Abstract
The number of patients receiving sodium–glucose cotransporter 2 inhibitors (SGLT2is), especially those with heart failure, is increasing worldwide. SGLT2is control glycaemia by triggering glycosuria with simultaneous facilitation of a more ketogenic metabolic profile. Patients therefore are more prone to develop euglycaemic diabetic ketoacidosis (euDKA), an entity largely unknown beyond diabetes care professionals. We present a heart failure with preserved ejection fraction (HFpEF) patient with known Type 2 diabetes. He was treated with dapagliflozin and presented acutely with dyspnoea, hyperglycaemia, and ketoacidosis. After standard treatment for diabetic ketoacidosis, hyperglycaemia was corrected, while metabolic ketoacidosis persisted, and thus, euDKA was suspected. With adequate therapy, the patient recovered completely and was discharged without any sequelae. To the best of our knowledge, our case is the first to describe SGLT2i‐induced euDKA in HFpEF patients. Regarding no previous reports of euDKA in heart failure with reduced ejection fraction, our report is highly relevant for ongoing SGLT2i trials in HFpEF and clinical practice in general.
Keywords: Heart failure, Diabetic ketoacidosis, Euglycaemic diabetic ketoacidosis, SGLT2 inhibitor
Introduction
Diabetic ketoacidosis (DKA) typically presents with a triad of hyperglycaemia, high anion gap metabolic acidosis, and ketonemia/ketonuria. 1 Although manifested mostly in Type 1 diabetes mellitus, the incidence in patients with Type 2 diabetes mellitus (T2DM) in both clinical practice 2 , 3 and randomized trials 4 is beyond negligible. The specific type, an euglycaemic DKA (euDKA), was first described by Munro et al. in 1973 5 ; this is less common manifestation of DKA for which only mild to moderate blood glucose elevations are reported, whereas there are other potentially lethal derangements in bodily fluids. Therefore, euDKA represents a diagnostic and therapeutic challenge and should be identified promptly to assure timely and adequate therapy.
In recent years, sodium–glucose cotransporter 2 inhibitors (SGLT2is) changed our aspects of diabetes mellitus (DM) therapy due to cardiovascular effects in DM patients. 6 , 7 , 8 , 9 This has extended to field of heart failure with reduced ejection fraction (HFrEF), even in the absence of DM. 9 , 10 , 11 , 12 , 13 , 14 Based on available evidence, the clinical practice has changed considerably, 15 , 16 , 17 but there are certain caveats clinicians need to be aware of, particularly those with acute and severe consequences.
Our report is particularly relevant with regard to heart failure (HF) epidemiology. One finds no euDKA reports in published HFrEF clinical trials 6 , 7 , 8 , 10 , 12 , 18 as well in a meta‐analysis of clinical trials DKA occurred only in 0.14% of patients [odds ratio 2.13; 95% confidence interval (1.38–3.27)]. 4 Therefore, scientific and clinical community may feel relaxed with regard to prevalence and potential life‐threatening complications of euDKA. When expanding from HFrEF to HFpEF, in whom DM is also prevalent, 9 , 14 , 19 attending physicians need to be aware of this potentially fatal condition. 20 We herein present a case of the patient with HFpEF and T2DM that was admitted because of dapagliflozin‐induced DKA that eventually was recognized and treated as euDKA.
Case presentation
A 49‐year‐old male patient with T2DM sought medical attention due to a 3 day history of dyspnoea and malaise that escalated with nausea and vomiting. No one from his family did have similar problems. He was not taking painkillers, alcohol, or illegal drugs. He denied having muscle pain, trauma, headache, coughing, fever, chest pains, diarrhoea, polyuria, and other symptoms suggesting an underlying condition that could have triggered the deterioration. He was a non‐smoker without known alcohol abuse. His regular therapy consisted of insulin, dapagliflozin, atorvastatin, pantoprazole, salbutamol, vilanterol/fluticasone, quetiapine, alprazolam, tizanidine, venlafaxine, pregabalin, and bisoprolol.
His medical history included T2DM, biliary pancreatitis, paranoid schizophrenia, bronchial asthma, and knee and spine surgery. HFpEF was diagnosed 4 years prior to current complaints.
