Abstract
Aims
As evidenced by scintigraphy imaging, the prevalence of transthyretin (TTR) cardiac amyloidosis in heart failure patients with preserved ejection fraction (HFpEF) and left ventricular hypertrophy (LVH) ranges between 13% and 19%. The natural evolution of cardiac amyloidosis begins with the deposition of amyloid material in the myocardium, with LVH ensuing at later stages. With current imaging modalities, it is possible to detect TTR cardiac amyloidosis before the hypertrophic stage. The aim of this study was to determine the prevalence of TTR cardiac amyloidosis in HFpEF patients without LVH.
Methods and results
The study prospectively enrolled patients admitted for HF with LV ejection fraction (LVEF) ≥ 50% and LV wall thickness <12 mm. TTR cardiac amyloidosis was diagnosed according to accepted criteria, which include positive cardiac 99‐Tc‐DPD scintigraphy in the absence of monoclonal protein expansion in blood. Transthyretin gene sequencing was performed in positive patients. From July 2017 to January 2020, 329 patients with HFpEF and LV thickness <12 mm were identified. After exclusions, 58 patients completed the study with cardiac scintigraphy (79 years, 54% men; median LVEF 60% and LV wall thickness 10.5 mm). Three patients (5.2%) were positive for TTR cardiac amyloidosis; genetic analysis excluded the presence of hereditary TTR amyloidosis. Positive patients baseline characteristics (84 years, 67% men, LVEF 60%, and LV wall thickness 11 mm) were similar to patients without TTR, except for troponin levels (0.05 vs. 0.02 ng/mL, P = 0.03) and glomerular filtration rate (82 vs. 60 mL/min, P = 0.032), which were higher in TTR patients.
Conclusions
In a cohort of patients with HFpEF without LVH, the prevalence of TTR cardiac amyloidosis was 5%. Early diagnosis of cardiac involvement in TTR amyloidosis (before manifest LVH) would seem recommendable because newly approved specific treatments can prevent additional deposition of amyloid material.
Keywords: Transthyretin cardiac amyloidosis, Heart failure with preserved ejection fraction, Cardiac amyloidosis
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a common condition whose prevalence is projected to increase further in the coming years due to population aging. HFpEF is a frequent manifestation of several underlying myocardial diseases. This etiological heterogeneity, combined with the lack of benefits from current treatments across the HFpEF spectrum, presents challenges for the management of these patients. Identifying the aetiology underlying HFpEF is therefore a crucial step toward improved patient management.
Cardiac amyloidosis is a frequent cause of HFpEF. Historically, light‐chain amyloidosis (AL) was considered the most frequent form; however, recent evidence has revealed transthyretin (TTR) amyloidosis to be more prevalent. Two forms of TTR cardiac amyloidosis (TTR‐CA) have been described, a genetic (hereditary) form and a wild‐type (sporadic) form, with the wild‐type form more frequent. Until recently, cardiac amyloidosis was diagnosed by positive histopathology after endomyocardial biopsy, but recent advances in non‐invasive imaging (technetium‐labelled cardiac scintigraphy) allow non‐invasive diagnosis with high specificity. 1 The new non‐invasive tests have revealed that the prevalence of wild‐type TTR‐CA is much higher than previously thought; according to a report, wild‐type TTR‐CA is present in up to 13% of HFpEF patients with left ventricular hypertrophy (LVH). 2 As a result of these advances, the proportion of HFpEF patients with a formal diagnosis of TTR amyloidosis has increased significantly. Identifying the aetiology of HFpEF is of high clinical interest because of the availability of new treatments for TTR‐CA, such as Tafamidis. 3 Tafamidis works by blocking amyloid deposition in the target organ (the heart in this case) but does not eliminate material that has already been deposited. Therefore, the sooner Tafamidis therapy is initiated in the course of the disease, the greater the benefit in terms of amyloid deposition inhibition.
Cardiac amyloidosis is suspected in patients with HFpEF and LVH. However, the disease begins with the deposition of amyloid material in the myocardium, with LVH appearing only at later stages as the result of the accumulation of material and associated inflammation and fibrosis. We hypothesized that current imaging techniques would permit the detection of cardiac involvement in TTR early in the course of the disease, long before manifest LVH. Early diagnosis before LVH onset would open a window of opportunity for therapy to curb the disease in its early stages by preventing further amyloid deposition. The earlier the disease is detected, the greater the benefit from the newly identified specific treatments. 3 The aim of this study was to determine the prevalence of TTR amyloidosis in early‐stage HFpEF patients without significant LVH.
Methods
We conducted a prospective, observational, single‐centre study. The study was approved by our institutional ethics committee. All participants provided written informed consent.
Study population
Patients were recruited during hospitalization at Fundación Jiménez Díaz University Hospital, Madrid, Spain. The screening included all consecutive patients undergoing echocardiography during admission to the Cardiac Imaging laboratory. All patients admitted to the hospital for heart failure (HF) undergo an echocardiography study in this laboratory.
