Abstract
Aims
Despite substantial improvements over the last three decades, heart failure (HF) remains associated with a poor prognosis. The sodium‐glucose co‐transporter‐2 inhibitor empagliflozin demonstrated significant reductions of HF hospitalization in patients with HF independent of the presence or absence of type 2 diabetes mellitus in the EMPEROR‐Reduced trial and cardiovascular mortality in the EMPA‐REG OUTCOME trial. To further elucidate the mechanisms behind these positive outcomes, this study aims to determine the effects of empagliflozin treatment on cardiac energy metabolism and physiology using magnetic resonance spectroscopy (MRS) and cardiovascular magnetic resonance (CMR).
Methods and results
The EMPA‐VISION trial is a double‐blind, randomized, placebo‐controlled, mechanistic study. A maximum of 86 patients with HF with reduced ejection fraction (n = 43, Cohort A) or preserved ejection fraction (n = 43, Cohort B), with or without type 2 diabetes mellitus, will be enrolled. Participants will be randomized 1:1 to receive either 10 mg of empagliflozin or placebo for 12 weeks. Eligible patients will undergo cardiovascular magnetic resonance, resting and dobutamine stress MRS, echocardiograms, cardiopulmonary exercise tests, serum metabolomics, and quality of life questionnaires at baseline and after 12 weeks. The primary endpoint will be the change in resting phosphocreatine‐to‐adenosine triphosphate ratio, as measured by 31Phosphorus‐MRS.
Conclusions
EMPA‐VISION is the first clinical trial assessing the effects of empagliflozin treatment on cardiac energy metabolism in human subjects in vivo. The results will shed light on the mechanistic action of empagliflozin in patients with HF and help to explain the results of the safety and efficacy outcome trials (EMPEROR‐Reduced and EMPEROR‐Preserved).
Keywords: Heart failure, Diabetes, SGLT2 inhibitors, 31P‐MRS, Trial design, Empagliflozin
Introduction
Chronic heart failure (HF) is a progressive syndrome caused by an imbalance of oxygen supply provided by the heart and metabolic demand by various tissues. Its symptoms and multi‐organ adverse effects lead to high morbidity, poor quality of life (QoL), poor survival rates, and significant economic strain on health care systems worldwide. 1 , 2 With estimates of over 60 million people affected globally, this epidemic is predicted to substantially increase over the next decade. 3 The global socio‐economic burden of HF comprises an annual cost of $108 billion and accounts for approximately 2% of total health care expenditure. 4
Heart failure phenotyping is based on echocardiographic measurement of left ventricular ejection fraction (LVEF) and categorizes the syndrome into two main groups: HF with reduced ejection fraction (HFrEF; LVEF < 40%) and preserved ejection fraction (HFpEF; LVEF ≥ 50%). 2 HFrEF and HFpEF have approximately equal prevalence and 5 year mortality rates. 5 HFpEF is more prevalent in older, female patients and shows a steep increase with advancing age, whereas HFrEF plateaus with advancing age and is more frequently seen in male patients. 6
In HFrEF, morbidity and mortality remain high despite the success of therapeutic interventions. 7 Given the ageing population, the number of patients with HFpEF is constantly rising and frequency of hospital admissions is comparable with those with HFrEF. 8 Even more concerning, the mainstay of treatment for HFpEF is symptom relief, while no outcome‐modifying treatment is available, a fact underscoring the unmet therapeutic need in this group.
Up to 40% of patients with HF have concomitant type 2 diabetes mellitus (T2DM), and a similar proportion presents with impaired glucose tolerance, often termed ‘pre‐diabetes’, both of which increase mortality. 9 Interestingly, even in the absence of diabetes, insulin sensitivity decreases as HF progresses, indicating a possible link between HF and gluco‐metabolic disturbances. 10
Empagliflozin is a selective sodium‐glucose co‐transporter‐2 inhibitor (SGLT2‐i) licensed for treating patients with T2DM by promoting urinary glucose excretion. 11 In EMPA‐REG OUTCOME, treatment with empagliflozin reduced the risk of death from cardiovascular (CV) causes by 38% and HF hospitalization by 35%, in patients with T2DM and established CV disease. 12 In the trial, the reduction in the composite primary outcome (3‐point major adverse CV events) was specifically driven by a reduction of endpoints associated with the progression of HF.
Dedicated outcome trials in patients with HFrEF confirmed benefits on HF outcomes for empagliflozin, 13 dapagliflozin, 14 and recently, sotagliflozin. 15 Subgroup analyses indicate similar benefits for canagliflozin 16 and ertugliflozin 17 ; hence, salutary effects in HFrEF should be considered a class effect for SGLT2‐is. More importantly, these effects are consistently observed irrespective of the presence or absence of T2DM. 18 Demonstrating a consistent reduction in CV mortality in HFrEF might require larger sample sizes as this effect was not present in the respective trials but a pooled meta‐analysis of EMPEROR‐Reduced and DAPA‐HF did indeed demonstrate significance. 19
Recent imaging trials using CV magnetic resonance (CMR) to measure treatment effects of empagliflozin in HFrEF have reported positive effects on markers of cardiac remodelling. 20 , 21 Nevertheless, to the best of our knowledge, no study has assessed effects on cardiac energy metabolism in patients with HF and none of the previous studies evaluated the impact of empagliflozin in HFrEF and HFpEF separately.
Heart failure is associated with altered myocardial substrate metabolism and impaired myocardial energy production; hence, modulating cardiac energy metabolism may denote a promising future approach to treat HF. 22 , 23 The creatine kinase reaction delivers adenosine triphosphate (ATP) via phosphocreatine (PCr) from the mitochondria to the myofibrils in cardiomyocytes. In chronic HF, ATP demand outweighs its synthesis and PCr levels fall while ATP is reduced to a lesser extent. This results in a detectable depletion of the ratio of concentrations of PCr relative to ATP (PCr/ATP), which reflects the severity of energy starvation in the failing heart. 24
The current study will evaluate whether treatment with empagliflozin leads to a measurable difference in PCr/ATP and thus to an increase in myocardial energy supply, which could improve resting and stress cardiac energetics, cardiac function, and exercise capacity. We aim to elucidate whether empagliflozin modulates cardiac energy metabolism, which in turn could contribute to the favourable effects observed in patients with HF.
