Abstract
Aims
Frailty may be found in heart failure patients especially in the elderly and is associated with a poor prognosis. However, assessment of frailty status is time‐consuming, and the electronic frailty indices developed using health records have served as useful surrogates. We hypothesized that an electronic frailty index developed using machine learning can improve short‐term mortality prediction in patients with heart failure.
Methods and results
This was a retrospective observational study that included patients admitted to nine public hospitals for heart failure from Hong Kong between 2013 and 2017. Age, sex, variables in the modified frailty index, Deyo's Charlson co‐morbidity index (≥2), neutrophil‐to‐lymphocyte ratio (NLR), and prognostic nutritional index at baseline were analysed. Gradient boosting, which is a supervised sequential ensemble learning algorithm with weak prediction submodels (typically decision trees), was applied to predict mortality. Variables were ranked in the order of importance with a total score of 100 and used to build the frailty models. Comparisons were made with decision tree and multivariable logistic regression. A total of 8893 patients (median: age 81, Q1–Q3: 71–87 years old) were included, in whom 9% had 30 day mortality and 17% had 90 day mortality. Prognostic nutritional index, age, and NLR were the most important variables predicting 30 day mortality (importance score: 37.4, 32.1, and 20.5, respectively) and 90 day mortality (importance score: 35.3, 36.3, and 14.6, respectively). Gradient boosting significantly outperformed decision tree and multivariable logistic regression. The area under the curve from a five‐fold cross validation was 0.90 for gradient boosting and 0.87 and 0.86 for decision tree and logistic regression in predicting 30 day mortality. For the prediction of 90 day mortality, the area under the curve was 0.92, 0.89, and 0.86 for gradient boosting, decision tree, and logistic regression, respectively.
Conclusions
The electronic frailty index based on co‐morbidities, inflammation, and nutrition information can readily predict mortality outcomes. Their predictive performances were significantly improved by gradient boosting techniques.
Keywords: Frailty index, Heart failure, Mortality, Inflammation, Nutrition, Machine learning
Introduction
Frailty refers to a reduced physiological reserve leading to an impairment in resilience from physical distress. Compared with highly functional community‐dwelling elders, frail older adults are more likely to experience falls and disability, contributing to frequent hospitalization and premature death. 1 Conventional evaluation of frailty relies on physical examination. However, this precludes its calculation using administrative data such as electronic health records. Recently, a claims‐based frailty scoring system has been validated against Fried and colleges' frailty phenotype using a claim database in the USA. 2 , 3 , 4 These electronic frailty indices do not normally include measures of chronic inflammation or nutrition status, which are both closely related to frailty syndrome and are strong determinants of adverse outcomes such as mortality. 5 , 6
Heart failure is a complex syndrome characterized by high prevalence in older patients and poor prognosis. 7 , 8 Heart failure and frailty have an overlapping phenotype, and their co‐existence is common. 9 Given these associations, there has been several studies exploring the intersections between heart failure and frailty. 9 Importantly, frailty has been recognized as a major prognostic indicator of heart failure, in which patients with concurrent frailty and heart failure have increased risks of hospitalizations and mortality. 10
Furthermore, inflammation and nutrition status are known independent predictors of heart failure outcomes. 11 , 12 , 13 Inflammation has a pivotal role in the pathogenesis of heart failure. 14 It can trigger cardiac remodelling and dysfunction that further induce cardiomyocyte damage that underlies heart failure. 15 Moreover, it has been proposed that co‐morbidities, such as diabetes and obesity, can induce a systemic pro‐inflammatory state that drives the myocardial structural and functional alterations in heart failure. 16 , 17 Conversely, inflammation can also be a consequence of established heart failure via the mechanisms of increased wall stress on endothelial cells, cell death, and oxidative stress. 18 In this regard, inflammation and heart failure are interconnected and mutually inducing. Elevated pro‐inflammatory cytokines were found to associate with worse clinical outcomes in patients with heart failure, 19 , 20 and some studies have demonstrated that neutrophil‐to‐lymphocyte ratio (NLR) can be used as a prognostic factor for heart failure. 21 Similar to inflammation, malnutrition is also an independent risk factor and prognostic factor for heart failure. 22 , 23 Various nutritional metrics, including prognostic nutritional index (PNI), associate well with the survival outcomes. 24 , 25 Both factors possess important predictive values for clinical outcomes in patients with heart failure. 26 , 27 , 28 Despite the multiple associations between frailty, heart failure, nutrition status, and inflammation, whether incorporating the measures of nutrition status and inflammation into the existing frailty index can enhance its predictive value on outcomes of heart failure remains unknown.
Machine learning techniques have gained popularity in medical research. Specifically, gradient boosting has recently been explored as a method to predict adverse outcomes in heart failure. 29 In this study, using a large cohort of patients with heart failure, we tested the hypothesis that incorporating NLR and PNI into an electronic frailty index using gradient boosting, a machine learning approach will improve prediction for short‐term mortality risks.
Methods
Data source and study population
This study received Ethical Approval from the local Ethics Committee. This is a retrospective cohort study nested within the territory‐wide Clinical Data Analysis and Reporting System, an electronic health record system managed by the Hong Kong Hospital Authority since 1995. The database included over seven million Hong Kong residents and has been used for producing high‐quality clinical studies. 30 , 31
Patient information was de‐identified with pseudo‐identity numbers. Clinical data available from Clinical Data Analysis and Reporting System include demographic information, diagnosis, procedure, prescription, laboratory test results, admission/discharge information, and death information.
