Abstract
Aims
Information on the prevalence, outcome and factors associated with heart failure in patients with adult congenital heart disease (CHD) (ACHD‐HF) is lacking. We aimed at assessing the prevalence and outcome of ACHD‐HF, the variables associated with ACHD‐HF, and the differences between major anatomical/pathophysiological ACHD subgroups.
Methods and results
We included 3905 patients (age 35.4 ± 13.2 years) under active follow‐up in our institution (last visit >2010). Outcome of ACHD‐HF cases was compared with sex‐ and age‐matched cases. Univariable and multivariable binary logistic regression with ACHD‐HF diagnosis as a dependent variable was performed. Overall prevalence of ACHD‐HF was 6.4% (mean age 49.5 ± 16.7 years), but was higher in patients with cyanotic CHD (41%), Fontan circulation (30%), and a systemic right ventricle (25%). All‐cause mortality was higher in ACHD‐HF cases when compared with controls (mortality rate ratio 4.67 (2.36–9.27); P = 0.0001). In multivariable logistic regression analysis, age at latest follow‐up [per 10 years; odds ratio (OR) 1.52; 95% confidence interval (CI) 1.31–1.77], infective endocarditis (OR 4.11; 95%CI 1.80–9.38), history of atrial arrhythmia (OR 3.52; 95%CI 2.17–5.74), pacemaker implantation (OR 2.66; 95% CI 1.50–4.72), end‐organ dysfunction (OR 2.41; 95% CI 1.03–5.63), New York Heart Association class (OR 9.28; 95% CI 6.04–14.25), heart rate (per 10 bpm; OR 1.27; 95% CI 1.08–1.50), ventricular dysfunction (OR 3.62; 95% CI 2.54–5.17), and pulmonary hypertension severity (OR 1.66; 95% CI 1.21–2.30) were independently related to the presence of ACHD‐HF. Some variables (age, atrial arrhythmia, pacemaker, New York Heart Association, and ventricular dysfunction) were related to ACHD‐HF in all anatomical/physiological subgroups, whereas others were not.
Conclusions
ACHD‐HF is prevalent especially in complex CHD and is associated with poor prognosis. Our data provide insight in the factors related to ACHD‐HF including differences between specific anatomical and physiological subgroups.
Keywords: Congenital, Heart failure, ACHD, All‐cause mortality
Introduction
As a result of a significant increase in the survival of patients with congenital heart disease (CHD), 88% of children with CHD nowadays survive into adulthood. 1 There are now more adults than children with CHD with a rapidly expanding cohort of survivors with complex lesions and even a new group of patients with ‘geriatric’ ACHD. 2 , 3 Even repaired, the hearts of adult patients with CHD (ACHD) remain abnormal, making them vulnerable to the development of heart failure (HF). 4 , 5 Unsurprisingly, ACHD‐HF has become a rapidly growing reason for hospitalization, 6 is associated with a high mortality rate, 7 and has become the leading cause of death for ACHD patients. 8 The associated increase in ACHD‐HF‐related admissions and procedures poses a significant burden on the health care system. 6 , 9 , 10 Simultaneously, the number of ACHD patients assessed for orthotopic heart transplantation increases, although few are listed with those unsuitable for transplantation having a poor prognosis. 11
Despite this, precise data on the prevalence of ACHD‐HF remain elusive. Detailed information on the factors associated with ACHD‐HF is needed in order to recognize patients at risk, trigger further investigations for modifiable structural and electrical issues and/or refer patients for consideration of advanced HF therapies. 12 Fundamental differences in anatomy and pathophysiology between subgroups need to be taken into account to understand similarities and differences in the ACHD‐HF phenotype. Therefore, this study aimed at evaluating the prevalence and outcome of ACHD‐HF, examining variables associated with ACHD‐HF and identifying differences between major anatomical/pathophysiological ACHD groups in a contemporary cohort of ACHD patients followed in a tertiary care centre.
Methods
Study population
All adult patients with CHD (ACHD) under active follow‐up at the University Hospitals Leuven (with a last follow‐up visit >1 January 2010) were included in the study and followed until the latest follow‐up visit or until death. For the purpose of the study, we defined the start of inclusion as age ≥16 years. Patients were divided into subgroups based on their major underlying heart defect, using a modification of the hierarchical classification developed for the Dutch Congenital Cor Vitia Project. 13 Patients were further classified according to the anatomic complexity of their heart defect into mild‐, moderate‐, or severe‐complexity heart defects 14 and based on the physiological severity as outlined in the 2018 American Heart Association (AHA)/American College of Cardiology (ACC) (AHA/ACC) guideline for the management of adults with CHD. 15 Finally, patients were categorized into the following mutually exclusive subgroups: (i) Cyanotic ACHD (including Eisenmenger physiology); (ii) Fontan circulation; (iii) biventricular circulation with a systemic right ventricle (RV); (iv) biventricular circulation with a systemic left ventricle (LV)—shunt lesions; (v) biventricular circulation with a systemic LV—predominant right‐sided residual lesions; and (vi) biventricular circulation with a systemic LV—predominant left‐sided residual lesions (Table S1). 4
Data on clinical status were obtained from medical records reviewed by one investigator (A. V. D. B.) at their latest follow‐up visit prior to censoring or death. Ventricular dilatation, ventricular dysfunction, and valvular dysfunction (systemic, pulmonic, or both) were recorded from echocardiography reports. Ventricular dysfunction was assessed qualitatively, reported on a scale from 1 (normal) to 4 (severely depressed). Right and left atrioventricular valve regurgitation was assessed by colour‐Doppler and reported on a scale from 1 (none) to 4 (severe). Data on overall mortality were established from the clinical working station of the hospital, which is linked to the national statistical office. Patients' records were reviewed after pseudonymizing patient data in accordance with the Global Data Protection Regulation. The institutional ethics committee approved the study protocol and the study was conducted in compliance with the principles of the Declaration of Helsinki.
Adult congenital heart disease‐heart failure definition
Heart failure related to ACHD was defined as signs and/or symptoms of HF requiring medical therapy 16 plus one of the following: (i) impaired ventricular function (systolic, diastolic or both) with elevated intracardiac pressures; (ii) elevated B‐type natriuretic peptide (BNP) or N‐terminal BNP; (iii) peak oxygen consumption in the lowest quartile according to published norms for ACHD subtype 17 or 4. Unique HF manifestations in patients with a Fontan circulation (protein loosing enteropathy, plastic bronchitis, Fontan systemic venous pressure ≥20 mmHg, and cardiac index <2 L/min m2). HF diagnosis was reviewed by one investigator (A. V. D. B.). In case of doubt, the case was discussed with colleagues (W. B., E. T., and P. D. M.).