On physical examination, he was alert and orientated, with a blood pressure of 120/77 mmHg and body temperature of 36.6°C. Acetone breath was observed, and he was tachypnoeic, with oxygen saturation of 90%, and tachycardic (115 per minute) and had bibasilar inspiratory rales. The rest of the physical examination was unremarkable. Initially, asthma worsening was suspected because of dyspnoea; thus, he received methylprednisolone 125 mg and ipratropium bromide/fenoterol hydrobromide inhalation.
The laboratory findings (Table 1 and Figure 1 ) revealed severe metabolic acidosis (pH 7.13) with an elevated anion gap and disproportionally low lactate (1 mmol/L). Blood glucose was elevated at 27.1 mmol/L, as was leucocyte count, C‐reactive protein, and N‐terminal prohormone of brain natriuretic peptide (at 765 ng/L). The remaining laboratory tests were unremarkable (see Table 1 ). We however detected elevated glycated haemoglobin of 12.6% (The International Federation of Clinical Chemistry and Laboratory Medicine 114 mmol/mol Hb). Chest X‐ray demonstrated no pathological findings.
Table 1.
Laboratory data of the patient during hospitalization
| Parameter | Reference range | At admission | 6 HPA | 14 HPA | 24 HPA | 33 HPA | 48 HPA | 96 HPA | 120 HPA | 216 HPA |
|---|---|---|---|---|---|---|---|---|---|---|
| V‐Haemoglobin (g/L) | 130–170 | 173 | 145 | 143 | 136 | 142 | 135 | |||
| V‐Leucocytes (giga/L) | 4–10 | 26 | 17.2 | 18.2 | 11.5 | 5.4 | 5.9 | |||
| V‐Creatine kinase (μkat/L) | 22–198 | 8.46 | 14.93 | 21.64 | 28.9 | 22 | 16.13 | |||
| V‐Creatinine (μmol/L) | 59–104 | 132 | 98 | 77 | 75 | 73 | 65 | 54 | 53 | 73 |
| V‐Urea (μmol/L) | 2.8–8.1 | 12 | 10.6 | 8.7 | 8.9 | 7.9 | 5.7 | 5.6 | 5.9 | |
| V‐Potassium (mmol/L) | 3.5–5.1 | 5.3 | 4.9 | 4.9 | 4.8 | 9.9 | 4.1 | 4.8 | 4.3 | 4.5 |
| V‐Sodium (mmol/L) | 135–145 | 134 | 138 | 137 | 143 | 148 | 141 | 141 | 141 | 139 |
| V‐Chloride (mmol/L) | 98–107 | 89 | 102 | 107 | 110 | 112 | 109 | 103 | 101 | 99 |
| V‐C‐reactive protein (mg/L) | <5 | 10 | 21 | 37 | 38 | 27 | 32 | 49 | 24 | 5 |
| A‐pCO2 (kPa) | 4.5–6 | 1.9 | 2 | 2.1 | 2.5 | 2.2 | 4.5 | 5.3 | ||
| A‐pO2 (kPa) | >10.5 | 14.5 | 12.2 | 13.8 | 12.2 | 12.7 | 9.9 | 6.7 | ||
| A‐HCO3 − (mmol/L) | 22–26 | 5 | 7 | 6 | 9 | 7 | 21 | 25 | ||
| U‐pH | 4.6–8 | 5.5 | 5 | 6 | 6.5 | |||||
| U‐Protein (poE) | 0 | 1 | 1 | 1 | 0 | |||||
| U‐Glucose (poE) | 0 | 4 | 4 | 4 | 3 | |||||
| U‐Methylketones (poE) | 0 | 3 | 3 | 3 | 3 | |||||
| U‐Leucocytes (poE) | 0 | 0 | 0 | 0 | 0 | |||||
| U‐Nitrites (poE) | 0 | 0 | 0 | 0 | 0 |
HPA, hours post‐admission.
Figure 1.