The inclusion criterion was patient admitted to any of the nine local hospitals during a 4 year period between July 2013 and July 2017 with a principal diagnosis of heart failure. The diagnosis of heart failure was defined as having a record with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9 CM) codes of 428.X.
Study variables
Variables that were previously included in the modified frailty index 4 were identified from the relevant ICD‐9 codes. These include depression, Parkinson's disease, arthritis, paranoia, chronic skin ulcer, pneumonia, falls, skin and subcutaneous tissue infection, mycoses, gouty arthropathy, and urinary tract infection. Laboratory test results on the measures of albumin level, neutrophil, and lymphocyte counts were extracted to calculate inflammatory and nutritional indices. NLR was given by the ratio of peripheral neutrophil count/mm3 to peripheral lymphocyte count/mm3. PNI was calculated by 10 × serum albumin value (g/dL) + 0.005 × peripheral lymphocyte count/mm3. NLR and PNI estimates nearest to the admission time of the first heart failure related hospitalization of the patients were used in the analysis. Baseline Deyo's Charlson co‐morbidity index incorporating 17 major medical conditions was also included as a single score. 32 A comparison of the included variables used in the modified frailty index, Charlson Deyo's Charlson co‐morbidity index, and our electronic frailty index is shown in the Supporting Information, Table S1 .
Outcomes and statistical analysis
The primary outcomes were 30 day and 90 day mortality, from the date of the first heart failure related‐hospitalization of the patients. The outcome of 30 day mortality is binary and equal to 1 for mortality within 30 days and 0 otherwise, and the same for 90 day mortality outcome. Continuous variables were presented as median (interquartile range [IQR]), and categorical variables were presented as count (%). The Mann–Whitney U test was used to compare continuous variables. The χ 2 test with Yates' correction was used for 2 × 2 contingency data, and Pearson's χ 2 test was used for contingency data for variables with more than two categories. To identify significant risk factors associated with 30 day and 90 day mortality, univariate logistic regression was used to determine odds ratios and 95% confidence intervals. Significant variables from the univariable logistic regression (P < 0.05) were further included in multivariable logistic regression to build the frailty model.
The idea of frailty is the cumulative deficits, each of which in isolation may not exert significant effects. To test this idea, we conducted an additional multivariable logistic regression analysis incorporating all risk variables, including the non‐significant variables from univariable logistic regression. Finally, to demonstrate the utility of NLR and PNI, both variables were excluded in sensitivity analysis to examine the effects on evaluation metrics.
A two‐sided α of <0.05 was considered statistically significant. All statistical analyses were performed using RStudio software (Version: 1.1.456).
Machine learning model development
Gradient boosting is a typical type of machine learning boosting, relying on the intuition that the best possible next model, when sequentially combined with previous weak models (e.g. decision trees) in a stage‐wise fashion, is able to minimize the overall prediction error measured by performance evaluators, for example, precision, recall, and the area under the curve (AUC). Weaker learning models are fitted through loss gradient minimization with gradient descent optimization algorithm. 33 This method was used for mortality prediction in heart failure based on administrative claims with electronic health records. 29 Variable importance ranking was generated to construct a machine learning based risk score for mortality prediction. Partial dependence plots were provided as low‐dimensional graphical renderings of marginal effects to assist in the interpretation of relationships between the most important variables and the mortality outcome. A five‐fold cross validation was performed to compare the performance in terms of precision, recall, and AUC of the gradient boosting model with decision tree model and logistic regression model. The R packages, gbm (Version 2.1.5) and ggplot2 (Version 3.3.2), were used to generate the mortality prediction results.
Results
In our heart failure cohort (n = 8893), the median age was 81 (IQR 71–87) years, and 45% (n = 4027) were men. The baseline characteristics, individual variables included in the modified frailty index, inflammatory and nutritional indices between the patients died within 90 days and the patients without 90 day mortality are shown in Table 1 . The median cell counts for lymphocytes was 1.2*109/L and for neutrophils was 5.4*109/L, yielding an NLR of 4.4 (IQR 2.7–7.8). Albumin took a median level of 37.8 g/L, yielding a PNI of 44.0 (IQR 39.8–48.5) (PNI, given by 10 × serum albumin value (g/dL) + 0.005 × peripheral lymphocyte count (per mm3)).
Table 1.
Baseline characteristics of the heart failure cohort
| 90‐day mortality N = 1472 | No mortality N = 7421 | P‐value | |
|---|---|---|---|
| Gender | |||
| Male (%) | 639 (43.4%) | 3388 (45.7%) | 0.114 |
| Age | 84.4 [77.5–90.1] | 80.0 [70.1–85.9] | <0.001 |
| Modified frailty index | |||
| Depression | 1 (0.1%) | 15 (0.2%) | 0.276 |
| Parkinson's disease | 18 (1.2%) | 55 (0.7%) | 0.061 |
| Arthritis | 7 (0.5%) | 33 (0.4%) | 0.872 |
| Paranoia | 0 (0.0%) | 7 (0.1%) | 0.239 |
| Skin ulcer | 35 (2.4%) | 71 (1.0%) | <0.001 |
| Pneumonia | 549 (37.3%) | 1492 (20.1%) | <0.001 |
| Falls | 36 (2.5%) | 154 (2.08%) | 0.369 |
| Skin and soft tissue infection | 17 (1.2%) | 82 (1.1%) | 0.868 |
| Mycoses | 2 (0.1%) | 17 (0.2%) | 0.479 |
| Gouty arthropathy | 87 (5.9%) | 354 (4.8%) | 0.066 |
| UTI | 209 (14.2%) | 560 (7.6%) | <0.001 |
| Charlson score ≥2 | 775 (52.7%) | 697 (47.4%) | <0.001 |
| Inflammatory and nutritional indices | |||
| PNI | 41.5 [37.0–46.0] | 44.5 [40.4–48.8] | <0.001 |
| NLR | 5.5 [3.1–9.8] | 4.2 [2.6–7.4] | <0.001 |
NLR, neutrophil‐to‐lymphocyte ratio; PNI, prognostic nutritional index; UTI, urinary tract infection.
