Abstract
Aims
Risk stratification in patients with advanced chronic heart failure (HF) is an unmet need. Circulating microRNA (miRNA) levels have been proposed as diagnostic and prognostic biomarkers in several diseases including HF. The aims of the present study were to characterize HF‐specific miRNA expression profiles and to identify miRNAs with prognostic value in HF patients.
Methods and results
We performed a global miRNome analysis using next‐generation sequencing in the plasma of 30 advanced chronic HF patients and of matched healthy controls. A small subset of miRNAs was validated by real‐time PCR (P < 0.0008). Pearson's correlation analysis was computed between miRNA expression levels and common HF markers. Multivariate prediction models were exploited to evaluate miRNA profiles' prognostic role. Thirty‐two miRNAs were found to be dysregulated between the two groups. Six miRNAs (miR‐210‐3p, miR‐22‐5p, miR‐22‐3p, miR‐21‐3p, miR‐339‐3p, and miR‐125a‐5p) significantly correlated with HF biomarkers, among which N‐terminal prohormone of brain natriuretic peptide. Inside the cohort of advanced HF population, we identified three miRNAs (miR‐125a‐5p, miR‐10b‐5p, and miR‐9‐5p) altered in HF patients experiencing the primary endpoint of cardiac death, heart transplantation, or mechanical circulatory support implantation when compared with those without clinical events. The three miRNAs added substantial prognostic power to Barcelona Bio‐HF score, a multiparametric and validated risk stratification tool for HF (from area under the curve = 0.72 to area under the curve = 0.82).
Conclusions
This discovery study has characterized, for the first time, the advanced chronic HF‐specific miRNA expression pattern. We identified a few miRNAs able to improve the prognostic stratification of HF patients based on common clinical and laboratory values. Further studies are needed to validate our results in larger populations.
Keywords: Biomarkers, Circulating microRNAs, Heart failure, Risk stratification
Introduction
Heart failure (HF) prevalence is approximately 1–2% in the adult population of developed countries and is expected to rise, reflecting the chronic course of the disease as well as population growth and ageing. 1 Accurate prognostication strategies to plan treatment and follow‐up are still lacking. 2 In particular, the optimal timing for a non‐medical intervention represents a conundrum in advanced stages when pharmacological treatment is no longer effective. So far, neither singular parameter nor risk score provides adequate guidance on whether the individual HF patient may benefit from left ventricular assist devices (LVADs). 3 The main challenge for the management of advanced HF patients should be to exploit new predictors, which, together with laboratory and clinical parameters, may allow to better stratify the risk of adverse cardiovascular events and, thus, correctly identify the patients for an invasive therapeutic strategy. This approach could reduce hospitalization rates and costs, improving patients' outcomes and quality of life.
MicroRNAs (miRNAs) are small non‐coding RNAs involved in the regulation of protein translation at the post‐transcriptional level. They can inhibit messenger RNA translation and/or induce messenger RNA degradation, playing a pivotal role as regulators of human physiology and development. MiRNAs are expressed in tissues, but they can also be isolated from blood plasma, where they are stable and present as cell‐free circulating molecules or packed in extracellular vesicles. 4 , 5 , 6 The potential role of miRNAs as diagnostic and prognostic biomarkers has been advocated in several diseases such as cancer, but there are also several studies showing promising results for some miRNAs in HF. 7 , 8 However, the majority investigated the expression of candidate miRNAs. 9 , 10 , 11
The aims of the present study are (i) to produce expression profiles of cell‐free circulating miRNAs in advanced HF patients, using next‐generation sequencing (NGS); (ii) to identify differentially expressed miRNAs between patients and matched controls; (iii) to correlate miRNA expression levels with common HF markers; and (iv) to evaluate their prognostic role in this complex pathological setting, eventually including miRNA profiles in a predictive cardiovascular risk model.
Methods
This prospective, single‐centre, case–control study was realized through the synergic work of three operational units: the clinical unit, namely, the local LVAD Hub Centre in the Città della Salute e della Scienza University Hospital of Turin, Northern Italy, organized the patients' screening and recruitment; the biotechnology unit, represented by the University of Turin, controlled the processing, handling, and storage of serum and plasma; and the genomic unit, represented by the Italian Institute for Genomic Medicine, Candiolo (TO), Italy, performed the NGS analyses and shared their expertise in miRNA detection in body fluids.
Study population
Consecutive ambulatory patients with stable advanced HF 12 admitted to our clinical unit from September 2016 to August 2019 were prospectively enrolled. Inclusion criteria were age ≥18 years old, all criteria for advanced HF as defined by Metra et al., 12 and INTERMACS profile between 4 and 7. To reduce the effect of the acute event on miRNA expression profile, a minimum time of 3 weeks of clinical stability after hospital admission for acute decompensated HF was required for the enrolment. Exclusion criteria were extracardiac pathologies with life expectancy lower than 2 years and hospitalization for acute coronary syndrome, percutaneous revascularization, or recent cardiac surgery (within 40 days). Patients with cancer disease including haematological neoplasm were also excluded because of possible interference with miRNA expression.
The control arm of the study included healthy volunteers matched by age and sex. All subjects provided written informed consent for study participation. The investigation conforms to the principles outlined in the Declaration of Helsinki and was approved by the local ethics committee.
Clinical and hemodynamic assessment
At the time of enrolment, clinical history (age, gender, cardiovascular risk factors, medications intake at home, and HF aetiology) was recorded, and physical examination and electrocardiogram (ECG) were performed in all patients. A cardiopulmonary exercise test was performed according to the clinician's decision. All control participants underwent a complete physical examination, risk factors assessment, and ECG.
Two‐dimensional transthoracic echocardiography was performed in all patients, and haemodynamic data of right heart catheterization were reported when available. Further details are described in Supporting Information, Methods section.
Blood samples for clinical blood tests [creatinine, Na+, K+, urea, high‐sensitivity cardiac troponin T (Hs‐cTnT), total bilirubin, aspartate aminotransferase/alanine transaminase, N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP), soluble ST2 (sST2), and copeptin] and for the analysis of miRNA expression profiling were obtained from each patient and control at the time of enrolment. The glomerular filtration rate was estimated by Modification of Diet in Renal Disease equation.
Finally, for each patient, the risk of cardiovascular death and rehospitalization was estimated by the Barcelona (BCN) Bio‐HF web calculator, a composite HF risk stratification tool suggested by the European Society of Cardiology. BCN Bio‐HF score outperformed other multiparametric scores. 13 , 14
Blood sampling and RNA preparation
Blood samples were processed in the biotechnology unit laboratory within 4 h after collection. Blood samples (5–8 mL per subject) were centrifuged for 10 min at 1000 g, and 200 μL aliquots of plasma and serum were stored in long‐term storage cryogenic vials at −80°C until use. Serum samples were used to measure laboratory values (Tables 1 and 2 ). Total RNA from plasma was extracted in the genomic unit laboratory with the miRNeasy plasma/serum mini kit (Cat No. 217184, Qiagen, Hilden, Germany) using the QiaCube extractor (Qiagen) and following the manufacturer's instructions. For all samples, RNA concentration was quantified by Qubit 2.0 Fluorometer with Qubit miRNA Assay Kit (Cat No. Q32880, Invitrogen, Thermo Fisher, Waltham, MA, USA).
Table 1.
