Abstract
Aims
Knowledge about the impact of epinephrine on the outcome in venoarterial (VA) extracorporeal membrane oxygenation (ECMO) patients is limited, and existing data are conflicting.
Methods and results
We conducted a retrospective cohort study in a 1500 bed tertiary university hospital. Five hundred and eighty‐nine VA‐ECMO patients were analysed. The median age was 57 years [47–65], 68% of male. The major indications for ECMO were post‐cardiotomy cardiogenic shock (CS) (38%) and medical CS (36%). Two hundred and sixty‐two (44.5%) patients received epinephrine alone or associated with another catecholamine while on ECMO. Baseline factors significantly associated with epinephrine administration were younger age, higher sequential organ failure assessment score, cardiac arrest at implantation, and intra‐aortic balloon pump support at implantation, whereas medical CS and dobutamine administration were significantly associated with a lower risk of epinephrine administration. Epinephrine administration was independently associated with death [hazard ratio = 1.68 (1.44–2.23); P < 0.01]. A sensitivity analysis with propensity score inverse probability weighting in complete cases confirmed a significant association of epinephrine administration with death [hazard ratio = 1.69 (1.43–2.00); P < 0.001].
Conclusions
Among patients who required VA‐ECMO, epinephrine administration was associated with an increased risk for death.
Keywords: Extracorporeal life support, Vasopressors, Inotropes, Catecholamines, Cardiogenic shock, Outcome
Introduction
The European and US guidelines support the use of vasopressors, such as norepinephrine or epinephrine, to increase blood pressure and vital organ perfusion in cardiogenic shock (CS). 1 , 2 However, several recent studies have highlighted potential negative effects of epinephrine on survival or organ failure in patients with medical causes of CS. 3 , 4 , 5 In contrast, a recent meta‐analysis of randomized trials did not find any worse outcome associated with the continuous administration of epi.nephrine in critically ill patients, including CS patients. 6
Data on the use of epinephrine in extracorporeal life support are limited. 4 , 7 The inflammatory response observed after extracorporeal membrane oxygenation (ECMO) implantation is similar to that seen in inflammatory shock, a state where efficacy and safety of epinephrine administration were found similar to the association of dobutamine and norepinephrine. 8 , 9 Others advocated a less detrimental effect of epinephrine in hearts with reduced wall stress and better coronary perfusion. 4 Therefore, the main goal of our study was to determine the impact of epinephrine administration on survival in a large cohort of venoarterial (VA) ECMO patients.
Methods
Setting and patients
We conducted a retrospective analysis of prospectively collected data, in accordance with the Strengthening The Reporting of Observational Studies in Epidemiology (STROBE) guidelines. The study took place in a 1500 bed tertiary university hospital. All patients who had ECMO during their stay from the 1 January 2005 to the 31 December 2019 were screened from our institutional ECMO database, and only patients with VA‐ECMO were included. However, patients supported with VA‐ECMO for primary graft failure following heart transplant were excluded. Indications for VA‐ECMO therapy included medical and surgical causes of refractory CS in whom satisfactory systemic perfusion (systolic artery pressure > 80 mmHg, left atrial pressure < 20 mmHg, cardiac index higher than 1.8 L/min/m2) could not be achieved despite optimal intravascular volume status, high‐dose inotropic medication (epinephrine > 0.2 μg/kg/min or dobutamine > 15 μg/kg/min with or without norepinephrine > 0.2 μg/kg/min), and/or other support. Our study complied with the Declaration of Helsinki. Our database was approved by the French data protection authority: Commission Nationale de l'Informatique et des Libertés (CNIL, Reference 1685088, 25 July 2013). The need for written consent was waived because of the observational design.
Surgical procedure
The standard protocol for VA‐ECMO implantation in our institution has been previously published. 10 Briefly, the implanting team included two surgeons (senior and resident), a scrub nurse, and a perfusionist. All required material was available on a dedicated trolley to allow ECMO implantation to be performed wherever required, including in the operative theatre, intensive care units, and catheterization room. Peripheral implantation through the femoral access was used when possible. In all peripheral VA cases, a reperfusion catheter was introduced to prevent limb ischaemia. VA‐ECMO implantation was performed within the cardiac surgery operating theatre if the patient could be safely transported, but in case of unstable haemodynamics or cardiac arrest, implantation was performed bedside. Removal of the VA‐ECMO cannula was performed in the operating theatre (except in case of death under support) to allow optimal vessel repair.