Study design
Study design and study population
EMPA‐VISION is a randomized, double‐blind, controlled trial (Figure 1 and Table 1 for trial design and eligibility criteria, respectively) comparing the effects of empagliflozin and placebo on cardiac physiology and energy metabolism. Participants will be recruited into two cohorts consisting of up to 43 patients with HFrEF (Cohort A) and 43 patients with HFpEF (Cohort B), and, allowing for a maximum of 30% withdrawal rate, the minimum sample size will be 30 evaluable datasets per cohort. Participants will be randomized 1:1 via an interactive response technology system to receive either placebo or 10 mg of empagliflozin. Cardiac energy metabolism, cardiac structure, volumes and function as well as various biomarkers will be assessed before and after 12 weeks of treatment with empagliflozin.
Figure 1.
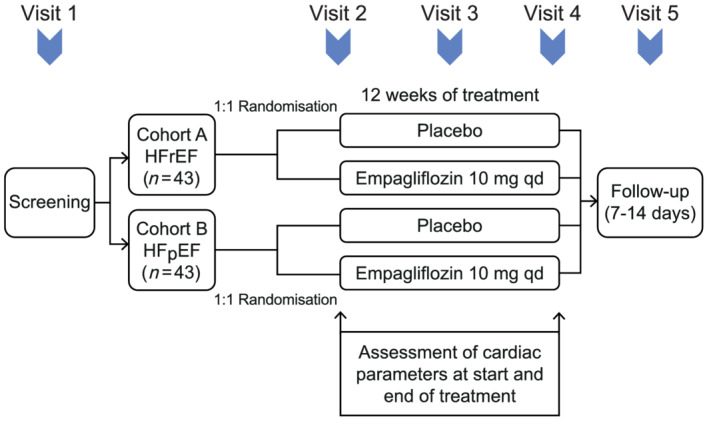
Schematic overview of the EMPA‐VISION study design. After screening (Visit 1; Day 0) and randomization, participants will be invited for dedicated assessment before randomization and treatment (Visit 2; Day 1). A safety assessment will be conducted after 2 weeks of treatment (Visit 3; Day 15 ± 1). Following treatment for 12 weeks, assessment will be repeated as described before (Visit 4; Day 84 ± 4). A final follow‐up will be carried out via telephone (Visit 5; Day 91 ± 7). HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Table 1.
Simplified overview of the inclusion (including specific criteria for each of the two cohorts) and exclusion criteria of the EMPA‐VISION trial
| Inclusion criteria | |
|---|---|
|
• CHF ≥ 3 months • NYHA II–IV at screening • BMI < 40 kg/m2 • Age ≥ 18 years • Written informed consent |
| Cohort A (HFrEF) | Cohort B (HFpEF) |
|---|---|
|
• LVEF < 40% (measured by echocardiogram) • NT‐proBNP (>125 pg/mL) no AF • NT‐proBNP (>600 pg/mL) if diagnosed AF • Optimal medical therapy |
• LVEF ≥ 50% • NT‐proBNP (>125 pg/mL) if free from AF • NT‐proBNP (>600 pg/mL) if diagnosed AF • LAVI > 34 mL/m2 • LVMI > 115 g/m2 (male) or >95 g/m2 (female) • Stable dose of oral diuretics > 1 week |
|
Exclusion criteria |
|---|
|
• Stroke or TIA < 6 months • Scars or non‐viable myocardium in the interventricular septum, unstable angina pectoris due to CAD, and major CV surgery (investigator opinion) • Any contraindication for CMR, CPET, and dobutamine stress test (including LVAD, ICD, CRT, or any cardiac device) • Heart transplant recipient or listed for heart transplant • Cardiomyopathy based on infiltrative diseases (e.g. amyloidosis), accumulation diseases (e.g. haemochromatosis and Fabry's disease), muscular dystrophies, cardiomyopathy with reversible causes (e.g. stress cardiomyopathy), hypertrophic obstructive cardiomyopathy, or known pericardial constriction • Moderate to severe uncorrected primary valvular heart disease • Acute decompensated HF (exacerbation of CHF) requiring i.v. diuretics, i.v. inotropes, or i.v. vasodilators, or LVAD or hospitalization • SBP ≥ 180 mmHg at screening. If SBP > 150 and <180 mmHg at screening on antihypertensive triple therapy • Symptomatic hypotension and/or an SBP < 100 mmHg at screening • Uncontrolled AF • Untreated ventricular arrhythmia with syncope documented within the 3 months prior to informed consent in patients without ICD • Diagnosis of cardiomyopathy induced by chemotherapy or peripartum <12 months prior to informed consent • Symptomatic bradycardia or second‐degree or third‐degree heart block in need of a pacemaker after adjusting beta‐blocker therapy or any other negative inotropic agents • Significant chronic pulmonary disease • Indication of liver disease, defined by serum levels of ALT, AST, or AP above 3 × upper limit of normal • Impaired renal function (creatinine clearance < 30 mL/min and/or dialysis) • Haemoglobin < 10 g/dL • T1DM • History of ketoacidosis • Major surgery <3 months prior or scheduled within trial • GI surgery or significant GI disorder • Active or suspected malignancy or history of malignancy within 2 years prior to informed consent • Any other disease than HF with a life expectancy of <1 year • Any drug considered likely to interfere with the safe conduct of the trial • Requirement for treatment with empagliflozin • Treatment with any SGLT2‐i or combined SGLT1‐i and SGLT2‐i • Currently enrolled in another investigational device or drug study, or <30 days between randomization and ending the other investigational trial • Known allergy or hypersensitivity to empagliflozin or other SGLT2‐is • Chronic alcohol or drug abuse • Women who are pregnant, breastfeeding, or who plan to become pregnant while in the trial |
AF, atrial fibrillation; ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; BMI, body mass index; CAD, coronary artery disease; CHF, chronic heart failure; CMR, cardiovascular magnetic resonance; CPET, cardiopulmonary exercise testing; CRT, cardiac resynchronization therapy; CV, cardiovascular; GI, gastrointestinal; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ICD, implantable cardioverter defibrillator; i.v., intravenous; LAVI, left atrial volume index; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure; SGLT1‐i, sodium‐glucose co‐transporter‐1 inhibitor; SGLT2‐i, sodium‐glucose co‐transporter‐2 inhibitor; T1DM, type 1 diabetes mellitus; TIA, transitory ischaemic attack.