Statistical analysis
Data were analysed using SPSS® for Windows (version 23, SPSS, Chicago) and tested for normality with the Kolmogorov–Smirnov test. Descriptive data for continuous variables are presented as means ± SD or as medians with interquartile ranges as appropriate. Descriptive data for discrete variables are presented as frequencies or percentages. For between‐group comparisons, the independent Student t test, χ 2, or Fisher exact test was used where applicable.
ACHD‐HF prevalence in the study cohort is reported for the entire cohort and for the individual subgroups. Patients with ACHD‐HF (cases) were matched with patients not developing ACHD‐HF (controls) according to sex, date of birth and ACHD subgroup. Baseline in the cases was defined as the date of ACHD‐HF diagnosis. Baseline in the controls was defined as the baseline date in the corresponding cases assuring approximately equal age at baseline in cases and controls. Patients with a follow‐up of at least 1 year after baseline were included. Mortality rates (deaths per 1000 patient years) were compared between cases and controls, and results are illustrated using inverse Kaplan–Meier plots and analysed using the log‐rank test.
Binary logistic regression analysis was performed with HF diagnosis as a dependent variable. For the entire cohort, a multivariable logistic regression was performed to determine variables related to HF diagnosis using the following covariates: age (per 10 years), sex, underlying genetic abnormality, prior intervention (surgical or percutaneous), smoking, alcohol consumption, history of infective endocarditis, coronary artery disease, a history of atrial, or ventricular arrhythmia, the presence of a pacemaker, end‐organ dysfunction (renal, pulmonary, or hepatic), New York Heart Association (NYHA) class, body mass index (BMI), mean arterial pressure (per 10 mmHg), heart rate (per 10 bpm), severity of cyanosis, ventricular dilatation, ventricular dysfunction, valvular dysfunction, aortic dilatation, pulmonary hypertension severity, the presence of venous or arterial stenosis, and shunt severity. For the anatomical/physiological subgroups, because of smaller numbers, only univariable logistic regression with these parameters was performed to assess consistency across subgroups.
Results
Demographics and heart failure prevalence
A total of 3905 [2032 male (52%)] ACHD patients were included in the study with a mean age at last follow up of 35.4 ± 13.2 years. Patient characteristics for the entire cohort are summarized in Table 1 and for the different subgroups in Supplementary Tables [Link] , [Link] , [Link] , [Link] , [Link] , [Link] . According to the Bethesda disease complexity classification, 33% of patients had simple defects, 56% moderate, and 11% severe complexity defects. Based on the physiological severity classification, 18% were in physiological Stage A, 36% in physiological Stage B, 42% in physiological Stage C, and 4% in physiological Stage D.
Table 1.
Patient characteristics
| Variable | Entire cohort n = 3905 | Heart failure n = 248 | No heart failure n = 3657 | P value |
|---|---|---|---|---|
| Bethesda classification | ||||
| Mild n (%) | 1306 (33.5) | 43 (17.3) | 1263 (34.5) | |
| Moderate n (%) | 2182 (56.0) | 114 (46.0) | 2068 (56.5) | |
| Severe n (%) | 417 (10.7) | 91 (36.7) | 326 (8.9) | <0.0001 |
| Physiological severity | ||||
| A n (%) | 701 (17.6) | 0 (0.0) | 701 (19.2) | |
| B n (%) | 1389 (35.6) | 4 (1.6) | 1385 (37.9) | |
| C n (%) | 1650 (42.2) | 171 (69.0) | 1479 (40.4) | |
| D n (%) | 165 (4.2) | 73 (29.4) | 92 (2.5) | <0.0001 |
| Age at last FU (years) | 35.4 ± 13.2 | 49.5 ± 16.7 | 34.4 ± 12.4 | <0.0001 |
| Male gender n (%) | 2032 (52.0) | 127 (51.2) | 1905 (52.1) | 0.793 |
| Any genetic abnormality n (%) | 441 (11.3) | 23 (9.3) | 418 (11.4) | 0.348 |
| Trisomy 21 n (%) | 142 (3.6) | 11 (4.4) | 131 (3.6) | 0.600 |
| 22q11 n (%) | 77 (2.0) | 6 (2.4) | 71 (1.9) | 0.633 |
| Noonan n (%) | 61 (1.6) | 1 (0.4) | 60 (1.6) | 0.182 |
| Williams n (%) | 25 (0.6) | 1 (0.4) | 24 (0.7) | 0.731 |
| Intervention n (%) | 2639 (67.6) | 206 (83.1) | 2433 (66.5) | <0.0001 |
| Smoking n (%) | 500 (12.8) | 25 (10.1) | 475 (13.0) | 0.200 |
| Missing n (%) | 216 (5.5) | 13 (5.2) | 203 (5.6) | |
| Alcohol | 0.001 | |||
| Never n (%) | 1323 (33.9) | 99 (39.9) | 1224 (33.5) | |
| Occasionally n (%) | 2023 (51.8) | 103 (41.5) | 1920 (52.5) | |
| Frequently n (%) | 343 (8.8) | 33 (13.3) | 310 (8.5) | |
| Missing n (%) | 216 (5.5) | 13 (5.2) | 203 (5.6) | |
| Infective endocarditis n (%) | 108 (2.8) | 23 (9.3) | 85 (2.3) | <0.0001 |
| Coronary artery disease n (%) | 23 (0.6) | 6 (2.4) | 17 (0.5) | 0.002 |
| Atrial arrhythmia n (%) | 346 (8.9) | 126 (50.8) | 220 (6.0) | <0.0001 |
| Ventricular arrhythmia n (%) | 120 (3.1) | 36 (14.5) | 84 (2.2) | <0.0001 |
| RF ablation n (%) | 160 (4.1) | 53 (21.4) | 107 (2.9) | <0.0001 |
