Abstract
Aims
Optimizing medical cardiac treatment for sleep apnoea (SA) in patients with chronic heart failure and reduced ejection fraction (HFrEF) is an expert Grade C recommendation based on six studies encompassing a total of 67 patients only. Whether sacubitril–valsartan (SV), a cornerstone of HFrEF medical treatment, impacts SA is unknown and requires evaluation.
Methods and results
The ENTRESTO‐SAS trial is a six‐centre, prospective, open‐label real‐life cohort study (NCT02916160). Ambulatory patients eligible for SV (i.e. HFrEF adults who remain symptomatic despite optimal treatment) were evaluated before and after 3 months of SV (including nocturnal ventilatory polygraphy); 118 patients were final analysed [median age was 66 (IQ25–75: 56–73) years, 81.4% male, 36.5% New York Heart Association III–IV, N‐terminal pro‐B‐type natriuretic peptide level of 1564 (701–3376) ng/L, left ventricular ejection fraction of 30 (25–34)%, 60.7% ischaemic HFrEF, 97.5% initially treated with angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers, 83.9% with beta‐blockers, 64.4% with mineralocorticoid receptor antagonists, and 74.6% with diuretics]. Three groups were defined according to initial central/obstructive apnoea–hypopnoea indices (AHIs): G1 (n = 49, AHIcentral ≥ 5/h and AHIobstructive < 15/h); G2 (n = 27, AHIobstructive ≥ 15/h); and G3 (n = 42, AHIcentral < 5/h and AHIobstructive < 15/h). At 3 months, the AHI (main predefined outcome) decreased significantly by −7.10/h (IQ25–75: −16.10 to 0.40; P < 0.001) in G1 + G2 without positive airway pressure treatment (45 patients, median initial AHI of 24.20 (IQ25–75: 16.40–43.50)/h). Of these, 24.4% presented an AHI decrease ≥50% and 37.78% had a final AHI < 15/h (tendency for improvement from an initial value of 20%: P = 0.0574). For G1 patients (n = 37), AHI significantly decreased from a median of 22.90 (16.00–43.50)/h to 19.20 (12.70–31.10)/h (P = 0.002). For G2 patients (n = 8), AHI decreased from a median of 30.10 (26.40–47.60)/h to 22.75 (14.60–36.90)/h (statistically non‐significant, P = 0.059).
Conclusions
In this real‐life population, SV treatment for 3 months in SA patients is associated with a significant decrease in AHI. These results support the current guidelines that recommend first an optimization of the HFrEF treatment in patients with HFrEF and central SA. A potential positive airway pressure sparing effect merits further investigation.
Keywords: Continuous positive airway pressure, Heart failure, Sacubitril–valsartan, Sleep apnoea, Sleep‐disordered breathing
Introduction
In developed countries, chronic heart failure (CHF) is a common disease affecting at least 1–2% of the adult population. 1 CHF patients still have a poor prognosis despite significant advances in therapy: more than half of elderly and/or HF hospitalized patients die within 5 years. 2 , 3 , 4
Sleep apnoea [SA, either predominantly obstructive SA (OSA) or predominantly central SA (CSA)] is a highly prevalent co‐morbidity in CHF patients associated with even worse outcomes. At least 50% of CHF patients have moderate to severe SA [i.e. SA with an apnoea–hypopnoea index (AHI) of ≥15/h], 5 , 6 reaching up to 76% in patients with heart failure and reduced ejection fraction (HFrEF). 7 While OSA is considered an independent risk factor increasing CHF morbidity and mortality, 8 , 9 CSA appears to be more a CHF severity marker, reflecting left ventricular dysfunction. 10 However, all SA phenotypes are associated with an increase in sympathetic activity leading to harmful conditions in this CHF context. Such may include renin–angiotensin–aldosterone system stimulation with salt and water retention, tachycardia, or peripheral vasoconstriction. 5 , 6 As a consequence, SA is considered as a potential therapeutic target in CHF as underlined by the 2017 European Respiratory Society Task Force. 11
Sleep apnoea treatment modalities in CHF are not supported by a high level of evidence, especially as concerns chronic HFrEF. Whereas treating OSA with continuous positive airway pressure (CPAP) is supported by non‐randomized/cohort studies reporting a decrease in mortality, 12 , 13 , 14 ventilatory treatment for CSA remains a matter of debate, in particular for patients with HFrEF. 11 , 15 , 16 In the context of the SERVE‐HF study 17 and pending the ADVENT‐HF study results, 18 optimization HFrEF management is first recommended to improve CSA in clinical practice (expert Grade C recommendation, 11 based on six studies encompassing a total of 67 patients only 19 ).
The combination therapy sacubitril–valsartan (SV) has known mechanisms of action likely to counteract the pathophysiology of both OSA and CSA in CHF patients (extracellular fluid overload, cardiac injury, and sympathetic nervous system activation). 19 Thereby, SV interferes with neurohumoral systems and improves CHF by decreasing renin–angiotensin–aldosterone and sympathetic activity, both possible actors also involved in the pathophysiology of SA. 20 , 21 In the current context, it is therefore a good candidate for correcting SA in CHF patients. To date, due to its beneficial effects in terms of mortality, hospitalization, or quality of life, SV is the cornerstone of medical treatment for HFrEF patients who remain symptomatic [i.e. patients defined as New York Heart Association (NYHA) Classes II–IV] despite optimal treatment with an angiotensin‐converting enzyme (ACE) inhibitor, a beta‐blocker, and a mineralocorticoid receptor antagonist. 1 , 22 , 23 , 24 Whether SV impacts SA in patients with HFrEF is unknown.
In the multicentre ENTRESTO‐SAS trial, we sought to assess whether SV initiation could improve SA outcomes in the HFrEF patients treated under real‐life conditions.
Methods
The ENTRESTO‐SAS trial is a 3 month, multicentre, prospective, open‐label, real‐life cohort study (NCT02916160) conducted from 22 September 2016 to 15 December 2019. The protocol complied with the Declaration of Helsinki and was reviewed and approved by an independent ethics committee (Comité de Protection des Personnes Sud Mediterranée IV; Reference Number 2016‐A00331‐50).