Inclusion criteria were (i) ≥18 years; (ii) hospital admission with signs and symptoms of HF according to current guidelines 4 (such as crackles on pulmonary auscultation, congestion on chest X‐ray, or a third heart sound) or elevated natriuretic peptides (BNP > 100 pg/mL or NT‐proBNP > 300 pg/mL); (iii) left ventricular ejection fraction (LVEF) ≥ 50%; and (iv) maximum wall thickness <12 mm.
Exclusion criteria were (i) a diagnosis of malignant forms of plasma cell dyscrasia, such as multiple myeloma or lymphoplasmacytic lymphoma; (ii) presence of cardiomyopathy, acute coronary syndrome, severe valvular heart disease, valvular prostheses, conduction disturbances, or severe anaemia (haemoglobin <8 g/dL) 5 ; (iii) a previous diagnosis of cardiac amyloidosis; (iv) any contraindication for scintigraphy (e.g. allergy and pregnancy); (v) or important systemic diseases or short life expectancy that the investigator considered could obstruct study completion.
Study protocol
All screened patients fulfilling the echocardiographic inclusion criteria were invited to participate. Once informed consent was obtained, a blood analysis was performed including a complete blood count, standard biochemical markers, serum free light chain assays, and serum immunofixation. Urine samples were also obtained and tested in urine free light chain and immunofixation assays.
Standard transthoracic echocardiography was performed by a physician with expertise in cardiac imaging using a commercially available system (IE33, HD15 and EPIQ 7C, Philips Medical Systems, Andover, USA). Echocardiography data were stored digitally.
All study participants were scheduled for post‐discharge 99m Tc‐3,3‐diphosphono‐1,2‐propanodicarboxylic acid (99‐Tc‐DPD) scintigraphy. Before scintigraphy, each patient was administered intravenously with 740 MBq of 99‐Tc‐DPD. After 3 h, patients were scanned using Sopha Medical Vision hybrid single photon emission computed tomography and computed tomography gamma cameras. Anterior and posterior whole body images were obtained, together with selective images in anterior, 45° left anterior oblique, and left lateral projections. Images were obtained using the following parameters: (i) whole‐body images, LEHR collimator, 512 × 256 matrix, 3000 kc; (ii) selective cardiac images, LEHR collimator, 256 × 256 matrix, 500 kc, zoom factor of 1.2. The resulting greyscale images were independently analysed by two experienced physicians. Cardiac 99‐Tc‐DPD retention was graded according to a reported visual scale (Perugini score) 6 from 0 to 3 points: 0 points indicates no myocardial uptake, 1 point indicates myocardial uptake below that of bone, 2 points indicates myocardial uptake equal to bone, and 3 points indicates myocardial uptake greater than bone. Diagnosis of TTR‐CA was established for scores >1, 1 and patients with a positive scintigraphy result were further analysed for complete DNA sequencing of the TTR gene.
Data collection and clinical follow‐up
Demographic and clinical characteristics were collected during admission. Hypertension, diabetes, and dyslipidaemia were defined according to current guidelines. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 Ischaemic cardiomyopathy was defined as the presence of coronary stenosis >70% or >50% in left main coronary artery, independently of the required treatment. Left atrial (LA) dilatation was considered when LA maximum diameter in a parasternal long‐axis view was ≥35 mm or when LA major length in an apical four‐chamber view was ≥53mm. 10
Statistical analysis
Quantitative variables are presented as mean ± standard deviation for normal distributions and as medians (interquartile range) for non‐normal distributions. Qualitative variables are presented as percentages.
Kolmogorov–Smirnov or Shapiro–Wilk tests were used to determine normal or non‐normal distribution for each variable. To compare baseline and final values between groups, χ 2 or Fisher tests were used for qualitative variables and Student's t or Mann–Whitney tests were used for quantitative variables, depending on normal or non‐normal distribution.
Analyses were performed with SPSS 19.0 (SPSS Inc., New York). Statistical differences were considered significant at P < 0.05 (two‐tailed).
Results
Study population
Between July 2017 and January 2020, 329 patients hospitalized due to HF and fulfilling the echocardiographic criteria (LVEF ≥ 50% and LV wall thickness < 12 mm) were identified. Of these patients, 161 (48.9%) were excluded for a variety of reasons (Figure 1 ): 1 patient was excluded because of a previous diagnosis of myeloma; 48 patients because of previous cardiac disease (21 patients with severe valvular heart disease; 9 patients with valvular prostheses; 9 patients with conduction disturbances) or severe anaemia (9); and 112 patients due to contraindicating clinical criteria. In addition, 1 patient (0.3%) died before inclusion, and 48 patients (14.6%) declined to participate. The study population therefore consisted of 119 patients. Of the included patients, 61 (51.2%) did not undergo the scintigraphy imaging study due to death before the scheduled procedure (n = 18) or withdrawal of consent (n = 43). The remaining 58 (48.7%) underwent scintigraphy (Figure 1 ), performed 30 (17–70.5) days after screening echocardiography.
Figure 1.