Clinical characteristics of chronic advanced heart failure
| HF group (n = 30) | Control group (n = 36) | |
|---|---|---|
| Clinical history and risk profile | ||
| Male gender | 26 (87) | 32 (89) |
| Age (years) | 65 [58–68] | 61.5 [57–65] |
| Body mass index | 23.9 [21.6–26.0] | |
| Hypertension | 18 (60) | |
| Diabetes mellitus | 7 (23) | |
| Familiarity for coronary artery disease | 7 (23) | |
| Current smoker | 18 (60) | |
| Dyslipidaemia | 15 (50) | |
| Ischaemic aetiology | 18 (60) | |
| INTERMACS class | 5 [5–5.7] | |
| VO2 max (mL/kg/min) a | 12.1 [10.4–14.2] | |
| Treatments | ||
| Furosemide (mg) | 75 [50–100] | |
| Beta‐blocker | 28 (93) | |
| ACE‐I | 15 (50) | |
| MRA | 25 (83) | |
| ICD | 29 (97) | |
| CRT | 11 (37) | |
| MitraClip | 7 (23) | |
| Echocardiographic data | ||
| Left ventricular EF (%) | 21 [19–25] | |
| LVEDD (mm) | 66.5 [61–70.7] | |
| RVEDD (mm) | 42 [40–48.7] | |
| Mean E/e′ (n) | 17 [14–28] | |
| Moderate‐to‐severe mitral regurgitation | 20 (67) | |
| Estimated systolic PAP (mmHg) | 50 [42–69] | |
| TAPSE (mm) | 16 [14–18] | |
| Haemodynamic variables b | ||
| CI (L/min/m2) | 1.8 [1.7–1.9] | |
| Mean WP (mmHg) | 23 [15–29] | |
| Mean PAP (mmHg) | 33 [22–39.5] | |
| TPG (mmHg) | 9 [6–13] | |
| PVR (WU) | 2.6 [1.6–3.7] | |
| Mean RAP (mmHg) | 7 [4–10] | |
| PAPi | 3.2 [2.6–4.5] | |
| Laboratory values | ||
| Na+ (mmol/L)*** | 135 [132–138] | 141 [140–142] |
| K+ (mmol/L)* | 4.2 [3.9–4.5] | 4.4 [4–4.6] |
| Creatinine (mg/dL)*** | 1.5 [1.2–1.8] | 0.88 [0.8–0.94] |
| MDRD | 50 [40–64] | |
| Urea (mg/dL)*** | 72 [52–97] | 34.5 [30–38.2] |
| Copeptin (pmol/L)*** | 29.9 [26.1–58.6] | 10.3 [6.5–15.1] |
| High‐sensitivity cardiac troponin T (ng/L)*** | 33.6 [23.8–65] | 6.1 [4.8–8.1] |
| Total bilirubin (mg/dL)*** | 0.8 [0.5–1.2] | 0.6 [0.5–0.8] |
| AST (IU/L)** | 19.5 [16–30.5] | 16 [14–19] |
| ALT (IU/L)*** | 17.5 [9–25.7] | 10.5 [8–14] |
| NT‐proBNP (pg/mL)*** | 4406 [2360–9385] | 32.9 [19.3–52.6] |
| sST2 (ng/mL)*** | 30.5 [20.8–44.6] | 22.9 [18.1–25.8] |
ACE‐I, angiotensin‐converting enzyme inhibitor; ALT, alanine transaminase; AST, aspartate aminotransferase; CI, cardiac index; CRT, cardiac resynchronization therapy; EF, ejection fraction; HF, heart failure; ICD, implantable cardiac defibrillator; LVEDD, left ventricular end‐diastolic diameter; MDRD, Modification of Diet in Renal Disease; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; PAP, pulmonary artery pressure; PAPi, pulmonary artery pulsatility index; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVEDD, right ventricular end‐diastolic diameter; sST2, soluble ST2; TAPSE, tricuspid annular plane systolic excursion; TPG, trans‐pulmonary gradient; WP, wedge pressure; WU, Wood units.
Data are presented as median [inter‐quartile range] or number (%).
VO2 max was available in 21 patients.
Right heart catheterization data were available in 23 patients.
Significant differences between HF patients and controls (P < 0.05).
Significant differences between HF patients and controls (P < 0.01).
Significant differences between HF patients and controls (P < 0.005).
Table 2.
Clinical characteristics of advanced HF patients with or without primary endpoint onset
| HF‐noPE group (n = 16) | HF‐PE group (n = 14) | |
|---|---|---|
| Clinical history and risk profile | ||
| Male gender | 14 (88) | 12 (86) |
| Age (years) | 66 [63–70] | 59 [55–65] |
| Body mass index | 24 [23–26] | 23 [21–26] |
| Hypertension | 8 (50) | 10 (71) |
| Diabetes mellitus | 5 (31) | 2 (14) |
| Familiarity for coronary artery disease | 5 (31) | 2 (14) |
| Current smoker | 9 (56) | 9 (64) |
| Dyslipidaemia | 9 (56) | 6 (43) |
| Ischaemic aetiology | 7 (44) | 4 (29) |
| INTERMACS class | 5 [5–5] | 5 [4–6] |
| VO2 max (mL/kg/min) a , *** | 14 [11–18] | 11.4 [10.3–14] |
| BCN Bio‐HF score* | 42 [13.9–78.6] | 25 [11.2–77.9] |
| Treatments | ||
| Furosemide (mg) | 75 [50–100] | 75 [50–120] |
| Beta‐blocker | 15 (94) | 13 (93) |
| ACE‐I | 7 (44) | 8 (57) |
| MRA | 13 (81) | 12 (86) |
| ICD | 15 (94) | 14 (100) |
| CRT | 6 (38) | 5 (36) |
| MitraClip | 4 (25) | 3 (21) |
| Echocardiographic data | ||
| Left ventricular EF (%) | 23.5 [19.5–25.7] | 20 [19.2–24.5] |
| LVEDD (mm) | 63 [60.7–70.2] | 67 [61.7–72.2] |
| RVEDD (mm) | 42 [39.2–49] | 43 [40.2–47.7] |
| Mean E/e′ (n) | 18.5 [15.2–25] | 16.3 [14.1–28.5] |
| Moderate‐to‐severe mitral regurgitation | 12 (75) | 8 (57) |
| Estimated systolic PAP (mmHg) | 49 [45–69.5] | 52.5 [40.5–61.5] |
| TAPSE (mm) | 15.5 [13.5–18] | 16.5 [14.2–19] |
| Haemodynamic variables b | ||
| CI (L/min/m2) | 1.9 [1.8–1.9] | 1.8 [1.7–1.9] |
| Mean WP (mmHg) | 22 [14–26] | 24.5 [16.5–29] |
| Mean PAP (mmHg) | 31 [19–36] | 34.5 [25–45] |
| TPG (mmHg)* | 7 [5–9] | 11.5 [8.2–14.7] |
| PVR (WU) | 2 [1.4–2.6] | 3 [2.22–5.32] |
| Mean RAP (mmHg) | 5 [4–6] | 8.5 [5.5–10] |
| PAPi | 4 [3.1–4.5] | 4 [2.5–5.2] |
| Laboratory values | ||
| Na+ (mmol/L) | 136 [133–138] | 134 [132–138] |
| K+ (mmol/L) | 4.2 [4–4.4] | 4.3 [3.8–4.6] |
| Creatinine (mg/dL) | 1.7 [1.3–2.3] | 1.5 [1.2–1.6] |
| MDRD* | 42 [29–54] | 56 [49–65] |
| Urea (mg/dL) | 75 [59–128] | 68 [53–86] |
| Copeptin (pmol/L) | 40.5 [27.3–66.8] | 29 [23–46] |
| High‐sensitivity cardiac troponin T (ng/L) | 39 [27.2–60.9] | 30 [15–61.2] |
| Total bilirubin (mg/dL) | 0.7 [0.5–1.4] | 0.9 [0.6–1.1] |
| AST (IU/L) | 19 [16–32] | 20 [16–26] |
| ALT (IU/L) | 18 [9–25] | 12 [9–24] |
| NT‐proBNP (pg/mL) | 5137 [2027–11334] | 3734 [2376–7293] |
| sST2 (ng/mL) | 32 [19–45] | 30 [27–45] |
ACE‐I, angiotensin‐converting enzyme inhibitor; ALT, alanine transaminase; AST, aspartate aminotransferase; BCN, Barcelona; CI, cardiac index; CRT, cardiac resynchronization therapy; EF, ejection fraction; HF, heart failure; ICD, implantable cardiac defibrillator; LVEDD, left ventricular end‐diastolic diameter; MDRD, Modification of Diet in Renal Disease; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; PAP, pulmonary artery pressure; PAPi, pulmonary artery pulsatility index; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVEDD, right ventricular end‐diastolic diameter; sST2, soluble ST2; TAPSE, tricuspid annular plane systolic excursion; TPG, trans‐pulmonary gradient; WP, wedge pressure; WU, Wood units.
