Abstract
Aims
The use of beta‐blocker therapy in cardiac amyloidosis (CA) is debated. We aimed at describing patterns of beta‐blocker prescription through a nationwide survey.
Methods and results
From 11 referral centres, we retrospectively collected data of CA patients with a first evaluation after 2016 (n = 642). Clinical characteristics at first and last evaluation were collected, with a focus on medical therapy. For patients in whom beta‐blocker therapy was started, stopped, or continued between first and last evaluation, the main reason for beta‐blocker management was requested. Median age of study population was 77 years; 81% were men. Arterial hypertension was found in 58% of patients, atrial fibrillation (AF) in 57%, and coronary artery disease in 16%. Left ventricular ejection fraction was preserved in 62% of cases, and 74% of patients had advanced diastolic dysfunction. Out of the 250 CA patients on beta‐blockers at last evaluation, 215 (33%) were already taking this therapy at first evaluation, while 35 (5%) were started it, in both cases primarily because of high‐rate AF. One‐hundred‐nineteen patients (19%) who were on beta‐blocker at first evaluation had this therapy withdrawn, mainly because of intolerance in the presence of heart failure with advanced diastolic dysfunction. The remaining 273 patients (43%) had never received beta‐blocker therapy. Beta‐blockers usage was similar between CA aetiologies. Patients taking vs. not taking beta‐blockers differed only for a greater prevalence of arterial hypertension, coronary artery disease, AF, and non‐restrictive filling pattern (P < 0.01 for all) in the former group.
Conclusions
Beta‐blockers prescription is not infrequent in CA. Such therapy may be tolerated in the presence of co‐morbidities for which beta‐blockers are routinely used and in the absence of advanced diastolic dysfunction.
Keywords: Cardiac amyloidosis, Beta‐blockers, Transthyretin, Light chains, Heart failure
Introduction
Cardiac amyloidosis (CA) is a condition increasingly recognized. 1 Progressive amyloid myocardial infiltration by light‐chain (AL) or transthyretin (ATTR, either mutant, ATTRv, or wild‐type, ATTRwt) proteins causes a variety of cardiac manifestations, including heart failure (HF) and arrhythmias. 2 , 3 Both atrial fibrillation (AF) and brady‐arrhythmias are common in CA. 4 , 5 , 6 This is particularly true for ATTRwt patients, who may present such conditions as co‐morbidities of their older age.
The use of beta‐blocker therapy in CA is debated. 7 , 8 CA often presents a restrictive HF pathophysiology with preserved left ventricular ejection fraction (LVEF), condition in which beta‐blockers lack of efficacy or may even be counterproductive. 9 Moreover, a previous study found worse outcomes in ATTRv patients taking beta‐blocker and angiotensin converting enzyme‐inhibitor (ACEi) therapy, and no benefit in ATTRwt. 10 Nevertheless, more recent real‐world analyses have shown that beta‐blockers in CA are frequently prescribed 11 and may be well‐tolerated, with accurate patients' selection. 12 Finally, CA may also coexist with age‐related co‐morbidities in which beta‐blockers are proven beneficial, in particular coronary artery disease (CAD).
The aim of this study was to investigate and describe patterns of beta‐blockers prescription in a contemporary cohort of CA.
