Abstract
Iron deficiency is a major heart failure co‐morbidity present in about 50% of patients with stable heart failure irrespective of the left ventricular function. Along with compromise of daily activities, it also increases patient morbidity and mortality, which is independent of anaemia. Several trials have established parenteral iron supplementation as an important complimentary therapy to improve patient well‐being and physical performance. Intravenous iron preparations, in the first‐line ferric carboxymaltose, demonstrated in previous clinical trials superior clinical effect in comparison with oral iron preparations, improving New York Heart Association functional class, 6 min walk test distance, peak oxygen consumption, and quality of life in patients with chronic heart failure. Beneficial effect of iron deficiency treatment on morbidity and mortality of heart failure patients is waiting for conformation in ongoing trials. Although the current guidelines for treatment of chronic and acute heart failure acknowledge importance of iron deficiency correction and recommend intravenous iron supplementation for its treatment, iron deficiency remains frequently undertreated and insufficiently diagnosed in setting of the chronic heart failure. This paper highlights the current state of the art in the pathophysiology of iron deficiency, associations with heart failure trajectory and outcome, and an overview of current guideline‐suggested treatment options.
Keywords: Iron deficiency, Heart failure, Ferric carboxymaltose, Quality of life, Exercise capacity
Epidemiology
Iron deficiency (ID) is one of the most common nutritional deficiencies worldwide, affecting approximately one‐third of the general population. 1 Reaching a prevalence of 30–50% in patients with stable chronic heart failure (HF), ID is recognized as major cause of anaemia in these patients, regardless of sex, race, and left ventricular ejection function. 2 , 3 , 4 At the same time, more than 40% of the patients with chronic HF without anaemia or abnormalities in haematological indices exhibit laboratory abnormalities of depleted iron stores. 5 Furthermore, ID in acute decompensated HF can be detected in up to 80% of evaluated patients, which further underlines the importance of research efforts towards understanding of aetiology of ID in HF. 6 , 7 , 8
Heart failure, as a chronic disabling syndrome, represents a leading cause of frequent hospitalizations and high health costs worldwide. 9 A myriad of co‐morbidities complicate the natural course of HF and exhibit deleterious impact on disease progression. Intuitively, these co‐morbidities also constitute targets for potential intervention to improve quality of life and patient outcome. As such, ID gained substantial importance as a part of comprehensive medical treatment of HF in past years. Remarkably, ID seems to have greater clinical repercussions on HF trajectory than anaemia per se, and the causal associations with worsened exercise capacity, oxygen consumption, hospitalization, and mortality are stronger for ID compared with anaemia in HF patients. 10 , 11
Pathophysiology of iron deficiency
Iron is an essential trace element, involved in plethora of crucial biochemical pathways and important for tissue metabolism. 12 , 13 In the human body, iron exists in the ferrous intracellular form (Fe2+) and in the ferric form (Fe3+), which is mainly extracellular and circulating. 14 , 15 The most prominent role of iron is in oxygen homeostasis, including oxygen transport and storage, oxidative metabolism in skeletal and cardiac muscle, mitochondrial function and metabolism of proteins, lipids, and ribonucleic acids. 16 , 17 , 18 As a cofactor for enzymes or an element of proteins with distinct cellular functions, iron is key factor in the maintenance of cellular energy and metabolism of extra‐haematopoietic tissues. 19
The main consequence of ID in HF is impaired erythropoiesis, while erythroblasts and reticulocytes are major iron consumers in human body. 20 , 21 Generally, cells with a high mitogenic potential (neoplastic and immune) are particularly sensitive to reduced iron supplies or dysregulated iron utilization. 22 Cardiac myocytes as tissue with high energy demand are especially sensitive to limited iron utilization and decreased iron supplies, which has potential impact on HF pathophysiology. 23
Skeletal muscle functioning is also affected by ID, mainly in the context of impaired energetic metabolism through switch from oxidative metabolism to glycolysis, as well as through alterations in both carbohydrate and fat catabolic processing. 24 ID leads to ultra‐structural alterations in skeletal muscle, as well. Histological examination of skeletal muscle in patients with HF reveals changes in fibre composition with an increased contribution of fast glycolytic fibres. 25 At the cellular level, decreased total number/volume of mitochondria, diminished mitochondrial volume density, and reduction of mitochondrial cristae surface density represent hallmark of rearrangement of myocyte energy centres induced through ID, where intravenous iron substitution restores muscle energetic and mitochondrial function. 26 In men with HF with reduced ejection fraction, low ferritin reflecting depleted iron stores is associated with inspiratory muscle weakness. 27
Average iron intake is 10–20 mg/day. 28 The iron homeostasis is exclusively regulated through iron absorption by the apical surface of enterocytes in the duodenum and the upper jejunum, because no means of iron excretion exists. Normally, only ~15% of dietary iron is absorbed. 29 Dietary iron is found in two distinct different molecular forms, inorganic (non‐haem) and organic (haem). Inorganic dietary iron is absorbed via the divalent metal transporter 1 on the surface of enterocytes, where in next step membrane ferrireductases reduce ferric to ferrous iron. On the other hand, for hem iron, there is specific haem carrier protein, associated with inducible hemoxigenase 1, which reduces iron before entering the cytosol. Intracellular iron is secreted to the circulation using ferroportin 1 after membrane enzyme hephaestin oxidizes iron to ferric form, which is in the circulation transported bond to transferrin. 30 , 31
There are in general two different iron transport pathways with inherent regulatory mechanisms. First embrace transport from site of absorption to different tissues (systemic iron metabolism), and second intracellular iron transport, is responsible for transport between organelles within the cell. 32
Systemic iron metabolism is predominantly regulated by hormone hepcidin and ferroportin, the only iron export protein known in mammals. 33 Hepcidin, a small peptide hormone synthesized mainly by hepatocytes, interacts with ferroportin on target cells in negative feedback fashion, causing (i) reduced expression of proteins involved in transmembrane iron import to enterocytes and (ii) internalization of ferroportin. 34 Hence, hepcidin blocks intestinal absorption of iron and diverts iron from the circulation, trapping it in enterocytes, hepatocytes, and macrophages. 33 Decreased intestinal iron absorption together with its accumulation in the reticuloendothelial stores reduces the availability of iron to target tissues (Figure 1 ). Major stimuli decreasing hepcidin expression in the liver and its release into the circulation are depleted iron stores, hypoxia, and ineffective erythropoiesis, whereas inflammation produces the opposite effect. 35 Conversely, Ganz et al. showed that even a mild transient increase in the serum iron is sufficient to dramatically rise hepcidin level, blocking iron absorption and release in extracellular compartment and circulation. 36
Figure 1.
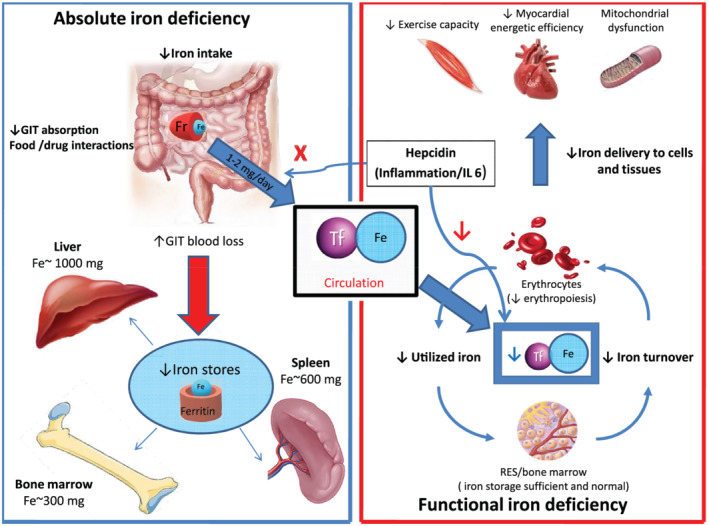
Basic physiological principles involved in iron absorption, transport and storage, as well aspathophysiological cascades responsible for absolute and functional iron deficiency. Fe, iron; Fr, ferroportin; GIT, gastrointestinal; RES, reticuloendothelial system; Tf, transferrin.