Data are presented as median [inter‐quartile range] or number (%).
VO2 max was available in 21 patients.
Right heart catheterization data were available in 23 patients.
Significant differences between HF patients with or without primary endpoint (P < 0.05).
Significant differences between HF patients with or without primary endpoint (P < 0.005).
MicroRNA profiling by next‐generation sequencing and real‐time PCR analysis validation
Small RNA transcripts were converted into barcoded cDNA libraries following the protocols developed in the genomic unit laboratory, as previously described. 15 , 16 , 17 To validate NGS results, we planned to measure by real‐time PCR the expression of a small subset of miRNAs identified to be differentially expressed between HF and control samples. This analysis was necessary to consolidate data obtained by NGS in a subgroup of patients and controls.
A more detailed description of the library preparation, Illumina sequencing protocols, and real‐time PCR analysis is given in Supporting Information, Methods section.
Computational and statistical analyses
The data from small RNA sequencing were computationally processed and analysed as previously described 18 and as specified in Supporting Information, Methods section.
Briefly, differential expression of miRNAs was tested with the DESeq2 package. Potential confounders were included as covariates: batch (library), sex, and age. P‐values were adjusted for multiple testing by the Benjamini–Hochberg procedure. Pearson's correlation was computed between the base 2 logarithms of normalized miRNA expression and clinical variables. Significance of differences in the median of clinical variables between endpoints was tested by the Wilcoxon–Mann–Whitney test.
Primary endpoint and follow‐up
Patients were prospectively followed with clinical examination, ECG, and transthoracic echocardiography for at least 12 months after the enrolment. The primary endpoint (PE) was a composite of cardiac death, elective or urgent heart transplantation, or LVAD implantation.
Prediction model for the diagnosis and prognosis of heart failure
Multivariate prediction models were based on logistic regression with ElasticNet penalty, and receiver operating characteristic curve and area under the curve (AUC) estimation was performed in cross‐validation. More in detail, prediction models were built in Python with the scikit‐learn module using the LogisticRegressionCV classifier. This classifier implements a logistic regression with ElasticNet penalty with an automatic selection of the best hyper‐parameters. We used AUC as a metric to assess the performance of the models that we built. To control for overfitting, we performed 10‐fold cross‐validation repeated with different random seeds 50 times. A non‐linear classifier such as the RandomForestClassifier did not provide better performance than results derived from the logistic regression.
Results
Baseline characteristics
Among 76 advanced HF patients screened, 46 did not meet the study criteria and were excluded as shown in Supporting Information, Figure S1 . Finally, 30 patients were enrolled in the study and matched by age and sex with healthy controls (Table 1 ). All patients (mean age 65 years, 87% male, and 60% with ischaemic aetiology of the cardiomyopathy) presented advanced chronic HF: 100% New York Heart Association III, median NT‐proBNP 4406 pg/mL, ejection fraction (EF) 21% (all patients showed a reduced EF), tricuspid annular plane systolic excursion 16 mm, VO2 max 12.1 mL/kg/min, and optimal guideline‐directed medical therapy including implantable cardioverter defibrillator in 97% and cardiac resynchronization therapy in 37% of patients. At right heart catheterization, performed in 23 patients, median cardiac index was 1.8 L/min/m2, mean pulmonary artery pressure was 33 mmHg [22–39.5], and pulmonary artery pulsatility index was 3.2 [2.6–4.5]. Other baseline characteristics are shown in Table 1 .
Clinical endpoint
Fourteen patients (46.7%) met the primary composite endpoint at 12 month follow‐up (HF‐PE group, Table 2 ). Of them, three patients died for cardiovascular causes, five patients underwent heart transplantation, and six patients received LVAD implantation. Twenty‐two patients were hospitalized for acute HF during the 12 month follow‐up. Notably, among echocardiographic parameters of right and left ventricular function, no differences were noted between HF‐PE group and patients' group without events (HF‐noPE group, Table 2 ). Moreover, commonly used HF biomarkers (copeptin, NT‐proBNP, sST2, and Hs‐cTnT) did not show any significant difference between the two HF cohorts (Table 2 ). The most significantly different parameters were VO2 max (although available in 21/30 patients) and BCN Bio‐HF score values, with an inverse correlation with prognosis, and trans‐pulmonary gradient (TPG, although available in 23/30 patients) with a direct correlation (P‐value ranging from 0.002 to 0.05, Table 2 ).
Receiver operating characteristic curves were then built for the main clinical and laboratory variables, as shown in Supporting Information, Figure S2 , to test their prediction capacity: VO2 max, TPG (though with many missing values: 9/30 for VO2 max and 7/30 for TPG), and the BCN Bio‐HF score were the most effective in our dataset (AUC = 0.88, 0.79, and 0.72, respectively).
MicroRNA profiling by next‐generation sequencing
MicroRNA expression profiles were obtained by NGS performed in 30 enrolled HF patients and 36 healthy controls. From Illumina deep sequencing, a median of 12.7 million reads per sample was generated, ranging from 4.04 to 49.48 million reads. After adapter trimming, reads were aligned to a reference of human small non‐coding RNAs as previously described. 18 Alignments correctly mapping to miRNA sequences annotated in miRbase release 22 were in median 1.79 million per sample, ranging from 0.21 to 9.66 million reads. After removing miRNAs with expression lower than 20 reads in more than 50% of samples, a total of 329 unique miRNA sequences were detected in plasma and inserted in a count matrix with 66 samples and 329 miRNAs (Supporting Information, Table S1 ).
Case–control comparison
Thirty‐two miRNAs out of the 329 detected were significantly differentially expressed in advanced HF patients when compared with healthy controls (adjusted P‐value <0.05; Table 3 and Supporting Information, Figure S3A ). Of them, 20 miRNAs were significantly up‐regulated and 12 down‐regulated in HF patients compared with controls (Table 3 ). The most differentially expressed miRNA was miR‐210‐3p that resulted up‐regulated in HF patients (log2 fold change = 0.8; adjusted P‐value = 5.4 × 10−7). The other significantly up‐regulated miRNAs were miR‐1287‐5p, miR‐148a‐3p and miR‐148a‐5p, miR‐629‐5p, miR‐22‐5p, miR‐339‐3p, miR‐1285‐3p, miR‐548o‐3p, miR‐22‐3p, miR‐550a‐5p, miR‐6515‐5p, miR‐152‐3p, miR‐330‐5p, miR‐3120‐5p, miR‐503‐5p, miR‐361‐3p, miR‐21‐3p, miR‐769‐5p, and miR‐576‐3p. Among the down‐regulated miRNAs, the most relevant were miR‐628‐3p and miR‐125a‐5p (log2 fold change = −0.5 and −0.7; adjusted P‐value = 0.0008 and 0.001, respectively). The other significantly down‐regulated miRNAs are reported in Table 3 .
Table 3.
Differentially expressed microRNAs between HF patients and healthy controls
| MiRNA name | Log2 fold change | P‐values | Mean reads |
|---|---|---|---|
| miR‐210‐3p | 0.85 | 0.00000054 | 129.46 |
| miR‐1287‐5p | 0.73 | 0.00030 | 29.25 |
| miR‐148a‐3p | 0.73 | 0.00033 | 74 769.39 |
| miR‐148a‐5p | 0.64 | 0.00077 | 160.43 |
| miR‐629‐5p | 0.68 | 0.00079 | 1464.03 |
| miR‐22‐5p | 0.38 | 0.0046 | 181.57 |
| miR‐339‐3p | 0.51 | 0.0071 | 169.04 |
| miR‐1285‐3p | 0.69 | 0.028 | 31.10 |
| miR‐548o‐3p | 0.44 | 0.028 | 99.51 |
| miR‐22‐3p | 0.37 | 0.028 | 6785.33 |
| miR‐550a‐5p | 0.58 | 0.034 | 52.85 |
| miR‐6515‐5p | 0.42 | 0.034 | 35.09 |
| miR‐152‐3p | 0.41 | 0.034 | 1656.89 |
| miR‐330‐5p | 0.34 | 0.040 | 55.25 |
| miR‐3120‐5p | 0.43 | 0.044 | 41.03 |
| miR‐503‐5p | 0.53 | 0.046 | 42.74 |
| miR‐361‐3p | 0.20 | 0.047 | 2601.47 |
| miR‐21‐3p | 0.61 | 0.048 | 36.70 |
| miR‐769‐5p | 0.24 | 0.048 | 197.93 |
| miR‐576‐3p | 0.41 | 0.049 | 126.85 |
| miR‐628‐3p | −0.49 | 0.0008 | 205.15 |
| miR‐125a‐5p | −0.69 | 0.0014 | 1245.26 |
| miR‐423‐3p | −0.39 | 0.0019 | 17 516.91 |
| miR‐485‐3p | −1.10 | 0.0052 | 135.97 |
| miR‐340‐3p | −0.44 | 0.0081 | 375.54 |
| miR‐6721‐5p | −0.51 | 0.012 | 45.93 |
| miR‐664a‐3p | −0.50 | 0.018 | 23.79 |
| miR‐505‐5p | −0.42 | 0.023 | 150.21 |
| miR‐181d‐5p | −0.35 | 0.028 | 176.91 |
| miR‐484 | −0.35 | 0.028 | 2545.13 |
| miR‐328‐3p | −0.42 | 0.028 | 2135.29 |
| miR‐30d‐5p | −0.16 | 0.034 | 75 352.14 |
HF, heart failure; miRNA, microRNA.