| Pacemaker n (%) | 206 (5.3) | 82 (33.1) | 124 (3.4) | <0.0001 |
| AICD n (%) | 71 (1.8) | 33 (13.3) | 38 (1.0) | <0.0001 |
| CRT n (%) | 23 (0.6) | 14 (5.6) | 9 (0.2) | <0.0001 |
| End‐organ dysfunction | <0.0001 | |||
| No n (%) | 3831 (98.1) | 193 (77.8) | 3638 (99.5) | |
| Mild n (%) | 63 (1.6) | 48 (19.4) | 15 (0.4) | |
| Severe n (%) | 11 (0.3) | 7 (2.8) | 4 (0.1) | |
| Clinical characteristics | ||||
| NYHA | ||||
| I n (%) | 3472 (88.9) | 43 (17.3) | 3429 (93.8) | |
| II n (%) | 365 (9.3) | 145 (58.5) | 220 (6.0) | |
| III n (%) | 59 (1.5) | 51 (20.6) | 8 (0.2) | |
| IV n (%) | 9 (0.2) | 9 (3.6) | 0 (0.00) | <0.0001 |
| Length (cm) | 170.1 ± 11.3 | 167.8 ± 11.3 | 170.2 ± 11.3 | 0.001 |
| Missing n (%) | 218 (5.6) | 13 (5.2) | 205 (5.6) | |
| Weight (kg) | 72.9 ± 16.1 | 72.1 ± 19.2 | 73.0 ± 15.9 | 0.491 |
| Missing n (%) | 249 (6.4) | 17 (6.9) | 232 (6.3) | |
| BMI (kg/m2) | 25.2 ± 4.9 | 25.4 ± 5.6 | 25.1 ± 4.8 | 0.465 |
| Missing n (%) | 257 (6.6) | 17 (6.9) | 240 (6.6) | |
| SBP (mmHg) | 128.4 ± 27.1 | 122.1 ± 19.8 | 128.8 ± 27.5 | <0.0001 |
| Missing n (%) | 228 (5.8) | 14 (5.6) | 214 (5.9) | |
| DBP (mmHg) | 73.5 ± 11.3 | 69.1 ± 13.1 | 73.8 ± 11.1 | <0.0001 |
| Missing n (%) | 231 (5.9) | 14 (5.6) | 217 (5.9) | |
| Heart rate (bpm) | 71.8 ± 13.4 | 74.2 ± 14.7 | 71.7 ± 13.3 | 0.005 |
| Missing n (%) | 270 (6.9) | 16 (6.5) | 254 (6.9) | |
| Cyanosis | ||||
| No n (%) | 3784 (96.9) | 199 (80.2) | 3585 (98.0) | |
| Mild n (%) | 61 (1.6) | 17 (6.9) | 44 (1.2) | |
| Severe n (%) | 60 (1.5) | 32 (12.9) | 28 (0.8) | <0.0001 |
| Echocardiography | ||||
| Ventricular dilatation | ||||
| No n (%) | 2766 (70.8) | 57 (23.0) | 2709 (74.1) | |
| Mild n (%) | 649 (16.6) | 55 (22.2) | 594 (16.2) | |
| Moderate–severe n (%) | 490 (12.5) | 136 (54.8) | 354 (9.7) | <0.0001 |
| Ventricular dysfunction | ||||
| No n (%) | 3193 (81.8) | 49 (19.8) | 3144 (86.0) | |
| Mild n (%) | 477 (12.2) | 74 (29.8) | 403 (11.0) | |
| Moderate–severe n (%) | 235 (6.0) | 125 (50.4) | 110 (3.0) | <0.0001 |
| Valvular dysfunction | ||||
| No n (%) | 940 (24.1) | 2 (0.8) | 938 (25.6) | |
| Mild n (%) | 1697 (43.4) | 80 (32.3) | 1617 (44.2) | |
| Moderate–severe n (%) | 1268 (32.5) | 166 (66.9) | 1102 (30.1) | <0.0001 |
| Aortic dilatation | ||||
| <35 mm n (%) | 3287 (84.2) | 217 (87.5) | 3070 (83.9) | |
| 35–39 mm n (%) | 313 (8.0) | 15 (6.0) | 298 (8.1) | |
| 40–49 mm n (%) | 269 (6.9) | 12 (4.8) | 257 (7.0) | |
| >50 mm n (%) | 36 (0.9) | 4 (1.6) | 32 (0.9) | 0.192 |
| Pulmonary hypertension | ||||
| <35 mmHg n (%) | 3695 (94.6) | 146 (58.9) | 3549 (97.0) | |
| 35–60 mmHg n (%) | 121 (3.1) | 57 (23.0) | 64 (1.8) | |
| >60 mmHg n (%) | 89 (2.8) | 45 (18.1) | 44 (1.2) | <0.0001 |
| Venous/arterial stenosis n (%) | 76 (1.9) | 8 (3.2) | 68 (1.9) | 0.147 |
| Persistent shunt n (%) | <0.0001 | |||
| No shunt | 3274 (83.8) | 170 (68.5) | 3104 (84.9) | |
| Mild/restrictive shunt | 493 (12.6) | 25 (10.1) | 468 (12.8) | |
| Significant/non‐restrictive shunt | 138 (3.5) | 53 (21.4) | 85 (2.3) | |
| Outcome | ||||
| Combined endpoint n (%) | 109 (2.8) | 64 (25.8) | 45 (1.2) | <0.0001 |
| Death n (%) | 96 (2.5) | 51 (20.6) | 45 (1.2) | <0.0001 |
| Transplant n (%) | 14 (0.4) | 14 (5.6) | 0 (0.0) | <0.0001 |
| VAD n (%) | 2 (0.1) | 2 (0.8) | 0 (0.0) | 0.004 |
AICD, automated cardioverter defibrillator; BMI, body mass index; CRT, cardiac resynchronization therapy; DBP, diastolic blood pressure; FU, follow‐up; NYHA, New York Heart Association; SBP, systolic blood pressure; VAD, ventricular assist device.
In total, 248 patients (6.4%) had a HF diagnosis at last follow‐up. Patients with moderate to severe complexity CHD and those in physiological severity Class C or D were more likely to have ACHD‐HF (Table 1 ). HF prevalence was significantly higher in cyanotic patients, patients with a Fontan circulation, and patients with a systemic RV when compared with patients with a biventricular circulation and a systemic LV, although absolute ACHD‐HF numbers were lower (Figure 1 ). With increasing age, ACHD‐HF prevalence increased from 2.3% in subjects aged 15–35 years to 6.8% in those age 35–50 years and 21.9% in those aged >50 years. This age‐related increase was consistent in all groups, but was especially evident in cyanotic patients, patients with a Fontan circulation, and patients with a systemic RV.
Figure 1.
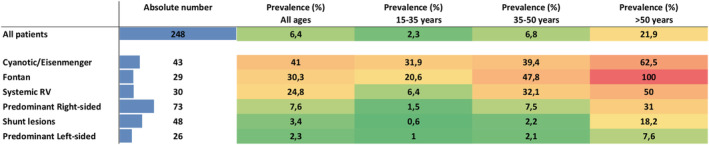
Adult congenital heart disease (ACHD)‐heart failure (HF) (ACHD‐HF) prevalence heat map. Heat map indicating the absolute number and proportion of patients with ACHD‐HF subdivided per age category and per ACHD subgroup. Although the proportion of patients with ACHD‐HF is substantially higher (at younger age) for cyanotic patients, Fontan patients and patients with a systemic right ventricle (RV), the absolute number of patients is higher in the ACHD subgroups with a biventricular circulation and systemic left ventricle (LV).