Because HF is characterized through high circulatory levels of inflammatory cytokines, it was initially postulated that, similar to chronic inflammatory states, HF patients have elevated levels of serum hepcidin. 37 , 38 However, recent studies in chronic as well as acute HF showed that hepcidin levels are actually decreased in HF. 39 , 40 Accordingly, initial premise that ID in HF is induced through limited circulatory iron availability due to metabolic mechanisms triggered by chronic inflammation is replaced with hypothesis that actually depleted iron stores are responsible for ID in HF. 33
Classification and diagnosis of iron deficiency in heart failure
For clinical and didactical purposes, two different types of ID are distinguished: absolute and functional (Figure 1 ). 41 Absolute ID is characterized through depleted iron stores, although iron transport, regulatory mechanisms, and erythropoiesis are undisturbed. 42 Contrary, functional ID represents a mismatch between iron demand and tissue supply, mostly due to iron utilization and iron maldistribution. 43
Although mechanisms underlying the development of ID in HF have not been rigorously investigated, generally ID arises as a consequence of impaired iron absorption, augmented gastrointestinal loss, and reduced availability of utilizable iron from the reticuloendothelial system. 44 In setting of HF, disease‐specific pathophysiological consequences (intestinal oedema, insufficient dietary intake, drug interactions due polypharmacy, and polymorbidity) further perpetuate depletion of iron storages. 45 , 46
In order to optimize the detection and classification of ID, as well as to ensure optimal and timely management of carefully selected candidates for iron replenishment, development of diagnostic algorithms advanced significantly over the last 10 years (Figure 2 ). 47 Albeit considered as a gold standard, due to its invasiveness and potential harmful side effects, a bone marrow biopsy for evaluating iron stores is replaced in clinical practice by the measurement of several blood biomarkers. 48
Figure 2.
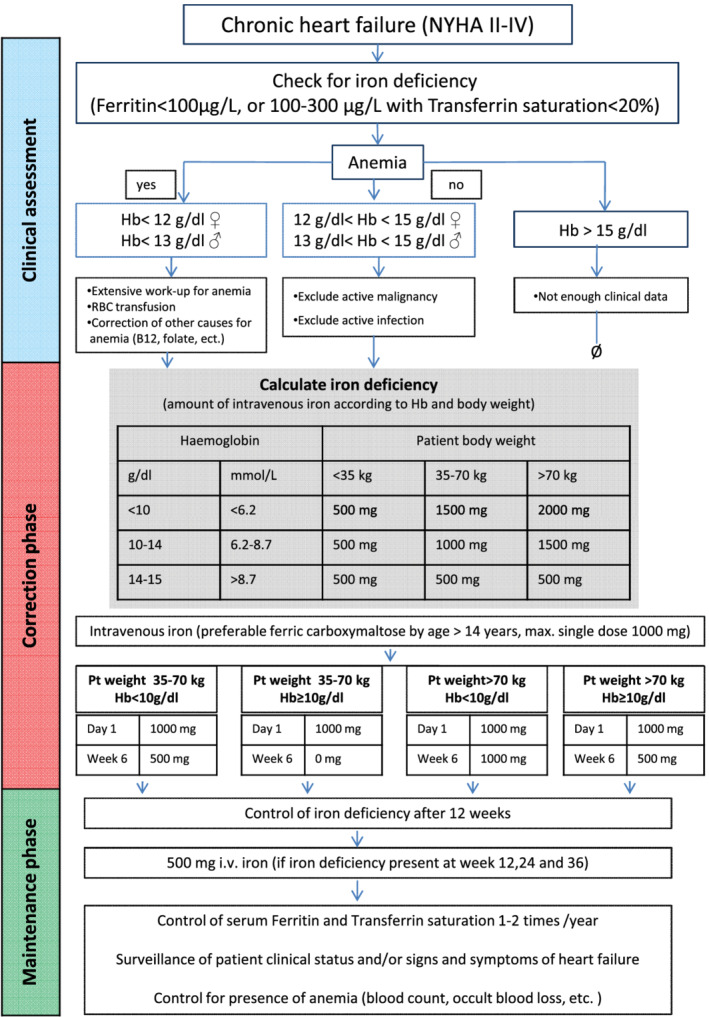
Proposed algorithm for the management of iron deficiency in heart failure. Algorithm adapted from McDonagh et al. 47 Hb, haemoglobin; i.v., intravenous; NYHA, New York Heart Association Functional Classification; Pt, patient.
As such, ferritin is one of the most commonly used laboratory measures of iron status worldwide. 49 There is a linear relationship between serum ferritin and ferritin expression in iron storage tissues, what enables usage of serum ferritin as surrogate marker of stored iron quantity and means that low circulating ferritin reflects depleted body iron stores. 50 However, serum ferritin levels are subjected to increase in chronic and inflammatory diseases, such as HF and chronic kidney disease. It is way cut‐off values for the diagnosis of ID in HF are arbitrarily set at a higher level (e.g. 100 mg/L). 51 It is worth mentioning that previous studies did not establish strong correlation between bone marrow iron and blood parameters of ID in HF. Nanas et al. found in 27 from 37 evaluated patients, hospitalized for decompensated HF, signs of ID in bone marrow biopsy despite normal levels of ferritin and erythropoietin in the serum. 52
Transferrin saturation (TSAT), representing the per cent of transferrin that has iron bound to it, is used as a marker of the availability of circulating iron to supply metabolizing cells. 43 Definition of ID solely based on TSAT < 20% in 42 patients with HF and a reduced left ventricular ejection fraction (LVEF ≤ 45%) undergoing median sternotomy for coronary artery bypass grafting showed compared with iron quantification in bone marrow aspirate a sensitivity of 94% and a specificity of 84%. 53 In HF, absolute ID is typically diagnosed with cut‐off ferritin values <100 mg/L and, distinguished from functional ID, diagnosed with normal serum ferritin (100–300 mg/L) and low TSAT (<20%). 48
Newly, soluble transferrin receptor (sTfR) and hepcidin were proposed as novel serum markers for ID in HF. The sTfR indicates reduced intracellular iron availability for metabolic needs and is a reliable diagnostic tool for confirmation of ID anaemia. 54 Importantly, the effect of chronic inflammation on circulating sTfR is minor, making sTfR a promising candidate for ID detection in chronic inflammatory states. 55 Jankowska et al. postulated a novel definition of ID based on the combined measurement of low circulation hepcidin (<14.5 ng/mL, the fifth percentile in healthy peers) and high circulation sTfR (≥1.59 mg/L, the 95th percentile in healthy peers). In multivariable analysis, this definition was strongly predictive of all‐cause mortality at 12 months. 39 Nevertheless, due to difficult and non‐standardized assessment of its circulating levels, hepcidin is currently used exclusively in research settings and mandates additional effort in overcoming specific methodological difficulties prior to its implementation in clinical routine.
Of note, mean corpuscular haemoglobin and mean corpuscular haemoglobin concentration have been found to be unreliable markers of ID status. Therefore, measuring these levels is not recommended for assessment of ID in patients with HF. 56 At the same time, serum transferrin alone does not represent iron storage or functional ID and should not be used in diagnostic work‐up in HF patients.
Serum iron levels ≤13 μmol/L showed an excellent diagnostic accuracy of 91% for ID compared with bone marrow biopsy. 53 However, as iron serum levels exhibit circadian variations, it cannot be used as reliable diagnostic tool for assessment of ID.