We validated by real‐time PCR the expression levels of four selected miRNAs, characterized by high significance (P‐value <0.0008) and large differential expression between case–control groups in the NGS analysis (log2 fold change >0.6). Validation was performed on 15 HF patients and their paired controls randomly chosen among all samples in the cohort. As shown in Figure 1 , miR‐210‐3p, miR‐1287‐5p, miR‐148a‐5p, and miR‐629‐5p were differentially expressed in advanced HF patients in comparison with healthy controls. These results confirmed miRNA profiles observed by NGS.
Figure 1.
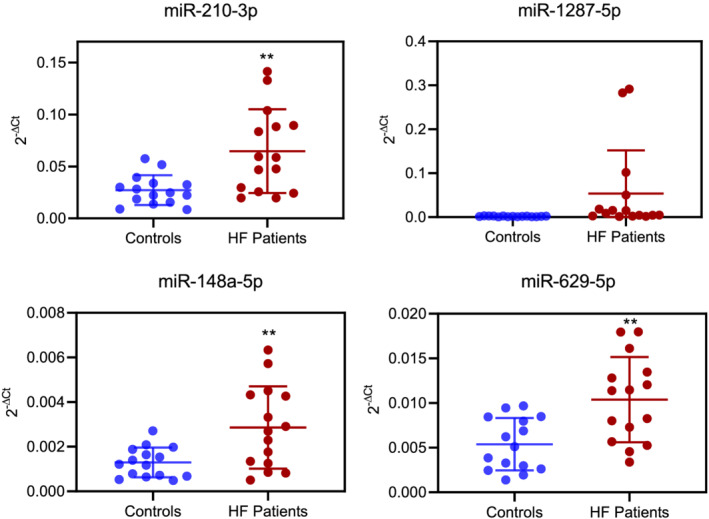
Validation by real‐time PCR of a subset of four microRNAs differentially expressed between heart failure (HF) cases and controls in the next‐generation sequencing analyses (P‐value <0.0008 and log2 fold change >0.6). Blue: controls. Red: HF patients. **P‐value ≤0.01.
MicroRNA profiles and heart failure markers
The 32 differentially expressed miRNAs between cases and controls were compared with established biomarkers of HF severity. Among the 32 differentially expressed miRNAs between cases and controls, six resulted associated by regression analysis with NT‐proBNP serological levels (Figure 2 and Supporting Information, Figure S3 ), which confirmed as the best common HF biomarker (AUC = 1, Supporting Information, Figure S4 ). The up‐regulated miR‐210‐3p, miR‐22‐5p, miR‐22‐3p, miR‐21‐3p, and miR‐339‐3p positively correlated with NT‐proBNP (R ≥ 0.30, Figure 2 ). Also, these miRNAs showed a positive correlation with at least another HF biomarker, among which copeptin, sST2, and hs‐cTnT (R ≥ 0.30, Figure 2 ). The down‐regulated miR‐125a‐5p negatively correlated with NT‐proBNP and sST2 (R < −0.30, Figure 2 ). Moreover, miR‐210‐3p, miR‐21‐3p, and miR‐339‐3p positively correlated with the BCN Bio‐HF score (R < 0.30), while miR‐125a‐5p showed a negative correlation (R = −0.36, Figure 2 ).
Figure 2.
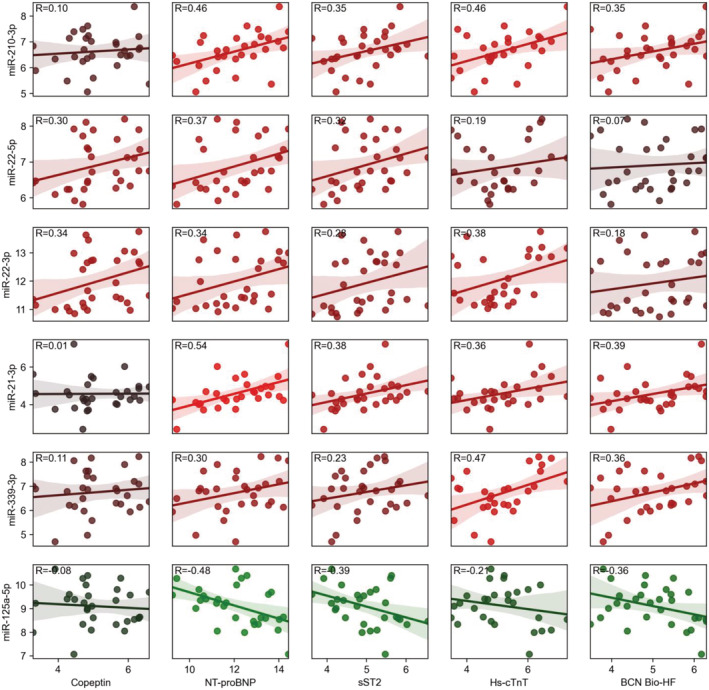
Differentially expressed microRNAs significantly correlated with heart failure (HF) biomarkers [copeptin, N‐terminal prohormone of brain natriuretic peptide (NT‐proBNP), soluble ST2 (sST2), and high‐sensitivity cardiac troponin T (Hs‐cTnT)] and Barcelona (BCN) Bio‐HF score. Bright red (R > 0.40) and bright green (R < −0.40) colours are associated with stronger positive and negative correlations, respectively. Dark red and dark green colours are weak correlation values. MicroRNA expression and clinical variable values are reported as base 2 logarithms. The correlation coefficients are shown (R).
However, the performance of these miRNAs alone as HF biomarkers was worse than that of common laboratory values when tested by AUC analysis, even in a prediction model including all 32 miRNAs (Supporting Information, Figure S4 ).
Circulating microRNAs related to clinical outcomes
Out of 329 miRNAs detected during the discovery phase, three miRNAs were significantly differentially expressed among HF patients experiencing primary endpoint (HF‐PE) and those without clinical events (HF‐noPE, Figure 3 A ). In particular, miR‐125a‐5p and miR‐10b‐5p were significantly down‐regulated (log2 fold change = −0.9 and −1.8, respectively; adjusted P‐value = 0.04 for both), while miR‐9‐5p was significantly up‐regulated (log2 fold change = 1; adjusted P‐value = 0.04; Figure 3 A ).
Figure 3.
