined as BMI ≥30 kg/m2. Body surface area (BSA) was calculated as the square root of the product of height (m) and weight (kg) divided by 3600. Tobacco use, menopause history, medications, and medical history including atrial fibrillation/flutter, hypertension, hyperlipidemic, diabetes, and obstructive sleep apnoea (OSA) were assessed from clinical documentation at the most recent clinic evaluation and verified with the patient at the initial study visit. Significant coronary artery disease was defined as history of myocardial infarction, ≥50% coronary stenosis on coronary angiography, or prior coronary revascularization including percutaneous coronary intervention or coronary artery bypass surgery. Laboratory data closest to the time of the initial study visit that were available at the time of the visit were recorded. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD‐EPI) equation, 13 and chronic kidney disease was defined as eGFR <60 mL/min/1.73 m2.
Magnetic resonance imaging
Subjects were scanned in supine position using 1.5‐T whole body magnetic resonance imaging (MRI) systems (Espree or Aera, Siemens, Erlangen, Germany). Regional scans of the heart, abdomen, and thigh were performed. Full details regarding the MRI acquisition protocols can be found in the supporting information.
For cardiac adipose tissue quantification, a balanced steady‐state free precession CINE was acquired in a four‐chamber orientation during a single breath‐hold. Epicardial adipose tissue (EAT) thickness was quantified during end‐diastole using two methods: (i) measuring the maximal thickness in the atrioventricular (AV) groove adjacent to the right ventricle (RV) and (ii) averaging the thickness measured at three points along the RV free wall, as previously described (Figure 1 A ). 14 Quantification was performed using OsiriX (Version 9.0, Pixmeo SARL, Geneva, Switzerland).
Figure 1.
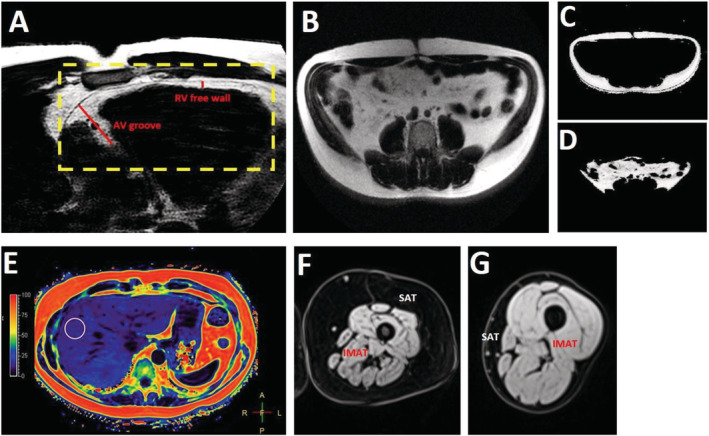
Adipose tissue quantification using cardiac magnetic resonance imaging. (A) Epicardial adipose tissue (EAT) was measured at end‐diastole in a cine image in the four‐chamber orientation at the atrioventricular (AV) groove and averaged at three points along the right ventricular (RV) free wall. (B) An axial slice at the fourth lumbar vertebra (L4) was used for abdominal adipose tissue quantification. A semi‐automated MATLAB algorithm was used to segment the abdominal fat into subcutaneous (C) and visceral (D) components. (E) Liver fat fraction was estimated by averaging the magnetic resonance imaging proton‐density fat fraction in three regions of interest drawn in three separate axial slices. (F) Thigh subcutaneous adipose tissue (SAT) was manually segmented, and a MATLAB tool was used to determine the intermuscular adipose tissue (IMAT). (G) The same process was repeated for controls.
Abdominal images were taken as axial sections every 3 cm from T10 to the sacral vertebra. A single slice at the level of L4 was used to determine abdominal subcutaneous adipose tissue (SAT) and intra‐abdominal visceral adipose tissue (VAT) (Figure 1 B ). This method has been shown to have high association with total visceral fat comparents. 15 A custom‐made MATLAB (MathWorks, Natick, MA, USA) tool was used to segment abdominal fat into separate SAT and VAT compartments using a semi‐automated algorithm (Figure 1 C,D ). Abdominal VAT was defined as fat bounded by the abdominal muscle wall, including fat within the mesentery and omentum. SAT was defined as fat lying outside the abdominal wall. Additional regions of interest (ROIs) were drawn on bilateral paraspinal and psoas muscles at the level of L4 to quantify skeletal muscle cross‐sectional area. 16 , 17
For liver fat quantification, MRI‐based proton‐density fat fraction (PDFF) has been shown to be an accurate method to quantify liver fat with high reproducibility and interobserver agreement. 18 ROIs measuring 5 cm2 were drawn over the liver in the MRI‐PDFF maps on three separate axial slices and averaged, avoiding blood vessels, the gallbladder, or discrete lesions (Figure 1 E ). PDFF is expressed as a percentage as a measure of degree of liver steatosis. 15
Thigh imaging was performed with a 3D multi‐echo Dixon acquisition in transverse orientation superior to the inferior head of the femur. A custom‐made MATLAB tool was used to determine the following thigh fat measures similar to measurements in prior studies of HFpEF. 8 , 19 Thigh SAT area was defined as the cross‐sectional area of fat exterior to the muscle in the subcutaneous space (Figure 1 F,G ). Total thigh muscle area was the total cross‐sectional muscle area inside the muscle‐subcutaneous fat border. The fat/water percentages of all muscle pixels were averaged to determine the thigh intermuscular adipose tissue (IMAT) fat fraction (FF). IMAT cross‐sectional area was calculated from IMAT FF multiplied by the total muscle area. The intermuscular fat (interMF) area, corresponding to the fat between the muscle bundles, was defined as the area of muscle pixels with a fat/water percentage >50%. 19 The intramuscular fat (intraMF) area was defined as the cross‐sectional area of the remaining fat within the muscles such that intraMF area + interMF area = IMAT area.
Clinical assessments and measures of functional capacity
Standardized 6 min walk test distance (6MWD) was administered in the HFpEF patients as previously described. 20 Overall functional status was assessed via patient self‐report using the global well‐being (GWB) visual analog scale. 21 HFpEF patients also underwent supine bicycle exercise testing, starting at 25 Watts (W) workload and increasing by 25 W every 3 min until exhaustion. Total time elapsed on during the test was recorded.