Since the 1970s, the iron deficit in chronically ill patients was calculated using Ganzoni's formula [iron deficit = body weight × 2.4 × (15 − haemoglobin in g/dL) + 500 mg], assuming that ideal haemoglobin concentration amounts 15.0 g/dL (or 13.0 g/dL by body weight <35 kg) and that additional 500 mg of iron is needed for replenishment of iron stores, Ganzoni's formula usually calculates iron deficit at 1000 mg or higher and was used for determination of iron deficit in the majority of randomized trials. 57 , 58
Important place in diagnostic work‐up of iron deficiency should be given to exclusion of treatable and reversible causes of iron depletion in HF patients. It is important to mention that depleted iron stores could be also a sign of undiagnosed malignancies, consequence of treatment with anticoagulants, or recurrent gastrointestinal bleedings. 59 It is why even decent lowering of haemoglobin values with concomitant laboratory signs of iron deficiency should trigger thorough medical evaluation of HF patients.
Oral iron therapy
Table 1 summarizes randomized clinical trials on ID in HF. Because of wide availability and low costs, oral iron replacement was considered as a promising strategy in ID management. Unfortunately, poor adherence, plethora of gastrointestinal side effects, and low intestinal absorption of iron additionally challenged investigation of feasibility of oral iron supplementation. Additionally, oral iron absorption test, which should evaluate plausibility and applicability of oral iron supplementation, identifying patients who are suitable for oral iron administration, finds rarely its place in clinical routine. Accordingly, so far, only two clinical randomized trials using oral iron preparation were conducted in patients with chronic HF. 61 , 64 , 68 , 69 , 70
Table 1.
Summary of studies evaluating iron supplementation in patients with heart failure
| Author | Study design | Sample size | Inclusion criteria | Iron deficiency/Hb | Iron supplementation | Duration | Primary endpoint | Major results in intervention arm |
|---|---|---|---|---|---|---|---|---|
| Silverberg et al. 60 | Single‐centre, uncontrolled, and open‐label | 26 | LVEF < 35% and NYHA class >III | Hb < 12 g/dL |
Erythropoietin Iron sucrose i.v. |
15–60 weeks | Retrospective/observational: no primary endpoint | NYHA class ↓, LVEF ↑, diuretic dose ↓, and hospitalization ↓ |
| Lewis et al. 61 (IRONOUT HF) | 1:1 randomized and double‐blind | 225 | NYHA class II–IV and LVEF ≤ 40% | Ferritin <100 ng/mL or serum ferritin 100–299 ng/mL with TSAT < 20% |
150 mg iron polysaccharide 2× a day p.o. |
16 weeks | Peak VO2 (from baseline to Week 16) | No change in peak VO2 |
| Toblli et al. 62 | 1:1 randomized, double‐blind, and placebo‐controlled | 40 | NYHA class II–IV and LVEF < 35% | TSAT < 20%, ferritin <100 μg/mL, and Hb < 12.5 g/dL | Iron sucrose 200 mg weekly for 5 weeks | 25 weeks | NT‐proBNP; inflammatory status | QoL ↑, NYHA class ↓, and hospitalization rate ↓ |
| Anker et al. 63 (FAIR‐HF) | 2:1 randomized, double‐blind, and placebo‐controlled | 459 | NYHA class II and LVEF < 40%; NYHA Class III and LVEF < 45% | Ferritin <100 μg/mL or serum ferritin 100–299 μg/mL with TSAT < 20% and Hb 9.5–13.5 g/dL | 200 mg ferric carboxymaltose until ferritin <500 μg/L, then 200 mg monthly | 24 weeks | Self‐reported PGA; NYHA class from baseline to Week 24 | PGA ↑, NYHA class ↓, and 6MWD ↑ |
| Beck‐da‐Silva et al. 64 (IRON‐HF) | 1:1:1 randomized, double‐blind, and placebo‐controlled | 23 | NYHA class II and LVEF < 40% | Ferritin 500 μg/mL with TSAT < 20% and Hb 9–12 g/dL |
200 mg ferrous sulfate p.o. for 8 weeks 200 mg/week iron sucrose i.v. |
5–22 weeks | Peak VO2 from baseline to Week 12 | Peak VO2—no significant ↑ in the i.v. iron group, ferritin ↑, and TSAT ↑ (in treated groups) |
| Ponikowski et al. 65 (CONFIRM‐HF) | 1:1 randomized, double‐blind, and placebo‐controlled | 304 | NYHA class II–III and LVEF < 40%, BNP > 100 pg/mL, and NT‐pro‐BNP > 400 pg/mL | Ferritin <100 ng/mL or serum ferritin 100–299 ng/mL with TSAT < 20% and Hb < 15 g/dL | 500–2000 mg ferric carboxymaltose at baseline and Week 6, then by persistent ID every 12 weeks | 52 weeks | Change in 6MWD from baseline to Week 24 | 6MWD ↑, PGA ↑, NYHA class ↓, QoL ↑, and hospitalization due to heart failure ↓ |
| Van Veldhuisen et al. 66 (EFFECT‐HF) | 1:1 randomized, double‐blind, and placebo‐controlled | 174 | NYHA class II–III LVEF < 40%, BNP > 100 pg/mL, and NT‐pro‐BNP > 400 pg/mL |
Ferritin <100 ng/mL or serum ferritin 100–299 ng/mL with TSAT < 20% and Hb < 15 g/dL Peak VO2 10–20 mL/kg/min |
500 mg ferric carboxymaltose at baseline, 6 weeks, and 12 weeks if ID persistent | 24 weeks | Change in peak VO2 from baseline to Week 24 | Peak VO2 ↑ (with imputation of deaths), NYHA class ↓, and PGA ↑ |
| Jankowska et al. 67 | Meta‐analysis | 509 | NYHA class II–III LVEF < 45% | Ferritin <100 ng/mL or serum ferritin 100–299 ng/mL with TSAT < 20% and Hb < 13 g/dL | Total iron dose: 1000–2000 mg i.v. (3 trials iron sucrose and 2 trials ferric carboxymaltose) | 5–36 weeks | All‐cause/cardiovascular death, cardiovascular hospitalization, quality of life (EQ‐5D, KCCQ, MLHFQ, and PGA), NYHA class, 6MWD, and LVEF | All‐cause death ↓, cardiovascular death and hospitalization ↓, NYHA class ↓, 6MWD ↑, and EQ‐5D, KCCQ, MLHFQ, and PGA ↑ |
6MWD, 6 min walk distance; BNP, brain natriuretic peptide; EQ‐5D, European Quality of Life‐5 Dimensions Questionnaire; ID, iron deficiency; Hb, haemoglobin; i.v., intravenous; ID, iron deficiency; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA class, New York Heart Association Functional Classification; p.o., per os.; peak VO2, peak oxygen consumption; PGA, patient global assessment; QoL, quality of life; TSAT, transferrin saturation.
This table summarizes clinical trials of ID correction in chronic heart failure.
In IRON‐HF study, suitable patients (LVEF < 40%, NYHA Class II–IV, haemoglobin 9–12 g/dL, TSAT < 20%, and ferritin <500 μg/L) were randomized in a double‐blind fashion to ferrous sulfate per os or intravenous therapy with iron sucrose combined with placebo. 50 The oral treatment arm of the study encompassed only seven patients, where 10 patients were assigned to intravenous iron supplementation. Three months after treatment, intravenous unlike oral iron therapy led to higher peak oxygen consumption. Compared with oral iron treatment group, intravenous supplementation resulted in numerically higher TSAT and ferritin increase. Although results and design of IRON‐HF study were an important step in research efforts towards better understanding of ID, because of highly limited number of patients (in total 23 patients included in the study), conclusions derived from this study should be interpreted with caution.
