Abstract
Aims
This study aimed to investigate differences in baseline and treatment characteristics, and in‐hospital mortality according to the aetiologies of cardiogenic shock in patients undergoing veno‐arterial‐extracorporeal membrane oxygenation (VA‐ECMO).
Methods and results
The RESCUE registry is a multicentre, observational cohort that includes 1247 patients with cardiogenic shock from 12 centres. A total of 496 patients requiring VA‐ECMO were finally selected, and the study population was stratified by cardiogenic shock aetiology [ischaemic cardiomyopathy (ICM, n = 342) and non‐ICM (NICM, n = 154)]. The primary outcome of interest was in‐hospital mortality. Sensitivity analyses including propensity‐score matching adjustments were performed. Mean age of the entire population was 61.8 ± 14.2, and 30.8% were women. There were significant differences in baseline characteristics; notable differences included the older age of patients with ICM (65.1 ± 13.7 vs. 58.2 ± 13.8, P < 0.001), preponderance of males [258 (75.4%) vs. 85 (55.2%), P < 0.001], and higher prevalence of diabetes mellitus [140 (40.9%) vs. 39 (25.3%), P = 0.001] compared with patients in the NICM aetiology group. Patients with ischaemic cardiogenic shock were more likely to have longer shock duration before VA‐ECMO implantation (518.7 ± 941.4 min vs. 292.4 ± 707.8 min, P = 0.003) and were less likely to undergo distal limb perfusion than those with NICM [108 (31.6%) vs. 79 (51.3%), P < 0.001]. In‐hospital mortality in the overall cohort was 52.2%; patients with ICM had a higher unadjusted risk of in‐hospital mortality [203 (59.4%) vs. 56 (36.4%); unadjusted hazard ratio, 2.295; 95% confidence interval, 1.698–3.100; P < 0.001]. There were no significant differences in the primary outcome between the two aetiologies following propensity‐score matching multiple adjustments (adjusted hazard ratio, 1.265; 95% confidence interval, 0.840–1.906; P = 0.260).
Conclusions
Results of the current study indicated among patients with cardiogenic shock undergoing VA‐ECMO, ischaemic aetiology does not seem to impact in‐hospital mortality. These findings underline that early initiation and appropriate treatment strategies of VA‐ECMO for patients with ICM shock are required.
Keywords: ECMO, Cardiogenic shock, Ischaemic cardiomyopathy, Non‐ischaemic cardiomyopathy
Introduction
Cardiogenic shock, the most severe form of acute heart failure (HF), is characterized by life‐threatening end‐organ hypo‐perfusion resulting from a low cardiac output state. 1 Improvement in survival has been observed over the past two decades, attributed to the introduction of routine use of early revascularisation in acute myocardial infarction (AMI) and modern intensive care. 2 Nevertheless, survival in cardiogenic shock is still dismal.
Mechanical circulatory support (MCS) devices provide a therapeutic option for patients with cardiogenic shock refractory to pharmacologic treatment. In the context of persistently poor cardiogenic shock outcomes and technological improvements in MCS, the number of patients treated with MCS, such as veno‐arterial‐extracorporeal membrane oxygenation (VA‐ECMO), has increased exponentially over the last decade. 3 , 4 Given the increased utilization of this therapeutic modality, it becomes clinically relevant to identify various determinants of clinical outcome. Although outcome data are limited to observational studies, there does appear to be differential short‐term outcomes according to indication for MCS. 5
Ischaemic cardiomyopathy (ICM) is the most common aetiology of HF. 4 , 6 Multiple studies have shown that patients with ICM have decreased survival compared to patients with non‐ICM (NICM). 7 , 8 However, few studies have assessed the impact of HF aetiology (ICM vs. NICM) on outcomes in patients with cardiogenic shock that underwent VA‐ECMO, leaving this field underexplored. Accordingly, the objective of this study was to describe the prevalence, clinical characteristics, in‐hospital treatment, and in‐hospital prognosis of a large cohort of patients with cardiogenic shock that underwent VA‐ECMO stratified by their aetiology.