Statistical analysis
Baseline patient characteristics were reported as mean (standard deviation), median [interquartile range], or frequency (percentage). Differences between groups were tested using the T test or Wilcoxon rank‐sum test for continuous variables and χ 2 or Fisher's exact tests for categorical variables.
Multivariable‐adjusted linear regression models were used to determine the associations between fat/muscle measures and HFpEF status, co‐morbidities, and functional capacity. Measures of fat/muscle composition were modelled as the outcome variables for the analyses comparing HFpEF to controls, adjusted for age, sex, and BSA. Within HFpEF patients, co‐morbidities (systolic BP, diabetes, OSA, and atrial fibrillation), and functional capacity measures (NYHA functional class, 6MWD, GWB, and exercise time) were modelled as the outcome variables for linear or logistic regression analyses, with measures of fat/muscle composition as the exposure variables. Logistic regression was used for the following variables: diabetes, OSA, atrial fibrillation, and NYHA class. Certain variables were log‐transformed due to skew: RV EAT, liver FF, abdominal VAT/SAT ratio, thigh SAT area, thigh IMAT area, thigh IMAT FF, thigh interMF area, thigh intraMF area, psoas muscle area, and exercise time. GWB score was transformed using inverse normal transformation. To explore sex differences, we compared differences in measures of fat/muscle composition, functional status, and exercise tolerance by sex, adjusted for age and BSA. We additionally tested for interactions with sex in the associations between fat/muscle measures and exercise tolerance.
Statistical analysis was performed using Statistical Analysis Software (Version 9.4, SAS Institute Inc., Cary, NC, USA).
Results
Participant characteristics
Clinical characteristics of our sample including 55 HFpEF patients and 33 controls are shown in Table 1 . HFpEF patients were on average older than controls. There was no significant sex difference between the two groups. Among HFpEF patients, 41 (75%) were women, compared with 21 (64%) women among controls (P = 0.28). Race was also similar between the two groups: 56% White and 44% Black participants in the HFpEF group and 70% White and 27% Black participants among controls (P = 0.11). HFpEF patients had a higher prevalence of co‐morbidities typically associated with HFpEF, including obesity, hypertension, diabetes, OSA, and CKD.
Table 1.
Participant characteristics
| HFpEF (n = 55) | Control (n = 33) | P | |
|---|---|---|---|
| Age, years | 67.5 (10.9) | 57.2 (10.1) | <0.001 |
| Female (%) | 41 (74.6) | 21 (63.6) | 0.28 |
| Race (%) | |||
| White | 31 (56.4) | 23 (69.7) | 0.11 |
| Black | 24 (43.6) | 9 (27.3) | |
| Body mass index, kg/m2 | 37.3 (9.8) | 27.3 (6.5) | <0.001 |
| Body surface area, m2 | 2.1 (0.3) | 1.9 (0.2) | <0.001 |
| Obesity (%) | 43 (78.2) | 9 (27.3) | <0.001 |
| Smoking (%) | 22 (40) | 8 (24.2) | 0.10 |
| Hypertension (%) | 53 (96.4) | 23 (69.7) | <0.001 |
| Systolic blood pressure, mmHg | 126.3 (15.8) | 133.3 (19.9) | 0.07 |
| Diastolic blood pressure, mmHg | 70.2 (7.5) | 76.2 (12.5) | 0.02 |
| Hyperlipidaemia (%) | 43 (79.6) | 20 (60.6) | 0.05 |
| Atrial fibrillation/flutter (%) | 15 (27.8) | 9 (27.3) | 0.96 |
| Diabetes (%) | 33 (60.0) | 5 (15.2) | <0.001 |
| Haemoglobin A1c, % | 6.8 (1.6) | 5.7 (0.5) | 0.001 |
| Coronary artery disease (%) | 7 (13.2) | 10 (30.3) | 0.05 |
| Obstructive sleep apnoea (%) | 35 (63.6) | 5 (15.2) | <0.001 |
| Chronic kidney disease (%) | 34 (61.8) | 12 (36.4) | 0.02 |
| eGFR, mL/min/1.73 m2 | 53.6 (21.1) | 77.4 (20.8) | <0.001 |
| Medications (%) | |||
| NT‐proBNP, pg/mL | 691.9 (1153.9) | N/A | |
| Beta‐blocker | 32 (58.2) | 9 (27.3) | 0.005 |
| Calcium channel blocker | 13 (23.6) | 5 (29.4) | 0.63 |
| ACE inhibitor | 15 (27.3) | 9 (27.3) | >0.99 |
| Angiotensin receptor blocker | 14 (25.9) | 4 (12.1) | 0.17 |
| Loop diuretic | 50 (90.9) | 0 (0) | <0.001 |
| Thiazide diuretic | 4 (7.3) | 3 (15.8) | 0.36 |
| Statin | 43 (78.2) | 18 (54.6) | 0.02 |
ACE, angiotensin‐converting enzyme; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; N/A, not available; NT‐proBNP, N terminal pro brain natriuretic peptide.
Data reported in mean (SD) or number (percentage).
Adipose tissue distribution and skeletal muscle composition
Compared with controls, HFpEF patients had higher EAT measured both along the RV free wall and in the AV groove; higher abdominal VAT, SAT, and VAT/SAT ratio; higher liver FF; higher thigh SAT, IMAT, IMAT FF, interMF, and intraMF; and higher paraspinal muscle area (Table 2 ). After adjusting for age, sex, and BSA, RV EAT, liver FF, thigh interMF area, and paraspinal muscle area remained significantly higher among HFpEF patients than controls. We found no interaction with sex in the association between HFpEF status and regional adiposity or muscle composition (Supporting Information, Table S1 ). We further evaluated for sex differences in adipose tissue distribution and muscle composition among HFpEF patients only (Table 3 ). Women had higher abdominal and thigh SAT area and lower abdominal VAT/SAT ratio and psoas muscle area than men. EAT and liver FF were not statistically significantly different between men and women with HFpEF. Analyses adjusting for BMI, instead of BSA, are shown in Supporting Information, Tables S2 and S3 and demonstrate similar results to those adjusting for BSA.