Outcome
Of 248 ACHD‐HF patients, 186 cases could be matched with one control according to sex, date of birth and ACHD anatomical/physiological subgroup. Mortality rate in the control group was 8.7 (95%CI 3.3–14.4) when compared to 40.6 (95%CI 28.6–52.7) in the ACHD‐HF group, resulting in a mortality rate ratio of 4.67 (95%CI 2.36–9.27). Figure 2 illustrates the inverse Kaplan–Meier plot for ACHD‐HF and matched control patients.
Figure 2.
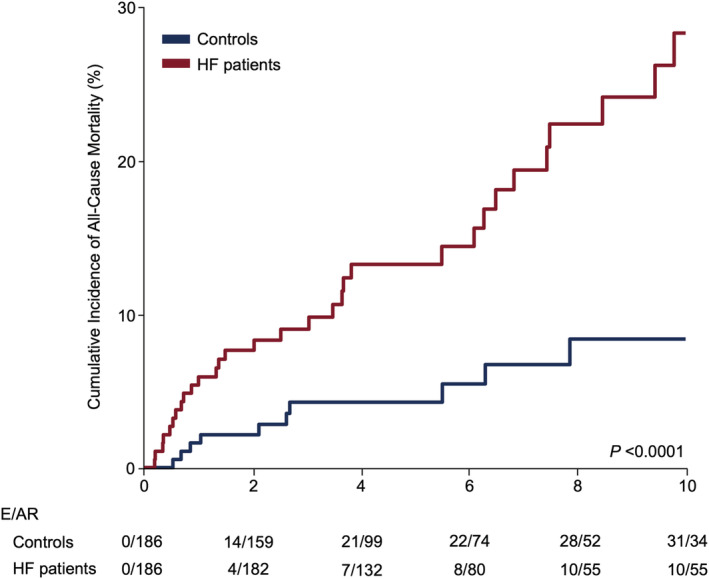
Adult congenital heart disease (ACHD)‐heart failure (HF) (ACHD‐HF) and all‐cause mortality. Inverse Kaplan–Meier plots for all‐cause mortality in 186 ACHD‐HF patients and 186 matched controls. All‐cause mortality was 4.67 times higher in ACHD‐HF patients as compared with their matched controls. AR, at risk; E, events; HF, heart failure.
Variables related to adult congenital heart disease‐heart failure
For the entire cohort, in multivariable logistic regression analysis (complete case analysis, missing cases 8.6%), age at latest follow‐up [per 10 years increment; odds ratio (OR) 1.52; 95% confidence interval (CI) 1.31–1.77; P < 0.0001], a history of infective endocarditis (OR 4.11; 95% CI 1.80–9.38; P = 0.001), a history of atrial arrhythmia (OR 3.52; 95% CI 2.17–5.74; P < 0.0001), need for pacemaker implantation (OR 2.66; 95% CI 1.50–4.72; P = 0.001), end‐organ dysfunction (OR 2.41; 95% CI 1.03–5.63; P = 0.042), NYHA class (OR 9.28; 95% CI 6.04–14.25; P < 0.0001), heart rate (per 10 bpm increase; OR 1.27; 95% CI 1.08–1.50; P = 0.003), ventricular dysfunction (OR 3.62; 95% CI 2.54–5.17; P < 0.0001), and pulmonary hypertension severity (OR 1.66; 95% CI 1.21–2.30; P = 0.002) were independently related to the presence of ACHD‐HF (Figure 3 ).
Figure 3.
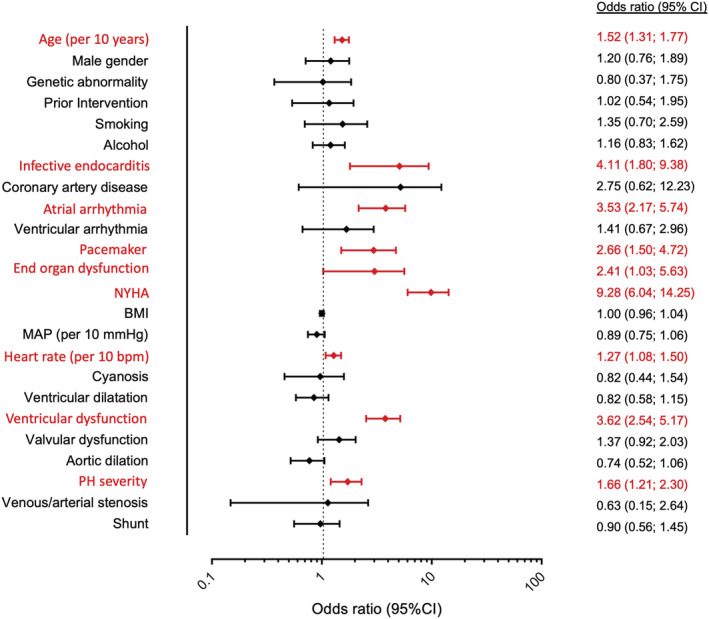
Multivariable binary logistic regression analysis. Multivariable analysis for the entire cohort indicating (in red) factors that are independently related with the presence of adult congenital heart disease‐heart failure (ACHD‐HF) [missing cases: 334 (8.6%)]. BMI, body mass index; NYHA, New York Heart Association.
For the anatomical/physiological subgroups, in univariable regression analysis, age at latest follow‐up, atrial arrhythmia, the presence of a pacemaker, NYHA, and ventricular dysfunction were consistently related to ACHD‐HF across all subgroups. A history of prior intervention, infective endocarditis, coronary artery disease, end‐organ dysfunction, ventricular dilatation, valvular dysfunction and pulmonary hypertension severity appeared important in some but not all subgroups (Figure 4 ). Of note, for patients with a Fontan circulation, there was no difference between different types of Fontan circulation and having a dominant systemic RV was not related to ACHD‐HF (Table S2B ).
Figure 4.