Methods
Study population
The RESCUE (REtrospective and prospective observational Study to investigate Clinical oUtcomes and Efficacy of left ventricular assist device for Korean patients with cardiogenic shock, NCT02985008 at www.clinicaltrials.gov) study is a multicentre, retrospective and prospective registry of patients with cardiogenic shock aged over 19 years. A total of 1247 patients from 12 tertiary centres were enrolled between January 2014 and December 2018 (954 enrolled retrospectively and 293 prospectively). Inclusion criteria were as follows: (i) age ≥ 19 years old, (ii) systolic blood pressure <90 mmHg for 30 min or a state that required inotrope or vasopressor support to achieve a systolic blood pressure >90 mmHg, and (iii) the presence of pulmonary congestion and signs of impaired organ perfusion (altered mental status, cold skin, urine output <0.5 mL/kg/h for the previous 6 h, or blood lactate >2.0 mmol/L). Major exclusion criteria were out‐of‐hospital cardiac arrest, other causes of shock apart from cardiogenic shock (hypovolemic, septic shock, or post‐cardiotomy shock), and those who refused active treatment. For the current study, 496 patients who underwent VA‐ECMO for cardiogenic shock were selected. The timeframe of the selected patients was the same as the original population. Among the selected, patients were classified according to cardiogenic shock aetiology (i.e. ICM or NICM), and we compared clinical profiles and in‐hospital outcomes as ICM vs. NICM (Figure S1 ).
Data collection and outcomes
The institutional review board of each hospital approved the study protocol and waived the requirement for written informed consent for patients enrolled in the retrospective registry. We obtained informed consent from the patients enrolled for prospective registry. The study was conducted according to the principles of the Declaration of Helsinki. For the RESCUE registry, information about patient demographics, laboratory data, procedural data, in‐hospital management, and outcomes were collected by independent clinical research coordinators via web‐based case report forms. Additional information was obtained from medical records, when necessary.
The primary outcome of the present study was determination of the impact of ICM on in‐hospital mortality. Secondary outcomes were in‐hospital cardiac mortality and VA‐ECMO related complications such as limb ischaemia, bleeding, in‐hospital cerebrovascular accident, and sepsis. All‐cause mortality was defined as death from any cause. All deaths were considered of cardiac cause unless an undisputed non‐cardiac cause could be established.
Procedures
The VA‐ECMO devices were initiated when patients in cardiogenic shock were unresponsive to the administration of vasopressors after the correction of hypovolaemia and hypoxaemia or when arrest was prolonged or recurrent. 9 VA‐ECMO device was inserted by percutaneous cannulation using the Seldinger technique or surgical cannulation using the cut‐down method at femoral vessels. Patients were heparinised to an activated clotting time of 180–200 s during the course of ECMO support. The decision to implant an intra‐aortic balloon pump was determined by experienced interventional cardiologists or cardiac surgeons. The intra‐aortic balloon pump was inserted percutaneously through the femoral artery with fluoroscopy guidance. If necessary, coronary interventions and best available medical treatment were performed in accordance with relevant standard guidelines at the time of each procedure. 10
Statistical analysis
Categorical variables were tested using the χ 2 test or Fisher's exact test, as appropriate, and presented as number and relative frequencies (percentages). Continuous variables were compared using the analysis of variance or the Kruskal–Wallis test, as appropriate, and presented as mean ± standard deviation or median with interquartile ranges, according to whether they were normally distributed. Cumulative event rates were calculated based on Kaplan–Meier censoring estimates, and comparison of clinical outcomes between the ICM and NICM groups was performed with the log‐rank test. Because differences in baseline characteristics could significantly affect outcomes, sensitivity analyses were performed to adjust for confounders as much as possible. Details of the statistical analysis are presented in the Supporting information. The Cox proportional hazard regression in a propensity‐score matched cohort was performed. All available covariates were included in the logistic regression model to generate propensity scores precisely following the recommendations of analysis using propensity score. 11 Propensity score matching yielded 84 patients in the ICM group and 84 control subjects in the NICM group. Balance between the two groups after propensity‐score matching was assessed by calculating per cent standardized mean differences. Per cent standardized mean differences after propensity‐score matching were within ±20% across all matched covariates, demonstrating achievement of successful balance between comparative groups (Table S1 ). To identify independent predictors of in‐hospital mortality, we used a multivariable Cox proportional hazard model. C‐statistics with 95% confidence intervals (CIs) were calculated to validate the discriminant function of the model. Statistical analyses were performed using R Statistical Software (version 3.2.5; R Foundation for Statistical Computing, Vienna, Austria) with P < 0.05 considered statistically significant.