Outcome
The primary outcome of the study was mortality within 30 days of ECMO implantation.
Statistical analysis
Statistical analysis was performed with the statistical software R 3.4.3 and the Statistical Package for Social Sciences Version 25 (SPSS Inc., IBM, Armonk, New York). Categorical variables were described as number (percentage), and continuous variables as median and interquartile range. The χ 2 test and Fisher's exact test were used to compare categorical variables as appropriate. The Mann–Whitney U test and the Kruskal–Wallis test were used to compare continuous variables. All univariate analyses were performed on complete cases. Multivariable logistic regression analysis was used to study variables associated with the use of epinephrine. The primary analysis of 30 days of mortality was performed using a multivariable Cox model.
Overall, 3.1% of data were missing (11.7% of patients had at least one missing value). For the purposes of the Cox multivariable analysis, missing data were assumed to be random and were handled by multiple imputation using chained equations. Ten imputed datasets were created, and the results were pooled according to Rubin's rule 11 and were reported as adjusted hazard ratios (HRs) with their 95% confidence intervals (CIs). All variables with a univariate association with the primary outcome at a P‐value < 0.20 level were included in the multivariable analysis.
Finally, to evaluate the robustness of the primary analysis, a sensitivity analysis with propensity score (PS) inverse probability weighting (IPW) was performed in the entire population in order to generate a weighted cohort in which baseline characteristic distributions were independent of epinephrine exposure. 12 , 13 PS calculation was based on variables associated with epinephrine administration and/or death with a P‐value < 0.20 in the univariate analysis.
All tests were two‐sided, and a P‐value < 0.05 was considered statistically significant.
Results
Population
During the study period, a total of 744 patients required ECMO support in our institution for other reasons than heart transplantation, including 589 patients who required VA‐ECMO (Figure 1 ). The median age was 57 years [47–65]. The major indications for ECMO were post‐cardiotomy CS (38%) and medical CS (36%). The median sequential organ failure assessment (SOFA) score on the day of ECMO implantation was 11 [9–12] (Table 1 and Supporting Information, Table S1 ). One‐hundred and sixteen patients (20%) were intubated for more than 24 h before ECMO implantation, but the median duration of mechanical ventilation before implantation was 0 days [0–1]. In the majority of patients (94%), ECMO was implanted peripherally (Table 1 ).
Figure 1.
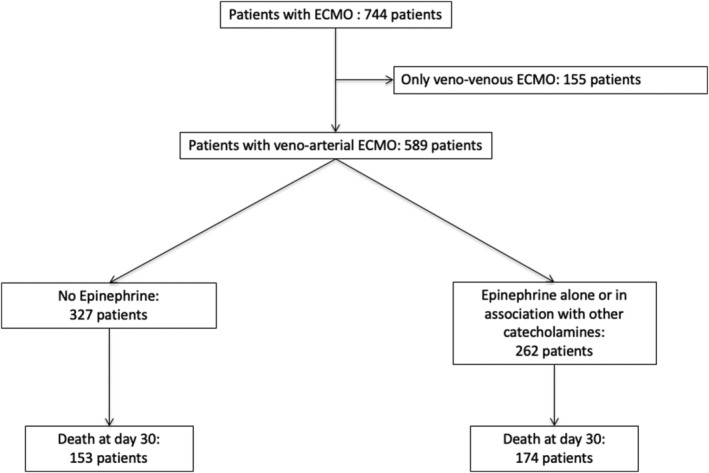
Flow chart of study population. ECMO, extracorporeal membrane oxygenation.
Table 1.