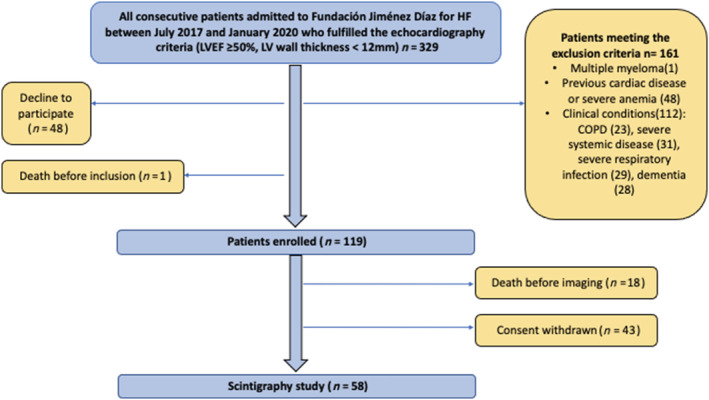
Recruitment and inclusion process. COPD, chronic obstructive pulmonary disease; HF, heart failure; LV, left ventricle; LVEF, left ventricular ejection fraction.
Baseline characteristics
Baseline characteristics are summarized in Table 1 . Median age was 79 (75–85) years, and 54% were male. Median LVEF was 60% (55–60%), and median LV wall thickness was 10.5 mm. Median BNP was 368 (170–967.8) pg/mL, and median NT‐proBNP was 1540 (978.5–2895) pg/mL, and median troponin levels were 0.02 ng/mL.
Table 1.
Baseline characteristics of total population and comparison of patients with positive and negative scintigraphy
| Population description | Total (n = 58) | Negative scintigraphy (n = 55) | Positive scintigraphy (n = 3) | P |
|---|---|---|---|---|
| Age, years | 79 (75–85) | 79 (75–85) | 84 | 0.487 |
| Age <65 years | 2 | 2 | 0 | 1.000 |
| Men | 54 | 54 | 67 | 1.000 |
| Diabetes | 36 | 36 | 33 | 1.000 |
| Smokers | 32 | 34 | 0 | 0.386 |
| Dyslipidaemia | 53 | 55 | 33 | 0.593 |
| Arterial hypertension | 83 | 86 | 33 | 0.074 |
| Atrial fibrillation | 64 | 64 | 100 | 0.971 |
| Ischaemic heart disease | 14 | 15 | 0 | 1.000 |
| Pacemaker carriers | 10 | 11 | 0 | 1.000 |
| Previous hospitalization for HF | 22 | 22 | 33 | 0.540 |
| MGUS | 19 | 20 | 0 | 1.000 |
| Medical treatments | ||||
| Aspirin | 28 | 27 | 33 | 1.000 |
| Anticoagulation | 64 | 64 | 100 | 1.000 |
| Clopidogrel | 5 | 6 | 0 | 1.000 |
| ACEIs | 41 | 42 | 33 | 1.000 |
| ARBs | 19 | 20 | 0 | 1.000 |
| Beta‐blockers | 62 | 64 | 33 | 0.551 |
| Calcium channel blockers | 18 | 19 | 0 | 1.000 |
| Thiazide diuretics | 19 | 20 | 0 | 1.000 |
| Loop diuretics | 81 | 82 | 33 | 0.474 |
| Mineral corticoid receptor antagonists | 17 | 18 | 0 | 1.000 |
| Digoxin | 9 | 9 | 0 | 1.000 |
| Statins | 45 | 44 | 33 | 0.582 |
| Antiarrhythmics | 12 | 13 | 0 | 1.000 |
| Laboratory values | ||||
| Creatinine, mg/dL | 1 (0.8–1.36) | 1 (0.8–1.37) | 0.8 | 0.080 |
| Estimated glomerular filtration rate, mL/min | 60 (49.7–74.5) | 60 (49–74) | 81.9 | 0.032 |
| Haemoglobin, g/dL | 12.4 (11.2–13.8) | 12 (11–14) | 12.9 | 0.549 |
| Platelets, n/mm3 | 214,500 (167,750–290,750) | 215,000 (161,000–289,000) | 214,000 | 0.715 |
| Leucocytes, n/mm3 | 6900 (5700–8887) | 6800 (5700–8850) | 7400 | 0.921 |
| Neutrophils | 66 (59–72) | 66 (60–72) | 52 | 0.324 |
| Sodium, mEq/L | 139 (137–141) | 139 (137–141) | 138 | 0.417 |
| Potassium, mEq/L | 4.1 (3.8–4.6) | 4.1 (3.8–4.7) | 3.9 | 0.232 |
| NT‐proBNP, pg/mL | 1540 (978.5–2895) | 1830 (1110–3800) | 1230 | 0.240 |
| BNP, pg/mL | 368 (170–967.8) | 312 (195–947) | 129 | 0.182 |
| Troponin I, ng/mL | 0.02 (0–0.03) | 0.02 (0–0.02) | 0.05 (0.04–0.05) | 0.003 |
| Echocardiographic parameters | ||||
| LVEF | 60 (55–60) | 60 (55–60) | 60 | 0.246 |
| Left atrium dilatation | 90 | 89 | 100 | 1.000 |
| Left atrium (PLA), mm | 41 (36.7–45) | 41 (36–45) | 44 | 0.432 |
| Left atrium (AFC), mm | 59 (55.7–63) | 58 (55–63) | 61 | 0.595 |
| Diastolic dysfunction or non‐evaluable | 96 | 96 | 100 | 0.543 |
| Left ventricle wall thickness, mm | 10.5 (9.5–11) | 10.5 (9.5–11) | 11 | 0.260 |
| E' wave (mm) | 7 (6–8) | 7 (6–8) | ||
| Right ventricle hypertrophy | 0 | 0 | 0 | |
| Interatrial septum hypertrophy | 2 | 2 | 0 | 1 |
| Pericardial effusion | 9 | 9 | 0 | 1 |
| Clinical signs | ||||
| Carpal tunnel syndrome | 5 | 4 | 33 | 0.15 |
| Lumbar spinal stenosis | 12 | 11 | 33 | 0.325 |
| Biceps tendon rupture | 0 | 0 | 0 | |
Data are presented as are median (interquartile range) or percentages. Interquartile ranges are not presented because of low number of patients. Data on natriuretic peptides above the median were only available for 100 patients.