Results
Overall (n = 496), 342 patients (69.0% of the total) were reported to have ICM and 154 (31.0%) to have NICM. Among patients with ischaemic shock (n = 342), 57.3% had ST‐segment elevation AMI, 28.1% non‐ST‐segment elevation AMI, and 14.6% had ICM without AMI (Figure S2 A). Figure S2 B shows the prevalence of various NICM aetiologies in the study population. Median age of the entire study population was 61.8 ± 14.2 years, and 30.8% were women.
Baseline characteristics
The baseline demographic and clinical characteristics of patients with ICM compared with those with NICM are shown in Table S2 . Notable differences included the older age of patients with ICM, preponderance of male patients, and higher prevalence of diabetes mellitus compared with patients in the NICM aetiology group. Patients with ICM had significantly higher levels of lactic acid before VA‐ECMO implantation compared with NICM patients. Patients with ICM were more likely to be in sustained cardiac arrest prior to initiation of support with VA‐ECMO. Table S3 lists various treatment characteristics recorded during index hospitalization among ICM and NICM patients with cardiogenic shock that underwent VA‐ECMO. Overall, patients with ICM were more likely to have had a higher vasoactive inotropic score and to have had a longer duration of cardiogenic shock before ECMO implantation. Vasoactive inotropic score was developed as an objective measure of the magnitude of vasopressor support and was demonstrated to be an independent predictor of clinical outcomes. 12 Patients with ICM had a higher prevalence of mechanical ventilation and continuous renal replacement therapy and had a lower prevalence of distal limb perfusion.
In‐hospital outcome
The rate of the primary outcome is shown in Table 1 , Figure 1 , and Table S3 . Patients with ICM had a higher unadjusted risk of in‐hospital mortality compared with NICM patients [hazard ratio (HR), 2.295; 95% CI, 1.698–3.100; P < 0.001]. However, the risk of in‐hospital mortality was not different between the two groups after propensity score matching multiple adjustments (adjusted HR, 1.265; 95% CI, 0.840–1.906; P = 0.260). Details of the in‐hospital outcome are presented in the Supporting information. Table 2 shows rates of secondary outcomes in patients with ICM compared with those with NICM. There were no significant differences between the two groups in the adjusted rates of cardiac mortality, ECMO cannula insertion site bleeding, limb ischaemia, gastrointestinal bleeding, cerebrovascular accident, and sepsis.
Table 1.
In‐hospital mortality according to ICM vs. NICM
| ICMP (n = 342) | NICMP (n = 154) | Unadjusted | PSM adjustment | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| In‐hospital mortality | 203 (59.4) | 56 (36.4) | 2.295 (1.698–3.100) | <0.001 | 1.265 (0.840–1.906) | 0.260 |
CI, confidence interval; HR, hazard ratio; ICM, ischaemic cardiomyopathy; NICM, non‐ischaemic cardiomyopathy; PSM, propensity‐score matching.
Values are expressed as n (%)
Figure 1.
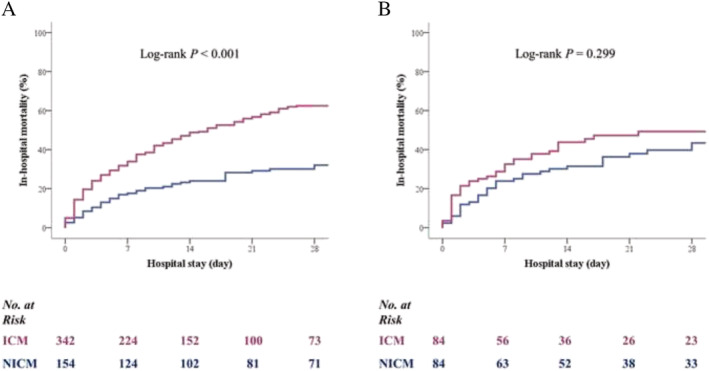
Time‐to‐event curves for in‐hospital mortality in the overall cohort of patients (A) and in the propensity‐score matched cohort of patients (B) managed with a VA‐ECMO. ICM, ischaemic cardiomyopathy; NICM, non‐ischaemic cardiomyopathy; VA‐ECMO, veno‐arterial‐extracorporeal membrane oxygenation.