Baseline characteristics
| Variables | All patients | Patients receiving epinephrine | Patients without epinephrine | P‐value |
|---|---|---|---|---|
| n = 589 | n = 262 | n = 327 | ||
| Simplified acute physiology score II [IQR] | 43 [33–59] | 46 [35–63] | 41 [31–53] | <0.01 |
| Age, years [IQR] | 57 [46–65] | 55 [44–63] | 58 [48–66] | <0.01 |
| Male—no. (%) | 402 (68) | 178 (68) | 224 (69) | 0.95 |
| Co‐morbidities | ||||
| Diabetes—no. (%) | 19 (3) | 7 (3) | 12 (4) | 0.66 |
| Arteriopathy—no. (%) | 6 (1) | 1 (0) | 5 (2) | 0.33 |
| Hypertension—no. (%) | 43 (7) | 15 (6) | 28 (9) | 0.25 |
| Reason for ECMO implantation | ||||
| Post‐cardiotomy cardiogenic shock—no. (%) | 221 (38) | 98 (37) | 123 (38) | 1 |
| Septic shock—no. (%) | 3 (1) | 1 (0) | 2 (1) | 1 |
| Medical cardiogenic shock—no. (%) | 212 (36) | 79 (30) | 133 (41) | 0.01 |
| Out of hospital cardiac arrest—no. (%) | 80 (14) | 47 (18) | 33 (10) | <0.01 |
| RV dysfunction during ARDS—no. (%) | 16 (3) | 7 (3) | 9 (3) | 1 |
| Other support at ECMO implantation | ||||
| Intra‐aortic balloon pump—no. (%) | 102 (17) | 53 (20) | 49 (15) | 0.12 |
| Number of days with mechanical ventilation [IQR] | 0 [0–1] | 0 [0–1] | 0 [0–1] | <0.01 |
| Extra‐renal epuration—no. (%) | 35 (6) | 23 (9) | 12 (4) | 0.02 |
| Dobutamine—no. (%) | 380 (66) | 142 (55) | 238 (74) | <0.01 |
| Norepinephrine—no. (%) | 377 (65) | 163 (63) | 214 (67) | 0.37 |
| Characteristics at ECMO implantation | ||||
| SOFA score [IQR] | 11 [9–12] | 11 [9–13] | 10 [8–12] | <0.01 |
| Cardiac arrest—no. (%) | 151 (26) | 90 (34) | 61 (19) | <0.01 |
| ECMO localization | ||||
| Central—no. (%) | 36 (6) | 16 (6) | 20 (6) | 1 |
| Periphery—no. (%) | 555 (94) | 246 (95) | 309 (95) | 0.89 |
| Central then periphery—no. (%) | 4 (1) | 1 (0) | 3 (1) | 0.63 |
ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; RV, right ventricular; SOFA, sequential organ failure assessment.
Factors associated with epinephrine administration
Of 589 included patients, 262 (44.5%) received epinephrine alone or associated with another catecholamine while on ECMO. Baseline factors significantly associated with epinephrine administration are displayed on Table 2 . A younger age was associated with epinephrine administration [adjusted odds ratio (OR) = 0.98 per each supplementary year, 95% CI (0.97–1.00); P < 0.01]. Patients with higher severity as suggested by higher SOFA score [adjusted OR = 1.33 per each supplementary point, 95% CI (1.21–1.45); P < 0.01] or cardiac arrest at ECMO implantation [adjusted OR = 2.14, 95% CI (1.26–3.65); P < 0.01] had a higher risk to receive epinephrine. Although medical CS and dobutamine administration were associated with a lower risk of epinephrine administration [adjusted OR = 0.22, 95% CI (0.13–0.38) and adjusted OR = 0.38, 95% CI (0.23–0.65); P < 0.01 for both], intra‐aortic balloon pump support at implantation was associated with an increased risk of epinephrine administration [OR = 2.66, 95% CI (1.53–4.64); P < 0.01].
Table 2.