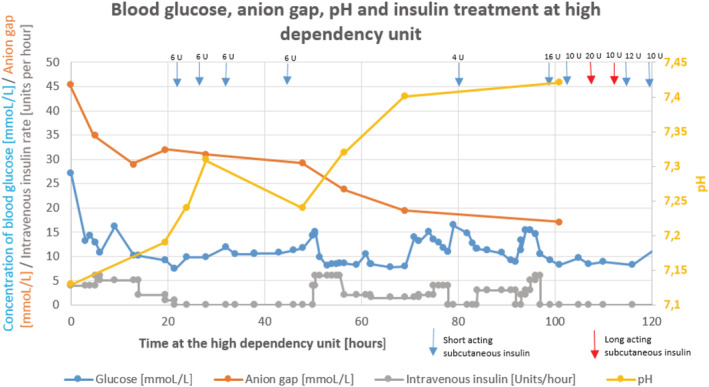
Blood glucose, anion gap, and pH depending on insulin infusion rate and subcutaneous insulin.
Based on available information and response to initial therapy, DKA was suspected, and patient was admitted to intensive care unit. We stopped his regular diabetes medications and initiated the therapy with 1000 mL of 0.9% saline solution with 30 mEq of KCl in the first hour, combined with human insulin (Actrapid®) as a continuous intravenous infusion (see Figure 1 ). Empirically, ceftriaxone due to suspected infection was initiated.
On the second day, blood glucose normalized, and we switched from intravenous to subcutaneous insulin (for details, see Figure 1 ). However, the patient still felt dyspnoeic and nauseated. Arterial blood analysis revealed metabolic acidosis with a high anion gap. As he was symptomatic and with low pH but high anion gap, he received 100 mmol of NaHCO3. Despite an improvement in pH, bicarbonate level along with anion gap was still abnormal. His calorie intake was inadequate, so we started parenteral nutrition support (Nutriflex® 1250 mL).
At regular checking of arterial blood on the third day, we found persistent acidosis (pH 7.24) with a high anion gap, while glucose was disproportionally low. He received 100 mmol of NaHCO3 again. Consultation with a diabetologist and clinical pharmacist raised the question about dapagliflozin as a trigger of the euDKA. We succeed to normalize blood glucose concentration with the standard DKA therapy. The acidosis, however, persisted, as well as glucosuria and ketonuria. The patient denied taking dapagliflozin and other medication on his own. As proposed, we started the treatment with 1500 mL intravenous 5% glucose/0.45% NaCl solution combined with 20 mEq of KCl along with human insulin initially 4 U/h and then adjusted to blood glucose concentration (see Figure 1 ).
On the fourth day hospitalized at our intensive care unit, we found both the pH and blood glucose concentration within normal ranges, while a high anion gap persisted (see Figure 1 ). Because of marked clinical improvement, we transferred him to the regular ward. We continued a 5% glucose solution with intravenous human insulin for the next 24 h, along with oral potassium. Then we started with his basal‐bolus insulin therapy. Eventually, the anion gap normalized, and he was clinically stable. The rest of hospitalization was uneventful, and the patient was discharged after 9 days of hospitalization. Within 3 months of follow‐up, the clinical course was without further encounters with medical system.
Discussion
Sodium–glucose cotransporter 2 inhibitors have changed the clinical landscape of DM therapy due to positive effects on cardiovascular outcomes in large clinical trials with SGLT2is (e.g. CANVAS, EMPA‐REG OUTCOME, and DECLARE–TIMI 58). 6 , 7 , 8 , 9 , 14 , 16 It has sparked the interest in evaluating their benefit in patients with HF, regardless of the DM status. 9 , 14 Three clinical trials (i.e. EMPEROR‐Reduced, DAPA‐HF, and SOLOIST‐WHF) showed a reduced combined risk of cardiovascular death or hospitalization for HFrEF with or without DM. However, no trial was powered enough to assess impacts on cardiovascular death or all‐cause death. Recently, a meta‐analysis of EMPEROR‐Reduced and DAPA‐HF showed a 13% reduction in all‐cause death [pooled hazard ratio (HR) 0.87, 95% confidence interval 0.77–0.98; P = 0.018], 14% reduction in cardiovascular death (pooled HR 0.86, 95% confidence interval 0.76–0.98; P = 0.027), and 26% reduction in the combined risk of cardiovascular death or hospitalization for HF (pooled HR 0.74, 95% confidence interval 0.68–0.82; P < 0.0001). 21 Those two trials showed reduced risk of hospitalization for HF or death from cardiovascular causes among patients with stable HF, while the SOLOIST‐WHF trial showed the benefit of sotagliflozin when initiated soon after an episode of decompensated HF. 11 Consequently, expectations of SGLT2is expanding the scarce area of treatment options for HFpEF are high; many clinical trials are ongoing in such patients (e.g. DELIVER and EMPEROR‐Preserved), 9 , 14 so we are awaiting the results of those trials with great interest.