Study visits
Figure 1 provides a schematic overview of the EMPA‐VISION study visit schedule.
Visit 1—screening visit (Day 0)
After patients have provided informed consent, assessments are conducted at this visit to determine eligibility. These include echocardiogram (Echo), serum N‐terminal pro‐B‐type natriuretic peptide levels, multi‐slice coronary computed tomography angiography, a detailed physical exam, 12‐lead electrocardiogram, New York Heart Association classification, and blood sampling. Women of childbearing potential (WOCBP) undergo a pregnancy test, and pregnant women will be excluded from the trial. Eligible patients will proceed to Visit 2.
Visit 2—randomization and cardiovascular magnetic resonance assessment (Day 1)
Participants are required to fast in preparation for the blood sampling and magnetic resonance spectroscopy (MRS) at this visit (no food or liquids except water for at least 6 h prior to sampling). Following confirmation of eligibility, participants will be randomized and allocated a treatment kit. Subsequently, Kansas City Cardiomyopathy Questionnaire (KCCQ) and five‐level EuroQol‐5D, blood sampling for various biomarkers and safety parameters, Echo, electrocardiogram, and body weight measurement will be repeated. Cardiopulmonary exercise testing (CPET) will be performed according to current recommendations. 25 Furthermore, participants will perform a 6 min walking test (6MWT) according to the guidelines of the conducting centre. CMR including MRS in resting state and with dobutamine stress will be carried out as outlined in Figure 2. Patients will start the study medication after all baseline results are obtained. WOCBP undergo repeated pregnancy testing on site to ensure compliance with safety recommendations before starting the medication.
Figure 2.
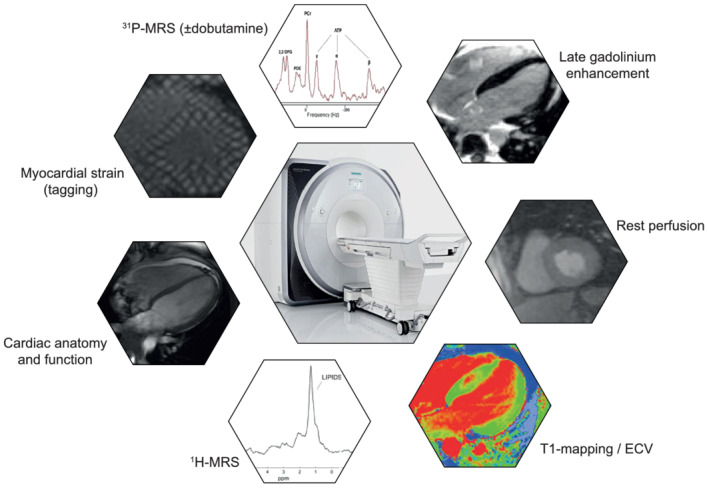
Overview of the cardiovascular magnetic resonance (CMR) techniques used in the EMPA‐VISION trial. All CMR sequences will be performed at a field strength of 3 Tesla (Siemens Healthineers, Erlangen, Germany). The CMR protocol is estimated to last approximately 2 h in total, split in two 1 h slots. After resting spectroscopy, dobutamine will be infused to target 65% of age maximal heart rate and stress spectroscopy will be acquired. 1H‐MRS, proton magnetic resonance spectroscopy; 31P‐MRS, phosphorus magnetic resonance spectroscopy; ECV, extracellular volume.
Visit 3—safety and compliance assessment (Day 15 ± 1)
The purpose of Visit 3 is to evaluate the participant's safety and confirm treatment adherence. The safety assessments include an Echo, urine testing for ketone body levels, body weight, vital signs, and a pregnancy test in WOCBP.
Visit 4—cardiovascular magnetic resonance assessment and end of treatment (Day 84 ± 4)
Representing the end of treatment, the assessments performed at Visit 2 are repeated following an overnight fast. All of the assessments must be performed within 48 h after participants have taken the last dose of their study medication. The final treatment adherence will be calculated before any excess medication is returned to pharmacy.
Visit 5—study completion (Day 91 ± 7)
Visit 5 marks the completion of the study for each individual participant. It is expected that this safety follow‐up will be performed via telephone although a clinic visit may be scheduled where required.
Cardiovascular magnetic resonance
A schematic overview of EMPA‐VISION's CMR protocol is outlined in Figure 2. All acquisitions will be carried out using a field strength of 3 Tesla (Siemens Healthcare, Erlangen, Germany) under fasting conditions (i.e. absence of food for >6 h). The same MR scanner will be used for Visit 2 and Visit 4 assessments for each patient. Further information on CMR acquisition, details on resting and dobutamine stress MRS, and image and spectral analysis are provided in the Supporting Information section.
Study endpoints
The primary endpoint of the EMPA‐VISION study is the change from baseline to Week 12 in PCr/ATP in the resting state measured by 31P‐MRS. An important exploratory endpoint is the difference from baseline to Week 12 in PCr/ATP under dobutamine stress, which may be more effective in identifying the mechanisms responsible for CV event prevention with empagliflozin. Other exploratory endpoints include changes in functional capacity (CPET and 6MWT), QoL questionnaires, cardiac structure and function, myocardial fat content, native T1‐mapping and extracellular volume, left atrial volume and emptying function, and metabolomics and biomarker assessments.
Safety
There are no safety endpoints defined for this trial. However, starting after obtaining written informed consent, clinical and laboratory testing‐related adverse events (AEs) will be collected and coded using the Medical Dictionary for Drug Regulatory Activities, until a period of 7 days after the last dose of trial medication (residual effect period). All serious AEs and AEs of special interest will be assessed and promptly (within 24 h) reported to the sponsor. In addition, pre‐defined AEs of special interest such as hepatic injury, decrease in renal function, and ketoacidosis will be recorded.
Ethical considerations
The study will be carried out in compliance with the ethical principles laid down in the Declaration of Helsinki, in accordance with the International Committee on Harmonisation's Guideline for Good Clinical Practice. The Clinical Trial Authorisation was obtained from the Medicines and Healthcare products Regulatory Agency, and ethical approval was granted by South Central Oxford C Research Ethics Committee (17/SC0262).