Baseline characteristics associated with epinephrine administration (logistic regression)
| Variables | Univariate | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P‐value | OR | 95% CI | P‐value | |
| Simplified acute physiology score II | 1.02 | 1.01–1.02 | <0.01 | 1.00 | 0.99–1.02 | 0.42 |
| Age | 0.99 | 0.97–1 | 0.03 | 0.98 | 0.97–1.00 | 0.01 |
| Male | 0.9 | 0.62–1.3 | 0.57 | |||
| Co‐morbidities | ||||||
| Diabetes | 0.85 | 0.32–2.27 | 0.75 | |||
| Arteriopathy | 0.3 | 0.03–2.72 | 0.26 | |||
| Hypertension | 0.75 | 0.38–1.46 | 0.39 | |||
| Other support at ECMO implantation | ||||||
| Intra‐aortic balloon pump | 1.5 | 0.95–2.35 | 0.08 | 2.66 | 1.53–4.64 | <0.01 |
| Number of days with mechanical ventilation | 0.99 | 0.94–1.04 | 0.65 | |||
| Extra‐renal epuration | 2.59 | 1.23–5.47 | 0.012 | 1.23 | 0.48–3.11 | 0.67 |
| Dobutamine | 0.36 | 0.25–0.53 | <0.01 | 0.22 | 0.13–0.38 | <0.01 |
| Norepinephrine | 0.75 | 0.52–1.07 | 0.11 | 0.43 | 0.26–0.70 | 0.12 |
| Reason for ECMO implantation | ||||||
| Post‐cardiotomy | Ref | Ref | Ref | Ref | Ref | Ref |
| Septic shock | 0.62 | 0.06–7.02 | 0.70 | 0.21 | 0.02–2.80 | 0.24 |
| Cardiogenic shock | 0.77 | 0.51–1.16 | 0.21 | 0.38 | 0.23–0.65 | <0.01 |
| Out of hospital cardiac arrest | 1.93 | 1.09–3.40 | 0.02 | 0.52 | 0.23–1.19 | 0.12 |
| RV dysfunction during ARDS | 0.97 | 0.35–2.72 | 0.96 | 0.62 | 0.11–1.16 | 0.08 |
| Characteristics at ECMO implantation | ||||||
| SOFA score | 1.13 | 1.06–1.21 | <0.01 | 1.33 | 1.21–1.45 | <0.01 |
| Cardiac arrest | 2.23 | 1.49–3.33 | <0.01 | 2.14 | 1.26–3.65 | <0.01 |
| ECMO localization | ||||||
| Central | 0.78 | 0.36–1.7 | 0.53 | |||
| Periphery | 1.12 | 0.51–2.49 | 0.78 | |||
| Central then periphery | 0.4 | 0.04–3.92 | 0.43 | |||
ARDS, acute respiratory distress syndrome; CI, confidence interval; ECMO, extracorporeal membrane oxygenation; OR, odds ratio; RV, right ventricular; SOFA, sequential organ failure assessment.
Outcomes
Factors associated with death after Cox model multivariable analysis are displayed on Table 3 . Epinephrine administration was significantly associated with death [adjusted HR = 1.68, 95% CI (1.44–2.23); P < 0.01] (Figure 2 ). Additionally, a sensitivity analysis with PS IPW was performed in complete cases, which confirmed a significant association of epinephrine administration with death [HR = 1.69, 95% CI (1.43–2.00); P < 0.001].
Table 3.
Factors associated with death in full population (589 patients)