Methods
From 11 Italian amyloidosis referral centres, we retrospectively collected data of all CA patients with a first evaluation after 1 January 2016 and at least one follow‐up visit, regardless of their vital status at the time of survey data collection. In the year 2016, Gillmore and colleagues 13 published an algorithm validating the use of bone scintigraphy for the non‐invasive diagnosis of ATTR CA, which is still considered the reference standard. Thus, we decided to include in our analysis only CA patients diagnosed according to this algorithm, which has been adopted by all centres participating in this survey. Collection started on 8 June 2020, and database was locked on 15 September 2020. Two larger centres contributed with 317 patients (Florence, n = 178; Bologna, n = 139), whereas the remaining nine with 325 patients, for a total of 642 CA patients. Demographics, clinical, and instrumental characteristics at first and last evaluation at each centre were collected, with a detailed section dedicated to HF medical therapy, including beta‐blockers, ACEi, angiotensin receptor blockers (ARB), mineral corticoid receptor antagonists (MRA), and loop diuretics. In particular, the status and dosage of beta‐blocker therapy at first and last evaluation were examined; for patients in whom beta‐blocker therapy was continued, started or stopped between first and last evaluation, the main reason for beta‐blocker management was requested. Among the proposed options, reasons for beta‐blocker therapy to be continued or started were the following: (i) high‐rate AF, as average heart rate per minute >100; (ii) ventricular arrhythmias, as the presence of sustained and/or non‐sustained ventricular tachycardia and/or frequent premature ventricular complexes at electrocardiography; (iii) left ventricular systolic dysfunction, as a LVEF ≤50%; (iv) arterial hypertension, defined as either antihypertensive medications use and/or systolicblood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg; (v) ischaemic heart disease, defined as a documented previous myocardial infarction and/or the existence of angiographic evidence of significant coronary stenosis. Reasons for beta‐blocker therapy to be stopped were the following: (i) intolerance in the context of HF signs and symptoms, with an advanced diastolic dysfunction pathophysiology defined by the presence of a pseudonormal or restrictive transmitral flow pattern, associated with increased left ventricular filling pressures and left atrial enlargement 14 ; (ii) bradycardia, defined as a heart rate below 60 b.p.m.; (iii) arterial hypotension, defined as systolic blood pressure <110 mmHg; (iv) atrioventricular conduction disturbances. All patients signed at each institution an informed consent for the use of their anonymized aggregated data for research purposes. Data were collected using the Research Electronic Data Capture (REDCap) data management platform. One‐way ANOVA, χ 2, and Fisher's exact tests were used as appropriate for descriptive statistics.
Results
Characteristics of the 642 patients at their last evaluation are displayed in Table 1 , overall and by type of CA. Date of the first evaluation spanned from 4 January 2016 to 7 June 2020, whereas date of last evaluation from 21 September 2016 to 14 September 2020, with a median time of follow‐up of 419 days (range 28–1662). ATTRwt was the most prevalent aetiology of CA (58%), with a steady yearly increase in the number of diagnoses (Table 1 ). Median age of overall population was 77 years, and 81% were men, with an older age and a greater male predominance in the ATTRwt group. More than a half of the study population had a history of arterial hypertension and of AF, with a significantly greater prevalence in the ATTRwt group. LVEF was preserved in 62% of the study sample, but 74% of patients had advanced diastolic dysfunction, which was particularly found in ATTRwt patients (Table 1 ). Diuretic therapy was prescribed to most patients, and especially to those with ATTRwt, whereas ACEi/ARB to 44% and MRA to 40%, without marked differences between groups. Pacemaker implantation was more common in ATTRwt (17%, vs. 7% in AL and 8% in ATTRv).
Table 1.
Characteristics of study population at last evaluation
| Overall n = 642 (%) | AL n = 167 (%) | ATTRv n = 100 (%) | ATTRwt n = 375 (%) | ||
|---|---|---|---|---|---|
| Age (median, range; years) | 77 (34–96) | 68 (44–96) | 72 (34–88) | 81 (54–96) | <0.0001 |
| Year of first evaluation | |||||
| 2016 | 81 | 23 | 20 | 38 | |