Sodium–glucose cotransporter 2 inhibitors are a game changer in HFrEF, with findings for HFpEF pending; thus, implementation and associated challenges in clinical practice will definitely increase. Currently, we do not have relevant safety concerns from clinical trials in terms of DKA, but the experience from clinical practice cannot be neglected. 4 , 10 While a few case reports consider euDKA associated with SGLT2is at admission, to the best of our knowledge, there are no other reports describing euDKA as a stage of treatment of DKA in a patient with HF.
Pathophysiologically, in both DKA and euDKA, the combination of insulin deprivation and stress hormone excess (glucagon, cortisol, and catecholamines) plays a central part. 22 Since under the normal physiological conditions, when the threshold of serum glucose that triggers its renal clearance is about 10 mmol/L, 23 SGLT2is induce iatrogenic glycosuria when serum glucose is around 8 mmol/L. 24 Therefore, when a patient is taking SGLT2is, the potential to correct hyperglycaemia in DKA is higher. We should keep in mind the two‐dimensional nature of DKA, the first one has enhanced the production of glucose, and the second one is enhanced ketogenesis. SGLT2is help the body to excrete glucose; on the contrary, they cannot remedy ketoacidosis. 1 They even trigger more ketogenic metabolic profile. 14 , 25
The reason is mostly attributable to hormone‐induced liver ketogenesis and partially to impaired kidney ammonia genesis. 26 We can explain enhanced liver ketogenesis through lack of insulin and excess of stress hormones; the situation leads to decreased production of malonyl‐CoA, a potent inhibitor of carnitine palmitoyltransferase I. Therefore, hepatic carnitine palmitoyltransferase I fosters promoting fatty acid entry into mitochondria and hepatic ketone body production. The capacity of peripheral tissues to utilize excess ketone bodies as an energy fuel is exceeded, so ketoacidosis finally occurs. 27
At admission, asthma exacerbation was the first suspected cause for dyspnoea, so corticosteroid was applied before laboratory results. When they were available, the present case met the criteria for DKA and aetiological therapy was initiated. A corticosteroid may have increased the level of glycaemia and masked pre‐existent euDKA. We speculated that acidosis started gradually before symptoms, and when pH exceeded a critical level, the patient felt nausea. However, we should consider the alternative explanation that he may have had nausea due to gastrointestinal infection and consequently developed DKA. The fact that the list of self‐measurements of capillary glucose was not available makes the definite conclusion challenging. As he felt sick, he did not eat nor take insulin but continued with dapagliflozin. Therefore, there was unbalanced glucose excretion. As he was using dapagliflozin, the excretion of glucose was unbalanced towards ketone bodies. In the conditions of severe insulin lack, both glucose and ketone bodies increased. The resulting acidosis compensation was evident as Kussmaul breathing.
When the diagnosis of metabolic acidosis with high anion gap is made, we should evaluate all other possible triggers; they encompass inadequate caloric intake and intoxication with cocaine, ethanol, salicylate, methanol, ethylene glycol, paraldehyde, 22 , 28 and drug–drug interactions. Based on the proven applicability and appropriateness of commercially available electronic databases assessing the prevalence of potentially harmful drug–drug interactions, 29 we carried out drug–drug interaction analysis with software Lexicomp®. We did not find any seriously harmful interactions; the software suggested only two considerations for the therapy modification and 16 possible interactions needed monitoring. Only quetiapine and salbutamol could potentially increase glycaemia. Because of the patient's mental disorder and asthma, we did not modify the treatment scheme. No other drug could induce acidosis. All other possible triggers were excluded, while the calorie deficit was covered by parenteral nutrition. Therefore, the most plausible trigger for euDKA was the previous use of dapagliflozin. The definite diagnosis of ketoacidosis is serum measurement of ketone bodies, which is extremely rare in clinical practice. In our institution, this is not a routine procedure; in addition, ketone body analysis needs to be performed promptly. However, with regard to high anion gap metabolic acidosis with disproportionally low lactate and acetone breath, ketoacidosis is a plausible explanation of the underlying aetiology.