Statistical considerations
To date, no data are available on the impact of SGLT2‐i treatment on PCr/ATP in patients with HF. Hence, sample size calculations in the current trial were based on previous results published by our own group, 26 literature by other groups investigating augmentation of cardiac energy metabolism with perhexiline 27 and trimetazidine, 28 and expert advice. The study is designed to detect a difference for the primary endpoint (PCr/ATP) of 0.3 with a standard deviation of 0.28 achieving a power of 80%. The significance level for the sample size calculation is 0.05 two‐sided for each cohort. Taking into account the calculated effect size of 1.07, each cohort requires a minimum of 30 evaluable participants in order to achieve the aforementioned power level. Given the complex study protocol, we allowed for a maximum of 30% dropout rate, which would enable us to randomize up to 43 patients per cohort (86 patients in total) if needed.
Discussion
EMPA‐VISION is the first randomized trial to assess cardiac energy metabolism in patients with HFrEF and HFpEF treated with empagliflozin.
Why a mechanistic sodium‐glucose co‐transporter‐2 inhibitor trial?
The prime mechanism of action for many established HF medications remains elusive despite clear evidence for their efficacy. More importantly, none of the current pharmacological treatments shown to reduce mortality in HF were specifically developed to treat this devastating syndrome. This is equally true for SGLT2‐i where beneficial effects were initially discovered in subgroup analyses of CV safety trials for T2DM for empagliflozin (EMPA‐REG OUTCOME), dapagliflozin (DECLARE‐TIMI 58), and canagliflozin (CANVAS). 12 , 29 , 30 The precise mechanisms of how SGLT2‐is exert their salubrious effects in patients with HF remain unclear and, even more importantly, might substantially differ in HFrEF and HFpEF, respectively. HF syndromes combine a broad variety of different aetiologies, but current drug treatment does not sufficiently reflect this underlying heterogeneity. 31 Determining a mechanism of action for novel treatments is an important factor in predicting individual treatment response and improving overall treatment compliance. 32 In addition, minimizing the time gap between diagnosis of HF and initiation of outcome‐improving treatment appears of particular importance for SGLT2‐i in the context of HFrEF. 33 Sequaciously, the major strength of EMPA‐VISION is a comprehensive understanding of the effects of empagliflozin treatment on energy metabolism, imaging biomarkers, exercise capacity, and serum metabolomics. Results of this trial will stimulate research to investigate novel biological targets for future HF treatments and may potentially help improve phenotyping of patients with HF by identifying a subset of metabolic responders to SGLT2‐i treatment.
Sodium‐glucose co‐transporter‐2 inhibitors and myocardial energy metabolism
The failing heart has previously been termed an ‘engine out of fuel’, and derangements in cardiac energy metabolism are considered a hallmark of cardiac failure. 23 , 34
Dedicated outcome trials in patients with HFrEF have confirmed a substantial risk reduction of HF events for empagliflozin, dapagliflozin, and sotagliflozin. 13 , 14 , 15 In HFrEF, myocardial PCr/ATP is significantly reduced, correlates with CV mortality, and predicts prognosis more accurately than LVEF. 35 Furthermore, the failing heart's ability to resynthesize ATP under increased workload is significantly blunted. 36
Evidence for salutary effects of SGLT2‐i in patients with HFpEF is limited to smaller sample sizes but has been shown for empagliflozin, canagliflozin, and dapagliflozin. 37 , 38 , 39 Additionally, subgroup analyses of CANVAS (canagliflozin) and DECLARE (dapagliflozin) provide larger‐scale evidence for a reduction of HF events in patients with HFpEF. 40 , 41 Patients with HFpEF exhibit similar reductions in myocardial energetics, and PCr/ATP correlates with the severity of diastolic dysfunction. 42 , 43 In small proof‐of‐principle studies, HF treatment and modulators of cardiac substrate metabolism improve PCr/ATP and cardiac function measurably within weeks, 28 , 44 , 45 findings that have also been reproduced in randomized controlled trials. 46 , 47
Sodium‐glucose co‐transporter‐2 inhibitors have the potential to mitigate these derangements in cardiac energy metabolism, 48 although this might be achieved via different pathways (Figure 3).
Figure 3.
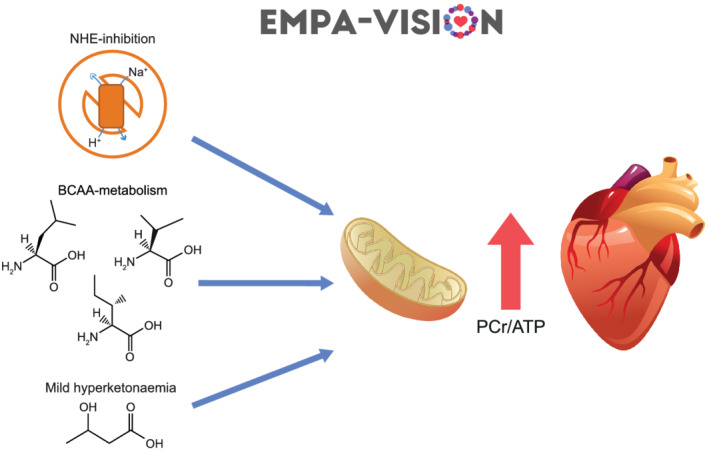
Postulated mechanisms by which empagliflozin might exert beneficial effects on patients with heart failure via manipulation of cardiac energy metabolism. Empagliflozin may enhance oxidative phosphorylation by inhibiting the cardiac isoform of the sodium/hydrogen‐exchanger 1 (NHE 1), promoting branched chain amino acid metabolism, and/or increasing ketone body oxidation. All of these effects would result in a measurable increase in cardiac energy production and storage, which in turn results in an increase in PCr/ATP as well as myocardial function. ATP, adenosine triphosphate; BCAA, branched chain amino acids; PCr, phosphocreatine.