ACEIs, angiotensin converting‐enzyme inhibitors; ARBs, angiotensin II receptor blockers; HF, heart failure; LVEF, left ventricular ejection fraction; MGUS, monoclonal gammopathy of uncertain significance.
Prevalence of transthyretin cardiac amyloidosis
Of the 58 scintigraphy studies, three (5.2%) were positive for TTR‐CA. In all three patients, radiotracer uptake was graded as 3 on the Perugini scale (myocardial uptake greater than bone uptake). Imaging results for the three affected patients are shown in Figures 2 , 3 , 4 . Positive patients baseline characteristics (84 years, 67% men, LVEF 60%, and LV wall thickness 11 mm) were similar to patients without TTR (Table 1 ), except for glomerular filtration rates, which were significantly better in positive patients (81.9 vs. 60 mL/min, P = 0.032) and troponin levels, which were higher in positive patients (0.05 vs. 0.02 ng/mL, P = 0.03). Serum and urine immunofixation and free light chains test were normal (Table 2 ).
Figure 2.
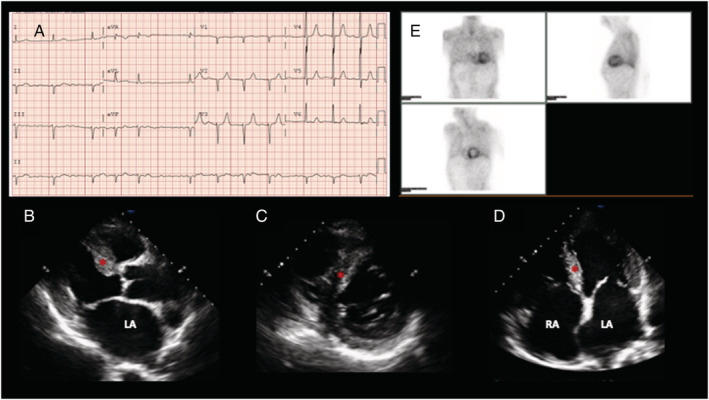
Positive Patient 1. (A) Electrocardiogram showing atrial fibrillation. The pseudoinfarction pattern is typical of TTR‐CA, evidenced by an anteroseptal QS wave. Left anterior hemiblock can be also observed. (B–D) Transthoracic echocardiogram. Long parasternal axis (B), short parasternal axis (C), and four‐chamber view (D). Bi‐atrial enlargement can be observed (LA, left atrium; RA, right atrium). Maximum septal wall‐thickness is 11 mm (red *). (E) 99‐Tc‐DPD scintigraphy: Intense and heterogeneous radiotracer uptake was detected predominantly in the left ventricle and was classified as grade 3 on the Perugini scale (uptake greater than that of bone). Uptake was also detected in the right ventricle.
Figure 3.
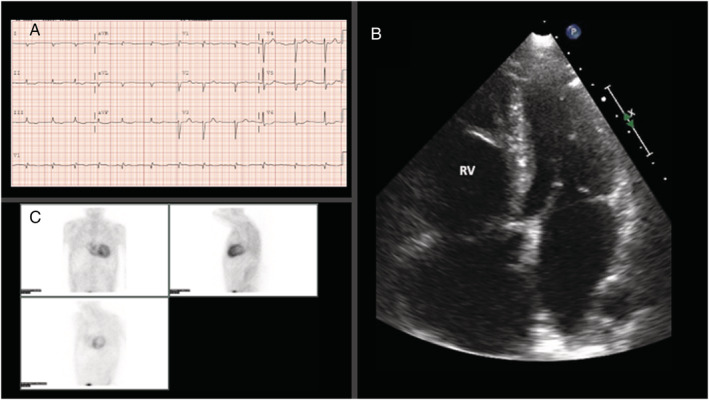
Positive Patient 2. (A) Electrocardiogram showing atrial fibrillation and an anterior QS wave suggestive of a pseudoinfarction pattern. Low voltage is observed in the limb leads. (B). Transthoracic echocardiography (4‐chamber view) showing right ventricular dilatation. (C) 99‐Tc‐DPD scintigraphy with radiotracer uptake (grade 3 on the Perugini scale). Radiotracer uptake is more marked in the right ventricle, consistent with the systolic dysfunction and right ventricular dilatation in this patient.