Study design
The ENTRESTO‐SAS study design is summarized in Figure 1 and has been previously reported. 19 Briefly, ambulatory patients eligible for SV treatment were invited to participate in the study [i.e. HFrEF patients who remain symptomatic (NYHA Classes II–IV) despite optimal treatment]. After inclusion and exclusion criteria verification, a pre‐therapeutic evaluation [including nocturnal ventilatory polygraphy (P)] was performed. SV was started after the P, and cardiological surveillance deployed to achieve the optimal SV treatment dose. At 3 months, a final evaluation was performed. For patients presenting with SA at baseline (i.e. AHIcentral ≥ 5/h and/or AHIobstructive ≥ 15/h), the latter included a second diagnostic P (without CPAP if applicable).
Figure 1.
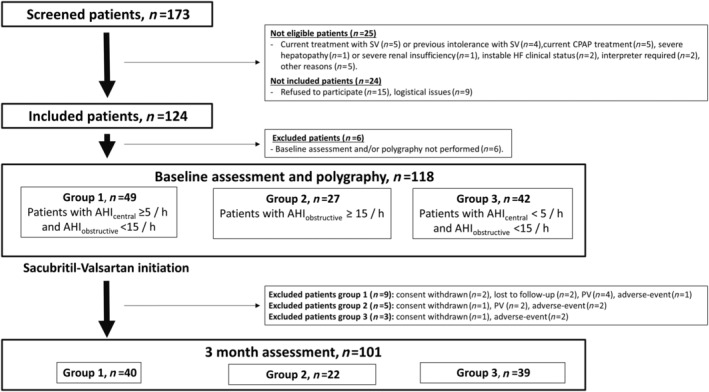
Study flow chart. Overview of screened, eligible, included, and excluded patients. Assessments included a physical examination, echocardiography, laboratory testing, the Minnesota Living with Heart Failure Questionnaire, the EQ‐5D‐3L questionnaire, the Epworth Sleepiness Scale, and the New York Heart Association score. Polygraphy was performed for Groups 1–3 at baseline and at 3 months for Groups 1 and 2. AHI, apnoea–hypopnoea index; CPAP, continuous positive airway pressure; HF, heart failure; PV, protocol violation; SV, sacubritil–valsartan.
Group definitions
Based on the initial P results, three groups were generated; G1: AHIcentral ≥ 5/h and AHIobstructive < 15/h; G2: AHIobstructive ≥ 15/h regardless of the AHIcentral; and G3: AHIcentral < 5/h and AHIobstructive < 15/h (see Supporting Information, Appendix S1 ).
Outcomes
The main predefined outcome was the change in AHI before vs. after 3 months of SV in G1 and G2 patients without positive airway pressure (PAP) treatment. The two main secondary outcomes were the proportion of these patients with a ≥50% decrease in their AHI or a final AHI < 15/h. For these AHI outcomes, the 2012 American Academy of Sleep Medicine recommendations were used to characterize not only apnoea and hypopnoea events but also the event phenotype (central, obstructive, and mixed). 25 The apnoea/hypopnoea criteria used as scoring rules are detailed in Supporting Information, Appendix S1 . CPAP‐treated patients were analysed separately as planned in the design paper. 19
For quality‐of‐life outcomes, the following validated scales and questionnaires were used: Minnesota Living with Heart Failure Questionnaire, 26 EQ‐5D‐3L Questionnaire, 27 and Epworth Sleepiness Scale. 28
Statistical methods
Continuous data were expressed as medians, inter‐quartile ranges (IQ25–75), and ranges [min–max]. Qualitative parameters were expressed as numbers and percentages. Three group comparisons were performed using ANOVA or Kruskal–Wallis tests for quantitative data. Qualitative variables were compared using χ 2 or Fisher's exact tests. In case of a significant global effect, pairwise comparisons were performed using Holm corrections for multiple comparisons. Evolutions between initial and final evaluations were studied using Student's paired tests or Wilcoxon paired test for quantitative variables and exact McNemar test for qualitative parameters. A bilateral P value of <0.05 was considered as indicating statistical significance. Missing data were not replaced. All analyses were conducted by the Clinical Research and Epidemiology Unit at the Montpellier University Hospitals using SAS (Enterprise Guide, Version 7.13; SAS Institute, Cary, NC, USA).
Results
The flow chart of the study is depicted in Figure 1 . A total of 118 patients were included in the analyses. Table 1 summarizes patient characteristics at baseline. Among these patients, 41.5% belonged to G1, 22.9% to G2, and 35.6% to G3. G2 patients presented a significant higher body mass ndex with a median of 28.96 (25.47–32.83) kg/m2 vs. G3 patients [median 24.69 (21.15–29.39) kg/m2, P = 0.048]. G1 patients presented not only a significant higher prevalence of atrial fibrillation (35.42%) than G3 patients (7.14%; P = 0.004) but also significantly higher serum N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) concentration levels [median 1816 (1004–3958) vs. 920.5 (248–2200) pg/mL, P = 0.037].
Table 1.