Table 2.
In‐hospital outcomes
| Overall (n = 496) | ICM (n = 342) | NICM (n = 154) | Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
|---|---|---|---|---|---|---|---|
| In‐hospital cardiac mortality | 225 (45.4) | 178 (52.0) | 47 (30.5) | 2.248 (1.627–3.108) | <0.001 | 1.360 (0.813–2.276) | 0.241 |
| ECMO site bleeding | 62 (12.5) | 45 (13.2) | 17 (11.0) | 1.221 (0.674–2.211) | 0.510 | 1.260 (0.655–2.423) | 0.489 |
| Limb ischaemia | 35 (7.1) | 25 (7.3) | 10 (6.5) | 1.136 (0.531–2.427) | 0.743 | 0.843 (0.377–1.885) | 0.678 |
| GI bleeding | 27 (5.4) | 19 (5.6) | 8 (5.2) | 1.074 (0.459–2.509) | 0.870 | 0.981 (0.396–2.434) | 0.967 |
| Cerebrovascular accident | 18 (3.6) | 15 (4.4) | 3 (1.9) | 2.309 (0.659–8.095) | 0.191 | 1.584 (0.417–60.13) | 0.500 |
| Sepsis | 21 (4.2) | 12 (3.5) | 9 (5.8) | 0.586 (0.242–1.421) | 0.237 | 0.562 (0.228–1.386) | 0.211 |
| Survival (n = 237) | ICM (n = 139) | NICM (n = 98) | Unadjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
|---|---|---|---|---|---|---|---|
| Poor neurological outcome | 19 (7.9) | 18 (12.9) | 1 (1.0) | 14.459 (1.897–110.219) | 0.010 | 14.891 (1.935–114.569) | 0.009 |
CI, confidence interval; CPC, Glasgow–Pittsburgh Cerebral Performance Categories; ECMO, extracorporeal membrane oxygenation; GI, gastrointestinal; ICM, ischaemic cardiomyopathy; HR, hazard ratio; NICM, non‐ischaemic cardiomyopathy.
Poor neurological outcomes = CPC scores of 3 (severe cerebral disability: conscious, dependent on others for daily support because of impaired brain function, ranges from ambulatory state to severe dementia or paralysis), 4 (coma or vegetative state: any degree of coma without the presence of all brain death criteria, unawareness, even if appears awake without interaction with environment; may have spontaneous eye opening and sleep/awake cycles, cerebral unresponsiveness), or 5 (brain death: apnoea, areflexia, or electroencephalogram silence).
Independent predictors of all‐cause mortality
Table 3 shows the independent predictors of in‐hospital mortality in sensitivity analyses using propensity score matching and inverse‐probability weighting analysis. Lactic acid level before ECMO implantation, time from shock to ECMO implantation, cardiopulmonary resuscitation, and distal limb perfusion were identified as independent predictors of in‐hospital mortality.
Table 3.