Ketone bodies provide an energy‐efficient fuel source for the failing heart, and the mild hyperketonaemia these drugs induce might thus be a reason for the effects observed with SGLT2‐is. 49 , 50 , 51 Although evidence for this ‘super fuel’ hypothesis is conflicting and mainly generated from animal models, it warrants investigation as ketones have been suggested to preserve cardiac energetics under conditions of metabolic stress. 52 , 53 , 54 Further studies investigating substrate metabolism in patients with HF suggest an increased breakdown of branched chain amino acids after treatment with empagliflozin, which could alleviate cardiac dysfunction. 55 , 56 However, impaired ATP production can also be ascribed to mitochondrial dysfunction, 57 a process that SGLT2‐is seem to alleviate via inhibition of the sodium/hydrogen‐exchanger 1, which in turn would increase overall mitochondrial energy generation. 58
EMPA‐VISION's additional serum metabolomics investigations add strength to our cardio‐metabolic assessments. The metabolome represents the totality of small metabolic intermediates of relevant biochemical pathways and with more than 40 000 molecules characterized already HFrEF and HFpEF each display a unique metabolic fingerprint. 59 , 60 Thus, metabolite biomarkers could assist clinical diagnosis, risk stratification, and identification of novel molecular targets. 61 Not only will our metabolomic assessments help to subtype distinct metabolic profiles in patients with HF at baseline but also clarify whether enhancement of cardiac energy metabolism with SGLT2‐is might be translated differently in HFrEF and HFpEF, respectively.
Regardless of the underlying mechanisms, a sufficient amount of evidence suggests that SGLT2‐is might influence overall cardiomyocyte energy production, although the exact mechanisms remain to be elucidated. If empagliflozin increases myocardial energy production in patients with chronic HF, this should be detected by the comprehensive assessments in the EMPA‐VISION trial (Figure 3).
Sodium‐glucose co‐transporter‐2 inhibitors, exercise physiology, and quality of life
Patients with HF experience a significant symptom burden that frequently leads to substantial reductions in QoL and exercise capacity. 62 Modulating cardiac energy metabolism in HF has been shown to improve exercise capacity. 63 Therefore, it is possible that SGLT2‐i treatment might lead to measurable differences in exercise capacity and perceived QoL. EMPA‐VISION will assess patient‐reported outcomes (KCCQ and five‐level EuroQol‐5D) and exercise capacity by means of CPET and 6MWT distance. Little is known about effects of SGLT2‐is on QoL and exercise capacity in HF. The EMPERIAL trials, investigating effects of empagliflozin treatment on exercise capacity and QoL in HFrEF and HFpEF, did not show a significant increase in 6MWT distance or QoL assessed by KCCQ. 64 Equally, DEFINE‐HF (using dapagliflozin in patients with HFrEF) failed to demonstrate meaningful changes in 6MWT distance despite significantly improving QoL (KCCQ). 65 It is worth noting that 6MWT distance was a secondary outcome in DEFINE‐HF but the ongoing DETERMINE trials (NCT03877224; NCT03877237) will investigate effects of dapagliflozin treatment on 6MWT distance and QoL as co‐primary endpoints in HFrEF and HFpEF. Of note, secondary analyses of EMPEROR‐Reduced and DAPA‐HF suggest a sustained improvement in KCCQ. 66 , 67
The 6 min walking tests entail a few limitations as they are highly effort dependent, are potentially falsely negative due to non‐cardiac reasons (e.g. injuries), and do not correlate specifically well with QoL. 68 A more reliable and accurate test to investigate exercise capacity and cardio‐respiratory fitness is CPET, which correlates well with both QoL and metabolic dysfunction. 69 More importantly, trials using empagliflozin in patients with HFrEF suggest positive effects on cardio‐respiratory fitness determined by CPET. 21 , 70 Thus, although these exercise test results in our EMPA‐VISION trial are considered exploratory, we are confident they will augment our CMR findings and provide deeper understanding on metabolic modulation by SGLT2‐is in HF.
Conclusions
EMPA‐VISION is the first randomized controlled trial to investigate effects of empagliflozin treatment on cardiac energy metabolism and cardiac physiology in patients with HFrEF and HFpEF, with or without T2DM. It is designed to provide a possible explanation for the benefits observed in CV outcome trials with SGLT2‐is such as EMPA‐REG OUTCOME, 12 CANVAS, 29 and DECLARE. 30 In addition, it may provide a mechanism of action for HF outcomes in DAPA‐HF 14 and EMPEROR‐Reduced, 13 the ongoing EMPEROR‐Preserved, 71 and DELIVER (NCT03619213) trials and further insights into results of the EMPERIAL exercise capacity and symptom burden trials. 64 Consequently, our trial may promote development of targeted treatments to modulate high‐energy phosphate metabolism in the failing heart and stimulate further research on SGLT2‐is and cardiac energy metabolism.
Conflict of interest
M.H., M.M., and S.N. are supported by an industrial grant provided by Boehringer Ingelheim. R.G. and H.N. are employees of Boehringer Ingelheim Pharma GmbH & Co. KG. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Funding
Boehringer Ingelheim is the sponsor of the EMPA‐VISION study and was involved in its study design. Boehringer Ingelheim also supported the preparation of this article. S.N. acknowledges support from the Oxford British Heart Foundation (BHF) Centre of Research Excellence. R.R.H. and S.N. were supported by the Oxford National Institute for Health Research (NIHR) Biomedical Research Centre (BRC). C.T.R. and L.V. are funded by a Sir Henry Dale Fellowship from the Wellcome Trust and the Royal Society (098436/Z/12/B and 221805/Z/20/Z, respectively). L.V. also gratefully acknowledges support of the Slovak Grant Agencies VEGA (2/0001/17) and APVV (15‐0029).
Supporting information
Data S1. Supporting information.
Acknowledgements
The authors thank the investigators, the Diabetes Trials Unit Team, specifically Irene Kennedy, Judith DeLos Santos, Catherine Krasopoulos, Marion Galley, and Claudia Nunes of the OCMR Nursing Team, and all patients who participated in this trial. R.R.H. and S.N. are Emeritus National Institute for Health Research Senior Investigators.
Hundertmark, M. J. , Agbaje, O. F. , Coleman, R. , George, J. T. , Grempler, R. , Holman, R. R. , Lamlum, H. , Lee, J. , Milton, J. E. , Niessen, H. G. , Rider, O. , Rodgers, C. T. , Valkovič, L. , Wicks, E. , Mahmod, M. , and Neubauer, S. (2021) Design and rationale of the EMPA‐VISION trial: investigating the metabolic effects of empagliflozin in patients with heart failure. ESC Heart Failure, 8: 2580–2590. 10.1002/ehf2.13406.