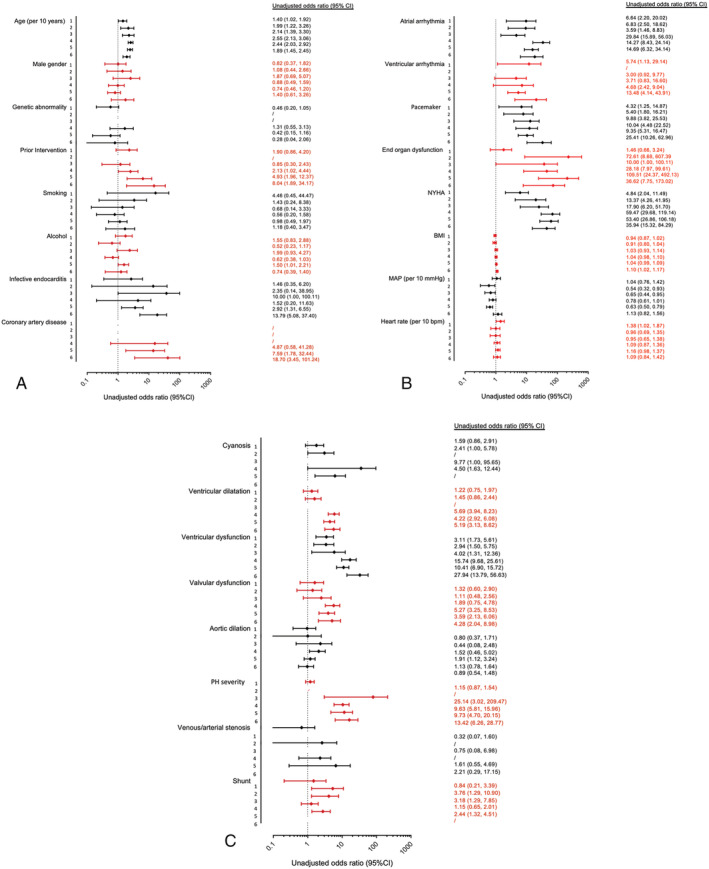
Univariable binary logistic regression analysis. Univariable analysis of factors related with the presence of adult congenital heart disease (ACHD)‐heart failure (ACHD‐HF) for individual ACHD anatomical/physiological subgroups (numbered 1–6 for cyanotic/Eisenmenger; Fontan; systemic right ventricle; predominant right‐sided residual lesions; shunt lesions; and predominant left‐sided residual lesions, respectively) for each risk factor assessed in the multivariable analysis of the entire cohort. BMI, body mass index; NYHA, New York Heart Association.
Discussion
This study evaluates the prevalence of HF in adult CHD based on a standardized definition for ACHD‐HF in a large contemporary ACHD cohort, providing an estimated ACHD‐HF prevalence of 6.4% at a median age of 35 years. ACHD‐HF prevalence varies across anatomical/physiological subgroups [largest prevalence in cyanotic patients (41%), patients with a Fontan circulation (30%) and patients with a systemic RV (25%)] and increases significantly with older age. An ACHD‐HF diagnosis increases all‐cause mortality risk almost fivefold. Age, infective endocarditis, atrial arrhythmia, pacemaker, end‐organ dysfunction, NYHA class, heart rate, ventricular dysfunction, and pulmonary hypertension severity were independently related to the presence of ACHD‐HF. Some variables (age, atrial arrhythmia, pacemaker, NYHA, and ventricular dysfunction) were related to ACHD‐HF in all anatomical/physiological subgroups, whereas others were not.
Adult congenital heart disease‐heart failure prevalence
In this cohort of 3905 ACHD patients, estimated prevalence of ACHD‐HF was 6.4% at a mean age of 35 years. Those results are comparable, albeit somewhat higher when compared with the prevalence of ACHD‐HF‐related admissions in the Dutch CONCOR study (4.6% of ACHD patients admitted over a 14 year period), 18 or the Swedish nation‐wide cohort study (3.3% of patients over a median follow‐up of 27.5 years). 5 Both studies used administrative HF labels to establish ACHD‐HF diagnosis, which may explain the higher prevalence in our study. ACHD‐HF prevalence is difficult to establish given the challenge to define ACHD‐HF. 19 Prior studies have used administrative diagnoses, 7 HF‐related hospital admission, 18 signs and symptoms of HF, 20 natriuretic peptides, and/or peak oxygen consumption 21 to define ACHD‐HF. In this study, we used a standardized, inclusive definition of ACHD‐HF using a combination of the following: signs and symptoms of HF, impaired ventricular function with elevated filling pressures, exercise intolerance, elevation of natriuretic peptides and/or unique ACHD‐HF manifestations in patients with a Fontan circulation (protein loosing enteropathy or plastic bronchitis). 22 This definition probably reduces the risk of underestimation (because only patients who require hospital admission were included) or overestimation of ACHD‐HF prevalence (because patients at risk for HF were also included 23 ). This study indicates a substantially earlier increase in HF prevalence in the ACHD population, when compared with the general population. In the general population, HF prevalence increases from 0.9% in subjects aged 55–64 to 17.4% in those aged ≥85 years, 24 which is quite different from our study cohort with ACHD‐HF prevalence increasing from 2.3% in subjects aged 15–35 years to 21.9% in subjects aged >50 years. ACHD‐HF prevalence was consistently higher in older patients for all anatomical/physiological ACHD subgroups (heat map). This is intuitive and the improved survival of ACHD patients will automatically increase ACHD‐HF prevalence over time. Maybe more importantly, ACHD‐HF prevalence in cyanotic patients, patients with a Fontan circulation and patients with a systemic RV was >30% from the age of 35 years and even >50% from the age of 50 years, consistent with prior studies. 25 , 26 Although ACHD‐HF prevalence is lower in patients with a biventricular circulation and systemic LV, absolute numbers in these subgroups exceed the combined number of cyanotic patients, patients with a Fontan circulation, and patients with a systemic RV.
Adult congenital heart disease‐heart failure outcome
In this study, patients with ACHD‐HF had worse outcome with 25.8% reaching the combined endpoint of death, heart transplant, or VAD implantation, compared with only 1.2% of ACHD patients without HF, which confirms prior reports. 18 , 27 Our case–control analysis indicates that all‐cause mortality was about five times higher in ACHD‐HF patients when compared with their matched controls. It also emphasizes the need to better understand factors associated with ACHD‐HF so that clinicians can recognize patients at risk for ACHD‐HF. 12 These risk factors should trigger a search for modifiable structural or electrical issues 16 , 28 and heighten ongoing vigilance. Because the ACHD‐HF phenotype will differ according to anatomical/physiological subgroups, 4 , 29 it is equally important to evaluate these risk factors separately for all subgroups.
Factors related to adult congenital heart disease‐heart failure
Infective endocarditis is a complication especially frequent in CHD patients with prosthetic valves or valve‐containing conduits 30 , 31 and is associated with increased mortality and morbidity, including HF. The need for high‐risk surgery in itself, or the sequelae of the infection/intervention may explain the relation with ACHD‐HF observed in this study. NYHA class is a strong predictor of outcome in the general ACHD population 32 , 33 as well as in ACHD‐HF patients. 4 Self‐reported NYHA class also has a strong association with presence of HF in ACHD patients. 22 Exercise intolerance is one of the cardinal symptoms of HF, so it's reassuring to confirm its value in ACHD patients who may have adapted to a gradually decreasing exercise capacity.