Patient characteristics at baseline
| N | Total | Group 1 | Group 2 | Group 3 | P value | |
|---|---|---|---|---|---|---|
| n = 118 | n = 49 | n = 27 | n = 42 | |||
| Age (years) | 118 | 66.00 [56.00–73.00] | 64.00 [55.00–75.00] | 69.00 [57.00–74.00] | 66.00 [55.00–72.00] | 0.628 c |
| Gender, n (%) | 118 | 0.318 d | ||||
| Male | 96 (81.36) | 43 (87.76) | 21 (77.78) | 32 (76.19) | ||
| Female | 22 (18.64) | 6 (12.24) | 6 (22.22) | 10 (23.81) | ||
| BMI (kg/m2) | 118 | 26.81 [23.18–30.76] | 27.38 [23.77–30.25] | 28.96 a [25.47–32.83] | 24.69 a [21.15–29.39] | 0.039 f |
| Systolic BP (mmHg) | 101 | 120 [110–130] | 120 [110–130] | 120 [109–125] | 120 [110–130] | 0.862 c |
| Diastolic BP (mmHg) | 101 | 70 [65–80] | 71 [65–80] | 70 [65–79] | 70 [64–80] | 0.932 f |
| Heart rate (b.p.m.) | 118 | 70 [63–80] | 69 [63–87] | 70 [64–78] | 71 [63–76] | 0.948 f |
| Co‐morbidities | ||||||
| Active smoking (or stop <1 year), n (%) | 118 | 25 (21.19) | 11 (22.45) | 6 (22.22) | 8 (19.05) | 0.914 d |
| Hypertension, n (%) | 118 | 45 (38.14) | 19 (38.78) | 13 (48.15) | 13 (30.95) | 0.354 d |
| Diabetes, n (%) | 118 | 25 (21.19) | 11 (22.45) | 9 (33.33) | 5 (11.90) | 0.100 d |
| Dyslipidaemia, n (%) | 118 | 42 (35.59) | 18 (36.73) | 11 (40.74) | 13 (30.95) | 0.692 d |
| ORD, n (%) | 118 | 10 (8.55) | 3 (6.12) | 2 (7.41) | 5 (12.20) | 0.641 d |
| PAD, n (%) | 118 | 16 (13.56) | 5 (10.20) | 7 (25.93) | 4 (9.52) | 0.142 e |
| eGFR Cockroft class, n (%) | 117 | 0.705 e | ||||
| <30 | 4 (3.42) | 1 (2.04) | 2 (7.41) | 1 (2.44) | ||
| [30–45] | 17 (14.53) | 8 (16.33) | 2 (7.41) | 7 (17.07) | ||
| [45–60] | 19 (16.24) | 8 (16.33) | 3 (11.11) | 8 (19.51) | ||
| ≥60 | 77 (65.81) | 32 (65.31) | 20 (74.07) | 25 (60.98) | ||
| eGFR Cockroft (mL/min/1.73 m2) | 117 | 74.64 [50.77–94.91] | 71.11 [54.68–99.80] | 80.25 [50.77–105.68] | 70.60 [48.98–90.41] | 0.268 c |
| Clinical features of HF | ||||||
| Ischaemic, n (%) | 117 | 71 (60.68) | 31 (63.27) | 18 (66.67) | 22 (53.66) | 0.499 d |
| Hypertensive, n (%) | 117 | 3 (2.56) | 2 (4.08) | 1 (3.70) | 0 (0.00) | 0.451 e |
| Valvulopathy, n (%) | 117 | 8 (6.84) | 4 (8.16) | 2 (7.41) | 2 (4.88) | 0.899 e |
| Primitive, n (%) | 117 | 30 (25.64) | 9 (18.37) | 7 (25.93) | 14 (34.15) | 0.232 d |
| Rhythmic, n (%) | 117 | 23 (19.66) | 13 (26.53) | 6 (22.22) | 4 (9.76) | 0.127 d |
| Atrial fibrillation, n (%) | 117 | 24 (20.51) | 17 (35.42) b | 4 (14.81) | 3 (7.14) b | 0.003 d |
| LVEF (%) | 118 | 30.00 [25.00–34.00] | 30.00 [25.00–33.00] | 30.00 [25.00–30.00] | 30.00 [25.00–35.00] | 0.853 f |
| NT‐proBNP (pg/mL) | 110 | 1564.5 [701–3376] | 1816 b [1004–3958] | 1721 [845–3333] | 920.5 b [248–2200] | 0.029 f |
| NYHA functional class, n (%) | 115 | |||||
| I/II | 9/64 [7.83/55.65] | 5/30 [10.42/62.50] | 2/14 [7.69/53.85] | 2/20 [4.88/48.78] | 0.141 d | |
| III/IV | 37/5 [32.17/4.35] | 9/4 [18.75/8.33] | 10/0 [38.46/0] | 18/1 [43.90/2.44] | ||
| Treatment | ||||||
| Loop diuretics | 118 | 88 (74.58) | 39 (79.59) | 21 (77.78) | 28 (66.67) | 0.335 d |
| Spironolactone | 118 | 76 (64.41) | 29 (59.18) | 21 (77.78) | 26 (61.90) | 0.246 d |
| ACE inhibitor or ARB | 118 | 115 (97.46) | 48 (97.96) | 27 (100.00) | 40 (95.24) | 0.608 e |
| Beta‐blocker | 118 | 99 (83.90) | 38 (77.55) | 26 (96.30) | 35 (83.33) | 0.083 e |
| Cardiac resynchronization therapy | 117 | 12 (10.26) | 6 (12.24) | 3 (11.54) | 3 (7.14) | 0.731 e |
| Pacemaker | 117 | 12 (10.26) | 4 (8.16) | 5 (18.52) | 3 (7.32) | 0.283 e |
| ICD | 118 | 55 (46.61) | 18 (36.73) b | 11 (40.74) | 26 (61.90) b | 0.044 d |
ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; HF, heart failure; ICD, implanted cardiac defibrillator; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; ORD, obstructive respiratory disease; PAD, peripheral arterial disease.
Quantitative variables were described by medians and [IQ25–75].
Significant pairwise comparisons after Holm corrections were presented for Group 2 vs. Group 3.
Significant pairwise comparisons after Holm corrections were presented for Group 1 vs. Group 3.
Statistical tests used were presented, on P values, for ANOVA.
Statistical tests used were presented, on P values, for χ 2 test.
Statistical tests used were presented, on P values, for Fisher's exact test.
Statistical tests used were presented, on P values, for Kruskal–Wallis test.
Primary outcome: apnoea–hypopnoea index change after 3 months of sacubitril–valsartan treatment
The main predefined outcome was the change in the AHI after 3 months of SV in G1 and G2 patients without PAP treatment (n = 37/40 patients for G1 and n = 8/22 patients for G2). Table 2 summarizes the P results for G1 + G2 patients. Individual data for the AHI are depicted in Figure 2 (for G1 and G2, see panels A and B, respectively). Specific P data for G1 and G2 are summarized in Tables 3 and 4 , respectively. At 3 months, the AHI primary outcome decreased significantly by a median −7.10/h (−16.10 to 0.40), P < 0.001, in G1 + G2 patients. G1 patients had mainly a central IAH pattern, whereas G2 patients have mainly an obstructive one. For G1 patients, AHI significantly decreased from a median of 22.90 (16.00–43.50)/h to 19.20 (12.70–31.10)/h (P = 0.002). The median AHI difference was −6.60 (−11.70 to 0.40)/h (see Table 3 ). For G2 patients, AHI decreased from a median of 30.10 (26.40–47.60)/h to 22.75 (14.60–36.90)/h (statistically non‐significant, P = 0.059). The median AHI difference was −12.40 (−23.60 to 0.35)/h (see Table 4 ).
Table 2.