In the second trial (IRONOUT HF—Oral Iron Repletion Effects On Oxygen Uptake in Heart Failure), patients underwent treatment with oral iron polysaccharide, 150 mg twice daily over 16 weeks, in a double‐blind, randomized, placebo‐controlled fashion. The primary endpoint was a change in peak oxygen uptake from baseline to 16 weeks. Secondary endpoints included changes in 6 min walk distance, N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels, and health status measured through Kansas City Cardiomyopathy Questionnaire. Oral iron therapy did not significantly improve any of the study endpoints and minimally influenced iron storages, implicating that route of administration, more than the dose regimen or treatment strategy, is essential for clinical benefit. 65
In conclusion, available evidence does not justify oral iron supplementation for ID in patients with chronic HF and due to unnecessary polypharmacy and possible side effects should be avoided.
Intravenous iron therapy
Over the past 20 years, we witnessed enormous development of parenteral iron preparations. 71 , 72 Currently, there are five different formulations available in the USA and Europe, suitable for intravenous iron supplementation (Table 2 ). All studies in patients with chronic HF have used either iron sucrose or ferric carboxymaltose (Figure 3 ). Intravenous iron proved to be safe in patients with HF and allows rapid correction of iron indices, particularly in instances in which intestinal absorption is compromised. The usually described side effects of intravenous iron therapy (such as hypotension, electrolyte imbalance, skin reactions, and musculoskeletal side effects) did not represent safety concern in published trials. 62 , 73 , 74
Table 2.
Most important clinical features and side effects of available intravenous iron preparations
| Iron preparation | Brand name | Maximal single dose (mg) | Plasma half‐life (h) | Side effects | Tested in heart failure |
|---|---|---|---|---|---|
| Sodium ferric gluconate | Ferrlicit® | 125 | 1 | Hypersensitivity, nausea, hypotension, cramps, hypertension, dizziness, and chest pain | No |
| Ferric carboxymaltose | Ferinject®/Injectafer® | 15 mg/kg (max 1000 mg in one infusion) | 16 | Hypersensitivity, hypertension, nausea, flushing, hypophosphatemia, and dizziness | Yes 56 , 57 , 58 |
| Iron sucrose | Venofer® | 200–300 | 6 | Hypersensitivity, hypotension, iron overload, diarrhoea, nausea, vomiting, headache, dizziness, hypotension, pruritus, and leg/back cramps | Yes 50 , 54 , 55 , 65 |
| Iron dextran | Dexferrum® | 20 mg/kg | 60 | Fatal and serious hypersensitivity–anaphylaxis, diarrhoea, nausea, vomiting, headache, and dizziness | No |
| Ferumoxytol | FeraHeme® | 510 | 15 | Fatal and serious hypersensitivity–anaphylaxis, diarrhoea, nausea, dizziness, hypotension, and constipation | No |
Figure 3.
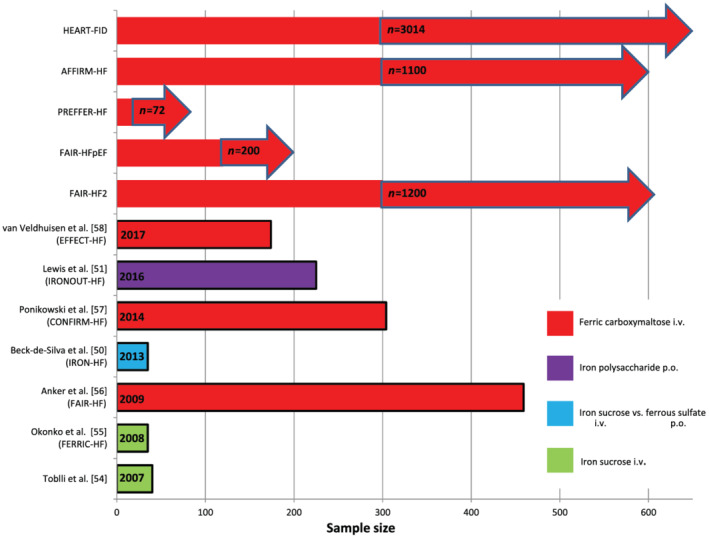
Overview of major publications and ongoing trials evaluating effects of treatment of iron deficiency in chronic heart failure. Ongoing trials are marked with an arrow. i.v., intravenous; p.o., per os.
FAIR‐HF (Ferinject Assessment in patients with IRon deficiency and chronic Heart Failure) was the first large multicentre trial of treatment with ferric carboxymaltose in patients with chronic HF. Patients with NYHA Class II and LVEF ≤ 40% or NYHA Class III and LVEF ≤ 45 were randomized in a 2:1 fashion to receive intravenous ferric carboxymaltose or placebo. Iron deficit was defined as serum ferritin of <100 mg/L or ferritin ranging from 100 to 300 mg/L, with TSAT of <20%. The presence of anaemia was not relevant for study inclusion, so that patients with haemoglobin concentration varying from 9.5 to 13.5 g/dL were eligible for study participation. Intravenous supplementation was conducted in two phases: first, correction phase with weekly intravenous administration of 200 mg of ferric carboxymaltose, followed with maintenance phase and monthly administration of 200 mg intravenous iron formulation according to individually calculated iron deficit. At follow‐up after 24 months, patients in the ferric carboxymaltose group reported improved self‐reported patient global assessment, NYHA functional class, 6 min walk distance, and quality of life as assessed by the Kansas City Cardiomyopathy Questionnaire. 63 Furthermore, the substudy of FAIR‐HF trial showed that intravenous ferric carboxymaltose was associated with early reductions in calculated plasma volume status and weight, implying that decongestion might be one mechanism via which iron repletion aids chronic HF patients. 75
Importance of iron treatment in setting of chronic HF with reduced LVEF found further affirmation in results of CONFIRM‐HF trial. In comparison with FAIR‐HF, in the CONFIRM‐HF trial, 6 min walk distance, as a more robust and objective method in assessment of the clinical status of HF patients, was used as primary endpoint over longer period of follow‐up from 52 weeks. Of note, the study protocol offered simplified dosing regimen for ferric carboxymaltose proposed by Evstatiev et al. 76 based on weight and haemoglobin levels, easily applicable in clinical routine compared with the traditional used Ganzoni formula. Patients with symptomatic HF (NYHA Class II or III, LVEF of ≤45%, and elevated level of either NT‐proBNP or BNP) were randomized in 1:1 manner to receive between 500 and 2000 mg of ferric carboxymaltose or placebo within the first 6 months following treatment with ferric carboxymaltose by persistent ID according to ferritin or TSAT at visits at 12, 24, and 36 weeks. Intravenous iron therapy with ferric carboxymaltose in patients with symptomatic HF and ID resulted in sustainable improvement in 6 min walk distance at follow‐up after 24 months. 65 One of the possible mechanisms, by which iron substitution induces improvement of cardiopulmonary exercise, could be its effect on cardiac remodelling. 77 Lacour et al. proved negative predictive value of iron deficiency on reverse cardiac remodelling after CRT therapy in patients with symptomatic HF and reduced LVEF. 78 Furthermore, Núñez et al. found that replenishment of iron myocardial amount in patients with chronic symptomatic HF with reduced ejection fraction (NYHA II–III) is strongly correlated with increase of LVEF at follow‐up. 79
Improvement of exercise capacity of HF patients under intravenous iron supplementation was additionally confirmed through results of EFFECT‐HF study. In this open‐label prospective study, a total of 174 patients were randomized to intravenous ferric carboxymaltose or no intervention. After 24 months, ferric carboxymaltose significantly increased serum ferritin and TSAT. Additionally, treatment with intravenous iron prevented decline in peak oxygen consumption, which was observed in non‐treated control group (−0.16 ± 0.375 vs. −0.63 ± 0.375 mL/min/kg; P = 0.23). 66
Current body of knowledge points out that ID can be frequently encountered in patients with acute decompensated HF. In the substudy of BIOSTAT‐CHF trial, which included patients either hospitalized for HF or presenting with worsening HF in the outpatient setting (LVEF ≤ 40% or, alternatively, BNP or NT‐proBNP levels of >400 or >2000 ng/L, respectively), ID was identified in 61.6% of patients, with highest prevalence in female (71.1% vs. 58.3%; P < 0.001). The presence of ID was significantly associated with lower estimated protein intake, chronic kidney disease, lower haemoglobin, higher C‐reactive protein levels, lower serum albumin levels, and P2Y12 inhibitor use (all P < 0.05), representing independent predictors of worse outcome in multivariable Cox proportional hazard regression analyses. These results imply that the aetiology of ID in worsening HF is complex and multifactorial and seems to consist of a combination of reduced iron uptake (malnutrition and fluid overload), impaired iron storage (inflammation and chronic kidney disease), and iron loss (anti‐platelets). 80
Upcoming results from AFFIRM‐AHF trial (ClinicalTrials.gov identifier: NCT02937454) will provide valuable insights concerning disease‐modifying potential of intravenous iron replenishment in patients hospitalized primary due to acute decompensated HF. In total, 1100 patients with NYHA II–III, LVEF < 50%, and clinical signs of volume overload or lung congestion have been enrolled in the study from April 2017 to 31 July 2019 and assigned to be treated with either 500–1500 mg of ferric carboxymaltose or placebo. The primary outcome of Affirm‐AHF trial presents the composite of recurrent HF hospitalizations and cardiovascular mortality. The main secondary outcomes include the composite of recurrent cardiovascular hospitalizations and cardiovascular mortality, recurrent HF hospitalizations, and safety‐related outcomes. 81 Each individual patient will be followed over a period of 52 weeks, so that first results are expected in 2021.