Clinical Trial Registration: https://clinicaltrials.gov/ct2/show/NCT03332212, NCT03332212.
References
- 1. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 2. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 3. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017; 3: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol 2014; 171: 368–376. [DOI] [PubMed] [Google Scholar]
- 5. Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA 2006; 296: 2209–2216. [DOI] [PubMed] [Google Scholar]
- 6. Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, van Veldhuisen DJ, van Gilst WH. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community‐based cohort: 11‐year follow‐up of PREVEND. Eur Heart J 2013; 34: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 7. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on E , Prevention Statistics C , Stroke Statistics S . Heart Disease and Stroke Statistics—2019 update: a report from the American Heart Association. Circulation 2019; 139: e56–e528. [DOI] [PubMed] [Google Scholar]
- 8. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC, Get With the Guidelines Scientific Advisory C, Investigators . Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012; 126: 65–75. [DOI] [PubMed] [Google Scholar]
- 9. Goode KM, John J, Rigby AS, Kilpatrick ES, Atkin SL, Bragadeesh T, Clark AL, Cleland JG. Elevated glycated haemoglobin is a strong predictor of mortality in patients with left ventricular systolic dysfunction who are not receiving treatment for diabetes mellitus. Heart 2009; 95: 917–923. [DOI] [PubMed] [Google Scholar]
- 10. Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin 2012; 8: 609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, Bakker RA, Mark M, Klein T, Eickelmann P. Empagliflozin, a novel selective sodium glucose cotransporter‐2 (SGLT‐2) inhibitor: characterisation and comparison with other SGLT‐2 inhibitors. Diabetes Obes Metab 2012; 14: 83–90. [DOI] [PubMed] [Google Scholar]
- 12. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E‐RO. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 13. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Bohm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner‐La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F, Investigators EM‐RT . Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 14. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM, Committees D‐HT, Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381: 1995–2008. [DOI] [PubMed] [Google Scholar]
- 15. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, Lewis JB, Riddle MC, Voors AA, Metra M, Lund LH, Komajda M, Testani JM, Wilcox CS, Ponikowski P, Lopes RD, Verma S, Lapuerta P, Pitt B, Investigators S‐WT. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021; 384: 117–128. [DOI] [PubMed] [Google Scholar]
- 16. Radholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, Fulcher G, Barrett TD, Shaw W, Desai M, Matthews DR, Neal B. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018; 138: 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cosentino F, Cannon CP, Cherney DZI, Masiukiewicz U, Pratley R, Dagogo‐Jack S, Frederich R, Charbonnel B, Mancuso J, Shih WJ, Terra SG, Cater NB, Gantz I, McGuire DK, Investigators VC. Efficacy of ertugliflozin on heart failure‐related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation 2020; 142: 2205–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Packer M. Lessons learned from the DAPA‐HF trial concerning the mechanisms of benefit of SGLT2 inhibitors on heart failure events in the context of other large‐scale trials nearing completion. Cardiovasc Diabetol 2019; 18: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, Packer M. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta‐analysis of the EMPEROR‐Reduced and DAPA‐HF trials. Lancet 2020; 396: 819–829. [DOI] [PubMed] [Google Scholar]
- 20. Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, Berry C, Chong V, Coyle L, Docherty KF, Dreisbach JG, Labinjoh C, Lang NN, Lennie V, McConnachie A, Murphy CL, Petrie CJ, Petrie JR, Speirits IA, Sourbron S, Welsh P, Woodward R, Radjenovic A, Mark PB, McMurray JJV, Jhund PS, Petrie MC, Sattar N. Effect of empagliflozin on left ventricular volumes in patients with type 2 diabetes, or prediabetes, and heart failure with reduced ejection fraction (SUGAR‐DM‐HF). Circulation 2021; 143: 516–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Santos‐Gallego CG, Vargas‐Delgado AP, Requena‐Ibanez JA, Garcia‐Ropero A, Mancini D, Pinney S, Macaluso F, Sartori S, Roque M, Sabatel‐Perez F, Rodriguez‐Cordero A, Zafar MU, Fergus I, Atallah‐Lajam F, Contreras JP, Varley C, Moreno PR, Abascal VM, Lala A, Tamler R, Sanz J, Fuster V, Badimon JJ, Investigators E‐T. Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J Am Coll Cardiol 2021; 77: 243–255. [DOI] [PubMed] [Google Scholar]
- 22. Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation 2007; 116: 434–448. [DOI] [PubMed] [Google Scholar]
- 23. Neubauer S. The failing heart—an engine out of fuel. N Engl J Med 2007; 356: 1140–1151. [DOI] [PubMed] [Google Scholar]
- 24. Ingwall JS. On the hypothesis that the failing heart is energy starved: lessons learned from the metabolism of ATP and creatine. Curr Hypertens Rep 2006; 8: 457–464. [DOI] [PubMed] [Google Scholar]
- 25. Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner VA, Coke LA, Fleg JL, Forman DE, Gerber TC, Gulati M, Madan K, Rhodes J, Thompson PD, Williams MA, American Heart Association Exercise Cardiac Rehabilitation and Prevention Committee of the Council on Clinical Cardiology, Council on Nutrition Physical Activity and Metabolism, Council on Cardiovascular Stroke Nursing, Council on Epidemiology and Prevention . Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 2013; 128: 873–934. [DOI] [PubMed] [Google Scholar]