| Variables | Number of patient with available data | Univariate analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | ||
| Simplified acute physiology score II | 527 | 1.02 | 1.01–1.03 | <0.01 | 1.01 | 1.00–1.02 | <0.01 |
| Age | 589 | 1.02 | 1.01–1.03 | <0.01 | 1.02 | 1.01–1.03 | <0.01 |
| Male | 589 | 0.96 | 0.76–1.21 | 0.72 | |||
| Co‐morbidities | |||||||
| Diabetes | 589 | 1.15 | 0.63–2.10 | 0.65 | |||
| Peripheral artery disease | 589 | 1.54 | 0.57–4.12 | 0.39 | |||
| Hypertension | 589 | 1.41 | 0.97–2.05 | 0.07 | 0.98 | 0.66–1.47 | 0.93 |
| Other support at ECMO implantation | |||||||
| Intra‐aortic balloon counter pulsation | 589 | 0.89 | 0.67–1.19 | 0.44 | |||
| Number of days with mechanical ventilation | 589 | 1.00 | 0.97–1.03 | 0.98 | |||
| Extra‐renal epuration | 589 | 1.64 | 1.09–2.47 | 0.02 | 1.15 | 0.70–1.88 | 0.59 |
| Dobutamine | 579 | 0.63 | 0.50–0.79 | <0.01 | 0.78 | 0.59–1.02 | 0.07 |
| Norepinephrine | 579 | 1.13 | 0.89–1.43 | 0.30 | |||
| Reason for ECMO implantation | |||||||
| Post‐cardiotomy | 532 | Ref | Ref | Ref | Ref | Ref | Ref |
| Septic shock | 532 | 1.09 | 0.53–2.22 | 0.82 | 1.45 | 0.38–5.61 | 0.58 |
| Cardiogenic shock | 532 | 3.44 | 0.91–12.99 | 0.07 | 0.98 | 0.74–1.30 | 0.89 |
| Out of hospital cardiac arrest | 532 | 1.01 | 0.49–2.07 | 0.98 | 0.98 | 0.64–1.49 | 0.93 |
| RV dysfunction during ARDS | 532 | 1.83 | 0.87–3.84 | 0.11 | 1.12 | 0.50–2.49 | 0.78 |
| Characteristics at ECMO implantation | |||||||
| SOFA score | 589 | 1.01 | 0.97–1.05 | 0.63 | |||
| Cardiac arrest | 589 | 2.19 | 1.74–2.76 | <0.01 | 1.82 | 1.38–2.40 | <0.01 |
| ECMO localization | |||||||
| Central | 589 | 1.58 | 1.05–2.38 | 0.03 | 1.58 | 1.03–2.44 | 0.04 |
| Catecholamine administration | |||||||
| Epinephrine administration | 589 | 1.79 | 1.44–2.23 | <0.01 | 1.68 | 1.33–2.11 | <0.01 |
ARDS, acute respiratory distress syndrome; CI, confidence interval; ECMO, extracorporeal membrane oxygenation; HR, hazard ratio; RV, right ventricular; SOFA, sequential organ failure assessment.
Figure 2.
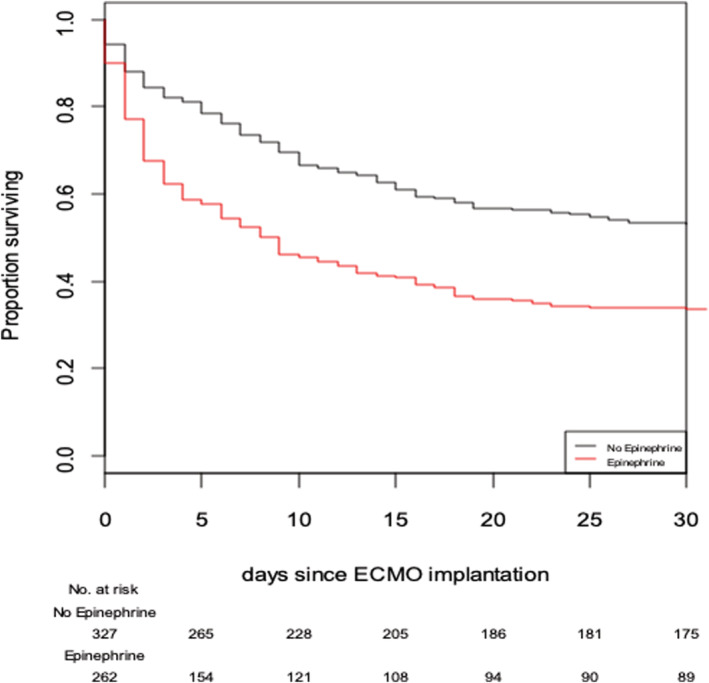
Survival curves for use of epinephrine with any catecholamine combination versus no epinephrine use (log‐rank test). ECMO, extracorporeal membrane oxygenation.
Other outcomes are reported on Table 4 . More patients of the epinephrine group died from persistent CS, although the difference was not statistically significant (16% vs. 10%, P = 0.06).
Table 4.