Table 2.
Adipose tissue distribution and skeletal muscle composition in HFpEF patients and controls
| HFpEF (N = 55) | Control (N = 33) | Unadjusted data | Adjusted data a | ||||
|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | P | β (SE) | P | |
| RV EAT, mm | 4.6 | 3.5–5.5 | 3.2 | 2.5–3.9 | <0.001 | 0.2 (0.1) | 0.03 |
| AV groove EAT, mm | 19.2 | 14.9–24.5 | 14.9 | 13.6–17.4 | 0.001 | 2.8 (1.6) | 0.08 |
| Liver FF, % | 6.4 | 4.2–10.3 | 4.1 | 0.9–6.4 | 0.001 | 0.5 (0.2) | 0.04 |
| Abdominal VAT, cm2 | 201.2 | 108.2–242.8 | 76.8 | 52.6–142.2 | <0.001 | 22.1 (20.0) | 0.27 |
| Abdominal SAT, cm2 | 388.0 | 267.1–517.1 | 247 | 154.3–328.8 | 0.001 | 52.6 (30.9) | 0.09 |
| Abdominal VAT/SAT ratio | 0.48 | 0.37–0.69 | 0.36 | 0.3–0.5 | 0.03 | 0.02 (0.15) | 0.87 |
| Thigh SAT, cm2 | 182.3 | 105.2–258.9 | 123.5 | 75.4–163 | 0.002 | 0.12 (0.12) | 0.34 |
| Thigh IMAT, cm2 | 27.0 | 16–42 | 15.8 | 15.1–21.6 | 0.06 | 0.29 (0.19) | 0.12 |
| Thigh IMAT FF, % | 22.3 | 16.2–28.9 | 14.3 | 11.8–17.4 | 0.006 | 0.33 (0.18) | 0.06 |
| Thigh interMF, cm2 | 11.0 | 7.2–18.1 | 5.0 | 4.1–6.8 | <0.001 | 0.60 (0.20) | 0.003 |
| Thigh intraMF, cm2 | 16.5 | 10.8–24.8 | 11.7 | 10.8–16 | 0.11 | 0.17 (0.13) | 0.18 |
| Thigh muscle, cm2 | 124.4 | 102.4–157.5 | 122.5 | 93.6–143.6 | 0.71 | −2.98 (15.32) | 0.85 |
| Psoas muscle, cm2 | 16.4 | 13.7–20.6 | 12.3 | 8.3–19.9 | 0.06 | 0.17 (0.10) | 0.10 |
| Paraspinal muscle, cm2 | 34.7 | 29.8–43.4 | 21.1 | 18.1–31.7 | <0.001 | 7.57 (3.01) | 0.01 |
AV, atrioventricular; EAT, epicardial adipose tissue; FF, fat fraction; HFpEF, heart failure with preserved ejection fraction; IMAT, intermuscular adipose tissue; interMF, intermuscular fat; intraMF, intramuscular fat; IQR, interquartile range; IQR, interquartile range; RV, right ventricular; SAT, subcutaneous adipose tissue; SE, standard error; VAT, visceral adipose tissue.
Analyses are adjusted for age, sex, and body surface area. Positive β coefficients indicate higher values in HFpEF patients than controls.
Table 3.
Adipose tissue distribution and skeletal muscle composition in male and female HFpEF patients
| Male (N = 14) | Female (N = 41) | β (SE) | P | |||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | |||
| RV EAT, mm | 4.0 | 3.6−5 | 4.7 | 3.5−5.9 | 0.17 (0.10) | 0.09 |
| AV groove EAT, mm | 19.3 | 17.5−27 | 19.0 | 14.5−21.7 | −1.57 (1.69) | 0.35 |
| Liver fat fraction, % | 5.2 | 3.8−10.3 | 6.7 | 4.2−13 | 0.11 (0.18) | 0.55 |
| Abdominal VAT, cm2 | 227.3 | 186.2−260 | 176.0 | 92.8−241 | 1.98 (22.02) | 0.93 |
| Abdominal SAT, cm2 | 297.3 | 249.7−344.5 | 443.9 | 278.4−535 | 182.51 (31.42) | <0.001 |
| Abdominal VAT/SAT ratio | 0.73 | 0.49−0.89 | 0.44 | 0.29−0.61 | −0.56 (0.15) | <0.001 |
| Thigh SAT, cm2 | 112.3 | 63−142 | 228.6 | 112.8−287.8 | 0.87 (0.11) | <0.001 |
| Thigh IMAT, cm2 | 29.8 | 25.3−35.9 | 26.7 | 15.8−42 | 0.09 (0.17) | 0.60 |
| Thigh IMAT FF, % | 20.1 | 16.5−25.6 | 23.3 | 12.7−29.2 | 0.21 (0.16) | 0.21 |
| Thigh interMF, cm2 | 12.4 | 7−15.5 | 11.9 | 8.4−19 | 0.32 (0.19) | 0.10 |
| Thigh intraMF, cm2 | 18.6 | 10.8−26.7 | 16.2 | 11.2−24.1 | 0.12 (0.12) | 0.30 |
| Thigh muscle, cm2 | 143.7 | 121.9−173.8 | 117.0 | 96.7−136.5 | −7.00 (13.26) | 0.60 |
| Psoas muscle, cm2 | 27.0 | 21.4−31.8 | 14.5 | 12.4−17.4 | −0.48 (0.07) | <0.001 |
| Paraspinal muscle, cm2 | 40.2 | 33.1−45.8 | 34.4 | 29.5−40.3 | −2.82 (2.55) | 0.27 |
AV, atrioventricular; EAT, epicardial adipose tissue; FF, fat fraction; HFpEF, heart failure with preserved ejection fraction; IMAT, intermuscular adipose tissue; interMF, intermuscular fat; intraMF, intramuscular fat; IQR, interquartile range; RV, right ventricular; SAT, subcutaneous adipose tissue; SE, standard error; VAT, visceral adipose tissue.
Analyses are adjusted for age and body surface area. Positive β coefficients indicate higher values in female HFpEF patients than male.