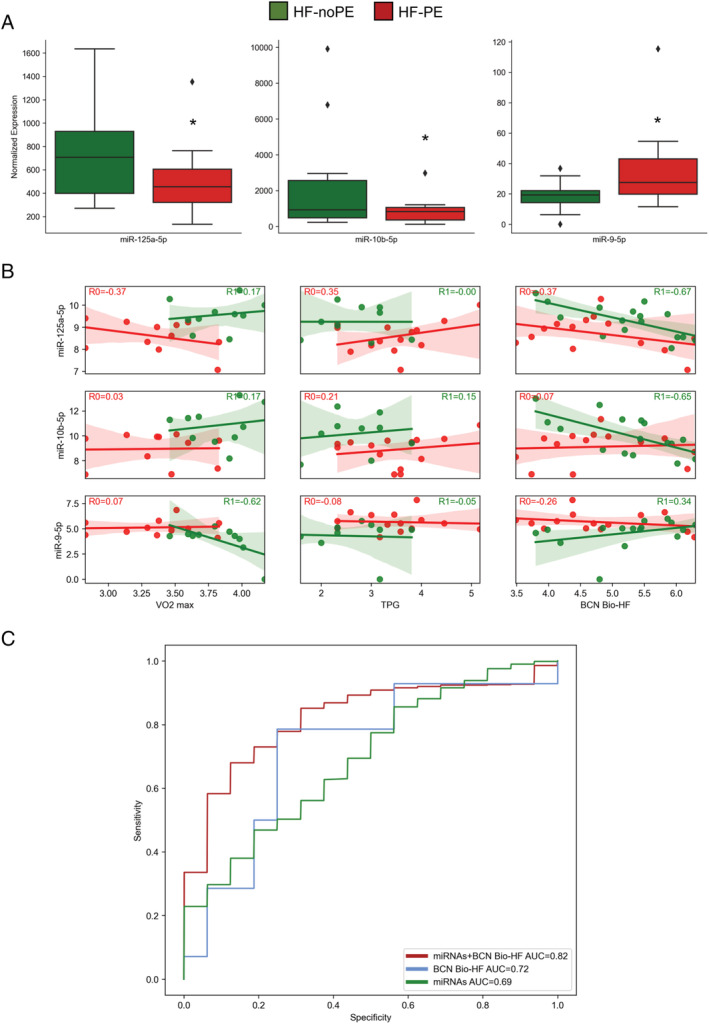
Circulating microRNAs (miRNAs) related to clinical outcomes. (A) miRNAs differentially expressed among heart failure (HF) patients experiencing primary endpoint (HF‐PE, red) and those without clinical events (HF‐noPE, green). *Adjusted P‐value = 0.04. ♦Outlier sample values. (B) Correlation levels of the three differentially expressed miRNAs in HF‐PE (red) vs. HF‐noPE (green) with clinical variable significant predictive markers (Table 2 ). miRNA expression and clinical variable values are reported as base 2 logarithms. The correlation coefficients are shown (R). (C) Receiver operating characteristic curves built for the three differentially expressed miRNAs among HF‐PE and HF‐noPE groups (green), Barcelona (BCN) Bio‐HF score (blue), and three miRNAs + BCN Bio‐HF score (red). The value of area under the curve (AUC) was reported. TPG, trans‐pulmonary gradient.
Therefore, we compared the levels of these three miRNAs with clinical variable significant predictive markers. As reported in Figure 3 B , in the group of HF‐noPE patients, miR‐9‐5p showed the best negative correlation with VO2 max (R = −0.62, Figure 3 B ). Interestingly, in the HF‐PE group, miR‐125a‐5p positively correlated with the TPG haemodynamic variable (R = 0.35, Figure 3 B ). In the HF‐noPE group, all the three miRNAs also correlated with BCN Bio‐HF score (Figure 3 B ).
The final objective of our work was to evaluate if some miRNAs could eventually improve a prognostic model solely based on clinical and laboratory values in advanced HF patients: we therefore tested the additive power of these three selected miRNAs to the variables showing the best AUC for endpoint prediction. Interestingly, while a moderate prognostic power characterized the model including all three miRNAs (AUC = 0.69, Figure 3 C ), their addiction to the model including BCN Bio‐HF (AUC = 0.82; Figure 3 C ), VO2 max (AUC = 0.90; Supporting Information, Figure S5A ), or TPG (AUC = 0.83; Supporting Information, Figure S5B ) remarkably increased the AUC: miRNAs eventually improved the prognostic stratification of advanced HF.
Discussion
To the best of our knowledge, this is the first study to characterize the complete miRNA expression profile through the NGS technology in a highly selected population of patients with advanced HF. Our main findings are the following: (i) on the global miRNome analysis, a unique profile of 32 miRNAs differentially expressed between advanced HF patients and controls; (ii) six miRNAs significantly correlated with HF markers; and (iii) among the advanced HF population, three miRNAs were significantly differentially expressed and correlated with the composite endpoint of cardiac death, heart transplantation or mechanical circulatory support implantation and implemented the prognostic prediction model solely based on clinical values.
In the puzzling setting of advanced HF, miRNAs still represent a mostly undiscovered chapter as compared with other diseases in which genomic medicine is confirming its promising perspectives. In particular, the idea of a serological parameter with a role in the gene regulation at a post‐transcriptional level and that could implement prognostic prediction in HF makes these non‐coding molecules exceptionally appealing. This is especially important when considering the relatively low prognostic power of the available markers and scores and the low availability, high costs, and complication burden of non‐pharmacological interventions, as mentioned earlier.
Our research relied on NGS application, a powerful forefront technique able to evaluate the entire miRNome with a hypothesis‐free approach. The majority of previous studies, performed in acute and chronic HF patients, used a candidate approach considering a limited and predefined number of miRNAs. 11 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 In contrast, only few papers exploited the NGS approach to determine the abundance of myocardial and circulating miRNAs in HF patients before and after LVAD implantation. 29 , 30 , 31
In the present study, we carefully selected an advanced HF population, as proven by clinical, echocardiographic, and laboratory parameters and by the high adverse event rate at 12 month follow‐up. In this population, we obtained an HF‐specific panel of 32 differentially expressed miRNAs in comparison with matched controls. Some (14) of these dysregulated miRNAs have already been described in HF or in other cardiovascular diseases. Five miRNAs (miR‐210‐3p, miR‐22‐5p, miR‐22‐3p, miR‐21‐3p, and miR‐339‐3p) were positively correlated to NT‐proBNP levels, and miR‐125a‐5p was negatively correlated. These miRNAs also correlated with at least one HF biomarker among copeptin, sST2, and Hs‐cTnT. As expected, though, they did not show a higher diagnostic force than those of biomarkers themselves, probably because the end‐stage HF disease involves multiple organs and is characterized by high inflammatory state, and miRNAs are expected to reflect more than the original cardiac pathology. While the aim of our work is not to improve diagnostics in HF, we intend to describe a correlation of the miRNA expression profile with the commonly available markers.
The major hypoxia‐inducible miR‐210 32 is dysregulated in several cardiovascular diseases, among which HF and myocardial infarction, 33 , 34 while up‐regulated levels of this miRNA have been found in patients with stable and end‐stage HF. 29 Intramyocardial injection of miR‐210 carrying vector during myocardial infarction improved cardiac function, reducing the infarct size and promoting angiogenesis, 35 therefore suggesting its association with a repair response to organ stress.
MiR‐21 was found dysregulated in different cardiovascular diseases, among which HF, cardiac hypertrophy, and myocardial ischaemia. 36 , 37 , 38 This important miRNA may attenuate HF damage by promoting angiogenesis and reducing fibrosis and apoptosis after myocardial infarction. 39 However, its role in the cardiac fibrotic process is controversial, because the majority of scientific results describe a pro‐fibrotic action in the infarcted heart tissue 38 and high levels have been found in the fibroblasts 40 and macrophages 41 of failing hearts.
Even if miR‐22, differently from miR‐210 and miR‐21, has been studied mainly in the cancer field, 42 it may be associated with regulation of cardiac remodelling 43 and has been recently reported as one of the most abundant miRNA in the heart, with higher levels in acute myocardial infarction and in stable chronic HF. 28 , 44 For these characteristics, it has been proposed as candidate diagnostic marker. 45
Conversely, miR‐339 has not been implicated in cardiac pathophysiology so far, only sparse data showed an up‐regulation of this miRNA after cardiac surgery with cardiopulmonary bypass and cardioplegic arrest 46 and in cardiac amyloidosis. 47
The role of miR‐125a‐5p in the cardiovascular system is poorly known, it has been proposed to differentiate HF patients with reduced left ventricular EF from those with preserved EF. 48
It is worthy to note that the muscle‐related myomiRs, such as miR‐499 and miR‐208, did not contribute to the HF signature of our patients. In the present study, these two specific miRNAs were not included in the count matrix because not expressed, whereas miR‐1 and miR‐133 did not show differences in the expression levels between HF patients and controls. These results are consistent with previous reports showing that myomiRs are biomarkers of the acute phase of the disease, returning to the baseline expression levels in the chronic and stable period. 29 , 49 Overall, a better understanding of the role of these miRNAs in HF may highlight molecular and cellular mechanisms of the cardiovascular disease and allow to improve HF patients' management and therapies.