Predictors for in‐hospital mortality
| Adjusted HR | 95% CI | P value | |
|---|---|---|---|
| Propensity score matched population | |||
| Body mass index | 1.104 | 1.037–1.175 | 0.002 |
| Left ventricular ejection fraction | 0.983 | 0.966–1.000 | 0.049 |
| Creatinine clearance rate | 0.413 | 0.270–0.631 | <0.001 |
| Pre‐ECMO lactic acid | 1.074 | 1.022–1.129 | 0.005 |
| Shock to ECMO time | 1.133 | 0.979–1.310 | 0.093 |
| Cardiopulmonary resuscitation | 3.712 | 2.257–6.104 | <0.001 |
| Distal limb perfusion | 0.539 | 0.338–0.862 | 0.010 |
| Mechanical ventilator | 2.497 | 1.138–5.482 | 0.023 |
| IPW‐adjusted | |||
| Pre‐ECMO lactic acid | 1.095 | 1.032–1.161 | 0.003 |
| Shock to ECMO time | 1.000 (1.0002) | 1.000–1.000 (1–1.000396) | 0.003 |
| Cardiopulmonary resuscitation | 1.711 | 1.110–2.636 | 0.015 |
| Initial pump flow | 0.704 | 0.531–0.934 | 0.015 |
| Distal limb perfusion | 0.486 | 0.325–0.726 | <0.001 |
| CRRT | 1.616 | 1.076–2.426 | 0.021 |
CI, confidence interval; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio; IPW, inverse‐probability weighting
Discussion
The objective of this study was to describe the prevalence, clinical characteristics, and in‐hospital outcomes of ischaemic and non‐ischaemic aetiologies of cardiomyopathy in a contemporary population of patients with cardiogenic shock that underwent VA‐ECMO. The findings may be summarized as follows: (i) the majority of patients with cardiogenic shock that underwent VA‐ECMO had ICM. (ii) There were significant differences in demographic and clinical characteristics of patients with ICM compared with those with NICM. (iii) Similarly, comparison of in‐hospital treatment characteristics between two groups of patients revealed clinically meaningful differences. (iv) However, in‐hospital mortality was similar for patients with ICM and NICM following multiple adjustments including in‐hospital treatment characteristics. Consistent results were retained in sensitivity analyses using propensity score matching and inverse‐probability weighting analysis (Figure S3 ).
Prevalence and baseline characteristics of ICM
We observed that ICM was the leading cause of cardiogenic shock in patients underwent VA‐ECMO (69.0% of the total). This was in agreement with previous studies; Gheorghiade et al. reported in a review of 24 trials that 62% of patients had an investigator reported ischaemic aetiology. 1 , 13
As expected, there were significant differences in demographic and clinical characteristics of patients with ischaemic as compared with NICM. Patients with NIC were older, and more often were male and current smokers, and more often had a history of diabetes mellitus, hypertension, and dyslipidaemia.
In‐hospital treatment
Comparison of treatment characteristics of patients with ICM and NICM revealed clinically meaningful differences; patients with ischaemic cardiogenic shock were more likely to have had a longer duration of shock prior to ECMO implantation and higher vasoactive inotropic score 3 , 9 , 12 than patients with NICM. On the other hand, a lower number of patients in the ICM cohort underwent distal limb perfusion than those in the NICM. The marked differences in baseline characteristics between ICM and NICM cases are considered to have a substantial impact on the treatment of cardiogenic shock, and differences in these treatment characteristics may affect clinical outcomes.
In‐hospital mortality
Despite intensive care and provision of MCS, in‐hospital mortality in the overall cohort of cardiogenic shock patients managed with a VA‐ECMO was 52.2%. This was in agreement with previous studies; Jaya Batra et al. reported that 30 day mortality was 52.2% in the overall cohort of patients that underwent ECMO in New York state. 14
Numerous studies have demonstrated an increased mortality in patients with ischaemic aetiology compared with non‐ischaemic aetiology in HF patients treated with guideline‐directed medical therapy. 7 , 8 This difference of mortality has been attributed to an older population and higher rates of comorbidities, suggesting that it may also influence outcomes in cardiogenic shock patients on VA‐ECMO. Furthermore, short‐term survival on VA‐ECMO is influenced by the indication for MCS as well as by comorbidities of patients. 3 , 5 However, our analysis demonstrated that there were no significant differences in in‐hospital mortality between two cardiogenic shock aetiologies following multiple adjustments including in‐hospital treatment. This is an unexpected result, but one possible explanation for why ischaemic aetiology was not associated with increased mortality rates in the SMART‐RESCUE registry may relate to the artificial improvement in systemic perfusion associated with the placement and appropriate management of VA‐ECMO which can compensate for the pathophysiological factors that contribute to decreased survival in patients with ICM. Recently, Acharya et al., using data from the ELSO registry, reported that patients with AMI complicated by cardiogenic shock VA‐ECMO achieved similar survival to hospital discharge; AMI complicated by cardiogenic shock was not a predictor of mortality in multivariable analysis (HR, 0.89; 95% CI, 0.768–1.050; P = 0.177). 15 This was consistent with our study, supporting an important early role for VA‐ECMO in this vulnerable population.