Associations between heart failure with preserved ejection fraction risk factors and measures of adipose tissue distribution and skeletal muscle composition
There were several cardiovascular risk factors observed to be associated with increased visceral fat stores and adverse adipose tissue distribution. In unadjusted analyses, diabetes was associated with higher EAT (AV groove EAT: 20.7 ± 6.6 vs. 17.6 ± 5.9 mm, P = 0.05) and abdominal VAT (217.9 ± 109.2 vs. 151.4 ± 78.5 cm2, P = 0.03). After adjustment for age, sex and BSA, higher systolic blood pressure and history of diabetes were associated with greater thigh interMF area (Table 4 ). Additionally, a history of OSA, common in obese HFpEF patients, was associated with lower thigh IMAT FF in adjusted analyses, and with higher abdominal VAT in unadjusted analyses (222.7 ± 98.5 vs. 136.0 ± 85.7 cm2, P = 0.006). Similar results were obtained after adjusting for BMI (Supporting Information, Table S4 ).
Table 4.
Associations a between HFpEF risk factors with adipose tissue and skeletal muscle measures
| Systolic blood pressure | Diabetes | Atrial fibrillation/flutter | Obstructive sleep apnoea | |||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | P | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | |
| RV EAT, mm | −0.64 (0.83) | 0.44 | 0.93 (0.71–1.23) | 0.62 | 0.70 (0.43–1.11) | 0.13 | 0.87 (0.64–1.20) | 0.40 |
| AV groove EAT, mm | 0.008 (0.31) | 0.98 | 1.06 (0.95–1.18) b | 0.29 | 1.07 (0.95–1.21) | 0.27 | 0.97 (0.86–1.10) | 0.67 |
| Liver fat fraction, % | 0.22 (0.27) | 0.42 | 0.99 (0.91–1.08) | 0.85 | 1.00 (0.90–1.11) | 0.97 | 1.01 (0.91–1.12) | 0.86 |
| Abdominal VAT, cm2 | −0.002 (0.03) | 0.93 | 1.00 (0.99–1.01) b | 0.48 | 1.00 (0.99–1.01) | 0.47 | 1.00 (1.00–1.01) b | 0.32 |
| Abdominal SAT, cm2 | −0.04 (0.02) | 0.06 | 1.00 (0.99–1.00) | 0.34 | 1.00 (0.99–1.01) | 0.51 | 1.00 (0.99–1.00) | 0.30 |
| VAT/SAT ratio | 6.25 (7.13) | 0.38 | 6.13 (0.44–85.58) | 0.18 | 1.30 (0.09–18.72) | 0.85 | 11.55 (0.45–298.80) | 0.14 |
| Thigh SAT, cm2 | 0.01 (0.03) | 0.74 | 0.99 (0.98–1.00) | 0.12 | 1.01 (1.00–1.02) | 0.11 | 1.00 (0.99–1.01) | 0.75 |
| Thigh IMAT, cm2 | 0.06 (0.12) | 0.61 | 1.01 (0.97–1.06) | 0.60 | 1.03 (0.97–1.09) | 0.40 | 0.96 (0.91–1.01) | 0.12 |
| Thigh IMAT FF, % | 0.30 (0.21) | 0.16 | 0.99 (0.92–1.06) | 0.69 | 1.04 (0.96–1.13) | 0.29 | 0.87 (0.77–0.98) | 0.02 |
| Thigh interMF, cm2 | 0.73 (0.17) | <0.001 | 1.22 (1.03–1.46) | 0.03 | 0.95 (0.83–1.09) | 0.46 | 0.96 (0.86–1.07) | 0.45 |
| Thigh intraMF, cm2 | 0.18 (0.29) | 0.53 | 1.01 (0.92–1.11) | 0.89 | 1.14 (0.99–1.31) | 0.07 | 0.96 (0.85–1.06) | 0.37 |
| Thigh muscle, cm2 | 0.003 (0.04) | 0.94 | 1.00 (0.98–1.01) | 0.67 | 0.98 (0.96–1.01) b | 0.17 | 1.02 (1.00–1.04) | 0.06 |
| Psoas muscle, cm2 | 0.81 (0.56) | 0.15 | 1.03 (0.84–1.25) | 0.79 | 0.95 (0.77–1.19) | 0.68 | 0.95 (0.77–1.17) | 0.62 |
| Paraspinal muscle, cm2 | −0.11 (0.33) | 0.74 | 1.03 (0.92–1.15) | 0.61 | 0.98 (0.85–1.12) | 0.75 | 0.97 (0.86–1.09) | 0.60 |
AV, atrioventricular; CI, confidence interval; EAT, epicardial adipose tissue; FF, fat fraction; HFpEF, heart failure with preserved ejection fraction; IMAT, intermuscular adipose tissue; interMF, intermuscular fat; intraMF, intramuscular fat; OR, odds ratio; RV, right ventricular; SAT, subcutaneous adipose tissue; SE, standard error; VAT, visceral adipose tissue.
Analyses are adjusted for age, sex, and body surface area. Positive β coefficients indicate higher values of adipose tissue/skeletal muscle measurement associated with the risk factor.
Associations significant in unadjusted analyses.
Associations of adipose tissue and skeletal muscle measures with functional status and exercise capacity
Several visceral fat depots were associated with more advanced symptoms and worse markers of exercise tolerance in HFpEF. Higher thigh intraMF was associated with a higher NYHA functional class and shorter 6MWD, even after adjusting for age, sex, and BSA (Table 5 ). Higher thigh IMAT FF, a quantitative measure of fat replacing skeletal muscle, was also associated with a higher NYHA class, corresponding to worse heart failure symptoms. Higher thigh SAT was associated with higher NYHA class (149.1 ± 70.6 cm2 for NYHA Class II vs. 223.0 ± 111.0 cm2 for NYHA Class III/IV, P = 0.03) and shorter 6MWD (R = −0.36, P = 0.01) in unadjusted analyses. RV EAT was associated with a lower GWB score (R = −0.37, P = 0.01) and shorter 6MWD (R = −0.32, P = 0.03); however, only the inverse association with GWB score remained after adjustment. Liver FF was associated with a lower GWB score. None of the adipose tissue or muscle measures were associated with bicycle exercise time in the adjusted model. Results were similar after adjusting for BMI instead of BSA (Supporting Information, Table S5 ). Men exercised significantly longer on supine bicycle testing than women, but there were no statistically significant differences in NYHA class, 6MWD, or GWB score by sex (Supporting Information, Table S6 ). We further assessed for interactions with sex among the associations found to be statistically significant in the above analyses. There was a significant interaction with sex in the association between RV EAT and GWBS (P = 0.007). The correlation coefficient between RV EAT and GWBS was −0.83 (P < 0.001) among men and −0.23 (P = 0.19) among women (Supporting Information, Table S7 ). Adjusted analyses to obtain sex‐specific model estimates using could not be performed due to the small sample size of men.