Concerning prognostication in our population, commonly available markers mostly did not help in the prognostic stratification; the parameters with the strongest predictive capacities were the VO2 max and TPG (AUC = 0.88 and 0.79, respectively). Noteworthy, also the validated BCN Bio‐HF score did not perform well (AUC = 0.72). The three differentially expressed miRNAs in the HF‐PE group (miR‐125a‐5p, miR‐10b‐5p, and miR‐9‐5p) added substantial prognostic power to the BCN Bio‐HF score. Little is known about the cardiovascular function of miR‐10b‐5p and miR‐9‐5p. On the other hand, miR‐125a‐5p has been found down‐regulated in HF patients vs. controls showing a potential role as HF biomarker. Interestingly, the three miRNAs are involved in the cardiac apoptotic process: miR‐125a‐3p has been associated with cell survival stimulation and apoptosis inhibition in cardiac myocytes. 50 miR‐10b‐5p was found to improve cardiac function in a murine model of myocardial infarction and exerted anti‐apoptotic function in cardiomyocytes in vitro. 51 Finally, for miR‐9, the role on apoptosis is controversial: the knockdown of this miRNA led to inhibition of hypoxia‐induced cardiomyocyte apoptosis, 52 but a recent work suggested its anti‐apoptotic function during myocardial infarction progression. 53 Overall, comparing our miRNA expression results with published data, we may speculate that they are involved in the worsening of HF progression through an effect on apoptosis and therefore play a prognostic role.
The main limitation of our paper is undoubtedly the small number of patients enrolled: this may lead to an overestimation of the role of some miRNAs and conversely to an underestimation of others, whose significance could have been blunted or enhanced in a larger sample size. For example, miR‐423‐5p, whose dysregulation has been previously associated with poor long‐term outcome in acute HF patients, 25 was found to be also down‐regulated in our HF‐PE group in comparison with HF‐noPE patients (fold change = −1, data not shown), but only with nominal significance (nominal P‐value = 0.003; adjusted P‐value = 0.06). Notwithstanding, this was conceived as a pilot ‘discovery’ study, it is the first of its kind, and other analogous works not investigating the whole miRNome enrolled a similar number of patients. Moreover, the authors faced the difficult task of standardizing the timing of sampling in phases of relative clinical stability of the complex setting of advanced HF.
Another limitation of the study is the lack of external validation of our predictive model. Even if the internal validation (10‐fold cross‐validation repeated with different random seeds 50 times) strengthens our results, further studies should investigate the role of our predictive model in an external cohort of selected advanced HF patients.
It could also be argued that the Seattle Heart Failure Model or Heart Failure Survival Score could have been used instead of the BCN Bio‐HF and may have produced higher AUC for prognostic prediction. However, BCN Bio‐HF is one of the scores recommended by the European Society of Cardiology and has been proven to outperform other risk models. Moreover, not all HF patients may be able to perform a cardiopulmonary exercise test, as was the case of 30% of our population, and this percentage may be higher in a real‐world setting.
We also acknowledge the low percentage of female included in our study: though in line with other registries and clinical trials of HF patients, 54 this may limit the generalizability of our results.
In conclusion, in this discovery study on patients with advanced HF, we sequenced the plasma miRNome and identified differentially expressed miRNAs between cases and controls with some miRNAs also altered between patients with and without adverse events at follow‐up. A prediction model including three of these miRNAs improved the prognostic stratification based on standard clinical and laboratory values. Further studies are needed to validate and confirm our results in larger populations.
Conflict of interest
None declared.
Funding
This work was supported by ‘Compagnia di San Paolo’ (ID ROL 17290).
Supporting information
Figure S1. Workflow of the study.
Figure S2. Receiver operating characteristic (ROC) curves built for the main clinical and laboratory variables that resulted differentially distributed among patients encountering primary endpoint (HF‐PE group) and those without events (HF‐noPE group). TPG:trans‐pulmonary gradient. The values of area under the curve (AUC) were reported.
Figure S3. (A) Volcano Plot reporting all miRNAs differentially expressed between cases and controls (red: up‐regulated, green: down‐regulated). The six miRNAs better correlated with NT‐proBNP are reported. (B) Heatmap reporting the six miRNAs better correlated with NT‐proBNP and significantly differentially expressed between cases (HF,red) and controls (CTL,blue).
Figure S4. Receiver operating characteristic (ROC) curves built for HF biomarkers (NT‐proBNP, Hs‐cTNT, Copeptin, and sST2) that resulted differentially distributed among cases and controls, for 32 differentially expressed miRNAs between cases and controls, and 6 miRNAs better correlated with NT‐proBNP and significantly differentially expressed between cases and controls. The values of area under the curve (AUC) were reported.
Figure S5. Receiver operating characteristic (ROC) curves built for the differentially expressed miRNAs among patients encountering primary endpoint (HF‐PE group) and those without events (HF‐noPE group), the 3 differentially expressed miRNAs alone or together with (A) VO2 max or (B) TPG. TPG: trans‐pulmonary gradient. The values of area under the curve (AUC) were reported.
Table S1. MiRNA raw counts metrics stratified for all samples included in the study.
Galluzzo, A. , Gallo, S. , Pardini, B. , Birolo, G. , Fariselli, P. , Boretto, P. , Vitacolonna, A. , Peraldo‐Neia, C. , Spilinga, M. , Volpe, A. , Celentani, D. , Pidello, S. , Bonzano, A. , Matullo, G. , Giustetto, C. , Bergerone, S. , and Crepaldi, T. (2021) Identification of novel circulating microRNAs in advanced heart failure by next‐generation sequencing. ESC Heart Failure, 8: 2907–2919. 10.1002/ehf2.13371.
Alessandro Galluzzo, Simona Gallo and Barbara Pardini contributed equally to the manuscript.