Values in bold indicate P < 0.05.
Predictors of adverse outcomes and frailty model
Of the 8893 patients with heart failure, 758 patients died within 30 days (9%), and 1472 died within 90 days (17%) of admission. The findings of univariate logistic regression are reported in the Supporting Information, Table S2 . Age, chronic skin ulcers, pneumonia, urinary tract infection, NLR, and PNI were significant predictors of 30 day mortality (Supporting Information, Table S2 , left). For 90 day mortality, the same variables that predicted 30 day mortality, as well as Charlson score ≥2 were significant predictors (Supporting Information, Table S2 , right). Subsequently, the significant variables from the univariable analysis were included in multivariable logistic regression. The results of multivariable logistic regression for 30 day and 90 day mortality prediction with all variables are reported in the Supporting Information, Tables S3 and S4 , respectively. Age, pneumonia, UTI, PNI, and NLR remained significant predictors of both 30 day and 90 day mortality (P < 0.05).
Gradient boosting learning results and frailty score
Five‐fold cross validation experiments were conducted with gradient boosting learning. The key to gradient boosting is to set the target outcomes to minimize the overall error in relation to precision, recall, and AUC. In this way, the gradient boosting model sequentially adds weak decision tree learning models to the ensemble where subsequent models correct the prediction errors of prior models (Supporting Information, Figure S1 ), from which we can see that probability of 30 day mortality and 90 day mortality increases drastically as age grows above 80 years old. Specifically, predictions given by the sequential models that are close to the actual outcome should reduce the overall error, and the process continues until minimized total prediction error is achieved.
A total of 1400 and 1500 trees for 30 day and 90 day mortality prediction were assigned, respectively. The optimal tree number was identified using sensitivity analysis by plotting the value of the out‐of‐bag (OOB) error rate according to the number of trees within the forest (Supporting Information, Figure S2 ). OOB samples are those samples that are not included in the bootstrap samples. Original training data are randomly sampled‐with‐replacement generating small subsets of data, also known as bootstrap samples. These bootstrap samples are then fed as training data to the forest model. The OOB approach was used for selecting the optimal tree number of the forest model in which four‐fifths (as training) of the data were used for constructing the predictive classifier, while the remaining was used for evaluating the performance of the forest model. Tree depth was set at 1 for both 30 day and 90 day mortality prediction according to tree depth parameter tuning (Supporting Information, Figure S3 ). The variable importance is reported in Table 2 and is used for building the frailty score.
Table 2.
Variable importance for 30‐day and 90‐day mortality prediction with gradient boosting learning
| 30‐day mortality | 90‐day mortality | ||
|---|---|---|---|
| Variable | Importance | Variable | Importance |
| PNI | 37.45 | Age | 36.25 |
| Age | 32.11 | PNI | 35.28 |
| NLR | 20.46 | NLR | 14.59 |
| Pneumonia | 6.04 | Pneumonia | 7.05 |
| Skin ulcer | 1.16 | UTI | 2.29 |
| UTI | 1.03 | Skin ulcer | 1.43 |
| Parkinson's disease | 0.49 | Male sex | 0.57 |
| Male sex | 0.45 | Falls | 0.48 |
| Skin and soft tissue infection | 0.25 | Parkinson's disease | 0.46 |
| Gout | 0.23 | Charlson score ≥2 | 0.46 |
| Falls | 0.19 | Arthritis | 0.35 |
| Charlson score ≥2 | 0.13 | Skin and soft tissue infection | 0.34 |
| Arthritis | 0.01 | Gout | 0.31 |
| Mycoses | 0.01 | Mycoses | 0.15 |
| Depression | 0 | Depression | 0 |
| Paranoia | 0 | Paranoia | 0 |
NLR, neutrophil‐to‐lymphocyte ratio; PNI, prognostic nutritional index; UTI, urinary tract infection.
The partial dependence relationships of the highest variable importance values for mortality prediction were also identified using gradient boosting learning. The probabilities of 30 day and 90 day mortality both increase as patient becomes older, and it increases sharply when patients are older than 80 (Figure 1 ). For PNI, the likelihood of mortality decreases sharply as PNI increases from 0 to 20 and remains almost constant when PNI increases beyond 65 (30 day mortality) or 70 (90 day mortality) (Figure 2 ). There is a non‐linear relationship between NLR and 30 day and 90 day mortality (Figure 3 ). Patients with pneumonia has high probability of mortality, 24% for 30 day mortality, and 14% for 90 day mortality.
Figure 1.