Heart failure with preserved ejection fraction (HFpEF) is increasingly the predominant form of HF in the developed world and tends to reach epidemic proportions. 48 ID negatively affects exercise tolerance and quality of life in HFpEF patients. 82 , 83 In stratified comparison of patients with reduced, mid‐range, and preserved LVEF in one prospective database of HF patients, ID was highly prevalent in all patient subgroups (64% in HFpEF cohort) and associated with decreased peak oxygen consumption. Furthermore, in HFpEF patients, baseline presence of ID led to worse clinical outcome, 10 although these results appear to be inconsistent across published systematic reviews and meta‐analyses. 84 These findings are suggestive of an important role of ID in HFpEF, indicating that it has a similar impact to that in HF with reduced ejection fraction. However, in the absence of randomized clinical trials of iron supplementation in HFpEF setting, we should focus on upcoming trials such as FAIR‐HFpEF (ClinicalTrials.gov identifier: NCT03074591) and PREFER‐HF (ClinicalTrials.gov identifier: NCT03833336), in order to fully comprehend the necessity and clinical effects of iron replenishment in HFpEF patients.
At the moment, we encounter gaps in evidence concerning effects of treatment of ID in HF concerning hard cardiovascular endpoints, such as mortality and hospitalization rates. Based on this, the results of FAIR‐HF2 trial (Intravenous Iron in Patients with Systolic Heart Failure and Iron Deficiency to Improve Morbidity and Mortality, ClinicalTrials.gov identifier: NCT03036462) are eagerly awaited. This trial aims at 1:1 double‐blind randomization of 1200 HF patients (NYHA II–III and LVEF ≤ 45%) with ID to ferric carboxymaltose or placebo. The primary endpoint includes a combined rate of recurrent hospitalizations for HF and cardiovascular death. The end on recruitment is planned for December 2021, while follow‐up will be event driven and should last for minimal 12 months. Focusing on patients with more severe HF (NYHA III–IV and LVEF 35%), HEART‐FID trial (ClinicalTrials.gov identifier: NCT03037931) aims at enrolling 3014 patients with ID. Effects of treatment with ferric carboxymaltose (1:1 randomization to placebo) will be tested on 12 month rate of death, hospitalization for worsening of HF, and 6 month change in 6 min walk test performance (primary endpoint). Only patients on maximally tolerated guideline‐recommended medical therapy of HF at least 2 weeks prior to randomization are included.
Available meta‐analyses and results from retrospective studies give us reason to believe that ID correction in HF patients improves clinical course of HF and reduces cardiovascular mortality. A meta‐analysis including patients with chronic HF included in four randomized trials of ID treatment (FER‐CARS‐01, FAIR‐HF, EFFICACY‐HF, and CONFIRM‐HF) suggested that treatment with intravenous ferric carboxymaltose reduces rates of recurrent cardiovascular hospitalizations and cardiovascular mortality [odds ratio (OR): 0.59, 95% (CI) 0.40–0.88; P = 0.009] as well as recurrent HF hospitalizations and cardiovascular mortality (OR: 0.53, 95% CI 0.33–0.86; P = 0.011). 58 In another meta‐analysis, Jankowska et al. found that intravenous iron therapy reduced the risk of the combined endpoint of all‐cause death or cardiovascular hospitalization [OR: 0.44, 95% CI 0.30–0.64, P < 0.0001], and the combined endpoint of cardiovascular death or hospitalization for worsening HF (OR 0.39, 95% CI 0.24–0.63, P = 0.0001) in iron‐deficient patients with systolic HF. 67
Management of ID in HF with combined treatment modalities did not prove to be clinically efficient and useful in HF patients. Silverberg et al. were among the first to treat anaemic HF patients with erythropoietin and intravenous ferric sucrose. After 6 months, treated patients reported improvements in mean haemoglobin concentration, LVEF, and NYHA functional class. 60 These results evoked high hopes and induced several clinical trials focusing on anaemia correction in HF. Unfortunately, long‐acting erythropoietin derivative alone did not show prognostic benefit in patients with HF. In the RED‐HF‐Trial (Reduction of Events by Darbepoetin Alfa in Heart Failure), anaemic patients with systolic HF treated with darbepoetin alfa did not show improvement concerning the primary endpoint in compared with non‐treated counterparts. Even more, darbepoetin alfa administration led to increase rate of thrombo‐embolic events (13.5% vs. 10.0%, respectively; P = 0.009) and an increased risk of ischaemic stroke (4.5% vs. 2.8%, respectively; P = 0.03), proving to be potentially harmful for treated patients. 85
Conclusions
In the last two decades, emerging importance of ID in symptomatic HF resulted in numerous very important clinical trials, which were able to underline necessity for iron supplementation and verify its impact on patient's quality of life.
In spite of certain inconsistency related to the cut‐off values for ID conformation across conducted clinical trials, ferritin of <100 mg/L or ferritin 100–300 mg/L when TSAT is <20% should be regarded as diagnostic for the presence of ID in HF patients.
Randomized clinical trials demonstrated that in HF patients with ID, intravenous iron repletion was well tolerated, safe, and associated with improvement in functional status and exercise capacity.
Although meta‐analyses and results from retrospective studies suggest beneficial effects of iron supplementation concerning hospitalization and cardiovascular mortality, definitive relationship of iron supplementation with mortality and morbidity in HF patients needs further validation in ongoing and future large‐scale, randomized prospective trials.
Conflict of interest
None declared.
Funding
M.L. was funded by the Slovenian Research Agency (grant no. J3‐9292, Burden of cachexia and sarcopenia in patients with chronic diseases: epidemiology, pathophysiology and outcomes, and grant no. J3‐9284, Epidemiology, pathophysiology and clinical relevance of anemia in chronic cardiopulmonary patients). G.L. was funded by Serbian Ministry of Science (grant no. 175033).
Acknowledgement
We would like to thank all authors for support and effort.
Loncar, G. , Obradovic, D. , Thiele, H. , von Haehling, S. , and Lainscak, M. (2021) Iron deficiency in heart failure. ESC Heart Failure, 8: 2368–2379. 10.1002/ehf2.13265.