Predictors for in‐hospital mortality
The timing of the ECMO cannulation appears to be associated with both morbidity and mortality. Several investigators have found that a longer delay to support initiation is associated with a much higher risk of end‐organ injury and patient death among those with refractory cardiogenic shock. 16 Our findings, in which the duration of shock prior to ECMO placement in survivors was shorter compared with those of non‐survivors, also lend support to these previous reports that early intervention with MCS is important to prevent the downward and complex spiral associated with cardiogenic shock. 1 In addition, the present analysis shows that the use of distal perfusion catheters reduced the incidence of limb ischaemia and in‐hospital mortality. These findings are in agreement with a prior meta‐analysis that reported the presence of a distal perfusion catheter was associated with at least a 15.7% absolute reduction in the incidence of limb ischaemia. 17
Taken together, it may be postulated that if appropriate therapy were taken with patients with cardiogenic shock, in‐hospital outcomes would be similar across aetiologies; therefore, this suggests that ICM should not necessarily affect the decision to initiate VA‐ECMO support in patients with cardiogenic shock.
Study limitations
Our study has several limitations that need to be acknowledged. First, limitations are inevitably linked to the observational design of our study, which does not make it possible to rule out unmeasured/residual confounding even after multiple adjustments, which might explain the significant differences in outcomes observed between patients with ICM and those with NICM. In addition, the RESCUE registry contains observational study data; therefore, the in‐hospital treatment reflects individual physicians' preferences, introducing confounding selection bias. Second, cardiogenic shock aetiology was reported by investigators and not verified in any way; however, the characteristics of the patients in the different aetiologic groups were consistent with what would be expected, suggesting valid categorization by investigators. 18 Third, appropriate caution must be taken when interpreting our findings, given the small sample size of our cohort. Furthermore, higher events per variable might be needed when low prevalence predictors are present in a model to eliminate bias in regression coefficients and improve predictive accuracy. 19 Fourth, the study is restricted to in‐hospital outcomes only, leading to the limitation of our ability to assess mid‐term to long‐term outcomes. Lastly, lack of information about some haemodynamic parameters such as central venous pressure, central venous oxygen saturation, cardiac output, and cardiac index renders a more comprehensive analysis unfeasible with this database.
Conclusions
Patients with an ischaemic cardiogenic shock were significantly older, had more comorbidities, including diabetes, and had different VA‐ECMO treatment characteristics. After adjustments for all these poor prognostic characteristics, the aetiology of cardiogenic shock was not in itself associated with worse clinical outcomes. More invasive and dedicated treatment strategies may be needed for patients with ICM cardiogenic shock.
Conflict of interest
The authors have no conflicts of interest to declare.
Funding
This work was supported by research fund of Chungnam National University Hospital.
Supporting information
Table S1. Percent Standardized Differences of Variables among Unadjusted, Propensity‐Score Matched, and IPW‐Adjusted Cohort, and Baseline Characteristics of Propensity‐Score Matched Cohort.
Table S2. Baseline Characteristics.
Table S2. In‐Hospital Treatment Characteristics.
Table S3. In‐Hospital Mortality According to ICM versus NICM.
Figure S1. Patient Flow
ICM = ischaemic cardiomyopathy, NICM = non‐ischaemic cardiomyopathy, VA‐ECMO = veno‐arterial‐extracorporeal membrane oxygenation.
Figure S2. Prevalence of ischaemic and non‐ischaemic cardiomyopathy (A). Prevalence of various non‐ischaemic aetiologies (B).