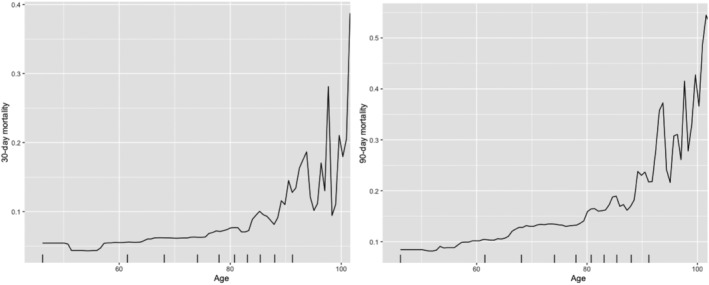
Partial dependence of patient age for 30 day (left) and 90 day (right) mortality risk probability prediction.
Figure 2.
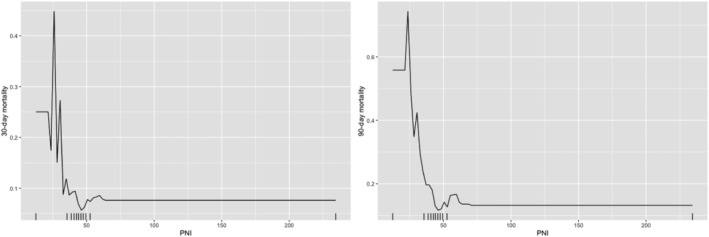
Partial dependence of PNI for 30 day (left) and 90 day (right) mortality risk probability prediction.
Figure 3.
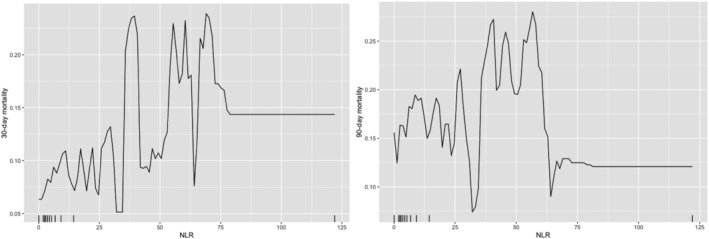
Partial dependence of NLR for 30 day (left) and 90 day (right) mortality risk probability prediction.
Comparative analyses of gradient boosting learning model, decision tree model, and multivariable logistic regression model for 30 day and 90 day mortality prediction are reported in the Supporting Information, Table S5 with five‐fold cross validation. Gradient boosting learning shows the best performance in prediction, recall, and AUC evaluation metrics.
The results from the sensitivity analysis excluding NLR and PNI are shown in the Supporting Information, Appendix S2 . The optimum tree numbers are shown in the Supporting Information, Figure S4 . Without NLR and PNI, age became the most important variable for predicting both 30 day and 90 day mortality (Supporting Information, Table S6 ; Figure S5 ), and some evaluation metrics were lower, but others were not affected (Supporting Information, Table S7 ). Five cross validations indicate that the machine learning model maintains comparable prediction performance as the case with NLR and PNI to predict 30 day mortality (precision = 0.90, recall = 0.89, and AUC = 0.90) and 90 day mortality (precision = 0.91, recall = 0.91, and AUC = 0.90).
Discussion
The main findings of this study are that (i) baseline PNI, NLR, and age at heart failure hospitalization in the modified electronic frailty index were the most predictive variables for the short‐term mortality outcomes in heart failure patients, and (ii) non‐linear partial dependence relationships between these predictors and outcomes were observed.
We developed a modified electronic frailty model after incorporating the inflammatory and nutritional indices into the conventional frailty scoring system based on the value of importance of each variable generated from gradient boosting learning model. Compared with multivariable logistic regression and decision tree, gradient boosting learning techniques improved the predictive performance of our frailty model. To enhance mortality prediction by capturing the non‐linear pattern within characteristics, we develop an interpretable machine learning model based on gradient boosting machine. 34 Machine learning models can be fitted to data individually or combined in an ensemble, resulting in an efficient combination of simple individual learning models that together create a more powerful model. 35
In this study, significant risk factors to predict 30 day and 90 day mortality are efficiently identified with gradient boosting learning model. The obtained rankings of important variables for mortality prediction can be used as an electronic heart failure frailty scoring tool for clinical use. The efficient identification of partial dependence for predictive variables provides more refined estimation of the likelihood of mortality. For example, effective estimations about the patient's mortality probability based on characteristics of smaller PNI, older age, larger NLR (below 60 or so), and pneumonia. All of these variables were associated with impaired mobility. Of these factors, pneumonia is a common nosocomial condition that also confers a significantly higher risk of 30 day post‐admission mortality. 36 In addition, we extensively conduct the analysis without PNI and NLR, and the results are provided in the supporting information. Variable importance ranking for 30 day identifies important variables age, pneumonia, skin ulcer, UTI, Parkinson's, gout, and male sex to predict 30 day mortality, while variables age, pneumonia, UTI, skin ulcer, Parkinson's, and Carlson score to predict 90 day mortality.