References
- 1. Andrews NC. Disorders of iron metabolism. N Engl J Med 1999; 341: 1986–1995. [DOI] [PubMed] [Google Scholar]
- 2. Yeo TJ, Yeo PS, Ching‐Chiew Wong R, Ong HY, Leong KT, Jaufeerally F, Sim D, Santhanakrishnan R, Lim SL, Chan MYM, Chai P, Low AF, Ling LH, Ng TP, Richards AM, Lam CS. Iron deficiency in a multi‐ethnic Asian population with and without heart failure: prevalence, clinical correlates, functional significance and prognosis. Eur J Heart Fail 2014; 16: 1125–1132. [DOI] [PubMed] [Google Scholar]
- 3. von Haehling S, Gremmler U, Krumm M, Mibach F, Schön N, Taggeselle J, Dahm JB, Angermann CE. Prevalence and clinical impact of iron deficiency and anaemia among outpatients with chronic heart failure: the PrEP Registry. Clin Res Cardiol 2017; 106: 436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jacob C, Altevers J, Barck I, Hardt T, Braun S, Greiner W. Retrospective analysis into differences in heart failure patients with and without iron deficiency or anaemia. ESC Heart Fail 2019; 6: 840–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klip IT, Comin‐Colet J, Voors AA, Ponikowski P, Enjuanes C, Banasiak W, Lok DJ, Rosentryt P, Torrens A, Polonski L, van Veldhuisen DJ, van der Meer P, Jankowska EA. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013; 165: 575–582. [DOI] [PubMed] [Google Scholar]
- 6. Van Aelst LNL, Abraham M, Sadoune M, Lefebvre T, Manivet P, Logeart D, Launay JM, Karim Z, Puy H, Cohen‐Solal A. Iron status and inflammatory biomarkers in patients with acutely decompensated heart failure: early in‐hospital phase and 30‐day follow‐up. Eur J Heart Fail 2017; 19: 1075–1076. [DOI] [PubMed] [Google Scholar]
- 7. Núñez J, García‐Blas S, Comín‐Colet J. Iron deficiency and risk of early readmission following hospitalization for acute heart failure. Reply. Eur J Heart Fail 2016; 18: 881–881. [DOI] [PubMed] [Google Scholar]
- 8. Allain F, Loizeau V, Chaufourier L, Hallouche M, Herrou L, Hodzic A, Blanchart K, Belin A, Manrique A, Milliez P, Sabatier R, Legallois D. Usefulness of a personalized algorithm‐based discharge checklist in patients hospitalized for acute heart failure. ESC Heart Fail 2020; 7: 1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rocha BML, Cunha GJL, Menezes Falcão LF. The burden of iron deficiency in heart failure: therapeutic approach. J Am Coll Cardiol 2018; 71: 782–793. [DOI] [PubMed] [Google Scholar]
- 10. Martens P, Nijst P, Verbrugge FH, Smeets K, Dupont M, Mullens W. Impact of iron deficiency on exercise capacity and outcome in heart failure with reduced, mid‐range and preserved ejection fraction. Acta Cardiol 2018; 73: 115–123. [DOI] [PubMed] [Google Scholar]
- 11. Kurz K, Lanser L, Seifert M, Kocher F, Pölzl G, Weiss G. Anaemia, iron status, and gender predict the outcome in patients with chronic heart failure. ESC Heart Fail 2020; 7: 1880–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kell DB. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genomics 2009; 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cvetinovic N, Loncar G, Isakovic AM, von Haehling S, Doehner W, Lainscak M, Farkas J. Micronutrient depletion in heart failure: common, clinically relevant and treatable. Int J Mol Sci 2019; 20: 5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hower V, Mendes P, Torti FM, Laubenbacher R, Akman S, Shulaev V, Torti SV. A general map of iron metabolism and tissue‐specific subnetworks. Mol Biosyst 2009; 5: 422–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. von Haehling S, Jankowska EA, van Veldhuisen DJ, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease. Nat Rev Cardiol 2015; 12: 659–669. [DOI] [PubMed] [Google Scholar]
- 16. Dunn LL, Suryo Rahmanto Y, Richardson DR. Iron uptake and metabolism in the new millennium. Trends Cell Biol 2007; 17: 93–100. [DOI] [PubMed] [Google Scholar]
- 17. Cairo G, Bernuzzi F, Recalcati S. A precious metal: iron, an essential nutrient for all cells. Genes Nutr 2006; 1: 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carrondo MA. Ferritins, iron uptake and storage from the bacterioferritin viewpoint. EMBO J 2003; 22: 1959–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rouault TA, Tong WH. Iron–sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat Rev Mol Cell Biol 2005; 6: 345–351. [DOI] [PubMed] [Google Scholar]
- 20. Nemeth E. Iron regulation and erythropoiesis. Curr Opin Hematol 2008; 15: 169–175. [DOI] [PubMed] [Google Scholar]
- 21. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005; 352: 1011–1023. [DOI] [PubMed] [Google Scholar]
- 22. Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr 2001; 131: 568S–579S. [DOI] [PubMed] [Google Scholar]
- 23. Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res 2009; 81: 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dziegala M, Josiak K, Kasztura M, Kobak K, von Haehling S, Banasiak W, Anker SD, Ponikowski P, Jankowska E. Iron deficiency as energetic insult to skeletal muscle in chronic diseases. J Cachexia Sarcopenia Muscle 2018; 9: 802–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zizola C, Schulze PC. Metabolic and structural impairment of skeletal muscle in heart failure. Heart Fail Rev 2013; 18: 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Drexler H, Riede U, Münzel T, König H, Funke E, Just H. Alterations of skeletal muscle in chronic heart failure. Circulation 1992; 85: 1751–1759. [DOI] [PubMed] [Google Scholar]
- 27. Tkaczyszyn M, Drozd M, Węgrzynowska‐Teodorczyk K, Flinta I, Kobak K, Banasiak W, Ponikowski P, Jankowska EA. Depleted iron stores are associated with inspiratory muscle weakness independently of skeletal muscle mass in men with systolic chronic heart failure. J Cachexia Sarcopenia Muscle 2018; 9: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang AS, Enns CA. Molecular mechanisms of normal iron homeostasis. Hematology Am Soc Hematol Educ Program 2009; 1: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Munoz M, Garcı'a‐Erce JA, Remacha AF. Disorders of iron metabolism. Part 1: molecular basis of iron homoeostasis. J Clin Pathol 2011; 64: 281–286. [DOI] [PubMed] [Google Scholar]
- 30. Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell 2010; 142: 24–38. [DOI] [PubMed] [Google Scholar]
- 31. Anderson GJ, Frazer DM, McLaren GD. Iron absorption and metabolism. Curr Opin Gastroenterol 2009; 25: 129–135. [DOI] [PubMed] [Google Scholar]
- 32. Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J 2011; 434: 365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghafourian K, Shapiro JS, Goodman L, Ardehali H. Iron and heart failure: diagnosis, therapies, and future directions. JACC Basic Transl Sci 2020; 5: 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Babitt JL, Lin HY. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis 2010; 55: 726–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Viatte L, Vaulont S. Hepcidin, the iron watcher. Biochimie 2009; 91: 1223–1228. [DOI] [PubMed] [Google Scholar]
- 36. Ganz T, Olbina G, Girelli D, Nemeth E, Westerman M. Immunoassay for human serum hepcidin. Blood 2008; 112: 4292–4297. [DOI] [PubMed] [Google Scholar]