Initial (baseline) and final (3 months) polygraphy data in G1 and G2 patients (restricted to patients without positive airway pressure treatment)
| G1 + G2 (n = 45) | |||||
|---|---|---|---|---|---|
| n | Initial | Final | Difference | P value | |
| AHI (events/h) | 45 | 24.20 (16.40–43.50) [8.10–67.00] | 20.40 (12.70–31.10) [1.00–55.00] | −7.10 (−16.10 to 0.40) [−30.50 to 32.20] | <0.001 a |
| AHI < 15, n (%) | 45 | 9 (20.00) | 17 (37.78) | — | 0.057 b |
| dAHI (events/h) | 41 | 29.40 (17.20–48.60) [0.00–66.00] | 27.30 (15.90–40.40) [0.00–58.30] | −2.30 (−13.30 to 3.80) [−66.00 to 54.70] | 0.095 a |
| AHIobstructive (events/h) | 45 | 4.20 (1.30–10.00) [0.00–27.00] | 5.60 (3.60–10.00) [0.00–49.20] | 0.40 (−3.50 to 5.20) [−20.90 to 44.80] | 0.611 a |
| AHIcentral (events/h) | 45 | 13.60 (8.20–31.80) [0.00–51.40] | 7.00 (3.10–16.60) [0.00–45.00] | −8.00 (−13.50 to −0.30) [−44.40 to 20.20] | <0.001 c |
| OAI (events/h) | 45 | 1.30 (0.10–4.00) [0.00–16.20] | 1.40 (0.60–4.00) [0.00–49.10] | 0.00 (−1.00 to 1.90) [−15.40 to 45.70] | 0.378 a |
| CAI (events/h) | 45 | 4.00 (1.50–20.50) [0.00–46.10] | 2.10 (0.50–5.50) [0.00–45.00] | −1.50 (−9.50 to 0.20) [−45.00 to 20.20] | <0.001 a |
| MAI (events/h) | 45 | 0.10 (0.00–1.40) [0.00–31.00] | 0.40 (0.00–1.50) [0.00–39.00] | 0.00 (−0.40 to 0.60) [−28.50 to 15.00] | 0.724 a |
| HI (events/h) | 44 | 13.45 (7.90–17.75) [2.50–38.70] | 8.45 (4.90–15.40) [0.40–42.80] | −3.55 (−7.85 to 0.45) [−18.90 to 33.20] | 0.014 a |
| HIobstructive (events/h) | 45 | 1.70 (0.50–6.70) [0.00–20.00] | 2.20 (0.70–6.20) [0.00–21.90] | 0.00 (−0.90 to 1.90) [−17.40 to 17.40] | 0.811 a |
| HIcentral (events/h) | 45 | 7.20 (2.10–11.30) [0.00–29.80] | 3.10 (0.50–7.40) [0.00–29.60] | −2.10 (−7.20 to 0.60) [−15.80 to 28.20] | 0.024 a |
| ODI (events/h) | 42 | −6.32 (±15.79) | −6.20 (−12.70 to 0.90) | [−43.50 to 39.20] | 0.013 c |
| Mean SpO2 (%) | 44 | 92.30 (91.35–94.55) [87.90–96.80] | 93.05 (91.60–94.70) [88.90–99.60] | 0.60 (−1.05 to 1.50) [−4.00 to 8.10] | 0.247 a |
| Minimum SpO2 (%) | 43 | 83.00 (78.00–86.00) [0.00–91.00] | 84.00 (81.00–87.00) [60.00–93.00] | 1.00 (−1.00 to 4.00) [−11.00 to 60.00] | 0.036 a |
| Time SpO2 < 90% (min) | 44 | 23.00 (5.00–96.50) [0.00–311.00] | 13.50 (2.50–67.50) [0.00–344.00] | −4.00 (−36.50 to 4.00) [−279.00 to 231.00] | 0.129 a |
| Time SpO2 < 90% (%) | 44 | 8.10 (1.00–22.95) [0.00–81.00] | 2.85 (0.40–12.75) [0.00–60.90] | −1.05 (−10.80 to 0.90) [−79.80 to 37.20] | 0.020 a |
AHI, apnoea–hypopnoea index; CAI, central apnoea index; dAHI, dorsal apnoea–hypopnoea index; HI, hypopnoea index, MAI, mixed apnoea index; OAI, obstructive apnoea index; ODI, oxygen desaturation index; SpO2, oxygen saturation on pulse oximetry.
Quantitative variables were described by medians and (IQ25–75) and [min–max].
The statistical tests used are presented on P values for Wilcoxon paired test.
The statistical tests used are presented on P values for McNemar's test with Yates' corrections.
The statistical tests used are presented on P values for Student's paired test.
Figure 2.
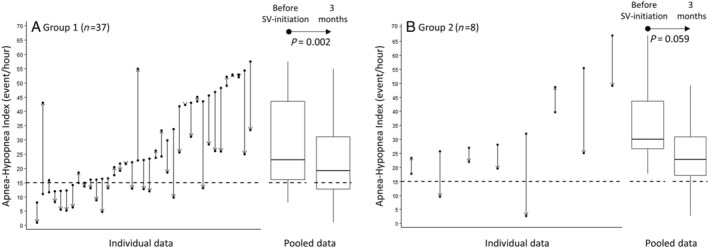
Apnoea–hypopnoea index before vs. after 3 months of sacubritil–valsartan (SV). Change in apnoea–hypopnoea index before vs. after 3 months of SV in (A) Group 1 and (B) Group 2 patients without positive airway pressure treatment.
Table 3.