- 26. Levelt E, Rodgers CT, Clarke WT, Mahmod M, Ariga R, Francis JM, Liu A, Wijesurendra RS, Dass S, Sabharwal N, Robson MD, Holloway CJ, Rider OJ, Clarke K, Karamitsos TD, Neubauer S. Cardiac energetics, oxygenation, and perfusion during increased workload in patients with type 2 diabetes mellitus. Eur Heart J 2016; 37: 3461–3469a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beadle RM, Williams LK, Kuehl M, Bowater S, Abozguia K, Leyva F, Yousef Z, Wagenmakers AJ, Thies F, Horowitz J, Frenneaux MP. Improvement in cardiac energetics by perhexiline in heart failure due to dilated cardiomyopathy. JACC Heart Fail 2015; 3: 202–211. [DOI] [PubMed] [Google Scholar]
- 28. Fragasso G, Perseghin G, De Cobelli F, Esposito A, Palloshi A, Lattuada G, Scifo P, Calori G, Del Maschio A, Margonato A. Effects of metabolic modulation by trimetazidine on left ventricular function and phosphocreatine/adenosine triphosphate ratio in patients with heart failure. Eur Heart J 2006; 27: 942–948. [DOI] [PubMed] [Google Scholar]
- 29. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR, Group CPC . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 30. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause‐Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS, Investigators D‐T. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357. [DOI] [PubMed] [Google Scholar]
- 31. Fordyce CB, Roe MT, Ahmad T, Libby P, Borer JS, Hiatt WR, Bristow MR, Packer M, Wasserman SM, Braunstein N, Pitt B, DeMets DL, Cooper‐Arnold K, Armstrong PW, Berkowitz SD, Scott R, Prats J, Galis ZS, Stockbridge N, Peterson ED, Califf RM. Cardiovascular drug development: is it dead or just hibernating? J Am Coll Cardiol 2015; 65: 1567–1582. [DOI] [PubMed] [Google Scholar]
- 32. Januzzi JL Jr, Ibrahim NE. Understanding the mechanistic benefit of heart failure drugs matters. J Am Coll Cardiol 2020; 76: 2752–2754. [DOI] [PubMed] [Google Scholar]
- 33. Berg DD, Jhund PS, Docherty KF, Murphy SA, Verma S, Inzucchi SE, Kober L, Kosiborod MN, Langkilde AM, Martinez FA, Bengtsson O, Ponikowski P, Sjostrand M, Solomon SD, McMurray JJV, Sabatine MS. Time to clinical benefit of dapagliflozin and significance of prior heart failure hospitalization in patients with heart failure with reduced ejection fraction. JAMA Cardiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wende AR, Brahma MK, McGinnis GR, Young ME. Metabolic origins of heart failure. JACC Basic Transl Sci 2017; 2: 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine‐to‐ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997; 96: 2190–2196. [DOI] [PubMed] [Google Scholar]
- 36. Nascimben L, Ingwall JS, Pauletto P, Friedrich J, Gwathmey JK, Saks V, Pessina AC, Allen PD. Creatine kinase system in failing and nonfailing human myocardium. Circulation 1996; 94: 1894–1901. [DOI] [PubMed] [Google Scholar]
- 37. Matsutani D, Sakamoto M, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Effect of canagliflozin on left ventricular diastolic function in patients with type 2 diabetes. Cardiovasc Diabetol 2018; 17: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Soga F, Tanaka H, Tatsumi K, Mochizuki Y, Sano H, Toki H, Matsumoto K, Shite J, Takaoka H, Doi T, Hirata KI. Impact of dapagliflozin on left ventricular diastolic function of patients with type 2 diabetic mellitus with chronic heart failure. Cardiovasc Diabetol 2018; 17: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verma S, Garg A, Yan AT, Gupta AK, Al‐Omran M, Sabongui A, Teoh H, Mazer CD, Connelly KA. Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA‐REG OUTCOME trial? Diabetes Care 2016; 39: e212–e213. [DOI] [PubMed] [Google Scholar]
- 40. Figtree GA, Radholm K, Barrett TD, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Matthews DR, Shaw W, Neal B. Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes mellitus. Circulation 2019; 139: 2591–2593. [DOI] [PubMed] [Google Scholar]
- 41. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Bonaca MP, Ruff CT, Desai AS, Goto S, Johansson PA, Gause‐Nilsson I, Johanson P, Langkilde AM, Raz I, Sabatine MS, Wiviott SD. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 2019; 139: 2528–2536. [DOI] [PubMed] [Google Scholar]
- 42. Lamb HJ, Beyerbacht HP, van der Laarse A, Stoel BC, Doornbos J, van der Wall EE, de Roos A. Diastolic dysfunction in hypertensive heart disease is associated with altered myocardial metabolism. Circulation 1999; 99: 2261–2267. [DOI] [PubMed] [Google Scholar]
- 43. Phan TT, Abozguia K, Nallur Shivu G, Mahadevan G, Ahmed I, Williams L, Dwivedi G, Patel K, Steendijk P, Ashrafian H, Henning A, Frenneaux M. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J Am Coll Cardiol 2009; 54: 402–409. [DOI] [PubMed] [Google Scholar]
- 44. Neubauer S, Krahe T, Schindler R, Horn M, Hillenbrand H, Entzeroth C, Mader H, Kromer EP, Riegger GA, Lackner K. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high‐energy phosphate metabolism in heart failure. Circulation 1992; 86: 1810–1818. [DOI] [PubMed] [Google Scholar]
- 45. Spoladore R, Fragasso G, Perseghin G, De Cobelli F, Esposito A, Maranta F, Calori G, Locatelli M, Lattuada G, Scifo P, Del Maschio A, Margonato A. Beneficial effects of beta‐blockers on left ventricular function and cellular energy reserve in patients with heart failure. Fundam Clin Pharmacol 2013; 27: 455–464. [DOI] [PubMed] [Google Scholar]
- 46. Fragasso G, Palloshi A, Puccetti P, Silipigni C, Rossodivita A, Pala M, Calori G, Alfieri O, Margonato A. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol 2006; 48: 992–998. [DOI] [PubMed] [Google Scholar]
- 47. Schmidt‐Schweda S, Holubarsch C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci (Lond) 2000; 99: 27–35. [PubMed] [Google Scholar]
- 48. Abdurrachim D, Manders E, Nicolay K, Mayoux E, Prompers JJ. Single dose of empagliflozin increases in vivo cardiac energy status in diabetic db/db mice. Cardiovasc Res 2018; 114: 1843–1844. [DOI] [PubMed] [Google Scholar]
- 49. Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA‐REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care 2016; 39: 1108–1114. [DOI] [PubMed] [Google Scholar]
- 50. Cahill GF Jr, Veech RL. Ketoacids? Good medicine? Trans Am Clin Climatol Assoc 2003; 114: 149–161 discussion 162‐143. [PMC free article] [PubMed] [Google Scholar]