| 2017 | 112 | 34 | 17 | 61 | |
| 2018 | 178 | 44 | 26 | 108 | |
| 2019 | 224 | 55 | 34 | 135 | |
| 2020 (January–June) | 47 | 11 | 3 | 33 | |
| Time from first evaluation (range; days) | 419 (28–1662) | 382 (34–1662) | 483 (113–1477) | 406 (28–1638) | 0.04 |
| Gender | <0.0001 | ||||
| Males | 519 (81) | 109 (65) | 71 (71) | 339 (90) | |
| Females | 123 (19) | 58 (35) | 29 (29) | 36 (10) | |
| History of arterial hypertension | 374 (58) | 80 (48) | 57 (57) | 237 (63) | 0.004 |
| History of coronary artery disease | 100 (16) | 15 (9) | 10 (10) | 75 (20) | 0.001 |
| History of atrial fibrillation | 364 (57) | 73 (44) | 38 (38) | 253 (68) | <0.0001 |
| Cardiac device | 127 (20) | 20 (12) | 13 (13) | 94 (25) | 0.02 |
| Pacemaker | 82 (13) | 11 (7) | 8 (8) | 63 (17) | |
| ICD | 31 (5) | 8 (5) | 3 (3) | 20 (5) | |
| CRT‐P/D | 14 (2) | 1 (<1) | 2 (2) | 11 (3) | |
| Clinical status | |||||
| NYHA functional class | 0.03 | ||||
| I/II | 405 (63) | 93 (56) | 71 (71) | 241 (64) | |
| III/IV | 237 (37) | 74 (44) | 29 (29) | 134 (36) | |
| Mean systolic blood pressure (mmHg, ±SD) | 120 ± 18 | 116 ± 20 | 122 ± 19 | 121 ± 17 | 0.02 |
| Mean heart rate (b.p.m., ±SD) | 75 ± 14 | 76 ± 13 | 74 ± 12 | 75 ± 14 | 0.78 |
| ECG and echocardiography | |||||
| Heart rhythm | <0.0001 | ||||
| Sinus rhythm | 348 (54) | 127 (76) | 71 (71) | 150 (40) | |
| Atrial fibrillation/flutter | 245 (38) | 35 (21) | 22 (22) | 188 (50) | |
| Pacemaker stimulated | 49 (8) | 5 (3) | 7 (7) | 37 (10) | |
| Left ventricular ejection fraction | 0.76 | ||||
| <40% | 95 (15) | 24 (14) | 13 (13) | 58 (16) | |
| 40–49% | 147 (23) | 36 (22) | 20 (20) | 91 (24) | |
| ≥50% | 400 (62) | 107 (64) | 67 (67) | 226 (60) | |
| Diastolic function | <0.0001 | ||||
| Normal | 27 (4) | 10 (6) | 11 (11) | 6 (2) | |
| Abnormal relaxation | 142 (22) | 48 (29) | 26 (26) | 68 (18) | |
| Pseudonormal | 215 (34) | 44 (26) | 31 (31) | 140 (37) | |
| Restrictive | 258 (40) | 65 (39) | 32 (32) | 161 (43) | |
| Cardiovascular therapy | |||||
| Loop diuretics | 545 (85) | 136 (81) | 70 (70) | 339 (90) | <0.0001 |
| Beta‐blocker | 250 (39) | 62 (37) | 37 (37) | 151 (40) | 0.72 |
| Beta‐blocker dose ≥50% | 52 (21) | 12 (19) | 3 (8) | 37 (25) | |
| ACEi/ARB | 276 (44) | 68 (41) | 51 (51) | 157 (42) | 0.21 |
| MRA | 258 (40) | 70 (41) | 28 (28) | 160 (43) | 0.03 |
| Combinations with beta‐blocker | 0.69 | ||||
| Beta‐blocker only | 60 (9) | 19 (11) | 9 (9) | 32 (8) | |
| Beta‐blocker and ACEi/ARB | 75 (12) | 16 (10) | 15 (15) | 44 (12) | |
| Beta‐blocker and MRA | 70 (11) | 17 (10) | 7 (7) | 46 (12) | |
| Beta‐blocker, ACEi/ARB and MRA | 45 (7) | 10 (6) | 6 (6) | 29 (8) | |
| Patterns of beta‐blocker prescription | <0.0001 | ||||
| Never beta‐blocker | 273 (43) | 87 (52) | 50 (50) | 136 (36) | |
| Stopped beta‐blocker | 119 (19) | 18 (11) | 13 (13) | 88 (24) | |
| Started beta‐blocker | 35 (5) | 14 (8) | 9 (9) | 12 (3) | |
| Continued beta‐blocker | 215 (33) | 48 (29) | 28 (28) | 139 (37) |
ACEi/ARB, ACE inhibitor/angiotensin receptor blocker; CRT‐P/D, cardiac resynchronization therapy without or with defibrillator; ICD, implantable cardioverter defibrillator; MRA, mineralocorticoid receptor antagonists; NYHA, New York heart Association.
Out of the 250 CA patients on beta‐blockers at last evaluation, 215 (33% of the overall population) were already taking this therapy at first evaluation (i.e. continued beta‐blocker), primarily because of high‐rate AF, ventricular arrhythmias, or left ventricular systolic dysfunction (Figure 1 ). In the remaining 35 CA patients (5%), beta‐blockers were started after first evaluation during follow‐up (i.e. started beta‐blocker), primarily because of high‐rate AF or ventricular arrhythmias. Of the 392 CA patients who were not taking beta‐blocker at last evaluation, 273 (43%) had never received beta‐blocker therapy. On the contrary, 119 (19%) patients who were on beta‐blocker at first evaluation had this therapy withdrawn at follow‐up (i.e. stopped beta‐blocker), mainly because of intolerance in the presence of HF with advanced diastolic dysfunction pathophysiology. Distribution of reasons for which beta‐blocker therapy was continued, started, or stopped are further detailed in Figure 1 . Other less frequent reasons for beta‐blocker interruption included asthenia (n = 3), initiation of therapy with digoxin (n = 1), and restoration of sinus rhythm after AF (n = 1), whereas for initiation or continuation were other supraventricular arrhythmias (n = 5), outflow tract obstruction (n = 1), and angina (n = 1).