Change in polygraphy data before (initial) vs. after (final) 3 months of sacubritil–valsartan in G1 patients without positive airway pressure treatment
| G1 (n = 37) | |||||
|---|---|---|---|---|---|
| n | Initial | Final | Difference | P value | |
| AHI (events/h) | 37 | 22.90 (16.00–43.50) [8.10–57.50] | 19.20 (12.70–31.10) [1.00–55.00] | −6.60 (−11.70 to 0.40) [−30.50 to 32.20] | 0.002 a |
| AHI < 15, n (%) | 37 | 9 (24.3) | 15 (40.5) | — | 0.146 b |
| dAHI (events/h) | 34 | 29.65 (16.00–48.60) [0.00–63.40] | 27.45 (15.90–40.40) [0.00–56.00] | −3.10 (−13.30 to 3.80) [−40.80 to 54.70] | 0.266 c |
| AHIobstructive (events/h) | 37 | 2.60 (1.20–6.60) [0.00–14.40] | 5.40 (2.20–8.50) [0.00–49.20] | 0.80 (−0.80 to 5.80) [−10.40 to 44.80] | 0.028 a |
| AHIcentral (events/h) | 37 | 13.60 (9.00–31.80) [5.10–51.40] | 7.00 (4.00–16.60) [0.10–45.00] | −9.00 (−15.00 to −3.40) [−44.40 to 20.20] | <0.001 c |
| OAI (events/h) | 37 | 0.60 (0.00–1.90) [0.00–8.50] | 1.40 (0.30–3.70) [0.00–49.10] | 0.60 (−0.40 to 2.60) [−4.60 to 45.70] | 0.030 a |
| CAI (events/h) | 37 | 4.90 (1.60–25.00) [0.00–46.10] | 2.30 (0.80–5.50) [0.00–45.00] | −1.50 (−11.40 to 0.20) [−45.00 to 6.70] | <0.001 a |
| MAI (events/h) | 37 | 0.10 (0.00–1.20) [0.00–31.00] | 0.50 (0.00–1.50) [0.00–39.00] | 0.00 (−0.40 to 0.70) [−28.50 to 15.00] | 0.651 a |
| HI (events/h) | 36 | 11.90 (7.10–14.65) [2.50–27.50] | 7.65 (4.90–13.65) [0.40–42.80] | −3.40 (−7.25 to −0.40) [−13.30 to 33.20] | 0.030 a |
| HIobstructive (events/h) | 37 | 1.20 (0.20–4.20) [0.00–12.50] | 1.90 (0.50–5.40) [0.00–18.70] | 0.00 (−0.70 to 1.90) [−9.10 to 17.40] | 0.234 a |
| HIcentral (events/h) | 37 | 7.20 (5.10–11.30) [0.00–29.80] | 3.10 (0.50–7.30) [0.00–29.60] | −4.70 (−7.50 to 0.60) [−15.80 to 28.20] | 0.018 a |
| ODI (events/h) | 36 | 11.90 (7.10–14.65) [2.50–27.50] | 7.65 (4.90–13.65) [0.40–42.80] | −3.40 (−7.25 to −0.40) [−13.30 to 33.20] | 0.030 a |
| Mean SpO2 (%) | 37 | 93.00 (91.80–94.60) [87.90–96.80] | 93.40 (92.20–94.90) [88.90–99.60] | 0.60 (−1.10 to 1.50) [−4.00 to 8.10] | 0.313 a |
| Minimum SpO2 (%) | 36 | 83.50 (78.00–86.00) [0.00–91.00] | 84.00 (80.50–86.50) [60.00–93.00] | 1.00 (−1.00 to 4.00) [−11.00 to 60.00] | 0.0621 a |
| Time SpO2 < 90% (min) | 37 | 17.00 (3.00–79.00) [0.00–274.00] | 7.00 (2.00–42.00) [0.00–344.00] | −3.00 (−20.00 to 4.00) [−203.0 to 231.0] | 0.220 a |
| Per cent time SpO2 < 90% (%) | 37 | 7.70 (0.60–19.00) [0.00–81.00] | 1.80 (0.30–8.60) [0.00–60.90] | −0.60 (−10.60 to 0.80) [−79.8 to 37.2] | 0.060 a |
AHI, apnoea–hypopnoea index; CAI, central apnoea index; dAHI, dorsal apnoea–hypopnoea index; HI, hypopnoea index, MAI, mixed apnoea index; OAI, obstructive apnoea index; ODI, oxygen desaturation index; SpO2, oxygen saturation on pulse oximetry.
Quantitative variables were described by medians and (IQ25–75) and [min–max].
The statistical tests used are presented on P values for Wilcoxon paired test.
The statistical tests used are presented on P values for McNemar's test with Yates' corrections.
The statistical tests used are presented on P values for Student's paired test.
Table 4.
Change in polygraphy data before (initial) vs. after (final) 3 months of sacubritil–valsartan in G2 patients without positive airway pressure treatment
| G2 (n = 8) | |||||
|---|---|---|---|---|---|
| n | Initial | Final | Difference | P value | |
| AHI (events/h) | 8 | 30.10 (26.40–47.60) [17.80–67.00] | 22.75 (14.60–36.90) [2.70–49.10] | −12.40 (−23.60 to 0.35) [−30.30 to 8.90] | 0.059 a |
| AHI < 15/h | 7 | 0 (0) | 2 (25.0) | — | 0.500 b |
| dAHI (events/h) | 8 | 28.00 (25.50–49.10) [16.70–66.00] | 26.00 (10.00–40.50) [0.00–58.30] | −2.10 (−15.50 to 9.20) [−66.00 to 11.50] | 0.375 c |
| AHIobstructive (events/h) | 8 | 22.85 (18.75–24.75) [17.30–27.00] | 11.45 (4.25–19.50) [0.90–23.00] | −11.20 (−16.15 to −6.40) [−20.90 to 5.70] | 0.010 a |
| AHIcentral (events/h) | 8 | 6.50 (0.90–25.60) [0.00–33.70] | 3.90 (1.10–25.10) [0.00–36.40] | 0.00 (−4.75 to 2.70) [−17.70 to 18.70] | 0.919 a |
| OAI (events/h) | 8 | 7.15 (5.60–12.60) [0.00–16.20] | 1.50 (1.05–4.10) [0.00–14.80] | −4.75 (−10.05 to −1.00) [−15.40 to 8.20] | 0.107 a |
| CAI (events/h) | 8 | 2.60 (0.75–5.65) [0.00–22.30] | 1.90 (0.00–7.60) [0.00–22.40] | −0.90 (−4.45 to 0.55) [−9.50 to 20.20] | 0.469 b |
| MAI (events/h) | 8 | 0.45 (0.00–3.25) [0.00–8.40] | 0.05 (0.00–0.95) [0.00–7.40] | 0.00 (−0.90 to 0.50) [−8.30 to 2.70] | 1.000 c |
| HI (events/h) | 8 | 19.85 (17.75–22.90) [9.80–38.70] | 17.25 (4.55–21.70) [2.20–32.20] | −9.00 (−14.90 to 3.05) [−18.90 to 12.50] | 0.170 a |
| HIobstructive (events/h) | 8 | 14.35 (9.30–19.05) [4.70–20.00] | 4.35 (1.55–15.90) [0.70–21.90] | −4.50 (−12.70 to 1.65) [−17.40 to 4.60] | 0.102 a |
| HIcentral (events/h) | 8 | 2.65 (0.00–13.40) [0.00–27.30] | 2.85 (0.25–13.80) [0.00–21.40] | 0.00 (−2.35 to 2.05) [−13.70 to 10.10] | 0.831 a |
| ODI (events/h) | 7 | 31.00 (15.30–55.90) [7.00–60.00] | 24.00 (11.00–45.90) [4.90–47.30] | −12.40 (−26.10 to 6.00) [−31.90 to 15.20] | 0.255 a |
| Mean SpO2 (%) | 7 | 91.30 (90.00–93.00) [89.10–93.00] | 91.80 (91.00–92.10) [89.50–94.40] | 0.40 (−1.00 to 1.80) [−1.60–4.40] | 0.439 a |
| Minimum SpO2 (%) | 7 | 83.00 (75.00–87.00) [71.00–89.00] | 83.00 (81.00–88.00) [79.00–91.00] | 2.00 (−3.00 to 8.00) [−4.00 to 12.00] | 0.291 a |
| Time SpO2 < 90% (min) | 7 | 182.00 (35.00–279.00) [12.00–311.00] | 54.00 (15.00–244.00) [0.00–310.00] | −33.00 (−69.00 to 42.00) [−279.00 to 128.0] | 0.403 a |
| Per cent time SpO2 < 90% (%) | 7 | 19.00 (2.00–48.50) [1.00–55.40] | 10.00 (4.00–27.70) [0.00–45.80] | −9.00 (−20.80 to 3.00) [−47.00 to 11.00] | 0.177 a |
AHI, apnoea–hypopnoea index; CAI, central apnoea index; dAHI, dorsal apnoea–hypopnoea index; HI, hypopnoea index, MAI, mixed apnoea index; OAI, obstructive apnoea index; ODI, oxygen desaturation index; SpO2, oxygen saturation on pulse oximetry.