Table 5.
Associations a between adipose tissue and skeletal muscle measures and exercise capacity
| NYHA class (II vs. III/IV) | 6 m walk distance | Global well‐being score | Exercise time | |||||
|---|---|---|---|---|---|---|---|---|
| β (SE) | P | β (SE) | P | β (SE) | P | β (SE) | P | |
| RV EAT, mm | 1.12 (0.97) | 0.25 | −4.56 (5.51) b | 0.41 | −0.17 (0.05) | 0.002 | −0.03 (0.04) | 0.36 |
| AV groove EAT, mm | −0.01 (0.06) | 0.80 | −1.18 (1.99) | 0.55 | −0.01 (0.02) | 0.49 | 0.01 (0.02) | 0.49 |
| Liver fat fraction, % | 0.34 (0.53) | 0.52 | 2.24 (1.75) | 0.20 | −0.43 (0.21) | 0.04 | 0.002 (0.02) | 0.89 |
| Abdominal VAT, cm2 | −0.003 (0.004) | 0.44 | 0.12 (0.17) | 0.49 | 0.002 (0.002) | 0.19 | 0.001 (0.001) | 0.38 |
| Abdominal SAT, cm2 | −0.002 (0.003) | 0.63 | 0.11 (0.13) | 0.41 | −0.001 (0.001) | 0.92 | −0.001 (0.001) | 0.23 |
| VAT/SAT ratio | 0.30 (0.59) | 0.62 | 0.12 (0.17) | 0.49 | 0.80 (0.48) | 0.09 | 0.34 (0.33) b | 0.30 |
| Thigh SAT, cm2 | −0.56 (0.84) b | 0.50 | −0.17 (0.19) b | 0.37 | 0.002 (0.002) | 0.39 | −0.002 (0.001) | 0.25 |
| Thigh IMAT, cm2 | 1.13 (0.62) b | 0.07 | −0.70 (0.80) | 0.38 | −0.01 (0.08) | 0.44 | 0.001 (0.01) | 0.84 |
| Thigh IMAT FF, % | 1.29 (0.65) | 0.047 | −1.49 (1.39) | 0.28 | −0.01 (0.01) | 0.68 | 0.001 (0.01) | 0.90 |
| Thigh interMF, cm2 | −0.58 (0.73) | 0.43 | 0.09 (1.22) | 0.94 | 0.002 (0.01) | 0.86 | 0.007 (0.01) | 0.41 |
| Thigh intraMF, cm2 | 2.46 (0.99) | 0.01 | −4.08 (1.88) | 0.03 | 0.002 (0.02) | 0.93 | 0.004 (0.01) | 0.77 |
| Thigh muscle, cm2 | 0.002 (0.01) | 0.77 | 0.36 (0.26) | 0.18 | 0.004 (0.003) | 0.89 | −0.002 (0.003) | 0.42 |
| Psoas muscle, cm2 | −2.79 (1.93) | 0.15 | −5.03 (3.45) | 0.15 | 0.02 (0.04) | 0.58 | −0.03 (0.03) | 0.20 |
| Paraspinal muscle, cm2 | 0.03 (0.060) | 0.63 | −2.44 (1.97) | 0.22 | −0.02 (0.31) | 0.31 | −0.02 (0.01) | 0.20 |
AV, atrioventricular; EAT, epicardial adipose tissue; FF, fat fraction; IMAT, intermuscular adipose tissue; interMF, intermuscular fat; intraMF, intramuscular fat; NYHA, New York Heart Association; RV, right ventricular; SAT, subcutaneous adipose tissue; SE, standard error; VAT, visceral adipose tissue.
Analyses are adjusted for age, sex, and body surface area. Positive β coefficients indicate higher values of adipose tissue/skeletal muscle measurement associated with the functional assessment.
Associations significant in unadjusted analyses
Discussion
In this study, we observed that adverse patterns of regional adiposity were greater in HFpEF patients compared with controls and that specific measures of visceral adipose tissue in the epicardial, liver, and thigh compartments remained higher in HFpEF patients even after accounting for age, sex, and body size. Women had more subcutaneous adiposity in both the abdominal and thigh compartments than men. We also found that there were significant associations between regional adiposity and common HFpEF co‐morbidities including diabetes, systolic blood pressure, and OSA. Lastly, our study demonstrated that measures of epicardial, liver, and thigh adiposity were associated with worse measures of functional status and exercise capacity. These results not only are consistent with prior important studies of the role of visceral fat in exercise tolerance but also further our current knowledge of the characterization and role of adiposity in HFpEF.
Regional adipose tissue distribution
In our study, we found that almost all measures of adiposity were higher among HFpEF patients than controls in unadjusted analyses, but that only certain adipose tissue measurements, namely, visceral fat stores reflected by RV EAT, liver FF, and thigh interMF, remained higher among HFpEF patients after adjustment for body size. This suggests that there are particular ectopic fat depots that are enriched in HFpEF patients beyond what can be accounted for with body size alone. Prior literature demonstrated that measures of EAT among a non‐HFpEF sample were similar with those among controls our study: 3.8 mm for RV EAT and 14.4–16.3 mm for AV groove EAT. 14 , 22 , 23 , 24 Additionally, measures of abdominal subcutaneous and visceral fat, as well as thigh subcutaneous and intra/intermuscular fat, in our control sample were comparable with those reported in prior literature. 8 , 25 , 26 Further research is needed to assess whether these findings contribute to the increased morbidity, cardiovascular events, and hospitalizations experienced by HFpEF patients.