References
- 1. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail 2020; 22: 1342–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lund LH, Stehlik J. Risk scores and biomarkers in heart failure: a journey to predictive accuracy and clinical utility. J Heart Lung Transplant 2016; 35: 711–713. [DOI] [PubMed] [Google Scholar]
- 3. Gustafsson F, Rogers JG. Left ventricular assist device therapy in advanced heart failure: patient selection and outcomes. Eur J Heart Fail 2017; 19: 595–602. [DOI] [PubMed] [Google Scholar]
- 4. Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell 2012; 148: 1172–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sala V, Bergerone S, Gatti S, Gallo S, Ponzetto A, Ponzetto C, Crepaldi T. MicroRNAs in myocardial ischemia: identifying new targets and tools for treating heart disease. New frontiers for miR‐medicine. Cell Mol Life Sci 2014; 71: 1439–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gomes CPC, Schroen B, Kuster GM, Robinson EL, Ford K, Squire IB, Heymans S, Martelli F, Emanueli C, Devaux Y, EU‐CardioRNA COST Action (CA17129) . Regulatory RNAs in heart failure. Circulation 2020; 141: 313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Divakaran V, Mann DL. The emerging role of microRNAs in cardiac remodeling and heart failure. Circ Res 2008; 103: 1072–1U47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Gonzalo‐Calvo D, Vea A, Bär C, Fiedler J, Couch LS, Brotons C, Llorente‐Cortes V, Thum T. Circulating noncoding RNAs in biomarker‐ guided cardiovascular therapy: a novel tool for personalized medicine? Eur Heart J 2019; 40: 1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu X, Wang H, Liu F, Chen L, Luo W, Su P, Li W, Yu L, Yang X, Cai J. Identification of micro‐RNA networks in end‐stage heart failure because of dilated cardiomyopathy. J Cell Mol Med 2013; 17: 1173–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scrutinio D, Conserva F, Passantino A, Iacoviello M, Lagioia R, Gesualdo L. Circulating microRNA‐150‐5p as a novel biomarker for advanced heart failure: a genome‐wide prospective study. J Heart Lung Transplant 2017; 36: 616–624. [DOI] [PubMed] [Google Scholar]
- 11. Masson S, Batkai S, Beermann J, Bar C, Pfanne A, Thum S, Magnoli M, Balconi G, Nicolosi GL, Tavazzi L, Latini R, Thum T. Circulating microRNA‐132 levels improve risk prediction for heart failure hospitalization in patients with chronic heart failure. Eur J Heart Fail 2018; 20: 78–85. [DOI] [PubMed] [Google Scholar]
- 12. Metra M, Ponikowski P, Dickstein K, McMurray JJV, Gavazzi A, Bergh CH, Fraser AG, Jaarsma T, Pitsis A, Mohancsi P, Böhm M, Anker S, Dargie H, Brutsaert D, Komajda M, Heart Failure Association of the European Society of Cardiology . Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2007; 9: 684–694. [DOI] [PubMed] [Google Scholar]
- 13. Crespo‐Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, Gustafsson F, Tsui S, Barge‐Caballero E, De Jonge N, Frigerio M, Hamdan R, Hasin T, Hülsmann M, Nalbantgil S, Potena L, Bauersachs J, Gkouziouta A, Ruhparwar A, Ristic AD, Straburzynska‐Migaj E, McDonagh T, Seferovic P, Ruschitzka F. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2018; 20: 1505–1535. [DOI] [PubMed] [Google Scholar]
- 14. Bayés‐Genís A, Lupón J. The Barcelona Bio‐HF calculator: a contemporary web‐based heart failure risk score. JACC Heart Fail 2018; 6: 808–810. [DOI] [PubMed] [Google Scholar]
- 15. Ferrero G, Cordero F, Tarallo S, Arigoni M, Riccardo F, Gallo G, Ronco G, Allasia M, Kulkarni N, Matullo G, Vineis P, Calogero RA, Pardini B, Naccarati A. Small non‐coding RNA profiling in human biofluids and surrogate tissues from healthy individuals: description of the diverse and most represented species. Oncotarget 2018; 9: 3097–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tarallo S, Ferrero G, Gallo G, Francavilla A, Clerico G, Realis Luc A, Manghi P, Thomas AM, Vineis P, Segata N, Pardini B, Naccarati A, Cordero F. Altered fecal small RNA profiles in colorectal cancer reflect gut microbiome composition in stool samples. mSystems 2019; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pardini B, Cordero F, Naccarati A, Viberti C, Birolo G, Oderda M, Di Gaetano C, Arigoni M, Martina F, Calogero RA, Sacerdote C, Gontero P, Vineis P, Matullo G. microRNA profiles in urine by next‐generation sequencing can stratify bladder cancer subtypes. Oncotarget 2018; 9: 20658–20669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sabo AA, Birolo G, Naccarati A, Dragomir MP, Aneli S, Allione A, Oderda M, Allasia M, Gontero P, Sacerdote C, Vineis P, Matullo G, Pardini B. Small non‐coding RNA profiling in plasma extracellular vesicles of bladder cancer patients by next‐generation sequencing: expression levels of miR‐126‐3p and piR‐5936 increase with higher histologic grades. Cancers (Basel) 2020; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Boven N, Kardys I, van Vark LC, Akkerhuis KM, de Ronde MWJ, Khan MAF, Merkus D, Liu Z, Voors AA, Asselbergs FW, van den Bos EJ, Boersma E, Hillege H, Duncker DJ, Pinto YM, Postmus D. Serially measured circulating microRNAs and adverse clinical outcomes in patients with acute heart failure. Eur J Heart Fail 2018; 20: 89–96. [DOI] [PubMed] [Google Scholar]
- 20. Bayés‐Genis A, Lanfear DE, de Ronde MWJ, Lupón J, Leenders JJ, Liu Z, Zuithoff NPA, Eijkemans MJC, Zamora E, de Antonio M, Zwinderman AH, Pinto‐Sietsma SJ, Pinto YM. Prognostic value of circulating microRNAs on heart failure‐related morbidity and mortality in two large diverse cohorts of general heart failure patients. Eur J Heart Fail 2018; 20: 67–75. [DOI] [PubMed] [Google Scholar]
- 21. Shah R, Ziegler O, Yeri A, Liu X, Murthy V, Rabideau D, Xiao CY, Hanspers K, Belcher A, Tackett M, Rosenzweig A, Pico AR, Januzzi JL, Das S. MicroRNAs associated with reverse left ventricular remodeling in humans identify pathways of heart failure progression. Circ Heart Fail 2018; 11: e004278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naga Prasad SV, Gupta MK, Duan ZH, Surampudi VS, Liu CG, Kotwal A, Moravec CS, Starling RC, Perez DM, Sen S, Wu Q, Plow EF, Karnik S. A unique microRNA profile in end‐stage heart failure indicates alterations in specific cardiovascular signaling networks. PLoS One 2017; 12: e0170456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ovchinnikova ES, Schmitter D, Vegter EL, ter Maaten JM, Valente MA, Liu LC, van der Harst P, Pinto YM, de Boer RA, Meyer S, Teerlink JR, O'Connor CM, Metra M, Davison BA, Bloomfield DM, Cotter G, Cleland JG, Mebazaa A, Laribi S, Givertz MM, Ponikowski P, van der Meer P, van Veldhuisen DJ, Voors AA, Berezikov E. Signature of circulating microRNAs in patients with acute heart failure. Eur J Heart Fail 2016; 18: 414–423. [DOI] [PubMed] [Google Scholar]
- 24. Lok SI, de Jonge N, van Kuik J, van Geffen AJP, Huibers MMH, van der Weide P, Siera E, Winkens B, Doevendans PA, de Weger RA, da Costa Martins PA. MicroRNA expression in myocardial tissue and plasma of patients with end‐stage heart failure during LVAD support: comparison of continuous and pulsatile devices. PLoS One 2015; 10: e0136404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seronde MF, Vausort M, Gayat E, Goretti E, Ng LL, Squire IB, Vodovar N, Sadoune M, Samuel JL, Thum T, Solal AC, Laribi S, Plaisance P, Wagner DR, Mebazaa A, Devaux Y, GREAT network . Circulating microRNAs and outcome in patients with acute heart failure. PLoS One 2015; 10: e0142237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watson CJ, Gupta SK, O'Connell E, Thum S, Glezeva N, Fendrich J, Gallagher J, Ledwidge M, Grote‐Levi L, McDonald K, Thum T. MicroRNA signatures differentiate preserved from reduced ejection fraction heart failure. Eur J Heart Fail 2015; 17: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vogel B, Keller A, Frese KS, Leidinger P, Sedaghat‐Hamedani F, Kayvanpour E, Kloos W, Backe C, Thanaraj A, Brefort T, Beier M, Hardt S, Meese E, Katus HA, Meder B. Multivariate miRNA signatures as biomarkers for non‐ischaemic systolic heart failure. Eur Heart J 2013; 34: 2812–2822. [DOI] [PubMed] [Google Scholar]
- 28. Goren Y, Kushnir M, Zafrir B, Tabak S, Lewis BS, Amir O. Serum levels of microRNAs in patients with heart failure. Eur J Heart Fail 2012; 14: 147–154. [DOI] [PubMed] [Google Scholar]