Heart failure has been recognized as predominately a syndrome that affects the geriatric population, with over 50% of incidence and 60% of heart failure‐associated mortality occurring in the population over 75 years old. 37 Age at diagnosis is also one of the most significant prognostic factors for subsequent survival. 38 In our cohort, the median age was 81 years old, and the risk of the short‐term mortality increased strikingly in those aged over 80. Age was ranked as the most important variable to predict 90 day mortality and the second most important variable for 30 day mortality. In 2011, it was reported that the 1 year mortality rates increased sharply from 20% to over 30% in patients 75–84 years and over 40% in patients aged over 85 years. 39 The high prevalence of important risk factors, such as hypertension and ischaemic heart disease, leads to the increasing incidence of heart failure in older patients. 40 Moreover, the survival outcomes of heart failure are closely related to the unfavourable age‐associated changes in cardiovascular structure and function, which compromise cardiac reverse capacity. 41 Therefore, it is not surprising to observe the strong prognostic value of age in our frailty model.
The frailty index was based on the concept that frailty is caused by the accumulation of health deficits. 42 The frailty state itself is considered as an individual variable that can predict mortality, 43 even independently of age in different settings. 44 The first electronic frailty index developed by Segal et al. was based on the same concept, in which the candidate variables were selected based on their potential correlation with the frailty state rather than mortality directly. 4 Therefore, the individual variables in the frailty index might not associate well with the mortality outcome, and the deficits cumulatively lead to an increased risk of mortality.
The specific value of frailty in heart failure cohort has been examined by many studies. A recent meta‐analysis has confirmed the association between pre‐frailty or frailty state and the worse clinical outcomes in patients with heart failure. 45 , 46 , 47 Indeed, recent guidelines have recommended the assessment of frailty status in heart failure patients to aid risk stratification. 48 The Identification of Senior At Risk scale is another frailty screening tool that can predict 30 day mortality in older patients with acute heart failure. 49 Among the current literature, a few studies utilized frailty indices and reached similar conclusions to other studies in which frailty was assessed as a phenotype, 50 , 51 and there is no consensus which method is more suitable in the cohort of heart failure patients. 45 The variables included in the various frailty indices used for heart failure were also largely different. A study in the UK combined the frailty index and nutritional index and found an improved prognostic power compared with the conventional frailty index, suggesting that nutrition and frailty are correlated but also remained as independent prognostic factors. 51 No previous study has attempted to incorporate inflammatory measures into frailty indices for heart failure prognosis despite the strong pathophysiological associations between these concepts. 52
Strength and limitations
To the best of our knowledge, this is the first study incorporating both the inflammatory marker and nutritional index into the conventional frailty index. The indices used in this study, NLR and PNI, can be easily calculated and incorporated into the decision‐making process in the clinical setting. We utilized a large patient cohort that is homogeneously Chinese from a real‐world database, and the final frailty score was derived from a machine learning model, which was shown to have a better performance than the baseline multivariate logistic regression for mortality prediction.
There are some limitations to our study. Firstly, this is a multicentre study conducted in Hong Kong, and external validation of our results using data from other countries is needed. Secondly, our study did not include information on the treatment prescribed during the acute phase and the postadmission period, which may affect the survival outcomes in patients. Thirdly, we cannot distinguish between subtypes of heart failure, such as heart failure with reduced rejection fraction or heart failure with reduced rejection fraction, as we do not have echocardiographic or relevant diagnostic codes available in our database. 53 Moreover, there are some challenges of machine learning in clinical setting. Our model was developed specifically using the variables available in the local database and may have generalizability issue in other clinical settings if these variables were measured differently. Furthermore, the relative predictive values of the variables in our model might be difficult to interpret in regard to their biological associations with the mortality due to the opaque nature of the machine‐learning techniques. Nevertheless, a previous systematic review and meta‐analysis found that machine learning techniques can aid the diagnosis, management, and prediction of outcomes in heart failure patients. 54 Nevertheless, frailty alone is a strong predictor of mortality, although it requires a thorough assessment that may be time‐consuming. 55 Our study complements previous findings that electronic frailty indices are useful for risk prediction in other settings. 56 , 57 , 58
Conclusions
In this study, we created an electronic frailty index that included co‐morbidity information, inflammatory, and nutritional indices. This was then used for short‐term mortality prediction in heart failure. Given that these variables can be determined or calculated automatically, their incorporation into clinical risk scores or prediction rules will facilitate clinicians to perform risk stratification more readily. Further prospective studies are warranted to validate the present model by combining other more comprehensive and complex inflammatory, nutritional, and frailty assessment tools to confirm its predictive power for clinical use.
Conflict of interest
All authors declare no conflict of interest.
Supporting information
Table S1. Variables used in the modified frailty index proposed by Segal et al. (2017), Deyo‐Charlson comorbidity index (1992), and our electronic frailty index.
Table S2. Univariable logistic regression for 30‐day and 90‐day mortality.
Table S3. Multivariable logistic regression for 30‐day mortality.
Table S4. Multivariable logistic regression for 90‐day mortality.
Table S5. Five‐fold cross validation model performance for 30‐day and 90‐day mortality prediction.
Figure S1. Sequential ensemble concept of gradient boosting learning model.
Figure S2. Optimal iteration tree number of gradient boosting learning model for 30‐day and 90‐day mortality prediction.
Figure S3. Tree in‐depth parameter selection for 30‐day and 90‐day mortality prediction.
Table S6. Variable importance for 30‐day and 90‐day mortality prediction with gradient boosting learning without NLR and PNI.
Table S7. Five‐fold cross validation model performance for 30‐day and 90‐day mortality prediction without NLR and PNI.
Figure S5. Variable importance ranking for 30‐day and 90‐day mortality prediction without NLR and PNI.