While we achieved blood glucose normalization, the acidosis with high anion gap persisted. We usually treat DKA with insulin/fluid/potassium infusion in routine clinical practice until glycaemia is within target range. In patients previously treated with SGLT2is, we should keep in mind that euDKA could be a phase of the initial treatment of DKA.
We discharged the patient without SGLT2i therapy. Although with the normal serum glycaemia, we still detected glycosuria at discharge. The prolonged effect of ipragliflozin and dapagliflozin compared with other SGLT2is is known in mice models; however, the effect is not known in humans yet. 30 As the patient was young but with poor control of glycaemia and already treated with insulin, the question arises about the beneficial effect of rechallenging with SGLT2is. The critical concern is pancreatitis in his medical history, raising caution in patients receiving SGLT2is. 31 However, all preventive measures such as regular food and fluid intakes, tight glycaemic control, omission of SGLT2is, and ketone measurement at states of increased insulin requirement (due to illness, surgery, or alcohol abuse) should be taken into account. 1 , 32 , 33 , 34 In the era of pandemics, it is important to suggest all treating physicians to temporarily omit SGLT2is, while the patients are recovering from the COVID‐19 disease. 35 Comprehensive patient education plays an essential role in preventing potentially life‐threatening euDKA.
The therapeutic approach of patients with euDKA does not differ significantly from those with DKA. Guidelines recommend rapid fluid replacement, awareness of electrolyte imbalances, and their prompt correction, followed by continuous insulin infusion after normalization of potassium and eventually glucose solution when its serum level is <13.9 mmol/L. The treatment should go on until the resolution of ketoacidosis (correction of acidosis and anion gap closure). Bicarbonate therapy can be inevitable for severe acidosis. 36
In clinical practice, euDKA seems far more frequent than observed in the standardized settings of clinical trials. 27 Until now, we do not have reports about euDKA in patients with HF without diabetes. 10 We believe that in the absence of routine measurement of ketones in blood and urine, the incidence is underestimated. One of the possible hypotheses of the SGLT2i cardioprotective role even argues that establishing a ketogenic state is beneficial and anti‐inflammatory. 14 , 25
In conclusion, our case is, to the best of our knowledge, the first to describe SGLT2i‐induced euDKA in HFpEF patients. Regarding the absence of euDKA in HFrEF clinical trials, many clinicians may be immersed into a false sense of security when prescribing SGLT2is to HFpEF patients. Therefore, it is of key importance to report the clinical cases such as ours and raise awareness about euDKA in clinical community at large. As we expect that SGLT2is will be recommended for clinical use in HFrEF patients by the next HF Association Guidelines and in a view of ongoing HFpEF clinical trials, 9 , 14 the cardiologists in particular should be aware of clinical presentation and appropriate management of this potentially life‐threatening situation.
Conflict of interest
None declared.
Cavka, L. , Bencak Ferko, U. , Pitz, N. , Trpkovski, Z. , and Lainscak, M. (2021) Sodium–glucose cotransporter 2 inhibitor‐induced euglycaemic diabetic ketoacidosis in heart failure with preserved ejection fraction. ESC Heart Failure, 8: 2631–2636. 10.1002/ehf2.13452.