Quantitative variables were described by medians and (IQ25–75) and [min–max].
The statistical tests used are presented on P values for Student's paired test.
The statistical tests used are presented on P values for McNemar's test with Yates' corrections.
The statistical tests used are presented on P values for Wilcoxon paired test.
As opposite to these AHI differences, weight differences before and after SV do not reached a statistical significant difference in any of the studied groups. The median weight difference was 1.00 (−2.30 to 3.00) kg, P = 0.667, for G1 patients; the median weight difference was 1.50 (−1.00 to 6.00) kg, P = 0.088, for G2 patients; and the median weight difference was 0.00 (−3.00 to 1.00) kg, P = 0.075, for G3 patients.
Univariate linear regressions with the AHI relative difference as the variable of interest in G1 and G2 patients are depicted in Supporting Information, Table S1 . Only initial left ventricular ejection fraction (LVEF) is significantly associated with (standardized β coefficient of 0.33, P = 0.038).
Main secondary outcomes: apnoea–hypopnoea index ≥50% decrease and final apnoea–hypopnoea index <15/h
The main secondary outcomes were the proportion of G1 and G2 patients with a ≥50% decrease in their AHI or a final AHI < 15/h (restricted to patients without PAP treatment); 24.4% of the patients presented a ≥50% decrease in their AHI (21.6% for G1 and 37.5% for G2), and 20% presented an initial AHI < 15/h vs. 37.78% at 3 months, P = 0.0574 (24.3% vs. 40.5% for G1 patients, P = 0.146; 0% vs. 25% for G2 patients, P = 0.5, respectively).
Secondary outcome: polygraphy and positive airway pressure data for the G1 + G2 populations regardless of positive airway pressure treatment status or sacubitril–valsartan adherence
Initial and final P data for G1 and G2 patients (without restrictions on PAP usage or SV adherence) are depicted in Supporting Information, Tables S2 and S3 , respectively. At the baseline assessment, 57.63% of the 118 patients presented with an initial AHI ≥ 15/h (41/49 in G1 patients, 27/27 G2 patients, and 0/42 G3 patients). PAP data are summarized in Supporting Information, Table S4 , and exclusively involved CPAP (n = 1/40 for G1 and n = 13/22 for G2). Two G1 patients and one G2 patient were not SV adherent at the P evaluation.
For these G1 patients, AHI significantly decreased from a median of 23.20 (16.00–43.55)/h to 20.95 (12.80–32.20)/h (P = 0.003). The median AHI difference was −4.10 (−11.65 to 1.0)/h (see Supporting Information, Table S2 ). For G2 patients, AHI significantly decreased from a median of 37.65 (28.40–45.30)/h to 23.95 (9.60–40.30)/h (P = 0.002). The median AHI difference was −16.15 (−27.70 to −1.60)/h (see Supporting Information, Table S3 ).
Secondary outcomes: cardiac assessment data
Supporting Information, Table S5 summarizes the changes in cardiac assessment between baseline and the 3 month evaluation. For the latter, the SV dosage was 24/26 mg for 33.7% of the patients, 49/51 mg for 31.7%, and 97/103 mg for 34.6%. After SV initiation, there was a trend towards a reduction in blood pressure in the three groups that did not reach statistical significance. NYHA class (I and II vs. III and IV) was down‐staged in the three groups (P = 0.070 for G1, P = 0.453 for G2, and P < 0.001 for G3). NT‐proBNP significantly decreased in the three groups [median change of −301.00 (−887.0 to −34.0) pg/mL for G1 (P = 0.001), −309.00 (−1281 to 164.0) pg/mL for OSA‐G2 (P = 0.043), and −299.50 (−802.50 to −44.00) pg/mL for G3 (P < 0.001)]; 51.72% of the whole population presented a change over 30% in NT‐proBNP values after SV initiation (without significant differences between groups). The LVEF significantly increased for G1 and G3 [median change of 2.00 (0.00–10.00)%, P = 0.001, and median change of 2.00 (0.00–7.00)%, P = 0.016, respectively]. The tricuspid annular plane systolic excursion significantly increased only in G1 [median change of 1.00 (−1.00 to 5.00) mm, P = 0.045] associated with a significant decrease in the pulmonary artery systolic pressure [median change of −7.00 (−16.00 to 0.00) mmHg, P = 0.001].
Supporting Information, Table S6 summarizes the changes in cardiological concomitant treatment between the initial and final P. No significant treatment changes occurred except for switching between SV and ACE inhibitors.
Supporting Information, Figure S1 depicts the correlations between the AHI relative difference and the relative differences for echocardiographic parameters. These correlations are weak (r = −0.15 to 0.13) and not statistically significant.
Secondary outcomes: quality‐of‐life data
No significant differences between initial and final Epworth Sleepiness Scale scores were observed (see Supporting Information, Table S7 ), regardless of group. On the contrary, the Minnesota Living with Heart Failure Questionnaire total score significantly and favourably decreased for G1 [from 24 (11–51) to 17 (6–33), P = 0.003] and G3 [from 31 (10–50) to 18 (6–31), P = 0.004]. Finally, the EQ‐5D‐3L Health Visual Analogue Scale score also favourably increased for G1 [from 60 (50–70) to 75 (60–80), P = 0.001] and G3 [from 52.5 (40–70) to 70 (60–80), P = 0.016].