Ju, C. , Zhou, J. , Lee, S. , Tan, M. S. , Liu, T. , Bazoukis, G. , Jeevaratnam, K. , Chan, E. W. Y. , Wong, I. C. K. , Wei, L. , Zhang, Q. , and Tse, G. (2021) Derivation of an electronic frailty index for predicting short‐term mortality in heart failure: a machine learning approach. ESC Heart Failure, 8: 2837–2845. 10.1002/ehf2.13358.
[Correction added on 08 June 2021, after first online publication: The last author's affiliations have been corrected in this version.]
Contributor Information
Qingpeng Zhang, Email: qingpeng.zhang@cityu.edu.hk.
Gary Tse, Email: g.tse@surrey.ac.uk.
References
- 1. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med 2011; 27: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Segal JB, Huang J, Roth DL, Varadhan R. External validation of the claims‐based frailty index in the national health and aging trends study cohort. Am J Epidemiol 2017; 186: 745–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research G . Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56: M146–M156. [DOI] [PubMed] [Google Scholar]
- 4. Segal JB, Chang HY, Du Y, Walston JD, Carlson MC, Varadhan R. Development of a claims‐based frailty indicator anchored to a well‐established frailty phenotype. Med Care 2017; 55: 716–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lorenzo‐Lopez L, Maseda A, de Labra C, Regueiro‐Folgueira L, Rodriguez‐Villamil JL, Millan‐Calenti JC. Nutritional determinants of frailty in older adults: a systematic review. BMC Geriatr 2017; 17: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, Sergi G, Isik AT, Manzato E, Maggi S, Maggio M, Prina AM, Cosco TD, Wu YT, Veronese N. Inflammation and frailty in the elderly: a systematic review and meta‐analysis. Ageing Res Rev 2016; 31: 1–8. [DOI] [PubMed] [Google Scholar]
- 7. Gastelurrutia P, Lupon J, Altimir S, de Antonio M, Gonzalez B, Cabanes R, Rodriguez M, Urrutia A, Domingo M, Zamora E, Diez C, Coll R, Bayes‐Genis A. Fragility is a key determinant of survival in heart failure patients. Int J Cardiol 2014; 175: 62–66. [DOI] [PubMed] [Google Scholar]
- 8. Sun Y, Wang N, Li X, Zhang Y, Yang J, Tse G, Liu Y. Predictive value of H2 FPEF score in patients with heart failure with preserved ejection fraction. ESC Heart Fail 2021; 8: 1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denfeld QE, Winters‐Stone K, Mudd JO, Gelow JM, Kurdi S, Lee CS. The prevalence of frailty in heart failure: a systematic review and meta‐analysis. Int J Cardiol 2017; 236: 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McNallan SM, Chamberlain AM, Gerber Y, Singh M, Kane RL, Weston SA, Dunlay SM, Jiang R, Roger VL. Measuring frailty in heart failure: a community perspective. Am Heart J 2013; 166: 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soukoulis V, Dihu JB, Sole M, Anker SD, Cleland J, Fonarow GC, Metra M, Pasini E, Strzelczyk T, Taegtmeyer H, Gheorghiade M. Micronutrient deficiencies an unmet need in heart failure. J Am Coll Cardiol 2009; 54: 1660–1673. [DOI] [PubMed] [Google Scholar]
- 12. Shirazi LF, Bissett J, Romeo F, Mehta JL. Role of inflammation in heart failure. Curr Atheroscler Rep 2017; 19: 27. [DOI] [PubMed] [Google Scholar]
- 13. Tse G, Zhou J, Woo SWD, Ko CH, Lai RWC, Liu T, Liu Y, Leung KSK, Li A, Lee S, Li KHC, Lakhani I, Zhang Q. Multi‐modality machine learning approach for risk stratification in heart failure with left ventricular ejection fraction ≤45. ESC Heart Fail 2020; 7: 3716–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lakhani I, Leung KSK, Tse G, Lee APW. Novel mechanisms in heart failure with preserved, midrange, and reduced ejection fraction. Front Physiol 2019; 10: 874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschope C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 2011; 4: 44–52. [DOI] [PubMed] [Google Scholar]
- 16. Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013; 62: 263–271. [DOI] [PubMed] [Google Scholar]
- 17. Roever L, Tse G, Biondi‐Zoccai G. Variability of metabolic parameters and risk of heart failure: can it be a marker of incident heart failure? Int J Cardiol 2019; 293: 183–184. [DOI] [PubMed] [Google Scholar]
- 18. Van Linthout S, Tschope C. Inflammation—cause or consequence of heart failure or both? Curr Heart Fail Rep 2017; 14: 251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edelmann F, Holzendorf V, Wachter R, Nolte K, Schmidt AG, Kraigher‐Krainer E, Duvinage A, Unkelbach I, Dungen HD, Tschope C, Herrmann‐Lingen C, Halle M, Hasenfuss G, Gelbrich G, Stough WG, Pieske BM. Galectin‐3 in patients with heart failure with preserved ejection fraction: results from the Aldo‐DHF trial. Eur J Heart Fail 2015; 17: 214–223. [DOI] [PubMed] [Google Scholar]
- 20. Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D'Agostino RB, Framingham Heart S . Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation 2003; 107: 1486–1491. [DOI] [PubMed] [Google Scholar]