Outcomes
| Variables | All patients | Patients receiving epinephrine | Patients without epinephrine | P‐value |
|---|---|---|---|---|
| n = 589 | n = 262 | n = 327 | ||
| Length of ECMO support, days [IQR] | 5 [2–9] | 4 [1–8] | 6 [3–10] | <0.001 |
| Survivor at 30 days* | 5 [2–9] | 7 [4–10] | 7 [4–10] | 0.97 |
| Length of vasopressor support, days [IQR] | 6 [2–12] | 5 [2–11] | 7 [3–13] | 0.006 |
| Survivor at 30 days* | 6 [2–12] | 5 [8–15] | 5 [8–13] | 0.43 |
| Length of stay in the ICU, days [IQR] | 18 [11–33] | 19 [12–35] | 18 [10–31] | 0.21 |
| Thrombosis—no. (%) | 316 (54) | 146 (56) | 170 (52) | 0.25 |
| Mesenteric ischaemia—no. (%) | 23 (4) | 12 (5) | 11 (3) | 0.59 |
| Acute renal failure—no. (%) | 244 (41) | 115 (44) | 129 (39) | 0.32 |
| Infection acquired during ECMO support—no. (%) | 191 (32) | 74 (28) | 117 (36) | 0.06 |
| Death at Day 30—no. (%) | 327 (55) | 174 (66) | 153 (47) | <0.001 |
| Reason for death | ||||
| Persistent cardiogenic shock—no. (%) | 74 (13) | 41 (16) | 33 (10) | 0.06 |
| Haemorrhage—no. (%) | 12 (2) | 3 (1) | 9 (3) | 0.28 |
| Thrombosis—no. (%) | 4 (1) | 3 (1) | 1 (0) | 0.33 |
| Mesenteric ischaemia—no. (%) | 4 (1) | 2 (1) | 2 (1) | 1 |
| Neurologic complication—no. (%) | 14 (2) | 9 (3) | 5 (2) | 0.22 |
| Infection acquired during ECMO support—no. (%) | 10 (2) | 4 (2) | 6 (2) | 1 |
ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; IQR, interquartile range.
Among patients who survived at 30 days (n = 269, 174 patients without epinephrine and 88 receiving epinephrine).
Discussion
In this large single‐centre observational study of 589 VA‐ECMO patients, epinephrine administration was independently associated with an increased risk of death. A high proportion of VA‐ECMO patients (44.5%) received epinephrine alone or associated with another catecholamine. Epinephrine use was associated with younger age, severity (higher SOFA score or cardiac arrest at implantation), and intra‐aortic balloon pump support at implantation, whereas medical CS and dobutamine administration were associated with a lower risk of epinephrine administration.
The available data on epinephrine use in VA‐ECMO are limited, and, to our knowledge only, one other study specifically compared the effects of epinephrine with other vasopressors in a VA‐ECMO population. The authors compared the effect of epinephrine alone, epinephrine plus an inodilator (levosimendan and/or dobutamine), or no vasopressors, in 231 patients with VA‐ECMO, implanted for CS or extracorporeal cardiopulmonary resuscitation. 7 As in our study, the authors found that the use of epinephrine with or without an inodilator was associated with increased 30 days of mortality. Of note, most of the patients received norepinephrine continuous infusion (90.5%).
The negative impact of epinephrine on survival has also been described in CS. The multinational CardShock study, which prospectively enrolled 219 patients with medical CS, demonstrated in several analyses using differing adjustment methods that epinephrine was independently associated with increased 90 days of mortality. 5 Moreover, an individual meta‐analysis of 2583 CS patients found a significantly higher risk of short‐term death in epinephrine‐treated patients, and again this result was confirmed after adjustment on selected variables in a subset of 1227 patients and after PS matching of two sets of 338 patients. 4 Notably, in the same study, sensitivity analyses confirmed the association with a poor outcome in several subgroups, except in the small subgroup (n = 124) that benefited from extracorporeal life support therapy (defined as ECMO and left ventricular assist device in that study). The only double‐blind multicentre randomized trial available compared the efficacy and safety of epinephrine versus norepinephrine for CS after acute myocardial infarction. When the vasopressor dose was adjusted to achieve equivalent cardiac index, epinephrine use was associated with a higher incidence of refractory CS. This preliminary finding led to early termination of the study, which included only 52 patients. 3 In contrast, a meta‐analysis of randomized trials in critically ill patients, including the latter study, showed that continuous infusion of epinephrine was not associated with a worse outcome. However, most of the 1277 patients included in this study were septic shock patients (n = 677) with only 168 CS or cardiac surgery patients. 6