Figure 1.
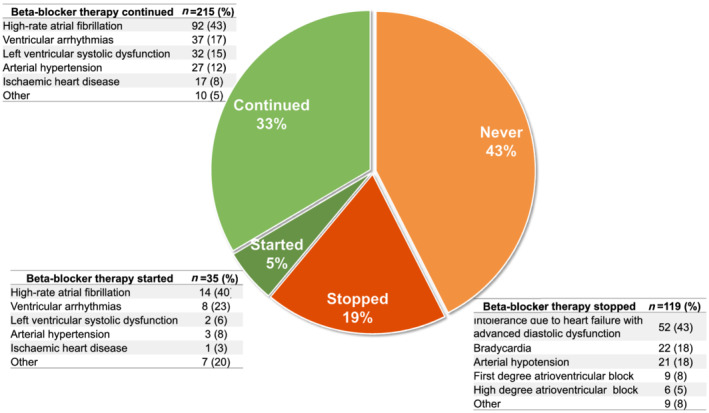
Patterns of beta‐blocker therapy prescription.
Beta‐blockers usage was similar between CA aetiologies at last evaluation. However, patterns of prescription varied by type of CA (Table 1 ). Specifically, half of patients in the AL and ATTRv groups never received beta‐blockers (vs. 36% in the ATTRwt), but beta‐blockers were more frequently stopped in the ATTRwt group (24%, vs. 11% in AL and 13% in ATTRv).
The distribution of cardiac amyloidosis aetiologies was similar in the two larger cohorts of Bologna and Firenze (in Bologna: 13% AL, 10% ATTRv, 77% ATTRwt; in Firenze: 16% AL, 14% ATTRv, 70% ATTRwt, P = 0.45), whereas a greater prevalence of AL was found in the cohort from other remaining centres (36% AL, 20% ATTRv, 44% ATTRwt, P = 0.001 as compared with Bologna and Firenze). These three cohorts were similar in terms of prevalence of atrial fibrillation (55% vs. 57% vs. 55%) and coronary artery disease (15% vs. 20% vs. 14%, P > 0.05 for all), with only a lower prevalence of arterial hypertension in Firenze (68% vs. 48% vs. 60%, P = 0.03). Nonetheless, rates of beta‐blockers prescription were similar across the three cohorts at last evaluation (45% vs. 33% vs. 40%, P = 0.25).
Patients taking vs. not taking beta‐blocker therapy at last evaluation differed only for a greater prevalence of arterial hypertension (67% vs. 53%), coronary artery disease (20% vs. 13%), AF (66% vs. 51%), and non‐restrictive filling pattern (64% vs. 57%, P < 0.01 for all) in the former group.
Discussion
In this sizable nationwide cohort, we found that prescription of beta‐blockers was not infrequent across all CA aetiologies. About 40% of patients were on beta‐blocker therapy at last evaluation, which is consistent with previous real‐world 11 and trial analyses. 15 It appears that prescription of beta‐blockers in CA is mainly driven by co‐morbidities and AF, and not by the amyloidotic cardiomyopathy per se.
Both AF and brady‐arrhythmias have been reported to be highly prevalent in CA, especially in ATTRwt patients. 4 , 6 , 16 High‐rate AF was the most common reason for beta‐blockers prescription in our study, followed by ventricular arrhythmias. On the contrary, the impact of brady‐arrhythmias on beta‐blocker therapy interruption was relatively limited. This finding highlights the importance of a better understanding of the CA arrhythmic profile.