There is growing evidence that in addition to the greater degree of adiposity, the location of fat depots is unique in HFpEF patients and may be important in their pathophysiology. 6 , 7 , 8 , 9 EAT has been postulated to release proinflammatory adipokines and has been associated with increased diastolic dysfunction, coronary endothelial dysfunction, and myocardial fibrosis. 27 There have been conflicting data in prior literature on whether EAT is higher or lower in HFpEF patients compared with controls. 7 , 8 , 9 In our study, only EAT measured at lateral to the RV free wall, and not EAT in the AV groove, was significantly higher in HFpEF patients after adjustment for BSA. Van Woerden et al. have previously shown that ventricular epicardial fat was higher in HFpEF patients than controls, but atrial epicardial fat was similar. 9 These data and ours suggest that local EAT associated with the ventricles, and not EAT associated with the atria, may be preferentially increased in HFpEF patients beyond what can be explained with increased body size alone.
In addition to increased EAT, we also found that liver FF was significantly higher among HFpEF patients than controls independent of BSA. To our knowledge, comparisons of liver fat content in HFpEF patients and controls have not previously been assessed. Prior population studies have demonstrated that individuals with non‐alcoholic fatty liver disease have more evidence of subclinical diastolic dysfunction and LV hypertrophy. 28 The increased liver FF in HFpEF patients suggests that adipose tissue deposition in the liver may be involved in the pathogenesis of HFpEF. Additionally, more severe liver fibrosis is associated with increased mortality in HFpEF. 29 Therefore, recognition of the increased possibility of liver dysfunction in HFpEF patients may be important clinically.
Sex differences in adipose tissue distribution and skeletal muscle composition
Our study showed that among HFpEF patients, women had higher abdominal and thigh SAT and lower abdominal VAT/SAT ratio than men. Women also had smaller psoas muscle area compared with men even after adjusting for BSA. Prior studies have shown that myocardial structure and function in women are different than that of men, and that women have poorer diastolic reserve and higher filling pressures with exercise. 11 Additionally, BMI has been shown to be differentially associated with HFpEF rather than HFrEF in women, but not in men. 30 However, sex differences in regional adipose tissue distribution and skeletal muscle composition in HFpEF patients are not well‐studied. In the general population, men are more likely to accumulate fat in the abdominal visceral region, and women are more likely to accumulate fat in the subcutaneous tissue, 31 as we confirmed in this study of a HFpEF cohort. Although HFpEF is nearly twice as prevalent among older women as men, visceral but not subcutaneous fat has previously been shown to be a predictor of incident HFpEF. 4 , 32 Additionally, despite the adverse exercise haemodynamics in women compared with those in men shown in prior studies, measures of epicardial, liver, and skeletal muscle fat were not different between men and women in our study. Moreover, we found no sex differences in the relationship between HFpEF status and regional adipose tissue distribution. Our results do not provide evidence that regional adiposity contributes to sex differences in HFpEF prevalence, and additional research will be necessary to further elucidate this relationship.
Heart failure with preserved ejection fraction risk factors and adiposity
Co‐morbid conditions and risk factors for HFpEF have been shown to contribute to exercise intolerance and may also be associated with increased regional adiposity. Insulin resistance has been associated with accumulation of VAT and SAT and dysfunctional fatty acid metabolism. 33 Among HFpEF patients, epicardial fat has been found to be significantly higher in those with diabetes compared with those without. 9 We found that presence of diabetes was associated with several measures of adiposity, including higher AV groove EAT, abdominal VAT, and thigh interMF, of which the association with thigh interMF remained significant after adjustment for body size. Diabetes is thought to contribute to HFpEF pathogenesis by inducing a proinflammatory milieu both systemically and locally in the coronary microvascular endothelium. 34 Whether excess VAT mediates this inflammation or is a result of a common inflammatory process underlying diabetes and HFpEF, and whether specific regional fat depots are transducers of more pronounced inflammation, should be clarified with further research. From a clinical perspective, given that diabetes and higher blood pressure are associated with certain regional adipose tissue depots in excess of their association with total body adiposity, clinicians may consider targeting patients with diabetes and/or hypertension for more aggressive adiposity‐reduction strategies.
Regional adipose tissue and functional capacity/exercise tolerance
There is a growing body of literature showing that excess adiposity is a major contributor to exercise intolerance in HFpEF. 4 , 7 , 8 In our study, we found that measures of skeletal muscle fat were associated with worse NYHA class and worse 6MWD performance. We also found that both RV EAT and liver FF were associated with lower GWB score. Adiposity is thought to mediate exercise intolerance by promoting systemic inflammation, along with endothelial dysfunction, arterial hypertension, mitochondrial impairment, and unfavourable cardiac mechanics. 6 , 7 , 8 , 9 The regionality and location of specific adipose tissue depots is increasingly being recognized as an important determinant of exercise tolerance. Haykowsky et al. have previously reported that regional depots of fat in the thigh intramuscular and abdominal subcutaneous compartments were associated with lower peak VO2 and 6MWD. 8 We similarly found that skeletal muscle fat in the thigh was associated with exercise capacity, and additionally that epicardial and liver fat were associated with patient‐reported measures of well‐being. Taken together, these data suggest that ectopic adipose tissue depots in specific localized regions in the body may play a key role in functional and exercise capacity in HFpEF.
Sex differences in the relationship between regional adipose tissue distribution and functional capacity are not well‐known. There is some evidence that women with HFpEF may have worse exercise capacity than men, 35 although this is not supported in other studies. 36 Women with HFpEF and increased VAT have been shown to have higher pulmonary capillary wedge pressure during exercise than those with normal VAT, whereas the difference was similar in men with our without excess VAT. 37 Whether sex differences exist in the relation between adiposity and clinical symptoms or functional capacity is not known. In our study, we observed an inverse correlation between RV EAT and GWBS in men, but not in women. Additional and larger clinical studies are needed to further evaluate these differences.