- 21. Yildiz A, Yuksel M, Oylumlu M, Polat N, Akil MA, Acet H. The association between the neutrophil/lymphocyte ratio and functional capacity in patients with idiopathic dilated cardiomyopathy. Anatol J Cardiol 2015; 15: 13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zapatero A, Barba R, Gonzalez N, Losa JE, Plaza S, Canora J, Marco J. Influence of obesity and malnutrition on acute heart failure. Rev Esp Cardiol (Engl Ed) 2012; 65: 421–426. [DOI] [PubMed] [Google Scholar]
- 23. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb‐Peploe KM, Harrington D, Kox WJ, Poole‐Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet 1997; 349: 1050–1053. [DOI] [PubMed] [Google Scholar]
- 24. Chien SC, Lo CI, Lin CF, Sung KT, Tsai JP, Huang WH, Yun CH, Hung TC, Lin JL, Liu CY, Hou CJ, Tsai IH, Su CH, Yeh HI, Hung CL. Malnutrition in acute heart failure with preserved ejection fraction: clinical correlates and prognostic implications. ESC Heart Fail 2019; 6: 953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng YL, Sung SH, Cheng HM, Hsu PF, Guo CY, Yu WC, Chen CH. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc 2017; 6: e004876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ueland T, Gullestad L, Nymo SH, Yndestad A, Aukrust P, Askevold ET. Inflammatory cytokines as biomarkers in heart failure. Clin Chim Acta 2015; 443: 71–77. [DOI] [PubMed] [Google Scholar]
- 27. Anker SD, Negassa A, Coats AJ, Afzal R, Poole‐Wilson PA, Cohn JN, Yusuf S. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin‐converting‐enzyme inhibitors: an observational study. Lancet 2003; 361: 1077–1083. [DOI] [PubMed] [Google Scholar]
- 28. Mano A, Fujita K, Uenomachi K, Kazama K, Katabuchi M, Wada K, Terakawa N, Arai K, Hori Y, Hashimoto S, Nakatani T, Kitamura S. Body mass index is a useful predictor of prognosis after left ventricular assist system implantation. J Heart Lung Transplant 2009; 28: 428–433. [DOI] [PubMed] [Google Scholar]
- 29. Desai RJ, Wang SV, Vaduganathan M, Evers T, Schneeweiss S. Comparison of machine learning methods with traditional models for use of administrative claims with electronic medical records to predict heart failure outcomes. JAMA Netw Open 2020; 3: e1918962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ju C, Lai RWC, Li KHC, Hung JKF, Lai JCL, Ho J, Liu Y, Tsoi MF, Liu T, Cheung BMY, Wong ICK, Tam LS, Tse G. Comparative cardiovascular risk in users versus non‐users of xanthine oxidase inhibitors and febuxostat versus allopurinol users. Rheumatology (Oxford) 2019; 59: 2340–2349. [DOI] [PubMed] [Google Scholar]
- 31. Lau WC, Chan EW, Cheung CL, Sing CW, Man KK, Lip GY, Siu CW, Lam JK, Lee AC, Wong IC. Association between dabigatran vs warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. JAMA 2017; 317: 1151–1158. [DOI] [PubMed] [Google Scholar]
- 32. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol 1992; 45: 613–619. [DOI] [PubMed] [Google Scholar]
- 33. Mason L, Baxter J, Bartlett P, Frean M. Boosting algorithms as gradient descent. In Advances in Neural Information Processing Systems. Cambridge, MA, United States: MIT Press; 2000: 512–518. [Google Scholar]
- 34. Friedman JH. Greedy function approximation: a gradient boosting machine. Ann Stat 2001; 29: 1189–1232. [Google Scholar]
- 35. Peter Bühlmann TH. Boosting algorithms: regularization, prediction and model fitting. Stat Sci 2007; 22: 477–505. [Google Scholar]
- 36. Kundi H, Wadhera RK, Strom JB, Valsdottir LR, Shen C, Kazi DS, Yeh RW. Association of frailty with 30‐day outcomes for acute myocardial infarction, heart failure, and pneumonia among elderly adults. JAMA Cardiol 2019; 4: 1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rich MW. Heart failure in the 21st century: a cardiogeriatric syndrome. J Gerontol A Biol Sci Med Sci 2001; 56: M88–M96. [DOI] [PubMed] [Google Scholar]
- 38. Taylor CJ, Ordonez‐Mena JM, Roalfe AK, Lay‐Flurrie S, Jones NR, Marshall T, Hobbs FDR. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000‐2017: population based cohort study. BMJ 2019; 364: l223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998‐2008. JAMA 2011; 306: 1669–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation 2003; 107: 139–146. [DOI] [PubMed] [Google Scholar]
- 41. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation 2003; 107: 346–354. [DOI] [PubMed] [Google Scholar]
- 42. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1: 323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013; 381: 752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hewitt J, Carter B, McCarthy K, Pearce L, Law J, Wilson FV, Tay HS, McCormack C, Stechman MJ, Moug SJ, Myint PK. Frailty predicts mortality in all emergency surgical admissions regardless of age. An observational study. Age Ageing 2019; 48: 388–394. [DOI] [PubMed] [Google Scholar]