Up to 60% of CA patients never used (mostly AL and ATTRv) or stopped beta‐blockers (most commonly ATTRwt). This happened primary due to intolerance in the presence of HF with advanced diastolic dysfunction pathophysiology. It is in fact well acknowledged that in the context of a fixed stroke volume due to restrictive left ventricular physiology, higher heart rate is required to maintain cardiac output. 8 , 9 Clearly, other mechanisms are also likely to play a role in the intolerance to beta‐blockers, because advanced diastolic dysfunction was present in 74% of our sample, and in some of these CA patients, beta‐blockers therapy was safely delivered. It is however worth of note that, when prescribed, beta‐blockers were effectively up‐titrated in only 21% of cases (Table 1 ). More in general, despite also ACEi/ARBs and MRA were prescribed similarly to beta‐blockers in about 40% of CA patients, the diverse combinations of these therapies were found in 6–15% of cases. Thus, overall, the tolerability of HF directed therapies in CA may be limited, and clearly, patients' selection and accurate dosage optimization are fundamental. 10 , 12 In the context of AL and ATTRv, autonomic dysfunction may also play a role. 2 , 3
Finally, using a contemporary cohort of CA, we describe for the first time how ATTRwt is becoming the most common CA aetiology in Italy, with a steadily yearly increase in the number of diagnoses. Despite this possibility had previously been hypothesized, data in this regard are lacking. 17 Our results, informative of HF medications patterns of use in CA, are even more important in consideration of this growing CA population.
Some shortcomings of our work should be acknowledged. This was a survey focused on assessing and comparing prescription of beta‐blockers at first vs. last evaluations, and as such, information regarding longitudinal modifications of that therapy (i.e., up‐titration or down‐titration over clinical course) were not retrieved. Dosage of drugs was reviewed only for beta‐blockers and not for other HF drugs. Due to the nature of data collection, we cannot exclude that unselected or undifferentiated variables influenced our results (i.e. AL patients' status and chemotherapy regimens; electrocardiographic parameters such as differentiation of premature ventricular complexes from non‐sustained or sustained ventricular tachycardia; echocardiographic indexes such as those of right ventricle function). Taking into considerations these aspects, the impact of beta‐blocker therapy on outcomes could not be appropriately judged and was therefore not investigated in the present survey.
Conclusions
Beta‐blockers prescription is not infrequent in CA. Such therapy may be tolerated particularly in the presence of co‐morbidities for which beta‐blockers are routinely used and in the absence of restrictive diastolic dysfunction. High‐rate AF and ventricular arrhythmias were the two main reason for beta‐blockers usage, while bradycardia or atrio‐ventricular blocks accounted for less than one third of beta‐blocker therapy interruption.
Conflict of interest
All authors declare they have no conflict of interest in relation to this study.
Tini, G. , Cappelli, F. , Biagini, E. , Musumeci, B. , Merlo, M. , Crotti, L. , Cameli, M. , Di Bella, G. , Cipriani, A. , Marzo, F. , Guerra, F. , Forleo, C. , Gagliardi, C. , Zampieri, M. , Carigi, S. , Vianello, P. F. , Mandoli, G. E. , Ciliberti, G. , Lichelli, L. , Mariani, D. , Porcari, A. , Russo, D. , Licordari, R. , Ponziani, A. , Porto, I. , Perfetto, F. , Autore, C. , Rapezzi, C. , Sinagra, G. , and Canepa, M. (2021) Current patterns of beta‐blocker prescription in cardiac amyloidosis: an Italian nationwide survey. ESC Heart Failure, 8: 3369–3374. 10.1002/ehf2.13411.
References
- 1. Gilstrap LG, Dominici F, Wang Y, El‐Sady MS, Singh A, Di Carli MF, Falk RH, Dorbala S. Epidemiology of cardiac amyloidosis‐associated heart failure hospitalizations among fee‐for‐service medicare beneficiaries in the United States. Circ Heart Fail 2019; 12: e005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falk RH, Alexander KM, Liao R, Dorbala S. AL (light‐chain) cardiac amyloidosis: a review of diagnosis and therapy. J Am Coll Cardiol 2016; 68: 1323–1341. [DOI] [PubMed] [Google Scholar]