Our study confirms that atrial arrhythmia is closely linked to ACHD‐HF. A diagnosis of atrial arrhythmia should raise awareness for ACHD‐HF (and vice versa) and should often trigger further investigations. Because atrial arrhythmia in itself could worsen haemodynamics, including worsening ventricular function, restoration, and maintenance of sinus rhythm is a valuable treatment strategy in these patients. 31 Pacemaker implantation has been associated with poor outcome in ACHD subgroups, which may be related to pacing‐induced dyssynchrony and subsequent ventricular and/or valvular dysfunction 34 similar to acquired HF. It has also been shown that the degree of structural ACHD complexity is a strong and independent predictor for late device complications, 35 suggesting that strict follow‐up should be planned in complex ACHD patients after pacemaker implantation. Ventricular dysfunction, which mainly relates to systolic systemic and/or pulmonic dysfunction, should always trigger consideration of ACHD‐HF. Because ventricular dysfunction alone was insufficient for an ACHD‐HF diagnosis, 11% of patients without ACHD‐HF had mild, and 3% had moderate/severe ventricular dysfunction. In these patients, who could be considered as having Stage B HF according to the ACC/AHA HF classification system, additional confirmation of an ACHD‐HF diagnosis with natriuretic peptides or invasive haemodynamics is of interest and has added prognostic significance, 4 , 36 especially in patients with moderate/severe complexity CHD. 22 , 23 , 25
Differences between anatomical/physiological subgroups
Coronary artery disease was related to ACHD‐HF in patients with a biventricular circulation and a systemic LV. Emergence of a ‘geriatric’ ACHD population has been described and in this population acquired morbidities like coronary artery disease likely play a role in HF development and outcome. 3 In (younger) patients with cyanotic heart disease, systemic RV or Fontan circulation, anatomical/physiological complexity still trumps ‘classic’ cardiovascular risk factors explaining the lack of association with coronary artery disease in these subgroups.
Other parameters will have limited discriminatory value in some anatomically/physiological subgroups if they are uniformly present or absent (for example ‘prior intervention’ or ‘pulmonary hypertension’ in patients with a Fontan circulation). Ventricular dilation and valvular dysfunction appear less related to ACHD‐HF in patients with cyanotic CHD, patients with a Fontan circulation and patients with a systemic RV. Although surprising for patients with a Fontan circulation, because the presence of atrioventricular valve regurgitation strongly relates to outcome, 37 dilated ventricles are often observed in the absence of ACHD‐HF in these patient groups. Since 2000, transcatheter percutaneous valve implantation (Melody) was described as an alternative to open‐heart surgery in patients with predominant right‐sided residual lesions (Fallot, pulmonary atresia). Infective endocarditis is significantly more incident in those patients after Melody stents in comparison with homografts. 38 Valve‐containing prosthetics is determined as a significant risk for infective endocarditis, whereas other prosthetics, including valve repair, are not associated with increased risk long term after implantation. 39 In any case, either infective endocarditis itself causing HF or residual lesions after infective endocarditis increase the risk for HF, explaining the stronger association with ACHD‐HF in these subgroups of patients.
Strengths and limitations
To the best of our knowledge, the current report represents one of the most detailed studies evaluating the prevalence of ACHD‐HF and its associated risk factors in the different subgroups of ACHD, using a uniform definition of ACHD‐HF, which provides more granular data when compared with other studies using patient data taken from administrative data. 40 Still, because of the retrospective nature of the study, and although only limited data was missing, our results are dependent on the accuracy of the recorded data. Our study underscores the complex nature of ACHD‐HF with involvement of multiple organ systems which require follow‐up in a dedicated ACHD‐HF clinic with support from interventional cardiologists, congenital cardiac surgeons, electrophysiologists, heart transplant team, and psychologists. 41 We did record data on HF admissions to the hospital, which will be included in future studies. Finally, our study was a single‐centre study, resulting in possible measurement of confounders that vary between centres.
Conclusions
Heart failure related to ACHD is prevalent especially in patients with complex CHD and is associated with poor prognosis. Our data provide insight in the factors related to ACHD‐HF including differences between specific anatomical/physiological subgroups.
Conflict of interest
No conflicts of interest.
Funding
None.
Supporting information
Table S2A. Patient characteristics Eisenmenger/Cyanotic CHD patients
Table S2B. Patient characteristics Fontan patients
Table S2C. Patient characteristics systemic RV patients
Table S2D. Patient characteristics shunt lesions patients
Table S2E. Patient characteristics predominant right‐sided lesions patients
Table S2F. Patient characteristics predominant left‐sided lesions patients
Acknowledgements
The authors would like to thank Ms Lutgarde Thijs for her invaluable help with the statistical analysis.
Arnaert, S. , De Meester, P. , Troost, E. , Droogne, W. , Van Aelst, L. , Van Cleemput, J. , Voros, G. , Gewillig, M. , Cools, B. , Moons, P. , Rega, F. , Meyns, B. , Zhang, Z. , Budts, W. , and Van De Bruaene, A. (2021) Heart failure related to adult congenital heart disease: prevalence, outcome and risk factors. ESC Heart Failure, 8: 2940–2950. 10.1002/ehf2.13378.