Several mechanisms may explain the worse outcomes observed with the use of epinephrine in CS patients, supported by VA‐ECMO or not. First, epinephrine infusion has been associated with an excess of myocardial work and myocardial oxygen consumption. Levy et al. found a significant increase of the double products (heart rate × systolic arterial pressure) and a higher heart rate during epinephrine infusion compared with the dobutamine–norepinephrine combination in CS from ischaemic aetiology or not. 3 , 14 Second, in the same studies, the authors found a transient increase in arterial lactate levels and tonometered PCO2 gap (difference between gastric mucosal PCO2 and arterial PCO2), a surrogate of splanchnic perfusion adequacy. These results might reflect previously described epinephrine‐related metabolic effects such as aerobic glycolysis or splanchnic thermogenic effects, but an excessive splanchnic vasoconstriction cannot be ruled out. 15 , 16 Moreover, epinephrine represses drug metabolism enzymes and induces a local inflammatory response via interleukin‐6 production in human hepatocytes in primary culture and in the human HepaRG cell line, respectively. 17 Finally, epinephrine‐related immune modulation might lead to immune paralysis with inhibition of tumour necrosis factor‐α and an increase of interleukin‐10 systemic productions, 18 , 19 as well as inhibition of nuclear factor‐κB in monocytes 20 or down‐regulation of toll‐like receptors in macrophages. 21
This study is, to date, the largest analysis evaluating the effects of epinephrine administration in VA‐ECMO patients. The robustness of our results was challenged by the use of two differing adjustment methods, which produced comparable estimates of epinephrine treatment effect. However, our study does have several limitations. First, while the data were prospectively collected, this was a retrospective observational analysis with its inherent limitations. Second, we acknowledge that sicker patients had higher chances to receive epinephrine, suggesting that this vasopressor could have been used as a rescue therapy in the most severely ill patients. However, a significant association between epinephrine administration and death was found after multivariable adjustment with a Cox model regression. The association held true after a second method of adjustment using PS with IPW. Although unmeasured confounding may persist despite statistical adjustment, our results suggest that the higher mortality observed among epinephrine recipients may not be solely explained by their more severe baseline profile. Third, some variables lacked granularity in the database such as ‘medical CS’, which included a wide range of ECMO indications. Finally, the single‐centre design may also limit the generalizability of our findings.
In conclusion, epinephrine administration was associated with an increased risk of death in patients on VA‐ECMO. These results support the findings of other recent studies that highlight possible detrimental effects of epinephrine in patients with CS. Together, these results support the need for a prospective randomized trial to address the optimum vasopressor strategy for patients on VA‐ECMO.
Conflict of interest
The authors declare that they have no competing interests.
Funding
None.
Supporting information
Table S1. Etiology of cardiogenic shock and association with death.
Acknowledgements
We would like to thank for their assistance the research nurses Véronique Desriac and Pascale Rouault.
Massart, N. , Mansour, A. , Ross, J. T. , Ecoffey, C. , Aninat, C. , Verhoye, J.‐P. , Launey, Y. , Tadie, J.‐M. , Auffret, V. , Flecher, E. , and Nesseler, N. (2021) Epinephrine administration in venoarterial extracorporeal membrane oxygenation patients is associated with mortality: a retrospective cohort study. ESC Heart Failure, 8: 2899–2906. 10.1002/ehf2.13370.