Figure 4.
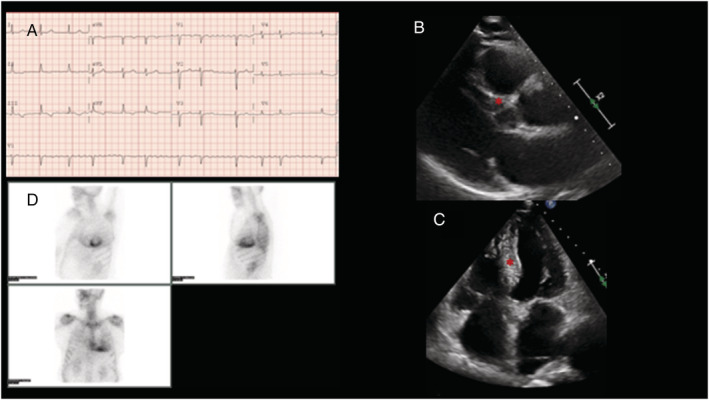
Positive Patient 3. (A) Electrocardiogram showing atrial fibrillation. Abnormal repolarization is evident in the inferior limb leads. (B, C) Transthoracic echocardiogram: long parasternal view (B) and 4‐chamber view (C). The interventricular septum (red *) shows areas with increased echogenicity. (D) 99‐Tc‐DPD scintigraphy. Left ventricle radiotracer uptake is observed, classified as grade 3 on the Perugini scale.
Table 2.
Patients with transthyretin cardiac amyloidosis
| Population description | Positive patient 1 | Positive patient 2 | Positive patient 3 |
|---|---|---|---|
| Age, years | 86 | 84 | 76 |
| Sex | Male | Male | Female |
| Diabetes | No | No | Yes |
| Smoker | No | No | No |
| Dyslipidaemia | No | Yes | No |
| Arterial hypertension | No | No | Yes |
| Atrial Fibrillation | Yes | Yes | Yes |
| Ischaemic heart disease | No | No | No |
| Severe aortic stenosis | No | No | No |
| Pacemaker carrier | No | No | No |
| Previous hospitalization for HF | No | Yes | No |
| MGUS | No | No | No |
| NYHA functional class | I | II | II |
| Carpal tunnel syndrome | No | No | Yes |
| Lumbar canal stenosis | No | No | Yes |
| Laboratory values | |||
| Creatinine, mg/dL | 0.9 | 0.8 | 0.78 |
| Estimated glomerular filtration rate, mL/min | 77 | 82 | 73.8 |
| NT‐proBNP, pg/mL | 1230 | 2070 | 856 |
| Troponin I, ng/mL | 0.04 | 0.10 | 0.05 |
| Serum immunofixation | Negative | Negative | Negative |
| Urine immunofixation | Negative | Negative | Negative |
| Kappa light chains (mg/dL) | 29 | 21 | 20 |
| Lambda light chains (mg/dL) | 25 | 19 | 13 |
| Ratio free light chains | 1.17 | 1.13 | 1.49 |
| Electrocardiography | |||
| Rhythm | AF | AF | AF |
| Low voltages | No | Yes | No |
| Right bundle branch block | No | No | No |
| Left bundle branch block | No | No | No |
| Left anterior hemiblock | Yes | No | No |
| Hypertrophy pattern | No | No | No |
| Pseudoinfarction pattern | Yes | Yes | No |
| Echocardiographic parameters | |||
| LVEF, % | 60 | 60 | 65 |
| Maximal LV wall thickness, mm | 11 | 9.5 | 11 |
| Left atrium (LPA), mm | 47 | 44 | 39 |
| Left atrium (4C), mm | 60 | 63 | 61 |
| Right atrium (4C), mm | 59 | 69 | 58 |
| Pericardial effusion | No | No | No |
| S wave (tissue Doppler right ventricle), mm | 11 | 8 | 11.5 |
| Scintigraphy | |||
| Uptake grade (Perugini) | 3 | 3 | 3 |
| Genetic study | |||
| Complete sequencing TTR gene | Negative | Negative | Negative |
| Events | |||
| HF readmissions | No | Yes | No |
| Death | No | No | No |
Cut‐off values for troponin I: upper limit of normality = 0.08 ng/mL; cut‐off value for myocardial infarction = 0.12 ng/mL.
AF, atrial fibrillation; HF, heart failure; LPA, long paraesternal axis; LV, left ventricle; LVEF, left ventricular ejection fraction; MGUS, monoclonal gammopathy of uncertain significance; NYHA, New York Heart Association classification; TTR, transthyretin; 4C, four‐chamber view.