Secondary outcomes: safety data
During the 3 months of the trial, one patient withdrew because of a side effect associated with the SV treatment (angioedema). At 3 months, 38.1% of the patients presented at least one adverse event, as summarized in Table 5 . The latter included unscheduled hospitalizations for cardiac failure for 17.72% of patients. Symptomatic hypotension was reported in 7.59%, a decrease in estimated glomerular filtration rate >30% in 3.80% and hyperkalaemia in 2.53% of patients.
Table 5.
Adverse effects at 3 months
| Patients, n (%) | Total | G1 | G2 | G3 | P value |
|---|---|---|---|---|---|
| n = 118 | n = 49 | n = 27 | n = 42 | ||
| Number of patients with at least 1 AE | 45 (38.1) | 13 (26.5) | 13 (48.2) | 19 (45.2) | 0.089 a |
| Number of patients with | |||||
| 1 AE | 24 (20.34) | 6 (12.24) | 6 (22.22) | 12 (28.57) | 0.126 b |
| 2 AE | 12 (10.17) | 2 (4.08) | 4 (14.81) | 6 (14.29) | |
| 3 AE | 7 (5.93) | 4 (8.16) | 2 (7.41) | 1 (2.38) | |
| ≥4 AE | 2 (1.69) | 1 (2.04) | 1 (3.70) | 0 (0.00) | |
| AE, n (%) | Total | G1 | G2 | G3 | P value |
| n = 79 | n = 26 | n = 26 | n = 27 | ||
| Unscheduled hospitalization for HF | 9 (11.39) | 3 (11.54) | 2 (7.69) | 4 (14.81) | 0.904 b |
| Scheduled hospitalization for HF | 3 (3.80) | 0 (0.00) | 3 (11.54) | 0 (0.00) | 0.066 b |
| Unscheduled hospitalization for cardiological causes other than HF | 5 (6.33) | 0 (0.00) | 1 (3.85) | 4 (14.81) | 0.120 b |
| Scheduled hospitalization for cardiological causes other than HF | 6 (7.59) | 4 (15.38) | 1 (3.85) | 1 (3.70) | 0.311 b |
| AE associated with SV intake | |||||
| Symptomatic hypotension | 6 (7.59) | 2 (7.69) | 2 (7.69) | 2 (7.41) | 1.000 b |
| Non‐symptomatic hypotension | 4 (5.06) | 3 (11.54) | 0 (0.00) | 1 (3.70) | 0.215 b |
| Decrease in renal function (>30% eGFR) (mL/min/1.73 m2) | 3 (3.80) | 1 (3.85) | 0 (0.00) | 2 (7.41) | 0.769 b |
| Hyperkalaemia (>5.5 mmol/L) | 2 (2.53) | 2 (7.69) | 0 (0.00) | 0 (0.00) | 0.211 b |
| Angioedema | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| Others | 41 (51.90) | 11 (42.31) | 17 (65.38) | 13 (48.15) | 0.223 a |
AE, adverse event; eGFR, estimated glomerular filtration rate; HF, heart failure; SV, sacubitril–valsartan.
Qualitative variables were described by numbers and percentages.
Statistical tests used were presented, on P values, for χ 2 test.
Statistical tests used were presented, on P values, for Fisher's exact test.
Discussion
To the best of our knowledge, we report the largest prospective, multicentre, real‐life study investigating the effects of an HFrEF‐targeting drug on AHI. Three months after starting SV treatment, we observed a significant decrease in AHI [median −7.10/h (IQ25–75: −16.10 to 0.40)] in SA HFrEF patients with SV adherence but who were not receiving PAP therapy. Of the latter, 24.4% had an AHI decrease ≥50%, and 17.78% more patients (for a total of 37.78%) had a final AHI < 15/h (suggesting a potential PAP sparing benefit).
Similarity with previously reported populations
Among HFrEF patients, the prevalence of SA varies in the literature. Research reports are heterogeneous not only in terms of design and population (HFrEF aetiology and HFrEF treatment) but also in the diagnostic criteria used for SA detection [i.e. polysomnography (PSG) vs. polygraphy], apnoea/hypopnoea coding rules, and AHI definitions (central/obstructive thresholds). 5 , 6 , 7 In our study, using P and the recommended 2012 American Academy of Sleep Medicine coding rules, 57.63% of the total population had an AHI > 15/h, 20.34% with >50% obstructive events, and 23.73% with >50% central events. These figures are consistent with previous reports as summarized in the 2017 European Respiratory Society Task Force report (CSA at a threshold of AHI ≥ 15/h occurs in 21–37% of stable HFrEF patients). 11
This real‐life study in patients with HFrEF and SV initiation is consistent with previous reports not only for baseline characteristics but also regarding impacts on cardiac function and related quality‐of‐life changes. At baseline, 97.46% were treated with ACE inhibitors or sartans, 83.9% with beta‐blockers (median heart rate of 70 b.p.m.), and 64.41% with mineralocorticoid receptor antagonist, as in previous reports. 29 , 30 , 31 , 32 , 33 After SV initiation, NT‐proBNP values decreased by 30% or more in 51.72% of the whole population (a threshold considered as clinically significant 22 , 34 ), which is near the 46.3% reported by Pharithi et al. 35 In G1 patients, SV initiation was associated with an improvement in right ventricular function as previously reported. 29 , 33 SV also improved the NYHA class, the Minnesota Living with Heart Failure Questionnaire scores, and the EQ‐5D VAS scores, as previously reported. 30 , 31
Central sleep apnoea treatment modalities in patients with heart failure and reduced ejection fraction
CSA treatment modalities in HFrEF patients are not supported by a high level of evidence, and importantly, poorly efficient CPAP has been demonstrated as harmful in CSA patients. 36 In the context of SERVE‐HF study 17 and pending the ADVENT‐HF study results, 18 optimizing HFrEF management is recommended to improve CSA in clinical practice (expert Grade C recommendation, 11 based on six studies encompassing a total of just 67 patients 19 ). For the 37/40 G1 patients with a mainly CSA pattern and an SV intake but without PAP treatment, we report a significant median AHI difference of −6.60 (−11.70 to 0.40)/h. If we consider that the AHI ≥ 15/h threshold is used to initiate a PAP treatment, our data suggest that SV is associated with a PAP sparing effect requiring further investigation (16.2% more G1 patients with a final AHI < 15/h in our study). Interestingly, 25% of our population presented an LVEF ≥ 35%, while 32% of the SERVE‐HF population presented an LVEF > 36%. 37 During the study, only one G1 patient has a CPAP trial as recommended by the 2017 European Respiratory Society statement (HFrEF patients with CSA can be treated with CPAP, if CPAP suppresses CSA and improves symptoms, Grade C recommendation). 11
Obstructive sleep apnoea treatment modalities in patients with heart failure and reduced ejection fraction