DCM = dilated cardiomyopathy, ICM = ischaemic cardiomyopathy, NICM = non‐ischaemic cardiomyopathy, PTE = pulmonary thromboembolism, SCMP = stress induced cardiomyopathy, VHD = valvular heart disease.
Figure S3. Cardiogenic Shock Treated with VA‐ECMO
ICM = ischaemic cardiomyopathy, IPW = inverse‐probability weighting, NICM = non‐ischaemic cardiomyopathy, PSM = propensity‐score matching, VA‐ECMO = veno‐arterial‐extracorporeal membrane oxygenation.
Seong, S.‐W. , Jin, G. , Kim, M. , Ahn, K. T. , Yang, J. H. , Gwon, H.‐C. , Ko, Y.‐G. , Yu, C. W. , Chun, W. J. , Jang, W. J. , Kim, H.‐J. , Bae, J.‐W. , Kwon, S. U. , Lee, H.‐J. , Lee, W. S. , Park, S.‐D. , Cho, S. S. , Ahn, J. H. , Song, P. S. , and Jeong, J.‐O. (2021) Comparison of in‐hospital outcomes of patients with vs. without ischaemic cardiomyopathy undergoing veno‐arterial‐extracorporeal membrane oxygenation. ESC Heart Failure, 8: 3308–3315. 10.1002/ehf2.13481.
Seok‐Woo Seong and Guiyue Jin contributed equally to the study
Registration: URL: https://www.clinicaltrials.gov. Unique identifier: NCT02985008.
Contributor Information
Pil Sang Song, Email: pssong73@gmail.com.
Jin‐Ok Jeong, Email: jojeong@cnu.ac.kr.
References
- 1. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG, American Heart Association Council on Clinical Cardiology , Council on Cardiovascular and Stroke Nursing , Council on Quality of Care and Outcomes Research , Mission: Lifeline . Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2017; 136: e232–e268. [DOI] [PubMed] [Google Scholar]
- 2. Jenča D, Melenovský V, Stehlik J, Staněk V, Kettner J, Kautzner J, Adámková V, Wohlfahrt P. Heart failure after myocardial infarction: incidence and predictors. ESC Heart Fail 2021; 8: 222–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mandawat A, Rao SV. Percutaneous mechanical circulatory support devices in cardiogenic shock. Circ Cardiovasc Interv 2017; 10: e004337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schrage B, Becher PM, Goßling A, Savarese G, Dabboura S, Yan I, Beer B, Söffker G, Seiffert M, Kluge S, Kirchhof P, Blankenberg S, Westermann D. Temporal trends in incidence, causes, use of mechanical circulatory support and mortality in cardiogenic shock. ESC Heart Fail 2021; 8: 1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eckman PM, Katz JN, El Banayosy A, Bohula EA, Sun B, van Diepen S. Veno‐arterial extracorporeal membrane oxygenation for cardiogenic shock: an introduction for the busy clinician. Circulation 2019; 140: 2019–2037. [DOI] [PubMed] [Google Scholar]
- 6. Lemogoum D, Kamdem F, Ba H, Ngatchou W, Hye Ndindjock G, Dzudie A, Monkam Y, Mouliom S, Hermans MP, Bika Lele EC, van de Borne P. Epidemiology of acutely decompensated systolic heart failure over the 2003‐2013 decade in Douala General Hospital, Cameroon. ESC Heart Fail 2021; 8: 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilcox JE, Fang JC, Margulies KB, Mann DL. Heart failure with recovered left ventricular ejection fraction: JACC Scientific Expert Panel. J Am Coll Cardiol 2020; 76: 719–734. [DOI] [PubMed] [Google Scholar]
- 8. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 9. Cook JL, Colvin M, Francis GS, Grady KL, Hoffman TM, Jessup M, John R, Kiernan MS, Mitchell JE, Pagani FD, Petty M, Ravichandran P, Rogers JG, Semigran MJ, Toole JM, American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology , Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation , Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing , Council on Cardiovascular Radiology and Intervention , Council on Cardiovascular Surgery and Anesthesia . Recommendations for the use of mechanical circulatory support: ambulatory and community patient care: a scientific statement from the American Heart Association. Circulation 2017; 135: e1145–e1158. [DOI] [PubMed] [Google Scholar]