Characteristics of patients with transthyretin cardiac amyloidosis
Of the three patients diagnosed with TTR‐CA, Patients 1 and 2 were men (84 and 86 years old, respectively), and Patient 3 was a 76‐year‐old woman. Baseline characteristics are shown in Table 2 . Only one patient (Patient 3) had clinical features of TTR amyloidosis (carpal tunnel syndrome and spinal canal stenosis). In all three patients, renal function was normal, whereas NT‐proBNP was elevated. Median NT‐proBNP for the TTR‐CA patients was 1230 pg/mL, compared with 1540 (978.5–28,955) pg/mL for all patients with a scintigraphy study. Troponin was elevated in 1 of the TTR‐CA patients. Electrocardiograms revealed atrial fibrillation in all three patients. The QRS complex was narrow in all three patients, and no patient had high QRS voltages. Median LVEF was 60%, and median LV wall thickness was 11 mm. All three patients showed bi‐atrial enlargement. Right ventricle (RV) systolic function was low‐normal in Patients 1 and 3, whereas Patient 2 had RV systolic dysfunction, consistent with higher radiotracer uptake in the RV in this patient. TTR gene sequencing excluded the hereditary form of TTR in all patients. Survival at 2 years was 100%.
Discussion
To the best of our knowledge, this is the first study to prospectively evaluate TTR amyloidosis prevalence in HFpEF patients without LVH. TTR amyloidosis prevalence in this population was 5.2%. Previous studies have been focused in patients with LVH, reporting TTR‐CA prevalence between 13% and 19%. 2 , 11 , 12 A recent study in 108 patients with HFpEF with LVH with endomyocardial biopsy estimated a prevalence of cardiac amyloidosis (TTR‐CA and AL amyloidosis) of 14%. 12 Thus, the combination of these rates in patients with LVH with the 5% estimated in our population without LVH yields an estimated prevalence of TTR amyloidosis in patients hospitalized for HFpEF between 5% and 19%.
Transthyretin cardiac amyloidosis begins with the deposition of amyloid material in the myocardium. Prefibrillar transthyretin oligomers infiltrate the myocardium, and the deposition of this material has been linked to an intramyocardial inflammatory response observed in up to 48% of patients with biopsy‐proven cardiac amyloidosis. 13 Moreover, animal experiments showed that inflammation can promote further amyloid deposition. 14 The inflammatory response leads to fibrosis, which can be detected by cardiac magnetic resonance. 15 The amyloid deposition thus begins much early than the later development of LVH, which manifests only in later stages. 16 Halting disease progression requires diagnosis in these early stages, before LVH develops. Tafamidis, the only drug demonstrated to improve survival in wild‐type TTR‐CA patients, acts by blocking new amyloid deposition, and the ATTR‐ACT study (Tafamidis in Transthyretin Cardiomyopathy Clinical Trial) showed that the drug is more effective in early stages of the disease. 3 In the trial, Tafamidis treatment of TTR amyloidosis patients was associated with a 30% lower all‐cause mortality and a lower cardiovascular hospitalization rate. 3 The reduction in mortality was observed at 30 months. Probably related to the mechanism of action of Tafamidis, Kaplan–Meier curves showed a parallel course until month 18, when they started to diverge. Tafamidis blocks the synthesis of new amyloid fibrils by preventing the unfolding of the transthyretin tetramer into monomers; it does not eliminate already deposited amyloid material. Given this mechanism of action and the progressive nature of the disease, Tafamidis is expected to have greater benefit in early stages of the disease, 3 which might in part explain the finding that Tafamidis was not cost‐effective in a later analysis in the USA. 17 Similarly, the benefits of Tafamidis in a 6 min walk test and in quality of life questionnaires were noticeable after just 6 months of treatment, again suggesting that diagnosis in the early stages of the disease, before LVH ensues, would increase the chances of treatment effectiveness. In our study, TTR amyloidosis was diagnosed in 5% of HFpEF patients even in the absence of significant LV echocardiographic abnormalities, demonstrating the feasibility of identification of the disease before LVH ensues. All patients included in this study met criteria for HFpEF according to ESC Guidelines, 4 and therefore, the results could be extrapolated to future studies. Pre‐LVH diagnosis of TTR‐CA is possible with technetium‐labelled cardiac scintigraphy. This imaging technique allows for screening of HFpEF patients without LVH and no etiological diagnosis, especially in specific subgroups such as elderly patients, with conduction abnormalities or other red flags for TTR‐CA. Scintigraphy has lower diagnostic performance in patients with lower pre‐test probabilities, such as younger patients. 99‐Tc‐DPD scintigraphy screening is feasible, because it is affordable (around 30€ per dose in Spain) and involves low radiation exposure for the patient (around 4 mSv, similar to 2 year background radiation in a city like Madrid). However, recent studies have highlighted some limitations of technetium‐labelled cardiac scintigraphy. 18 Misdiagnosis can occur when light‐chain amyloid cardiomyopathy is not excluded; moreover, false‐positive results have been described in the presence of acute or subacute myocardial infarction, which can cause focal uptake, and false‐negative results may occur when myocardial deposits are minimal and in certain pathogenic transthyretin mutations. 18 In our study, we tested for and excluded patients with amyloid light‐chain amyloidosis; moreover, none of the patients with a positive scintigraphy result had a history of myocardial infarction, and hereditary forms were excluded in the three diagnosed patients. We can therefore have confidence in the diagnosis of sporadic TTR‐CA.