- 29. Akat KM, Moore‐McGriff D, Morozov P, Brown M, Gogakos T, Correa Da Rosa J, Mihailovic A, Sauer M, Ji R, Ramarathnam A, Totary‐Jain A, Williams Z, Tuschl T, Schulze PC. Comparative RNA‐sequencing analysis of myocardial and circulating small RNAs in human heart failure and their utility as biomarkers. Proc Natl Acad Sci U S A 2014; 111: 11151–11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barsanti C, Trivella MG, D'Aurizio R, El Baroudi M, Baumgart M, Groth M, Caruso R, Verde A, Botta L, Cozzi L, Pitto L. Differential regulation of microRNAs in end‐stage failing hearts is associated with left ventricular assist device unloading. Biomed Res Int 2015; 2015: 592512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang KC, Yamada KA, Patel AY, Topkara VK, George I, Cheema FH, Ewald GA, Mann DL, Nerbonne JM. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation 2014; 129: 1009–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chan YC, Banerjee J, Choi SY, Sen CK. miR‐210: the master hypoxamir. Microcirculation 2012; 19: 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao DS, Chen Y, Jiang H, Lu JP, Zhang G, Geng J, Zhang Q, Shen JH, Zhou X, Zhu W, Shan QJ. Serum miR‐210 and miR‐30a expressions tend to revert to fetal levels in Chinese adult patients with chronic heart failure. Cardiovasc Pathol 2013; 22: 444–450. [DOI] [PubMed] [Google Scholar]
- 34. Guan Y, Song X, Sun W, Wang Y, Liu B. Effect of hypoxia‐induced microRNA‐210 expression on cardiovascular disease and the underlying mechanism. Oxid Med Cell Longev 2019; 2019: 4727283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, Fasanaro P, Sun N, Wang X, Martelli F, Robbins RC, Wu JC. MicroRNA210 as a novel therapy for treatment of ischemic heart disease. Circulation 2010; 122: S124–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheng Y, Zhang C. MicroRNA‐21 in cardiovascular disease. J Cardiovasc Transl Res 2010; 3: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kumarswamy R, Volkmann I, Thum T. Regulation and function of miRNA‐21 in health and disease. RNA Biol 2011; 8: 706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kura B, Kalocayova B, Devaux Y, Bartekova M. Potential clinical implications of miR‐1 and miR‐21 in heart disease and cardioprotection. Int J Mol Sci 2020; 21: 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bejerano T, Etzion S, Elyagon S, Etzion Y, Cohen S. Nanoparticle delivery of miRNA‐21 mimic to cardiac macrophages improves myocardial remodeling after myocardial infarction. Nano Lett 2018; 18: 5885–5891. [DOI] [PubMed] [Google Scholar]
- 40. Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JTR, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA‐21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008; 456: 980–984. [DOI] [PubMed] [Google Scholar]
- 41. Hinkel R, Ramanujam D, Kaczmarek V, Howe A, Klett K, Beck C, Dueck A, Thum T, Laugwitz KL, Maegdefessel L, Weber C, Kupatt C, Engelhardt S. AntimiR‐21 prevents myocardial dysfunction in a pig model of ischemia/reperfusion injury. J Am Coll Cardiol 2020; 75: 1788–1800. [DOI] [PubMed] [Google Scholar]
- 42. Xu D, Takeshita F, Hino Y, Fukunaga S, Kudo Y, Tamaki A, Matsunaga J, Takahashi RU, Takata T, Shimamoto A, Ochiya T, Tahara H. miR‐22 represses cancer progression by inducing cellular senescence. J Cell Biol 2011; 193: 409–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang ZP, Wang DZ. miR‐22 in cardiac remodeling and disease. Trends Cardiovasc Med 2014; 24: 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li H, Zhang P, Li F, Yuan G, Wang X, Zhang A, Li F. Plasma miR‐22‐5p, miR‐132‐5p, and miR‐150‐3p are associated with acute myocardial infarction. Biomed Res Int 2019; 2019: 5012648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang YM, Li WW, Wu J, Han M, Li BH. The diagnostic value of circulating microRNAs in heart failure. Exp Ther Med 2019; 17: 1985–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Saddic LA, Chang TW, Sigurdsson MI, Heydarpour M, Raby BA, Shernan SK, Aranki SF, Body SC, Muehlschlegel JD. Integrated microRNA and mRNA responses to acute human left ventricular ischemia. Physiol Genomics 2015; 47: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Derda AA, Pfanne A, Bär C, Schimmel K, Kennel PJ, Xiao K, Schulze PC, Bauersachs J, Thum T. Blood‐based microRNA profiling in patients with cardiac amyloidosis. PLoS One 2018; 13: e0204235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wong LL, Armugam A, Sepramaniam S, Karolina DS, Lim KY, Lim JY, Chong JPC, Ng JYX, Chen JT, Chan MMY, Chen Z, Yeo PSD, Ng TP, Ling LH, Sim D, Leong KTG, Ong HY, Jaufeerally F, Wong R, Chai P, Low AF, Lam CSP, Jeyaseelan K, Richards AM. Circulating microRNAs in heart failure with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail 2015; 17: 393–404. [DOI] [PubMed] [Google Scholar]
- 49. Weiland M, Gao XH, Zhou L, Mi QS. Small RNAs have a large impact: circulating microRNAs as biomarkers for human diseases. RNA Biol 2012; 9: 850–859. [DOI] [PubMed] [Google Scholar]
- 50. Díaz I, Calderón‐Sánchez E, del Toro R, Ávila‐Médina J, Sánchez de Rojas‐de Pedro E, Domínguez‐Rodríguez A, Rosado JA, Hmadcha A, Ordóñez A, Smani T. miR‐125a, miR‐139 and miR‐324 contribute to Urocortin protection against myocardial ischemia‐reperfusion injury. Sci Rep 2017; 7: 8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wu L, Chen Y, Chen Y, Yang W, Han Y, Lu L, Yang K, Cao J. Effect of HIF‐1α/miR‐10b‐5p/PTEN on hypoxia‐induced cardiomyocyte apoptosis. J Am Heart Assoc 2019; 8: e011948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zheng J, Peng B, Zhang Y, Ai F, Hu X. miR‐9 knockdown inhibits hypoxia‐induced cardiomyocyte apoptosis by targeting Yap1. Life Sci 2019; 219: 129–135. [DOI] [PubMed] [Google Scholar]
- 53. Yang D, Yu J, Liu HB, Yan XQ, Hu J, Yu Y, Guo J, Yuan Y, Du ZM. The long non‐coding RNA TUG1‐miR‐9a‐5p axis contributes to ischemic injuries by promoting cardiomyocyte apoptosis via targeting KLF5. Cell Death Dis 2019; 10: 908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lainščak M, Milinković I, Polovina M, Crespo‐Leiro MG, Lund LH, Anker SD, Laroche C, Ferrari R, Coats AJS, McDonagh T, Filippatos G, Maggioni AP, Piepoli MF, Rosano GMC, Ruschitzka F, Simić D, Ašanin M, Eicher JC, Yilmaz MB, Seferović PM, European Society of Cardiology Heart Failure Long‐Term Registry Investigators Group . Sex‐ and age‐related differences in the management and outcomes of chronic heart failure: an analysis of patients from the ESC HFA EORP Heart Failure Long‐Term Registry. Eur J Heart Fail 2020; 22: 92–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Workflow of the study.
Figure S2. Receiver operating characteristic (ROC) curves built for the main clinical and laboratory variables that resulted differentially distributed among patients encountering primary endpoint (HF‐PE group) and those without events (HF‐noPE group). TPG:trans‐pulmonary gradient. The values of area under the curve (AUC) were reported.
Figure S3. (A) Volcano Plot reporting all miRNAs differentially expressed between cases and controls (red: up‐regulated, green: down‐regulated). The six miRNAs better correlated with NT‐proBNP are reported. (B) Heatmap reporting the six miRNAs better correlated with NT‐proBNP and significantly differentially expressed between cases (HF,red) and controls (CTL,blue).
Figure S4. Receiver operating characteristic (ROC) curves built for HF biomarkers (NT‐proBNP, Hs‐cTNT, Copeptin, and sST2) that resulted differentially distributed among cases and controls, for 32 differentially expressed miRNAs between cases and controls, and 6 miRNAs better correlated with NT‐proBNP and significantly differentially expressed between cases and controls. The values of area under the curve (AUC) were reported.
Figure S5. Receiver operating characteristic (ROC) curves built for the differentially expressed miRNAs among patients encountering primary endpoint (HF‐PE group) and those without events (HF‐noPE group), the 3 differentially expressed miRNAs alone or together with (A) VO2 max or (B) TPG. TPG: trans‐pulmonary gradient. The values of area under the curve (AUC) were reported.
Table S1. MiRNA raw counts metrics stratified for all samples included in the study.