References
- 1. Moons P, Bovijn L, Budts W, Belmans A, Gewillig M. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation 2010; 122: 2264–2272. [DOI] [PubMed] [Google Scholar]
- 2. Baumgartner H. Geriatric congenital heart disease: a new challenge in the care of adults with congenital heart disease? Eur Heart J 2014; 35: 683–685. [DOI] [PubMed] [Google Scholar]
- 3. Tutarel O, Kempny A, Alonso‐Gonzalez R, Jabbour R, Li W, Uebing A, Dimopoulos K, Swan L, Gatzoulis MA, Diller GP. Congenital heart disease beyond the age of 60: emergence of a new population with high resource utilization, high morbidity, and high mortality. Eur Heart J 2014; 35: 725–732. [DOI] [PubMed] [Google Scholar]
- 4. Bruaene A, Van De Hickey EJ, Kovacs AH, Crean AM, Wald RM, Silversides CK, Redington AN, Ross HJ, Alba AC, Billia F, Nair K, Benson L, Horlick E, Osten M, Colman J, Heggie J, Oechslin EN, Roche SL. Phenotype, management and predictors of outcome in a large cohort of adult congenital heart disease patients with heart failure. Int J Cardiol 2018; 252: 80–87. [DOI] [PubMed] [Google Scholar]
- 5. Gilljam T, Mandalenakis Z, Dellborg M, Lappas G, Eriksson P, Skoglund K, Rosengren A. Development of heart failure in young patients with congenital heart disease: A nation‐wide cohort study. Open Hear 2019; 6: e000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burchill LJ, Gao L, Kovacs AH, Opotowsky AR, Maxwell BG, Minnier J, Khan AM, Broberg CS. Hospitalization trends and health resource use for adult congenital heart disease‐related heart failure. J Am Heart Assoc 2018; 7: e008775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zomer AC, Vaartjes I, Van Der Velde ET, De Jong HMY, Konings TC, Wagenaar LJ, Heesen WF, Eerens F, Baur LHB, Grobbee DE, Mulder BJM. Heart failure admissions in adults with congenital heart disease; risk factors and prognosis. Int J Cardiol 2013; 168: 2487–2493. [DOI] [PubMed] [Google Scholar]
- 8. Diller GP, Kempny A, Alonso‐Gonzalez R, Swan L, Uebing A, Li W, Babu‐Narayan S, Wort SJ, Dimopoulos K, Gatzoulis MA. Survival prospects and circumstances of death in contemporary adult congenital heart disease patients under follow‐up at a large tertiary centre. Circulation 2015; 132: 2118–2125. [DOI] [PubMed] [Google Scholar]
- 9. Mackie AS, Tran DT, Marelli AJ, Kaul P. Cost of congenital heart disease hospitalizations in canada: a population‐based study. Can J Cardiol 2017; 33: 792–798. [DOI] [PubMed] [Google Scholar]
- 10. VanderPluym CJ, Cedars A, Eghtesady P, Maxwell BG, Gelow JM, Burchill LJ, Maltais S, Koehl DA, Cantor RS, Blume ED. Outcomes following implantation of mechanical circulatory support in adults with congenital heart disease: an analysis of the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS). J Heart Lung Transplant 2018; 37: 89–99. [DOI] [PubMed] [Google Scholar]
- 11. Crossland DS, Jansen K, Parry G, Harper A, Perri G, Davidson A, De Rita F, Hermuzi A, Nassar M, Seller N, Macgowan GA, Hasan A, O'Sullivan JJ, Coats L. Outcome following heart transplant assessment in adults with congenital heart disease. Heart 2019; 105: 1741–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang F, Harel‐Sterling L, Cohen S, Liu A, Brophy JM, Paradis G, Marelli AJ. Heart failure risk predictions in adult patients with congenital heart disease: a systematic review. Heart 2019; 105: 1661–1669. [DOI] [PubMed] [Google Scholar]
- 13. van der Velde ET, Vriend JW, Mannens MM, Uiterwaal CS, Brand R, Mulder BJ. CONCOR, an initiative towards a national registry and DNA‐bank of patients with congenital heart disease in the Netherlands: rationale, design, and first results. Eur J Epidemiol 2005; 20: 549–557. [DOI] [PubMed] [Google Scholar]
- 14. Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, Somerville J, Williams RG, Webb GD. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol 2001; 37: 1170–1175. [DOI] [PubMed] [Google Scholar]
- 15. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM, Crumb SR, Dearani JA, Fuller S, Gurvitz M, Khairy P, Landzberg MJ, Saidi A, Valente AM, Van Hare GF. 2018 AHA/ACC Guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2019; 73: e81–e192. [DOI] [PubMed] [Google Scholar]
- 16. Budts W, Roos‐Hesselink J, Rädle‐Hurst T, Eicken A, McDonagh TA, Lambrinou E, Crespo‐Leiro MG, Walker F, Frogoudaki AA. Treatment of heart failure in adult congenital heart disease: a position paper of the Working Group of Grown‐Up Congenital Heart Disease and the Heart Failure Association of the European Society of Cardiology. Eur Heart J 2016; 37: 1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kempny A, Dimopoulos K, Uebing A, Moceri P, Swan L, Gatzoulis MA, Diller GP. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily lifesingle centre experience and review of published data. Eur Heart J 2012; 33: 1386–1396. [DOI] [PubMed] [Google Scholar]
- 18. Lal S, Kotchetkova I, Cao J, Jackson D, Cordina R, Celermajer DS. Heart failure admissions and poor subsequent outcomes in adults with congenital heart disease. Eur J Heart Fail 2018; 20: 812–815. [DOI] [PubMed] [Google Scholar]
- 19. Alshawabkeh LI, Opotowsky AR. Burden of heart failure in adults with congenital heart disease. Curr Heart Fail Rep 2016; 13: 247–254. [DOI] [PubMed] [Google Scholar]
- 20. Khairy P, Fernandes SM, Mayer JE, Triedman JK, Walsh EP, Lock JE, Landzberg MJ. Long‐term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008; 117: 85–92. [DOI] [PubMed] [Google Scholar]
- 21. Norozi K, Buchhorn R, Bartmus D, Alpers V, Arnhold JO, Schoof S, Zoege M, Binder L, Geyer S, Wessel A. Elevated brain natriuretic peptide and reduced exercise capacity in adult patients operated on for tetralogy of Fallot is due to biventricular dysfunction as determined by the myocardial performance index. Am J Cardiol 2006; 97: 1377–1382. [DOI] [PubMed] [Google Scholar]
- 22. McLarry J, Broberg C, Opotowsky AR, Kaufman T, Stout K, Burchill LJ. Defining heart failure in adult congenital heart disease. Prog Pediatr Cardiol 2014; 38: 3–7. [Google Scholar]
- 23. Burchill LJ, Lee MGY, Nguyen VP, Stout KK. Heart failure in adult congenital heart disease. Cardiol Clin 2020; 38: 457–469. [DOI] [PubMed] [Google Scholar]