Strengths and limitations
Our study has several limitations. First, our sample size was relatively small, which may have limited the power to detect more subtle associations. We were additionally unable to perform more comprehensive multivariable adjustment due to the limitations of sample size. Second, we had a limited sample of control participants that did now allow for matching of the controls on baseline characteristics. Third, although we did not find significant sex interactions between adiposity and functional status, we cannot fully exclude the possibility of sex differences in these associations due to the small sample size, particularly of men with HFpEF. Future investigations will be needed to further explore these associations. Fourth, given the observational cross‐sectional nature of our study, we cannot make conclusions about the directionality or causality of our associations. Lastly, we did not adjust for multiple comparisons due to the exploratory, hypothesis‐generating nature of this work. Our study also should be considered in the context of its strengths, including comprehensive assessment of regional fat depots including novel measurements not previously studied in HFpEF.
Conclusions
Heart failure with preserved ejection fraction patients have increased epicardial, liver, and skeletal muscle fat compared with controls out of proportion to their increased body size; and men and women with HFpEF differ in their adipose tissue distribution. Regional adipose tissue measures reflective of visceral fat redistribution are associated with HFpEF co‐morbidities, including diabetes and hypertension, as well as measures of functional status and exercise capacity. Excess adiposity may thus play a key role in HFpEF symptoms and its associated conditions. Future research should be directed at further characterizing regional adipose tissue depots, sex differences, and whether targeting specific depots have therapeutic potential in HFpEF.
Conflict of interest
The authors report no relevant conflicts of interest.
Funding
This work was funded by the American Heart Association Go Red for Women Strategically Focused Research Network (grants 16SFRN27870000 and 16SFRN28780016). Dr Hays is also supported by the Magic that Matters Fund of Johns Hopkins Medicine and National Institutes of Health/NHLBI (1R01HL147660).
Supporting information
Figure S1. Outline of study design.
Table S1. Interaction analysis of HFpEF status and sex in the regression between HFpEF status and regional adiposity/muscle composition.
Table S2. Adipose tissue distribution and skeletal muscle composition in HFpEF patients and controls, adjusting for BMI.
Table S3. Adipose tissue distribution and skeletal muscle composition in male and female HFpEF patients, adjusted for BMI.
Table S4. Associations* between HFpEF risk factors with adipose tissue and skeletal muscle measures, adjusted for BMI.
Table S5. Associations* between adipose tissue and skeletal muscle measures and exercise capacity, adjusted for BMI.
Table S6. Measures of functional capacity and exercise tolerance in men and women.
Table S7. Sex‐stratified correlations between RV EAT and global well‐being score.
Ying, W. , Sharma, K. , Yanek, L. R. , Vaidya, D. , Schär, M. , Markl, M. , Subramanya, V. , Soleimani, S. , Ouyang, P. , Michos, E. D. , Shah, S. J. , and Hays, A. G. (2021) Visceral adiposity, muscle composition, and exercise tolerance in heart failure with preserved ejection fraction. ESC Heart Failure, 8: 2535–2545. 10.1002/ehf2.13382.
References
- 1. Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res 2014; 115: 79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC, Get With the Guidelines Scientific Advisory C and Investigators . Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012; 126: 65–75. [DOI] [PubMed] [Google Scholar]
- 3. Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol 2011; 58: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol 2014; 113: 1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neeland IJ, Poirier P, Despres JP. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation 2018; 137: 1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kitzman DW, Shah SJ. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol 2016; 68: 200–203. [DOI] [PubMed] [Google Scholar]
- 7. Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017; 136: 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haykowsky MJ, Nicklas BJ, Brubaker PH, Hundley WG, Brinkley TE, Upadhya B, Becton JT, Nelson MD, Chen H, Kitzman DW. Regional adipose distribution and its relationship to exercise intolerance in older obese patients who have heart failure with preserved ejection fraction. JACC Heart Fail 2018; 6: 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid‐range and preserved ejection fraction. Eur J Heart Fail 2018; 20: 1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Packer M. Atrial fibrillation and heart failure with preserved ejection fraction in patients with nonalcoholic fatty liver disease. Am J Med 2020; 133: 170–177. [DOI] [PubMed] [Google Scholar]
- 11. Beale AL, Nanayakkara S, Segan L, Mariani JA, Maeder MT, van Empel V, Vizi D, Evans S, Lam CSP, Kaye DM. Sex differences in heart failure with preserved ejection fraction pathophysiology: a detailed invasive hemodynamic and echocardiographic analysis. JACC Heart Fail 2019; 7: 239–249. [DOI] [PubMed] [Google Scholar]
- 12. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iantorno M, Soleimanifard S, Schär M, Brown TT, Bonanno G, Barditch‐Crovo P, Mathews L, Lai S, Gerstenblith G, Weiss RG, Hays AG. Regional coronary endothelial dysfunction is related to the degree of local epicardial fat in people with HIV. Atherosclerosis 2018; 278: 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cunha GM, Correa de Mello LL, Hasenstab KA, Spina L, Bussade I, Prata Mesiano JM, Coutinho W, Guzman G, Sajoux I. MRI estimated changes in visceral adipose tissue and liver fat fraction in patients with obesity during a very low‐calorie‐ketogenic diet compared to a standard low‐calorie diet. Clin Radiol 2020; 75: 526–532. [DOI] [PubMed] [Google Scholar]
- 16. Schweitzer L, Geisler C, Pourhassan M, Braun W, Gluer CC, Bosy‐Westphal A, Muller MJ. Estimation of skeletal muscle mass and visceral adipose tissue volume by a single magnetic resonance imaging slice in healthy elderly adults. J Nutr 2016; 146: 2143–2148. [DOI] [PubMed] [Google Scholar]