- 51. Nielsen R, Moller N, Botker HE, Eiskjaer H, Pryds K, Mellemkjaer S, Hansson NH, Harms HJ, Gormsen LC, Froekiaer J, Tolbod LP, Sorensen J. The ketone body 3‐hydroxybutyrate increases cardiac output without affecting myocardial external efficiency. Eur Heart J. [Abstract] 2018; 39: ehy563‐P3193. [Google Scholar]
- 52. Santos‐Gallego CG, Ibanez JAR, Antonio RS, Ishikawa K, Watanabe S, Botija MBP, Salvo AJS, Hajjar R, Fuster V, Badimon J. Empagliflozin induces a myocardial metabolic shift from glucose consumption to ketone metabolism that mitigates adverse cardiac remodeling and improves myocardial contractility. J Am Coll Cardiol 2018; 71: 674–674. [Google Scholar]
- 53. Lopaschuk GD, Verma S. Empagliflozin's fuel hypothesis: not so soon. Cell Metab 2016; 24: 200–202. [DOI] [PubMed] [Google Scholar]
- 54. Bruer J, Chung KJ, Pesonen E, Haas RH, Guth BD, Sahn DJ, Hesselink JR. Ketone bodies maintain normal cardiac function and myocardial high energy phosphates during insulin‐induced hypoglycemia in vivo. Basic Res Cardiol 1989; 84: 510–523. [DOI] [PubMed] [Google Scholar]
- 55. Lopaschuk GD, Ussher JR. Evolving concepts of myocardial energy metabolism: more than just fats and carbohydrates. Circ Res 2016; 119: 1173–1176. [DOI] [PubMed] [Google Scholar]
- 56. Kappel BA, Lehrke M, Schutt K, Artati A, Adamski J, Lebherz C, Marx N. Effect of empagliflozin on the metabolic signature of patients with type 2 diabetes mellitus and cardiovascular disease. Circulation 2017; 136: 969–972. [DOI] [PubMed] [Google Scholar]
- 57. Rosca MG, Hoppel CL. Mitochondria in heart failure. Cardiovasc Res 2010; 88: 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, Jancev M, Hollmann MW, Weber NC, Coronel R, Zuurbier CJ. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 2018; 61: 722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lanfear DE, Gibbs JJ, Li J, She R, Petucci C, Culver JA, Tang WHW, Pinto YM, Williams LK, Sabbah HN, Gardell SJ. Targeted metabolomic profiling of plasma and survival in heart failure patients. JACC Heart Fail 2017; 5: 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zordoky BN, Sung MM, Ezekowitz J, Mandal R, Han B, Bjorndahl TC, Bouatra S, Anderson T, Oudit GY, Wishart DS, Dyck JR, Alberta H. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS ONE 2015; 10: e0124844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bayes‐Genis A, Liu PP, Lanfear DE, de Boer RA, Gonzalez A, Thum T, Emdin M, Januzzi JL. Omics phenotyping in heart failure: the next frontier. Eur Heart J 2020; 41: 3477–3484. [DOI] [PubMed] [Google Scholar]
- 62. Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long‐term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 1999; 99: 1173–1182. [DOI] [PubMed] [Google Scholar]
- 63. Abozguia K, Elliott P, McKenna W, Phan TT, Nallur‐Shivu G, Ahmed I, Maher AR, Kaur K, Taylor J, Henning A, Ashrafian H, Watkins H, Frenneaux M. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation 2010; 122: 1562–1569. [DOI] [PubMed] [Google Scholar]
- 64. Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, Filippatos G, Gniot J, Fu M, Gullestad L, Howlett JG, Nicholls SJ, Redon J, Schenkenberger I, Silva‐Cardoso J, Stork S, Krzysztof Wranicz J, Savarese G, Brueckmann M, Jamal W, Nordaby M, Peil B, Ritter I, Ustyugova A, Zeller C, Salsali A, Anker SD. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J 2021; 42: 700–710. [DOI] [PubMed] [Google Scholar]
- 65. Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, McGuire DK, Pitt B, Scirica BM, Austin B, Drazner MH, Fong MW, Givertz MM, Gordon RA, Jermyn R, Katz SD, Lamba S, Lanfear DE, LaRue SJ, Lindenfeld J, Malone M, Margulies K, Mentz RJ, Mutharasan RK, Pursley M, Umpierrez G, Kosiborod M. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE‐HF trial. Circulation 2019; 140: 1463–1476. [DOI] [PubMed] [Google Scholar]
- 66. Butler J, Anker SD, Filippatos G, Khan MS, Ferreira JP, Pocock SJ, Giannetti N, Januzzi JL, Pina IL, Lam CSP, Ponikowski P, Sattar N, Verma S, Brueckmann M, Jamal W, Vedin O, Peil B, Zeller C, Zannad F, Packer M, Committees EM‐RT, Investigators . Empagliflozin and health‐related quality of life outcomes in patients with heart failure with reduced ejection fraction: the EMPEROR‐Reduced trial. Eur Heart J 2021; 42: 1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, Inzucchi SE, Kober L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjostrand M, Langkilde AM, McMurray JJV. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA‐HF trial. Circulation 2020; 141: 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Flynn KE, Lin L, Moe GW, Howlett JG, Fine LJ, Spertus JA, McConnell TR, Pina IL, Weinfurt KP. Relationships between changes in patient‐reported health status and functional capacity in outpatients with heart failure. Am Heart J 2012; 163: 88–94 e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Contaifer D Jr, Buckley LF, Wohlford G, Kumar NG, Morriss JM, Ranasinghe AD, Carbone S, Canada JM, Trankle C, Abbate A, Van Tassell BW, Wijesinghe DS. Metabolic modulation predicts heart failure tests performance. PLoS ONE 2019; 14: e0218153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nunez J, Palau P, Dominguez E, Mollar A, Nunez E, Ramon JM, Minana G, Santas E, Facila L, Gorriz JL, Sanchis J, Bayes‐Genis A. Early effects of empagliflozin on exercise tolerance in patients with heart failure: a pilot study. Clin Cardiol 2018; 41: 476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Anker SD, Butler J, Filippatos GS, Jamal W, Salsali A, Schnee J, Kimura K, Zeller C, George J, Brueckmann M, Zannad F, Packer M, Committees EM‐PT, Investigators . Evaluation of the effects of sodium‐glucose co‐transporter 2 inhibition with empagliflozin on morbidity and mortality in patients with chronic heart failure and a preserved ejection fraction: rationale for and design of the EMPEROR‐Preserved Trial. Eur J Heart Fail 2019; 21: 1279–1287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information.