- 24. Bleumink GS, Knetsch AM, Sturkenboom MCJM, Straus SMJM, Hofman A, Deckers JW, Witteman JCM, Stricker BHC. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure—the Rotterdam Study. Eur Heart J 2004; 25: 1614–1619. [DOI] [PubMed] [Google Scholar]
- 25. Van De Bruaene A, Toh N, Hickey EJ, Benson L, Horlick E, Granton JT, Williams WG, Roche SL. Pulmonary hypertension in patients with a subaortic right ventricle: prevalence, impact and management. Heart 2019; 105: 1471–1478. [DOI] [PubMed] [Google Scholar]
- 26. Daley M, Du Plessis K, Zannino D, Hornung T, Disney P, Cordina R, Grigg L, Radford DJ, Bullock A, D'Udekem Y. Reintervention and survival in 1428 patients in the Australian and New Zealand Fontan Registry. Heart 2020; 106: 751–757. [DOI] [PubMed] [Google Scholar]
- 27. Rodriguez FH, Moodie DS, Parekh DR, Franklin WJ, Morales DLS, Zafar F, Adams GJ, Friedman RA, Rossano JW. Outcomes of heart failure‐related hospitalization in adults with congenital heart disease in the United States. Congenit Heart Dis 2013; 8: 513–519. [DOI] [PubMed] [Google Scholar]
- 28. Van De Bruaene A, Meier L, Droogne W, De Meester P, Troost E, Gewillig M, Budts W. Management of acute heart failure in adult patients with congenital heart disease. Heart Fail Rev 2018; 23: 1–14. [DOI] [PubMed] [Google Scholar]
- 29. Menachem JN, Schlendorf KH, Mazurek JA, Bichell DP, Brinkley DM, Frischhertz BP, Mettler BA, Shah AS, Zalawadiya S, Book W, Lindenfeld JA. Advanced heart failure in adults with congenital heart disease. JACC Hear Fail 2020; 8: 87–99. [DOI] [PubMed] [Google Scholar]
- 30. Verheugt CL, Uiterwaal CSPM, Van Der Velde ET, Meijboom FJ, Pieper PG, Van Dijk APJ, Vliegen HW, Grobbee DE, Mulder BJM. Mortality in adult congenital heart disease. Eur Heart J 2010; 31: 1220–1229. [DOI] [PubMed] [Google Scholar]
- 31. Crossland DS, Van De Bruaene A, Silversides CK, Hickey EJ, Roche SL. Heart failure in adult congenital heart disease: from advanced therapies to end‐of‐life care. Can J Cardiol 2019; 35: 1723–1739. [DOI] [PubMed] [Google Scholar]
- 32. Ahmed A, Aronow WS, Fleg JL. Higher New York Heart Association classes and increased mortality and hospitalization in patients with heart failure and preserved left ventricular function. Am Heart J 2006; 151: 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bredy C, Ministeri M, Kempny A, Alonso‐Gonzalez R, Swan L, Uebing A, Diller GP, Gatzoulis MA, Dimopoulos K. New York Heart Association (NYHA) classification in adults with congenital heart disease: Relation to objective measures of exercise and outcome. Eur Hear J ‐ Qual Care Clin Outcomes 2018; 4: 51–58. [DOI] [PubMed] [Google Scholar]
- 34. Vejlstrup N, Sørensen K, Mattsson E, Thilén U, Kvidal P, Johansson B, Iversen K, Søndergaard L, Dellborg M, Eriksson P. Long‐term outcome of mustard/senning correction for transposition of the great arteries in Sweden and Denmark. Circulation 2015; 132: 633–638. [DOI] [PubMed] [Google Scholar]
- 35. Midha D, Chen Z, Jones DG, Williams HJ, Lascelles K, Jarman J, Clague J, Till J, Dimopoulos K, Babu‐Narayan SV, Markides V, Gatzoulis MA, Wong T. Pacing in congenital heart disease—a four‐decade experience in a single tertiary centre. Int J Cardiol 2017; 241: 177–181. [DOI] [PubMed] [Google Scholar]
- 36. Geenen LW, Baggen VJM, Koudstaal T, Boomars KA, Eindhoven JA, Boersma E, Roos‐Hesselink JW, van den Bosch AE. The prognostic value of various biomarkers in adults with pulmonary hypertension: a multi‐biomarker approach. Am Heart J 2019; 208: 91–99. [DOI] [PubMed] [Google Scholar]
- 37. D'Udekem Y, Xu MY, Galati JC, Lu S, Iyengar AJ, Konstantinov IE, Wheaton GR, Ramsay JM, Grigg LE, Millar J, Cheung MM, Brizard CP. Predictors of survival after single‐ventricle palliation: the impact of right ventricular dominance. J Am Coll Cardiol 2012; 59: 1178–1185. [DOI] [PubMed] [Google Scholar]
- 38. Van Dijck I, Budts W, Cools B, Eyskens B, Boshoff DE, Heying R, Frerich S, Vanagt WY, Troost E, Gewillig M. Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart 2015; 101: 788–793. [DOI] [PubMed] [Google Scholar]
- 39. Kuijpers JM, Koolbergen DR, Groenink M, Peels KCH, Reichert CLA, Post MC, Bosker HA, Wajon EMCJ, Zwinderman AH, Mulder BJM, Bouma BJ. Incidence, risk factors, and predictors of infective endocarditis in adult congenital heart disease: focus on the use of prosthetic material. Eur Heart J 2017; 38: 2048–2056. [DOI] [PubMed] [Google Scholar]
- 40. Cohen S, Liu A, Wang F, Guo L, Brophy JM, Abrahamowicz M, Therrien J, Beauchesne LM, Bédard E, Grewal J, Khairy P, Oechslin E, Roche SL, Silversides CK, Muhll IFV, Marelli AJ. Risk prediction models for heart failure admissions in adults with congenital heart disease. Int J Cardiol 2020; 322: 149–157. [DOI] [PubMed] [Google Scholar]
- 41. Baumgartner H, De Backer J, Babu‐Narayan SV, Budts W, Chessa M, Diller G‐P, Lung B, Kluin J, Lang IM, Meijboom F, Moons P, Mulder BJM, Oechslin E, Roos‐Hesselink JW, Schwerzmann M, Sondergaard L, Zeppenfeld K, Ernst S, Ladouceur M, Aboyans V, Alexander D, Christodorescu R, Corrado D, D'Alto M, de Groot N, Delgado V, Di SG, Dos SL, Eicken A, Fitzsimons D, Frogoudaki AA, Gatzoulis M, Heymans S, Hörer J, Houyel L, Jondeau G, Katus HA, Landmesser U, Lewis BS, Lyon A, Mueller CE, Mylotte D, Petersen SE, Petronio AS, Roffi M, Rosenhek R, Shlyakhto E, Simpson IA, Sousa‐Uva M, Torp‐Pedersen CT, Touyz RM, Van De Bruaene A, Babu‐Narayan SV, Budts W, Chessa M, Diller G‐P, Iung B, Kluin J, Lang IM, Meijboom F, Moons P, Mulder BJM, Oechslin E, Roos‐Hesselink JW, Schwerzmann M, Sondergaard L, Zeppenfeld K, ESC Scientific Document Group . 2020 ESC guidelines for the management of adult congenital heart disease. Eur Heart J 2020; 42: 563–645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S2A. Patient characteristics Eisenmenger/Cyanotic CHD patients
Table S2B. Patient characteristics Fontan patients
Table S2C. Patient characteristics systemic RV patients
Table S2D. Patient characteristics shunt lesions patients
Table S2E. Patient characteristics predominant right‐sided lesions patients
Table S2F. Patient characteristics predominant left‐sided lesions patients