Despite the clear value of pre‐LVH diagnosis of TTR‐CA, establishing the clinical grounds for suspecting the disease presents a challenge at these early stages. Aside from LVH, other echocardiographic features that might suggest TTR‐CA include loss of atrial function, bi‐atrial enlargement, diastolic dysfunction with elevated filling pressures, RV dysfunction, and pericardial effusion. Among the three TTR amyloidosis patients identified in our population, three had bi‐atrial dilatation, two had low‐normal RV function, and one had RV systolic dysfunction. The presence of HFpEF with right‐sided signs of HF in combination with poor tolerance to antihypertensive drugs may be a strong indication of TTR‐CA. More specific features of the disease may also be present, such as bilateral carpal tunnel syndrome (as in Patient 3), spontaneous biceps tendon rupture, spinal canal stenosis (Patient 3), and peripheral neuropathy with lower limb paresthesia or dysautonomia. TTR amyloidosis is also suggested by a pseudoinfarction pattern in the electrocardiogram (as in Patients 1 and 2), conduction disturbances (Patient 1), or low voltage criteria (Patient 2). The presence of any of these echocardiographic and clinical signs might be a previous step to identify those patients with higher level of suspicion who would be better candidates for cardiac scintigraphy screening.
Limitations
This study has the limitations inherent to an observational single‐centre study, with its potential for selection bias. Second, the screened population was elderly, and some of the patients died or abandoned the study due to physical limitations that prevented them from completing the tests. This limited the number of completed scintigraphy studies (n = 58) and may have limited the detection of the prevalence of TTR‐CA in this HFpEF population. Nevertheless, these limitations would lead to an underestimation of disease and therefore do not reduce the clinical impact of the results. A third consideration is the use of the ESC guideline definition of HFpEF; American guidelines define HFpEF in patients with LVEF >40%. 19 The use of the American definition would have included more patients in the study population, and this might have resulted in a higher disease prevalence given that TTR‐CA sometimes produces mild left ventricular dysfunction. Another potential limitation is the use of non‐invasive criteria to diagnose TTR‐CA. These criteria were described by Gillmore et al. 1 and the positive predictive value for TTR amyloidosis diagnosis was 100%; however, endomyocardial biopsy remains the gold standard. A further issue with scintigraphy is that a false‐negative result is possible when myocardial infiltration is minimal at a very early disease stage. Finally, patients with TTR‐CA were not given a follow‐up echocardiography examination. Future studies are needed to address important questions about when patients with early‐stage TTR‐CA will develop LVH and what influence this would have on their prognosis.
Conclusions
In a population of HFpEF patients without LVH, the prevalence of transthyretin cardiac amyloidosis was 5.2%. These results suggest that TTR amyloidosis screening is justified in HFpEF patients lacking an etiological diagnosis, even in the absence of LVH, particularly in specific subgroups such as elderly patients. Early diagnosis (before significant LVH ensues) is important because treatments shown to increase survival work by preventing the deposition of additional amyloid material and are therefore more effective at early disease stages.
Conflict of interest
J.T. participates in talks and Advisory Boards for Sanofi in the area of PCSK9 inhibitors. The remaining authors declare no conflict of interest.
Funding
This study received funding from the Instituto de Salud Carlos III (ISCIII; PI19/00655), with cofunding from the European Regional Development Fund (ERDF) ‘A way of making Europe’ (A.A.). B.I. is funded by the European Research Council (ERC) under the European Union Horizon 2020 Research and Innovation Programme (ERC‐Consolidator Grant agreement No. 819775) and by the Ministry of Science and Innovation (‘RETOS 2019’ grant no. PID2019‐107332RB‐I00) The CNIC is supported by the ISCIII, the MCN, and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (SEV‐2015‐0505).
Acknowledgement
Simon Bartlett (CNIC) provided English editing.
Devesa, A. , Camblor Blasco, A. , Pello Lázaro, A. M. , Askari, E. , Lapeña, G. , Gómez Talavera, S. , Taibo Urquía, M. , Rodríguez Olleros, C. , Tuñón, J. , Ibáñez, B. , and Aceña, Á. (2021) Prevalence of transthyretin amyloidosis in patients with heart failure and no left ventricular hypertrophy. ESC Heart Failure, 8: 2856–2865. 10.1002/ehf2.13360.
Contributor Information
Borja Ibáñez, Email: bibanez@cnic.es.
Álvaro Aceña, Email: aacena@fjd.es.