- 10. Neumann F‐J, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J‐P, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, ESC Scientific Document Group . 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019; 40: 87–165. [DOI] [PubMed] [Google Scholar]
- 11. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015; 34: 3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han J, Pinsino A, Sanchez J, Takayama H, Garan AR, Topkara VK, Naka Y, Demmer RT, Kurlansky PA, Colombo PC, Takeda K, Yuzefpolskaya M. Prognostic value of vasoactive‐inotropic score following continuous flow left ventricular assist device implantation. J Heart Lung Transplant 2019; 38: 930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shore S, Grau‐Sepulveda MV, Bhatt DL, Heidenreich PA, Eapen ZJ, Hernandez AF, Yancy CW, Fonarow GC. Characteristics, treatments, and outcomes of hospitalized heart failure patients stratified by etiologies of cardiomyopathy. JACC Heart Fail 2015; 3: 906–916. [DOI] [PubMed] [Google Scholar]
- 14. Batra J, Toyoda N, Goldstone AB, Itagaki S, Egorova NN, Chikwe J. Extracorporeal membrane oxygenation in New York State: trends, outcomes, and implications for patient selection. Circ Heart Fail 2016; 9: e003179. [DOI] [PubMed] [Google Scholar]
- 15. Acharya D, Torabi M, Borgstrom M, Rajapreyar I, Lee K, Kern K, Rycus P, Tonna JE, Alexander P, Lotun K. Extracorporeal membrane oxygenation in myocardial infarction complicated by cardiogenic shock: analysis of the ELSO registry. J Am Coll Cardiol 2020; 76: 1001–1002. [DOI] [PubMed] [Google Scholar]
- 16. Schmidt M, Burrell A, Roberts L, Bailey M, Sheldrake J, Rycus PT, Hodgson C, Scheinkestel C, Cooper DJ, Thiagarajan RR, Brodie D, Pellegrino V, Pilcher D. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno‐arterial‐ECMO (SAVE)‐score. Eur Heart J 2015; 36: 2246–2256. [DOI] [PubMed] [Google Scholar]
- 17. Juo YY, Skancke M, Sanaiha Y, Mantha A, Jimenez JC, Benharash P. Efficacy of distal perfusion cannulae in preventing limb ischemia during extracorporeal membrane oxygenation: a systematic review and meta‐analysis. Artif Organs 2017; 41: E263–E273. [DOI] [PubMed] [Google Scholar]
- 18. Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol 2002; 39: 210–218. [DOI] [PubMed] [Google Scholar]
- 19. Ogundimu EO, Altmar DG, Collins GS. Adequate sample size for developing prediction models is not simply related to events per variable. J Clin Epidemiol 2016; 76: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Percent Standardized Differences of Variables among Unadjusted, Propensity‐Score Matched, and IPW‐Adjusted Cohort, and Baseline Characteristics of Propensity‐Score Matched Cohort.
Table S2. Baseline Characteristics.
Table S2. In‐Hospital Treatment Characteristics.
Table S3. In‐Hospital Mortality According to ICM versus NICM.
Figure S1. Patient Flow
ICM = ischaemic cardiomyopathy, NICM = non‐ischaemic cardiomyopathy, VA‐ECMO = veno‐arterial‐extracorporeal membrane oxygenation.
Figure S2. Prevalence of ischaemic and non‐ischaemic cardiomyopathy (A). Prevalence of various non‐ischaemic aetiologies (B).
DCM = dilated cardiomyopathy, ICM = ischaemic cardiomyopathy, NICM = non‐ischaemic cardiomyopathy, PTE = pulmonary thromboembolism, SCMP = stress induced cardiomyopathy, VHD = valvular heart disease.
Figure S3. Cardiogenic Shock Treated with VA‐ECMO
ICM = ischaemic cardiomyopathy, IPW = inverse‐probability weighting, NICM = non‐ischaemic cardiomyopathy, PSM = propensity‐score matching, VA‐ECMO = veno‐arterial‐extracorporeal membrane oxygenation.