- 45. Zhang Y, Yuan M, Gong M, Tse G, Li G, Liu T. Frailty and clinical outcomes in heart failure: a systematic review and meta‐analysis. J Am Med Dir Assoc 2018; 19: 1003–1008 e1001. [DOI] [PubMed] [Google Scholar]
- 46. Zhang Y, Yuan M, Gong M, Li G, Liu T, Tse G. Associations between prefrailty or frailty components and clinical outcomes in heart failure: a follow‐up meta‐analysis. J Am Med Dir Assoc 2019; 20: 509–510. [DOI] [PubMed] [Google Scholar]
- 47. Zhang Y, Yuan M, Gong M, Li G, Tse G, Liu T. Reply to the letter to editor. J Am Med Dir Assoc 2018; 19: 1146–1148. [DOI] [PubMed] [Google Scholar]
- 48. Díez‐Villanueva P, Arizá‐Solé A, Vidán MT, Bonanad C, Formiga F, Sanchis J, Martín‐Sánchez FJ, Ruiz Ros V, Sanmartín Fernández M, Bueno H, Martínez‐Sellés M. Recommendations of the geriatric cardiology section of the Spanish Society of Cardiology for the assessment of frailty in elderly patients with heart disease. Rev Esp Cardiol (English Edition) 2019; 72: 63–71. [DOI] [PubMed] [Google Scholar]
- 49. Martin‐Sanchez FJ, Llopis Garcia G, Gonzalez‐Colaco Harmand M, Fernandez Perez C, Gonzalez Del Castillo J, Llorens P, Herrero P, Jacob J, Gil V, Dominguez‐Rodriguez A, Rossello X, Miro O, en representacion de los investigadores del Registro OAK , Resto de investigadores del registro OAK . Identification of senior at risk scale predicts 30‐day mortality among older patients with acute heart failure. Med Intensiva 2020; 44: 9–17. [DOI] [PubMed] [Google Scholar]
- 50. Dunlay SM, Park SJ, Joyce LD, Daly RC, Stulak JM, McNallan SM, Roger VL, Kushwaha SS. Frailty and outcomes after implantation of left ventricular assist device as destination therapy. J Heart Lung Transplant 2014; 33: 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sze S, Zhang J, Pellicori P, Morgan D, Hoye A, Clark AL. Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin Res Cardiol 2017; 106: 533–541. [DOI] [PubMed] [Google Scholar]
- 52. Bellumkonda L, Tyrrell D, Hummel SL, Goldstein DR. Pathophysiology of heart failure and frailty: a common inflammatory origin? Aging Cell 2017; 16: 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pathadka S, Yan VKC, Li X, Tse G, Wan EYF, Lau H, Lau WCY, Siu DCW, Chan EW, Wong ICK. Hospitalization and mortality in patients with heart failure treated with sacubitril/valsartan vs. enalapril: a real‐world, population‐based study. Front Cardiovasc Med 2020; 7: 602363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bazoukis G, Stavrakis S, Zhou J, Bollepalli SC, Tse G, Zhang Q, Singh JP, Armoundas AA. Machine learning versus conventional clinical methods in guiding management of heart failure patients—a systematic review. Heart Fail Rev 2021; 26: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kojima G, Iliffe S, Walters K. Frailty index as a predictor of mortality: a systematic review and meta‐analysis. Age Ageing 2018; 47: 193–200. [DOI] [PubMed] [Google Scholar]
- 56. Li CKH, Xu Z, Ho J, Lakhani I, Liu YZ, Bazoukis G, Liu T, Wong WT, Cheng SH, Chan MTV, Zhang L, Gin T, Wong MCS, Wong ICK, Wu WKK, Zhang Q, Tse G. Association of NPAC score with survival after acute myocardial infarction. Atherosclerosis 2020; 301: 30–36. [DOI] [PubMed] [Google Scholar]
- 57. Callahan KE, Clark CJ, Edwards AF, Harwood TN, Williamson JD, Moses AW, Willard JJ, Cristiano JA, Meadows K, Hurie J, High KP, Meredith JW, Pajewski NM. Automated Frailty Screening At‐Scale for Pre‐Operative Risk Stratification Using the Electronic Frailty Index. J Am Geriatr Soc 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fan J, Yu C, Guo Y, Bian Z, Sun Z, Yang L, Chen Y, Du H, Li Z, Lei Y, Sun D, Clarke R, Chen J, Chen Z, Lv J, Li L. Frailty index and all‐cause and cause‐specific mortality in Chinese adults: a prospective cohort study. Lancet Public Health 2020; 5: e650–e660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Variables used in the modified frailty index proposed by Segal et al. (2017), Deyo‐Charlson comorbidity index (1992), and our electronic frailty index.
Table S2. Univariable logistic regression for 30‐day and 90‐day mortality.
Table S3. Multivariable logistic regression for 30‐day mortality.
Table S4. Multivariable logistic regression for 90‐day mortality.
Table S5. Five‐fold cross validation model performance for 30‐day and 90‐day mortality prediction.
Figure S1. Sequential ensemble concept of gradient boosting learning model.
Figure S2. Optimal iteration tree number of gradient boosting learning model for 30‐day and 90‐day mortality prediction.
Figure S3. Tree in‐depth parameter selection for 30‐day and 90‐day mortality prediction.
Table S6. Variable importance for 30‐day and 90‐day mortality prediction with gradient boosting learning without NLR and PNI.
Table S7. Five‐fold cross validation model performance for 30‐day and 90‐day mortality prediction without NLR and PNI.
Figure S5. Variable importance ranking for 30‐day and 90‐day mortality prediction without NLR and PNI.