- 37. Mehta JL, Pothineni NV. Inflammation in heart failure: the holy grail? Hypertension 2016; 68: 27–29. [DOI] [PubMed] [Google Scholar]
- 38. Briasoulis A, Androulakis E, Christophides T, Tousoulis D. The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Fail Rev 2016; 21: 169–176. [DOI] [PubMed] [Google Scholar]
- 39. Jankowska EA, Kasztura M, Sokolski M, Bronisz M, Nawrocka S, Oleśkowska‐Florek W, Zymliński R, Biegus J, Siwołowski P, Banasiak W, Anker SD, Filippatos G, Cleland JG, Ponikowski P. Iron deficiency defined as depleted iron stores accompanied by unmet cellular iron requirements identifies patients at the highest risk of death after an episode of acute heart failure. Eur Heart J 2014; 35: 2468–2476. [DOI] [PubMed] [Google Scholar]
- 40. Shirazi LFBJ, Romeo F, Mehta JL. Role of inflammation in heart failure. Curr Atheroscler Rep 2017; 19: 27. [DOI] [PubMed] [Google Scholar]
- 41. Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol 2006; 1: S4–S8. [DOI] [PubMed] [Google Scholar]
- 42. Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron‐restricted erythropoiesis. Blood 2010; 116: 4754–4761. [DOI] [PubMed] [Google Scholar]
- 43. Cappellini MD, Comin‐Colet J, de Francisco A, Dignass A, Doehner W, Lam CS, Macdougall IC, Rogler G, Camaschella C, Kadir R, Kassebaum NJ, Spahn DR, Taher AT, Musallam KM, IRON CORE . Group. Iron deficiency across chronic inflammatory conditions: international expert opinion on definition, diagnosis, and management. Am J Hematol 2017; 92: 1068–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Veldhuisen DJ, Anker SD, Ponikowski P, Macdougall IC. Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches. Nat Rev Cardiol 2011; 8: 485–493. [DOI] [PubMed] [Google Scholar]
- 45. Hughes CM, Woodside JV, McGartland C, Roberts MJ, Nicholls DP, McKeown PP. Nutritional intake and oxidative stress in chronic heart failure. Nutr Metab Cardiovasc Dis 2012; 22: 376–382. [DOI] [PubMed] [Google Scholar]
- 46. Lourenço BH, Vieira LP, Macedo A, Nakasato M, Marucci Mde F, Bocchi EA. Nutritional status and adequacy of energy and nutrient intakes among heart failure patients. Arq Bras Cardiol 2009; 93: 541–548. [DOI] [PubMed] [Google Scholar]
- 47. McDonagh T, Damy T, Doehner W, Lam CSP, Sindone A, van der Meer P, Cohen‐Solal A, Kindermann I, Manito N, Pfister O, Pohjantähti‐Maaroos H, Taylor J, Comin‐Colet J. Screening, diagnosis and treatment of iron deficiency in chronic heart failure: putting the 2016 European Society of Cardiology heart failure guidelines into clinical practice. Eur J Heart Fail 2018; 20: 1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van Meer P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 49. Mei Z, Cogswell ME, Parvanta I, Lynch S, Beard JL, Stoltzfus RJ, Grummer‐Strawn LM. Hemoglobin and ferritin are currently the most efficient indicators of population response to iron interventions: an analysis of nine randomized controlled trials. J Nutr 2005; 135: 1974–1980. [DOI] [PubMed] [Google Scholar]
- 50. Koulaouzidis A, Said E, Cottier R, Saeed AA. Soluble transferrin receptors and iron deficiency, a step beyond ferritin. A systematic review. J Gastrointestin Liver Dis 2009; 18: 345–352. [PubMed] [Google Scholar]
- 51. Pasricha SR, Flecknoe‐Brown SC, Allen KJ, Gibson PR, McMahon LP, Olynyk JK, Roger SD, Savoia HF, Tampi R, Thomson AR, Wood EM, Robinson KL. Diagnosis and management of iron deficiency anaemia: a clinical update. Med J Aust 2010; 193: 525–532. [DOI] [PubMed] [Google Scholar]
- 52. Nanas JN, Matsouka C, Karageorgopoulos D, Leonti A, Tsolakis E, Drakos SG, Tsagalou EP, Maroulidis GD, Alexopoulos GP, Kanakakis JE, Anastasiou‐Nana MI. Etiology of anemia in patients with advanced heart failure. J Am Coll Cardiol 2006; 48: 2485–2489. [DOI] [PubMed] [Google Scholar]
- 53. Grote Beverborg N, Klip IT, Meijers WC, Voors AA, Vegter EL, van der Wal HH, Swinkels DW, van Pelt J, Mulder AB, Bulstra SK, Vellenga E, Mariani MA, de Boer RA, van Veldhuisen DJ, van der Meer P. Definition of iron deficiency based on the gold standard of bone marrow iron staining in heart failure patients. Circ Heart Fail 2018; 11: e004519. [DOI] [PubMed] [Google Scholar]
- 54. Kemna EH, Tjalsma H, Willems HL, Swinkels DW. Hepcidin: from discovery to differential diagnosis. Haematologica 2008; 93: 90–97. [DOI] [PubMed] [Google Scholar]
- 55. Jankowska EA, Malyszko J, Ardehali H, Koc‐Zorawska E, Banasiak W, von Haehling S, Macdougall IC, Weiss G, McMurray JJ, Anker SD, Gheorghiade M, Ponikowski P. Iron status in patients with chronic heart failure. Eur Heart J 2013; 34: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tkaczyszyn M, Comin‐Colet J, Voors AA, van Veldhuisen DJ, Enjuanes C, Moliner‐Borja P, Rozentryt P, Polonski L, Banasiak W, Ponikowski P, van der Meer P, Jankowska EA. Iron deficiency and red cell indices in patients with heart failure. Eur J Heart Fail 2018; 20: 114–122. [DOI] [PubMed] [Google Scholar]
- 57. Ganzoni AM. Intravenous iron‐dextran: therapeutic and experimental possibilities. Schweiz Med Wochenschr 1970; 100: 301–303. [PubMed] [Google Scholar]
- 58. Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin‐Colet J, Ruschitzka F, Lüscher TF, Arutyunov GP, Motro M, Mori C, Roubert B, Pocock SJ, Ponikowski P. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron‐deficient heart failure patients: an individual patient data meta‐analysis. Eur J Heart Fail 2018; 20: 125–133. [DOI] [PubMed] [Google Scholar]
- 59. Martens P, Minten L, Dupont M, Mullens W. Prevalence of underlying gastrointestinal malignancies in iron‐deficient heart failure. ESC Heart Fail 2019; 6: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Silverberg DS, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E, Brosh D, Laniado S, Schwartz D, Yachnin T, Shapira I, Gavish D, Baruch R, Koifman B, Kaplan C, Steinbruch S, Iaina A. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol 2000; 35: 1737–1744. [DOI] [PubMed] [Google Scholar]
- 61. Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, Tang WHW, LaRue SJ, Redfield MM, Semigran MJ, Givertz MM, Van Buren P, Whellan D, Anstrom KJ, Shah MR, Desvigne‐Nickens P, Butler J, Braunwald E, NHLBI Heart Failure Clinical Research Network . Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: the IRONOUT HF randomized clinical trial. JAMA 2017; 317: 1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Toblli JE, Lombraña A, Duarte P, Di Gennaro F. Intravenous iron reduces NT‐pro‐brain natriuretic peptide in anemic patients with chronic heart failure and renal insufficiency. J Am Coll Cardiol 2007; 50: 1657–1665. [DOI] [PubMed] [Google Scholar]
- 63. Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, Lüscher TF, Bart B, Banasiak W, Niegowska J, Kirwan BA, Mori C, von Eisenhart RB, Pocock SJ, Poole‐Wilson PA, Ponikowski P, FAIR‐HF Trial Investigators . Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448. [DOI] [PubMed] [Google Scholar]
- 64. Beck‐da‐Silva L, Piardi D, Soder S, Rohde LE, Pereira‐Barretto AC, de Albuquerque D, Bocchi E, Vilas‐Boas F, Moura LZ, Montera MW, Rassi S, Clausell N. IRON‐HF study: a randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol 2013; 168: 3439–3442. [DOI] [PubMed] [Google Scholar]