- 3. Gertz MA, Benson MD, Dyck PJ, Grogan M, Coelho T, Cruz M, Berk JL, Plante‐Bordeneuve V, Schmidt HHJ, Merlini G. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol 2015; 66: 2451–2466. [DOI] [PubMed] [Google Scholar]
- 4. Cappelli F, Vignini E, Martone R, Perlini S, Mussinelli R, Sabena A, Morini S, Gabriele M, Taborchi G, Bartolini S, Lossi A, Nardi G, Marchionni N, Di Mario C, Olivotto I, Perfetto F. Baseline ECG features and arrhythmic profile in transthyretin versus light chain cardiac amyloidosis. Circ Heart Fail 2020; 13: e006619. [DOI] [PubMed] [Google Scholar]
- 5. Cappelli F, Tini G, Russo D, Emdin M, Del Franco A, Vergaro G, Di Bella G, Mazzeo A, Canepa M, Volpe M, Perfetto F, Autore C, Di Mario C, Rapezzi C, Musumeci MB. Arterial thrombo‐embolic events in cardiac amyloidosis: a look beyond atrial fibrillation. Amyloid 2021; 28: 12–18. [DOI] [PubMed] [Google Scholar]
- 6. Donnellan E, Wazni OM, Saliba WI, Hanna M, Kanj M, Patel DR, Wilner B, Kochar A, Jaber WA. Prevalence, incidence, and impact on mortality of conduction system disease in transthyretin cardiac amyloidosis. Am J Cardiol 2020; 128: 140–146. [DOI] [PubMed] [Google Scholar]
- 7. Emdin M, Aimo A, Rapezzi C, Fontana M, Perfetto F, Seferović PM, Barison A, Castiglione V, Vergaro G, Giannoni A, Passino C, Merlini G. Treatment of cardiac transthyretin amyloidosis: an update. Eur Heart J 2019; 40: 3699–3706. [DOI] [PubMed] [Google Scholar]
- 8. Giancaterino S, Urey MA, Darden D, Hsu JC. Management of arrhythmias in cardiac amyloidosis. JACC Clin Electrophysiol 2020; 6: 351–361. [DOI] [PubMed] [Google Scholar]
- 9. Seferović PM, Polovina M, Bauersachs J, Arad M, Gal TB, Lund LH, Felix SB, Arbustini E, Caforio ALP, Farmakis D, Filippatos GS, Gialafos E, Kanjuh V, Krljanac G, Limongelli G, Linhart A, Lyon AR, Maksimović R, Miličić D, Milinković I, Noutsias M, Oto A, Oto Ö, Pavlović SU, Piepoli MF, Ristić AD, Rosano GMC, Seggewiss H, Ašanin M, Seferović JP, Ruschitzka F, Čelutkiene J, Jaarsma T, Mueller C, Moura B, Hill L, Volterrani M, Lopatin Y, Metra M, Backs J, Mullens W, Chioncel O, Boer RA, Anker S, Rapezzi C, Coats AJS, Tschöpe C. Heart failure in cardiomyopathies: a position paper from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2019; 21: 553–576. [DOI] [PubMed] [Google Scholar]
- 10. aus dem Siepen F, Hein S, Bauer R, Katus HA, Kristen AV. Standard heart failure medication in cardiac transthyretin amyloidosis: useful or harmful? Amyloid 2017; 24: 132–133. [DOI] [PubMed] [Google Scholar]
- 11. Canepa M, Tini G, Musumeci B, Cappelli F, Milandri A, Mussinelli R, Autore C, Perfetto F, Rapezzi C, Perlini S. Real‐world versus trial patients with transthyretin amyloid cardiomyopathy. Eur J Heart Fail 2019; 21: 1479–1481. [DOI] [PubMed] [Google Scholar]
- 12. Aimo A, Vergaro G, Castiglione V, Rapezzi C, Emdin M. Safety and tolerability of neurohormonal antagonism in cardiac amyloidosis. Eur J Intern Med 2020; 80: 66–72. [DOI] [PubMed] [Google Scholar]
- 13. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AW, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016; 133: 2404–2412. [DOI] [PubMed] [Google Scholar]
- 14. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD, Houston, Texas; Oslo, Norway; Phoenix, Arizona; Nashville, Tennessee; Hamilton, Ontario, Canada; Uppsala, Sweden; Ghent and Liège, Belgium; Cleveland, Ohio; Novara, Italy; Rochester, Minnesota; Bucharest, Romania; and St. Louis, Missouri . Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016; 17: 1321–1360. [DOI] [PubMed] [Google Scholar]
- 15. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C, ATTR‐ACT Study Investigators . Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018; 379: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 16. Longhi S, Quarta CC, Milandri A, Lorenzini M, Gagliardi C, Manuzzi L, Bacchi‐Reggiani ML, Leone O, Ferlini A, Russo A, Gallelli I, Rapezzi C. Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid 2015; 22: 147–155. [DOI] [PubMed] [Google Scholar]
- 17. Rapezzi C, Lorenzini M, Longhi S, Milandri A, Gagliardi C, Bartolomei I, Salvi F, Maurer MS. Cardiac amyloidosis: the great pretender. Heart Fail Rev 2015; 20: 117–124. [DOI] [PubMed] [Google Scholar]