References
- 1. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AWJM, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016; 133: 2404–2412. [DOI] [PubMed] [Google Scholar]
- 2. González‐López E, Gallego‐Delgado M, Guzzo‐Merello G, De Haro‐Del Moral FJ, Cobo‐Marcos M, Robles C, Bornstein B, Salas C, Lara‐Pezzi E, Alonso‐Pulpon L, Garcia‐Pavia P. Wild‐type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015; 36: 2585–2594. [DOI] [PubMed] [Google Scholar]
- 3. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 2018; 379: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, Van Der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016; 37: 2129–2200.27206819 [Google Scholar]
- 5. WHO . Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. 2011;1–6.
- 6. Perugini E, Guidalotti PL, Salvi F, Cooke RMT, Pettinato C, Riva L, Leone O, Farsad M, Ciliberti P, Bacchi‐Reggiani L, Fallani F, Branzi A, Rapezzi C. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc‐3,3‐diphosphono‐1,2‐propanodicarboxylic acid scintigraphy. J Am Coll Cardiol Elsevier Masson SAS 2005; 46: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 7. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J 2018; 39: 3021–3104. [DOI] [PubMed] [Google Scholar]
- 8. Cosentino F, Grant PJ, Aboyans V, Bailey CJ, Ceriello A, Delgado V, Federici M, Filippatos G, Grobbee DE, Hansen TB, Huikuri HV, Johansson I, Jüni P, Lettino M, Marx N, Mellbin LG, Östgren CJ, Rocca B, Roffi M, Sattar N, Seferović PM, Sousa‐Uva M, Valensi P, Wheeler DC. 2019 ESC Guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force for diabetes, pre‐diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Associ. Eur Heart J 2019; 41: 255–323. [DOI] [PubMed] [Google Scholar]
- 9. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen M‐R, Tokgozoglu L, Wiklund O. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J 2019; 41: 111–188. [DOI] [PubMed] [Google Scholar]
- 10. Kou S, Caballero L, Dulgheru R, Voilliot D, De Sousa C, Kacharava G, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Gomez De Diego JJ, Hagendorff A, Henri C, Hristova K, Lopez T, Magne J, De LMG, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, Salustri A, Van De Veire N, Von Bardeleben RS, Vinereanu D, Voigt JU, Zamorano JL, Donal E, Lang RM, Badano LP, Lancellotti P. Echocardiographic reference ranges for normal cardiac chamber size: results from the NORRE study. Eur Heart J Cardiovasc Imaging 2014; 15: 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Presti SL, Horvath SA, Mihos CG, Rajadhyaksha C, McCloskey V, Santana O. Transthyretin cardiac amyloidosis as diagnosed by 99mTc‐PYP scanning in patients with acute heart failure and preserved ejection fraction. Crit Pathw Cardiol 2019; 18: 195–199. [DOI] [PubMed] [Google Scholar]
- 12. Hahn VS, Yanek LR, Vaishnav J, Ying W, Vaidya D, Lee YZJ, Riley SJ, Subramanya V, Brown EE, Hopkins CD, Ononogbu S, Perzel Mandell K, Halushka MK, Steenbergen C, Rosenberg AZ, Tedford RJ, Judge DP, Shah SJ, Russell SD, Kass DA, Sharma K. Endomyocardial biopsy characterization of heart failure with preserved ejection fraction and prevalence of cardiac amyloidosis. JACC Heart Fail 2020; 8: 712–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siegismund CS, Escher F, Lassner D, Kühl U, Gross U, Fruhwald F, Wenzel P, Münzel T, Frey N, Linke RP, Schultheiss HP. Intramyocardial inflammation predicts adverse outcome in patients with cardiac AL amyloidosis. Eur J Heart Fail 2018; 20: 751–757. [DOI] [PubMed] [Google Scholar]
- 14. Gorevic PD. Amyloid and inflammation. Proc Natl Acad Sci U S A 2013; 110: 16291–16292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dorbala S, Cuddy S, Falk RH. How to image cardiac amyloidosis: a practical approach. JACC Cardiovasc Imaging 2019; 13: 1368–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation 2012; 126: 1286–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kazi DS, Bellows BK, Baron SJ, Shen C, Cohen DJ, Spertus JA, Yeh RW, Arnold SV, Sperry BW, Maurer MS, Shah SJ. Cost‐effectiveness of tafamidis therapy for transthyretin amyloid cardiomyopathy. Circulation 2020; 141: 1214–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hanna M, Ruberg FL, Maurer MS, Dispenzieri A, Dorbala S, Falk RH, Hoffman J, Jaber W, Soman P, Witteles RM, Grogan M. Cardiac scintigraphy with technetium‐99m‐labeled bone‐seeking tracers for suspected amyloidosis: JACC Review Topic of the Week. J Am Coll Cardiol 2020; 75: 2851–2862. [DOI] [PubMed] [Google Scholar]
- 19. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJV, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WHW, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Am Coll Cardiol 2013; 62: e147–e239. [DOI] [PubMed] [Google Scholar]