References
- 1. Barski L, Eshkoli T, Brandstaetter E, Jotkowitz A. Euglycemic diabetic ketoacidosis. Eur J Intern Med 2019; 63: 9–14. [DOI] [PubMed] [Google Scholar]
- 2. Tittel SR, Sondern KM, Weyer M, Poeplau T, Sauer BM, Schebek M, Ludwig KH, Hammer F, Fröhlich‐Reiterer E, Holl RW. Multicentre analysis of hyperglycaemic hyperosmolar state and diabetic ketoacidosis in type 1 and type 2 diabetes. Acta Diabetol 2020; 57: 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meyer EJ, Gabb G, Jesudason D. SGLT2 inhibitor‐associated euglycemic diabetic ketoacidosis: a South Australian clinical case series and Australian spontaneous adverse event notifications. Diabetes Care 2018; 41: e47–e49. [DOI] [PubMed] [Google Scholar]
- 4. Liu J, Li L, Li S, Wang Y, Qin X, Deng K, Liu Y, Zou K, Sun X. Sodium‐glucose co‐transporter‐2 inhibitors and the risk of diabetic ketoacidosis in patients with type 2 diabetes: a systematic review and meta‐analysis of randomized controlled trials. Diabetes Obes Metab 2020; 22: 1619–1627. [DOI] [PubMed] [Google Scholar]
- 5. Munro JF, Campbell IW, McCuish AC, Duncan LJ. Euglycaemic diabetic ketoacidosis. Br Med J 1973; 2: 578–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 7. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 8. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 9. Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Zannad F, Packer M, EMPEROR‐Preserved Trial Committees and Investigators , Packer M, Anker SD, Butler J, Filippatos GS, Zannad F, George J, Brueckmann M, Perrone S, Nicholls S, Janssens S, Bocchi E, Giannetti N, Verma S, Jian Z, Gomez Mesa JE, Spinar J, Böhm M, Merkely B, Chopra V, Senni M, Taddi S, Tsutsui H, Chuquiure E, la Rocca HPB, Ponikowski P, Vinereanu D, Sim D, Choi DJ, Juanatey JRG, Squire I, Butler J, Januzzi J, Pina I, Pocock SJ, Carson P, Doehner W, Miller A, Haas M, Pehrson S, Komajda M, Anand I, Teerlink J, Rabinstein A, Steiner T, Kamel H, Tsivgoulis G, Lewis J, Freston J, Kaplowitz N, Mann J, Petrie M, Bernstein R, Cheung A, Green J, Januzzi J, Kaul S, Ping CLS, Lip G, Marx N, McCullough P, Mehta C, Ponikowski P, Rosenstock J, Sattar N, Scirica B, Tsutsui H, Verma S, Wanner C, Welty FK, Parhofer KG, Clayton T, Pedersen TR, Lees KR, Konstam MA, Greenberg B, Palmer M. Evaluation of the effects of sodium–glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR‐Preserved Trial. Eur J Heart Fail 2019; 21: 1279–1287. [DOI] [PubMed] [Google Scholar]
- 10. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 11. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2020. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure; 384: 117–128. [DOI] [PubMed] [Google Scholar]
- 12. Packer M, Butler J, Filippatos G, Zannad F, Ferreira JP, Zeller C, Brueckmann M, Jamal W, Pocock SJ, Anker SD, for the EMPEROR Trial Committees and Investigators . Design of a prospective patient‐level pooled analysis of two parallel trials of empagliflozin in patients with established heart failure. Eur J Heart Fail 2020, doi: , Design of a prospective patient‐level pooled analysis of two parallel trials of empagliflozin in patients with established heart failure; 22: 2393–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pellicori P, Ofstad AP, Fitchett D, Zeller C, Wanner C, George J, Zinman B, Brueckmann M, Lindenfeld JA. Early benefits of empagliflozin in patients with or without heart failure: findings from EMPA‐REG OUTCOME. ESC Heart Failure 2020, doi: , Early benefits of empagliflozin in patients with or without heart failure: findings from EMPA‐REG OUTCOME; 7: 3401–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Williams DM, Evans M. Dapagliflozin for heart failure with preserved ejection fraction: will the DELIVER study deliver? Diabetes Ther 2020; 11: 2207–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seferović PM, Fragasso G, Petrie M, Mullens W, Ferrari R, Thum T, Bauersachs J, Anker SD, Ray R, Çavuşoğlu Y, Polovina M, Metra M, Ambrosio G, Prasad K, Seferović J, Jhund PS, Dattilo G, Čelutkiene J, Piepoli M, Moura B, Chioncel O, Ben Gal T, Heymans S, Boer RA, Jaarsma T, Hill L, Lopatin Y, Lyon AR, Ponikowski P, Lainščak M, Jankowska E, Mueller C, Cosentino F, Lund L, Filippatos GS, Ruschitzka F, Coats AJS, Rosano GMC. Sodium‐glucose co‐transporter 2 inhibitors in heart failure: beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020; 22: 1495–1503. [DOI] [PubMed] [Google Scholar]