- 65. Ponikowski P, van Veldhuisen DJ, Comin‐Colet J, Ertl G, Komajda M, Mareev V, McDonagh T, Parkhomenko A, Tavazzi L, Levesque V, Mori C, Roubert B, Filippatos G, Ruschitzka F, Anker SD, CONFIRM‐HF Investigators . Beneficial effects of long‐term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur Heart J 2015; 36: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. van Veldhuisen DJ, Ponikowski P, van der Meer P, Metra M, Böhm M, Doletsky A, Voors AA, Macdougall IC, Anker SD, Roubert B, Zakin L, EFFECT‐HF Investigators . Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 2017; 136: 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Jankowska EA, Tkaczyszyn M, Suchocki T, Drozd M, von Haehling S, Doehner W, Banasiak W, Filippatos G, Anker SD, Ponikowski P. Effects of intravenous iron therapy in iron‐deficient patients with systolic heart failure: a meta‐analysis of randomized controlled trials. Eur J Heart Fail 2016; 18: 786–795. [DOI] [PubMed] [Google Scholar]
- 68. von Haehling S, Ebner N, Evertz R, Ponikowski P, Anker SD. Iron deficiency in heart failure: an overview. JACC Heart Fail 2019; 7: 36–46. [DOI] [PubMed] [Google Scholar]
- 69. Ebner N, Jankowska EA, Ponikowski P, Lainscak M, Elsner S, Sliziuk V, Steinbeck L, Kube J, Bekfani T, Scherbakov N, Valentova M, Sandek A, Doehner W, Springer J, Anker SD, von Haehling S. The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. Results from the Studies Investigating Co‐morbidities Aggravating Heart Failure. Int J Cardiol 2016; 205: 6–12. [DOI] [PubMed] [Google Scholar]
- 70. Comin‐Colet J, Lainscak M, Dickstein K, Filippatos GS, Johnson P, Lüscher TF, Mori C, Willenheimer R, Ponikowski P, Anker SD. The effect of intravenous ferric carboxymaltose on health‐related quality of life in patients with chronic heart failure and iron deficiency: a subanalysis of the FAIR‐HF study. Eur Heart J 2013; 34: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bourguignon S, Faller M, Champs FO, Moutier H, Levesque K, Caranhac G, Cohen‐Solal A. Budget impact of intravenous ferric carboxymaltose in patients with chronic heart failure and iron deficiency in France. ESC Heart Fail 2019; 6: 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Martin‐Malo A, Borchard G, Flühmann B, Mori C, Silverberg D, Jankowska EA. Differences between intravenous iron products: focus on treatment of iron deficiency in chronic heart failure patients. ESC Heart Fail 2019; 6: 241–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Avni TBA, Grossman A, Green H, Leibovici L, Gafter‐Gvili A. The safety of intravenous iron preparations: systematic review and meta‐analysis. Mayo Clin Proc 2015; 90: 12–23. [DOI] [PubMed] [Google Scholar]
- 74. Okonko DO, Grzeslo A, Witkowski T, Mandal AK, Slater RM, Roughton M, Foldes G, Thum T, Majda J, Banasiak W, Missouris CG, Poole‐Wilson PA, Anker SD, Ponikowski P. Effect of intravenous iron sucrose on exercise tolerance in anemic and nonanemic patients with symptomatic chronic heart failure and iron deficiency FERRIC‐HF: a randomized, controlled, observer‐blinded trial. J Am Coll Cardiol 2008; 51: 103–112. [DOI] [PubMed] [Google Scholar]
- 75. Okonko DO, Jouhra F, Abu‐Own H, Filippatos G, Colet JC, Suki C, Mori C, Ponikowski P, Anker SD, FAIR‐HF Investigators . Effect of ferric carboxymaltose on calculated plasma volume status and clinical congestion: a FAIR‐HF substudy. ESC Heart Fail 2019; 6: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Evstatiev R, Marteau P, Iqbal T, Khalif IL, Stein J, Bokemeyer B, Chopey IV, Gutzwiller FS, Riopel L, Gasche C, FERGI Study Group. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141 3:846–853. [DOI] [PubMed] [Google Scholar]
- 77. Martens P, Dupont M, Dauw J, Somers F, Herbots L, Timmermans P, Verwerft J, Mullens W. Rationale and design of the IRON‐CRT trial: effect of intravenous ferric carboxymaltose on reverse remodelling following cardiac resynchronization therapy. ESC Heart Fail 2019; 6: 1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lacour P, Dang PL, Morris DA, Parwani AS, Doehner W, Schuessler F, Hohendanner F, Heinzel FR, Stroux A, Tschoepe C, Haverkamp W, Boldt LH, Pieske B, Blaschke F. The effect of iron deficiency on cardiac resynchronization therapy: results from the RIDE‐CRT Study. ESC Heart Fail 2020; 7: 1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Núñez J, Monmeneu JV, Mollar A, Núñez E, Bodí V, Miñana G, García‐Blas S, Santas E, Agüero J, Chorro FJ, Sanchis J, López‐Lereu MP. Left ventricular ejection fraction recovery in patients with heart failure treated with intravenous iron: a pilot study. ESC Heart Fail 2016; 3: 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. van der Wal HH, Grote Beverborg N, Dickstein K, Anker SD, Lang CC, Ng LL, van Veldhuisen DJ, Voors AA, van der Meer P. Iron deficiency in worsening heart failure is associated with reduced estimated protein intake, fluid retention, inflammation, and antiplatelet use. Eur Heart J 2019; 40: 3616–3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ponikowski P, Kirwan BA, Anker SD, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Haboubi T, Keren A, Khintibidze I, Kragten H, Martinez FA, McDonagh T, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parhomenko A, Pascual‐Figal DA, Ruschitzka F, Sim D, Skouri H, van der Meer P, Jankowska EA. Rationale and design of the AFFIRM‐AHF trial: a randomised, double‐blind, placebo‐controlled trial comparing the effect of intravenous ferric carboxymaltose on hospitalisations and mortality in iron‐deficient patients admitted for acute heart failure. Eur J Heart Fail 2019; 21: 1651–1658. [DOI] [PubMed] [Google Scholar]
- 82. Comín‐Colet J, Enjuanes C, González G, Torrens A, Cladellas M, Meroño O, Ribas N, Ruiz S, Gómez M, Verdú JM, Bruguera J. Iron deficiency is a key determinant of health‐related quality of life in patients with chronic heart failure regardless of anaemia status. Eur J Heart Fail 2013; 15: 1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bekfani T, Pellicori P, Morris D, Ebner N, Valentova M, Sandek A, Doehner W, Cleland JG, Lainscak M, Schulze PC, Anker SD, von Haehling S. Iron deficiency in patients with heart failure with preserved ejection fraction and its association with reduced exercise capacity, muscle strength and quality of life. Clin Res Cardiol 2019; 108: 203–211. [DOI] [PubMed] [Google Scholar]
- 84. Beale AL, Warren JL, Roberts N, Meyer P, Townsend NP, Kaye D. Iron deficiency in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Open Heart 2019; 6: e001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. van der Meer P, Grote Beverborg N, Pfeffer MA, Olson K, Anand IS, Westenbrink BD, McMurray JJV, Swedberg K, Young JB, Solomon SD, van Veldhuisen DJ. Hyporesponsiveness to darbepoetin alfa in patients with heart failure and anemia in the RED‐HF study (Reduction of Events by Darbepoetin Alfa in Heart Failure): clinical and prognostic associations. Circ Heart Fail 2018; 11: e004431. [DOI] [PubMed] [Google Scholar]