References
- 1. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG, for the American Heart Association Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Mission: Lifeline . Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2017; 136: e232–e268. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld JA, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
- 3. Levy B, Clere‐Jehl R, Legras A, Morichau‐Beauchant T, Leone M, Frederique G, Quenot J‐P, Kimmoun A, Cariou A, Lassus J. Epinephrine versus norepinephrine for cardiogenic shock after acute myocardial infarction. J Am Coll Cardiol 2018; 72: 173–182. [DOI] [PubMed] [Google Scholar]
- 4. Léopold V, Gayat E, Pirracchio R, Spinar J, Parenica J, Tarvasmäki T, Lassus J, Harjola V‐P, Champion S, Zannad F. Epinephrine and short‐term survival in cardiogenic shock: an individual data meta‐analysis of 2583 patients. Intensive Care Med 2018; 44: 847–856. [DOI] [PubMed] [Google Scholar]
- 5. Tarvasmäki T, Lassus J, Varpula M, Sionis A, Sund R, Køber L, Spinar J, Parissis J, Banaszewski M, Cardoso JS, Carubelli V. Current real‐life use of vasopressors and inotropes in cardiogenic shock—adrenaline use is associated with excess organ injury and mortality. Crit Care 2016; 20: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belletti A, Nagy A, Sartorelli M, Mucchetti M, Putzu A, Sartini C, Morselli F, de Domenico P, Zangrillo A, Landoni G, Lembo R. Effect of continuous epinephrine infusion on survival in critically ill patients: a meta‐analysis of randomized trials. Crit Care Med 2020; 48: 398–405. [DOI] [PubMed] [Google Scholar]
- 7. Zotzmann V, Rilinger J, Lang CN, Kaier K, Benk C, Duerschmied D, Biever PM, Bode C, Wengenmayer T, Staudacher DL. Epinephrine, inodilator, or no inotrope in venoarterial extracorporeal membrane oxygenation implantation: a single‐center experience. Crit Care 2019; 23: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care 2016; 20: 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Annane D, Vignon P, Renault A, Bollaert P‐E, Charpentier C, Martin C, Troché G, Ricard JD, Nitenberg G, Papazian L, Azoulay E, Bellissant E. Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet 2007; 370: 676–684. [DOI] [PubMed] [Google Scholar]
- 10. Flecher E, Anselmi A, Corbineau H, Langanay T, Verhoye JP, Felix C, Leurent G, le Tulzo Y, Malledant Y, Leguerrier A. Current aspects of extracorporeal membrane oxygenation in a tertiary referral centre: determinants of survival at follow‐up. Eur J Cardiothorac Surg 2014; 46: 665–671. [DOI] [PubMed] [Google Scholar]
- 11. Rubin D. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley; 1987. [Google Scholar]
- 12. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983; 70: 41–55. [Google Scholar]
- 13. Austin PC, Stuart EA. The performance of inverse probability of treatment weighting and full matching on the propensity score in the presence of model misspecification when estimating the effect of treatment on survival outcomes. Stat Methods Med Res 2017; 26: 1654–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levy B, Bollaert P‐E, Charpentier C, Nace L, Audibert G, Bauer P, Nabet P, Larcan A. Comparison of norepinephrine and dobutamine to epinephrine for hemodynamics, lactate metabolism, and gastric tonometric variables in septic shock: a prospective, randomized study. Intensive Care Med 1997; 23: 282–287. [DOI] [PubMed] [Google Scholar]
- 15. De Backer D, Creteur J, Silva E, Vincent JL. Effects of dopamine, norepinephrine, and epinephrine on the splanchnic circulation in septic shock: which is best? Crit Care Med 2003; 31: 1659–1667. [DOI] [PubMed] [Google Scholar]
- 16. Levy B, Gibot S, Franck P, Cravoisy A, Bollaert PE. Relation between muscle Na+K+ ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet 2005; 365: 871–875. [DOI] [PubMed] [Google Scholar]
- 17. Aninat C, Seguin P, Descheemaeker PN, Morel F, Malledant Y, Guillouzo A. Catecholamines induce an inflammatory response in human hepatocytes. Crit Care Med 2008; 36: 848–854. [DOI] [PubMed] [Google Scholar]
- 18. van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor‐alpha and potentiates interleukin 10 production during human endotoxemia. J Clin Invest 1996; 97: 713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van der Poll T, Lowry SF. Epinephrine inhibits endotoxin‐induced IL‐1β production: roles of tumor necrosis factor‐α and IL‐10. Am J Physiol 1997; 273: R1885–R1890. [DOI] [PubMed] [Google Scholar]
- 20. Farmer P, Pugin J. β‐Adrenergic agonists exert their “anti‐inflammatory” effects in monocytic cells through the IκB/NF‐κB pathway. Am J Physiol Lung Cell Mol Physiol 2000; 279: L675–LL82. [DOI] [PubMed] [Google Scholar]
- 21. Du Q, Min S, Chen LY, Ma YD, Guo XL, Wang Z, Wang ZG. Major stress hormones suppress the response of macrophages through down‐regulation of TLR2 and TLR4. J Surg Res 2012; 173: 354–361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Etiology of cardiogenic shock and association with death.