- 16. Lan NSR, Fegan PG, Yeap BB, Dwivedi G. The effects of sodium‐glucose cotransporter 2 inhibitors on left ventricular function: current evidence and future directions. ESC Heart Fail; 6: 927–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chang HY, Su YW, Feng AN, Fong MC, Huang KC, Chong E, Chen KC, Yin WH. Prescription patterns of diabetes medications influencing clinical outcomes of heart failure patients with reduced ejection fraction. ESC Heart Fail 2020; 7: 604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021; 384: 117–128. [DOI] [PubMed] [Google Scholar]
- 19. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo‐Leiro MG, Laroche C, Seferovic PM, Anker SD, Ferrari R, Ruschitzka F, Lopez‐Fernandez S, Miani D, Filippatos G, Maggioni AP, ESC Heart Failure Long‐Term Registry Investigators . Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 1242–1254. [DOI] [PubMed] [Google Scholar]
- 20. Wang KM, Isom RT. SGLT2 inhibitor‐induced euglycemic diabetic ketoacidosis: a case report. Kidney Med 2020. Mar‐Apr 2020; 2: 218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. Lancet 2020; 396: 819–829. [DOI] [PubMed] [Google Scholar]
- 22. Bonora BM, Avogaro A, Fadini GP. Euglycemic ketoacidosis. Curr Diab Rep 2020; 20: 25. [DOI] [PubMed] [Google Scholar]
- 23. Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol 2020. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. [DOI] [PubMed] [Google Scholar]
- 24. Osaki A, Okada S, Saito T, Yamada E, Ono K, Niijima Y, Hoshi H, Yamada M. Renal threshold for glucose reabsorption predicts diabetes improvement by sodium‐glucose cotransporter 2 inhibitor therapy. J Diabetes Investig 2016; 7: 751–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perry RJ, Shulman GI. Sodium‐glucose cotransporter‐2 inhibitors: understanding the mechanisms for therapeutic promise and persisting risks. J Biol Chem, 08 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Barsotti E, Clerico A, Muscelli E. Renal handling of ketones in response to sodium–glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care 2017; 40: 771–776. [DOI] [PubMed] [Google Scholar]
- 27. Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig 2016; 7: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abu‐Abed Abdin A, Hamza M, Khan MS, Ahmed A. Euglycemic diabetic ketoacidosis in a patient with cocaine intoxication. Case Rep Crit Care 2016; 2016: 4275651–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roblek T, Vaupotic T, Mrhar A, Lainscak M. Drug‐drug interaction software in clinical practice: a systematic review. Eur J Clin Pharmacol 2015; 71: 131–142. [DOI] [PubMed] [Google Scholar]
- 30. Choi MK, Nam SJ, Ji HY, Park MJ, Choi JS, Song IS. Comparative pharmacokinetics and pharmacodynamics of a novel sodium‐glucose cotransporter 2 inhibitor, DWP16001, with dapagliflozin and ipragliflozin. Pharmaceutics 2020; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilding J, Bailey C, Rigney U, Blak B, Kok M, Emmas C. Dapagliflozin therapy for type 2 diabetes in primary care: changes in HbA1c, weight and blood pressure over 2 years follow‐up. Prim Care Diabetes 2017; 11: 437–434. [DOI] [PubMed] [Google Scholar]
- 32. Pontes JPJ, de Melo CS, Arantes FBB, de Souza Ramos JTG, Módolo NSP, Navarro LH, Lima E. Perioperative euglycemic diabetic ketoacidosis following use of SGLT‐2 inhibitors after cardiac surgery. J Clin Anesth 2021; 71: 110201. [DOI] [PubMed] [Google Scholar]
- 33. Seger CD, Xing H, Wang L, Shin JS. Intraoperative diagnosis of sodium‐glucose cotransporter 2 inhibitor‐associated euglycemic diabetic ketoacidosis: a case report. A A Pract 2021; 15: e01380. [DOI] [PubMed] [Google Scholar]
- 34. Lindsay PJ, Gibson LE, Bittner EA, Berg S, Chang MG. Sodium‐glucose cotransporter‐2 (SGLT2) inhibitor‐induced euglycemic diabetic ketoacidosis complicating the perioperative management of a patient with type 2 diabetes mellitus (T2DM) and Fournier's gangrene: a case report. Int J Surg Case Rep 2020; 77: 463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vitale RJ, Valtis YK, McDonnell ME, Palermo NE, Fisher NDL. Euglycemic diabetic ketoacidosis with COVID‐19 infection in patients with type 2 diabetes taking SGLT2 inhibitors. AACE Clin Case Rep 2021. Jan‐Feb 2021; 7: 10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009; 32: 1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]