- 17. Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross‐sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis 2015; 17: O20–O26. [DOI] [PubMed] [Google Scholar]
- 18. Yokoo T, Serai SD, Pirasteh A, Bashir MR, Hamilton G, Hernando D, Hu HH, Hetterich H, Kuhn JP, Kukuk GM, Loomba R, Middleton MS, Obuchowski NA, Song JS, Tang A, Wu X, Reeder SB, Sirlin CB, Committee R‐QPB. Linearity, bias, and precision of hepatic proton density fat fraction measurements by using MR imaging: a meta‐analysis. Radiology 2018; 286: 486–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karampinos DC, Baum T, Nardo L, Alizai H, Yu H, Carballido‐Gamio J, Yap SP, Shimakawa A, Link TM, Majumdar S. Characterization of the regional distribution of skeletal muscle adipose tissue in type 2 diabetes using chemical shift‐based water/fat separation. J Magn Reson Imaging 2012; 35: 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6‐minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985; 132: 919–923. [PMC free article] [PubMed] [Google Scholar]
- 21. Sharma K, Vaishnav J, Kalathiya R, Hu JR, Miller J, Shah N, Hill T, Sharp M, Tsao A, Alexander KM, Gupta R, Montemayor K, Kovell L, Chasler JE, Lee YJ, Fine DM, Kass DA, Weiss RG, Thiemann DR, Ndumele CE, Schulman SP, Russell SD. Randomized evaluation of heart failure with preserved ejection fraction patients with acute heart failure and dopamine: the ROPA‐DOP trial. JACC Heart Fail 2018; 6: 859–870. [DOI] [PubMed] [Google Scholar]
- 22. Liang KW, Tsai IC, Lee WJ, Lee IT, Lee WL, Lin SY, Wan CJ, Fu CP, Ting CT, Sheu WH. MRI measured epicardial adipose tissue thickness at the right AV groove differentiates inflammatory status in obese men with metabolic syndrome. Obesity (Silver Spring, Md) 2012; 20: 525–532. [DOI] [PubMed] [Google Scholar]
- 23. Flüchter S, Haghi D, Dinter D, Heberlein W, Kühl HP, Neff W, Sueselbeck T, Borggrefe M, Papavassiliu T. Volumetric assessment of epicardial adipose tissue with cardiovascular magnetic resonance imaging. Obesity (Silver Spring, Md) 2007; 15: 870–878. [DOI] [PubMed] [Google Scholar]
- 24. Bertaso AG, Bertol D, Duncan BB, Foppa M. Epicardial fat: definition, measurements and systematic review of main outcomes. Arq Bras Cardiol 2013; 101: e18–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Culvenor AG, Boeth H, Diederichs G, Wirth W, Duda G, Eckstein F. Longitudinal bone, muscle and adipose tissue changes in physically active subjects—sex differences during adolescence and maturity. J Musculoskelet Neuronal Interact 2016; 16: 237–246. [PMC free article] [PubMed] [Google Scholar]
- 26. Ding J, Visser M, Kritchevsky SB, Nevitt M, Newman A, Sutton‐Tyrrell K, Harris TB. The association of regional fat depots with hypertension in older persons of white and African American ethnicity. Am J Hypertens 2004; 17: 971–976. [DOI] [PubMed] [Google Scholar]
- 27. Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. J Am Coll Cardiol 2018; 71: 2360–2372. [DOI] [PubMed] [Google Scholar]
- 28. VanWagner LB, Wilcox JE, Ning H, Lewis CE, Carr JJ, Rinella ME, Shah SJ, Lima JAC, Lloyd‐Jones DM. Longitudinal association of non‐alcoholic fatty liver disease with changes in myocardial structure and function: the CARDIA study. J Am Heart Assoc 2020; 9: e014279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoshihisa A, Sato Y, Yokokawa T, Sato T, Suzuki S, Oikawa M, Kobayashi A, Yamaki T, Kunii H, Nakazato K, Saitoh SI, Takeishi Y. Liver fibrosis score predicts mortality in heart failure patients with preserved ejection fraction. ESC Heart Fail 2018; 5: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Savji N, Meijers WC, Bartz TM, Bhambhani V, Cushman M, Nayor M, Kizer JR, Sarma A, Blaha MJ, Gansevoort RT, Gardin JM, Hillege HL, Ji F, Kop WJ, Lau ES, Lee DS, Sadreyev R, van Gilst WH, Wang TJ, Zanni MV, Vasan RS, Allen NB, Psaty BM, van der Harst P, Levy D, Larson M, Shah SJ, de Boer RA, Gottdiener JS, Ho JE. The association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail 2018; 6: 701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bredella MA. Sex differences in body composition. Adv Exp Med Biol 2017; 1043: 9–27. [DOI] [PubMed] [Google Scholar]
- 32. Rao VN, Zhao D, Allison MA, Guallar E, Sharma K, Criqui MH, Cushman M, Blumenthal RS, Michos ED. Adiposity and incident heart failure and its subtypes: MESA (Multi‐Ethnic Study of Atherosclerosis). JACC Heart Fail 2018; 6: 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yazıcı D, Sezer H. Insulin resistance, obesity and lipotoxicity. Adv Exp Med Biol 2017; 960: 277–304. [DOI] [PubMed] [Google Scholar]
- 34. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 35. Mauricio R, Patel KV, Agusala V, Singh K, Lewis A, Ayers C, Grodin JL, Berry JD, Pandey A. Sex differences in cardiac function, biomarkers and exercise performance in heart failure with preserved ejection fraction: findings from the RELAX trial. Eur J Heart Fail 2019; 21: 1476–1479. [DOI] [PubMed] [Google Scholar]
- 36. Honigberg MC, Lau ES, Jones AD, Coles A, Redfield MM, Lewis GD, Givertz MM. Sex differences in exercise capacity and quality of life in heart failure with preserved ejection fraction: a secondary analysis of the RELAX and NEAT‐HFpEF trials. J Card Fail 2020; 26: 276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sorimachi H, Obokata M, Takahashi N, Reddy YNV, Jain CC, Verbrugge FH, Koepp KE, Khosla S, Jensen MD, Borlaug BA. Pathophysiologic importance of visceral adipose tissue in women with heart failure and preserved ejection fraction. Eur Heart J 2020: ehaa823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Outline of study design.
Table S1. Interaction analysis of HFpEF status and sex in the regression between HFpEF status and regional adiposity/muscle composition.
Table S2. Adipose tissue distribution and skeletal muscle composition in HFpEF patients and controls, adjusting for BMI.
Table S3. Adipose tissue distribution and skeletal muscle composition in male and female HFpEF patients, adjusted for BMI.
Table S4. Associations* between HFpEF risk factors with adipose tissue and skeletal muscle measures, adjusted for BMI.
Table S5. Associations* between adipose tissue and skeletal muscle measures and exercise capacity, adjusted for BMI.
Table S6. Measures of functional capacity and exercise tolerance in men and women.
Table S7. Sex‐stratified correlations between RV EAT and global well‐being score.