OSA treatment with CPAP is supported by non‐randomized/cohort studies reporting a decrease in mortality. 12 , 13 , 14 Pending the ADVENT‐HF study results, 18 symptomatic patients are eligible for CPAP treatment. 38 As a consequence, only 9/22 G2 patients were not ‘CPAP treated’, and for the eight patients with SV intake at the 3 month P evaluation, the median AHI difference between initial and final assessment was −12.40 (−23.60 to 0.35)/h, P = 0.059. Because this difference was mainly the consequence of the change in the obstructive component of the AHI (−11.20 (−16.15 to −6.40), we can hypothesized that SV may act via its properties (both diuretic effect and improvement in the global cardiovascular status, hence a decrease in volaemia) and a decrease in upper airway oedema/rostral fluid shift. 39 , 40 As a matter of fact, HFrEF patients have an increased risk for OSA due to extracellular fluid overload. Achieving fluid homeostasis is a potential point of care because pharyngeal oedema and narrowing may develop during supine sleep with redistribution of fluid from the legs and subsequent pharyngeal collapsibility and airway obstruction in HFrEF patients with OSA. This paves the way for similar approaches regarding all the interventions able to improve globally the fluid homeostasis. These data support the hypothesis of a potential PAP sparing effect of the SV treatment in these patients requiring further research. Unfortunately, our study was not designed to collect the number of G2 patients accepting secondarily the CPAP treatment because of a final AHI ≥ 15/h despite the SV treatment.
Safety
The safety profile of SV in this real‐life population was in line with previous reports even though the short‐term design of our study (3 months) limited dose escalation. The short titration period may partly explain why high doses were obtained for only 34.6% of the whole population. This appears similar to the 35.7% of patients reported at 7 months in a non‐selected cohort, 32 but low compared with a further report mentioning >60% of the patients with a 97/103 mg SV dosage at 6 months. 31 We also report that 12.65% of our patients presented an SV‐associated hypotension, which is similar to the fraction (10.3%) reported in the PARASAIL study. 31 Only 3.80% of our whole population presented a 30% decrease in eGFR in comparison, which is quite comparable with the 3.03% reported by Pharithi et al. 35
Limits and strengths of the study
A limit, but also a strength, of our study is the real‐life design. Although randomized controlled trials are the gold standard for evaluating treatment effects, they may not always fully represent what happens in real life because of inherent selection bias. 41 , 42 , 43 A real‐life design can also become the only possible design for ethical reasons, as in our case for a treatment that had previously demonstrated a beneficial effect on mortality (SV improves cardiovascular mortality in HFrEF patients) but nevertheless requires investigation.
A 3 month rather than 4 month study was recommended by our ethics committee. As a consequence, only 36.36% of G1 + G2 patients reached the SV high dose. Nevertheless, we observed an SV effect on AHI, reinforcing the potential strength of our approach. At 3 months, a second P was not performed for G3 patients based on our ethics committee's recommendations.
Apnoea–hypopnoea index was determined by a P and not a PSG, which evidently underestimates AHI in comparison. In addition to real‐life study design constraints and ethics requirements, we further chose to perform a P rather than a PSG because (i) this reflects current practice around the world when PSG access is limited (the average waiting time for PSG is >2 months in industrialized countries) 44 , 45 , 46 and (ii) delaying SV initiation can worsen outcomes due to the SV effect on mortality.
The sample size of group G1 and G2 should be taken into account to explain some of the non‐significant statistical differences for patient characteristics/co‐morbidities or cardiological features at baseline.
Our study was not designed to specifically evaluate the effect of SV on PAPS. The observed PAPS decrease with SV in G1 is a matter of debate. These results are only preliminary as the impact on right cavities is an unexpected result deserving to be more deeply investigated.
The concomitant CPAP treatment for 13/22 G2 patients with an OSA pattern limits our conclusions in this group, and the interest of our study mainly concerns the 37/40 G1 patients with CSA patterns. Importantly, we report no significant changes in cardiological medical treatments in G1, which could also explain the benefit on AHI (inducing a bias in our analyses). Considering the AHI ≥ 15/h threshold used to initiate a PAP treatment, our data suggest that SV may be associated with a PAP sparing effect. But these data need to be confirmed by specifically designed studies.
Conclusions
In this real‐life population of HFrEF patients with an initial SA diagnostic but no PAP treatment, SV is associated with a significant decrease in AHI at 3 months. Our results support the current guidelines that recommend first an optimization of the HFrEF treatment in patients with HFrEF and CSA. 11 A potential PAP sparing benefit merits further research.
Conflict of interest
D.J. reports personal fees from Philips Healthcare, ResMed, GSK, Boehringer Ingelheim, AstraZeneca, Chiesi Farmaceutici, Sanofi, Novartis, and Bastide Le Confort Médical; personal fees and non‐financial support from SEFAM; grants, personal fees, and non‐financial support from Lowenstein and Nomics; grants and personal fees from APARD; and grants from ADENE, outside the submitted work. P.B. reports personal fees from Novartis, outside the submitted work. M.G. reports personal fees from Novartis, outside the submitted work. P.F. reports personal fees from Novartis and AstraZeneca and non‐financial support from Abbot, outside the submitted work. J.‐E.R. reports personal fees from AstraZeneca, Novartis, and Vifor, outside the submitted work. M.‐P.C. reports non‐financial support from Eole Santé and SOS Oxygène, outside the submitted work. F.P. reports personal fees from Novartis, Pfizer, Actelion, and Vifor, outside the submitted work. J.‐P.M. reports grants from APARD and Novartis, outside the submitted work. C.M.S. reports grants from AstraZeneca, outside the submitted work. N.M. reports personal fees from AstraZeneca and grants from GSK, outside the submitted work. A.B. reports grants, personal fees, non‐financial support, and other from AstraZeneca and Boehringer Ingelheim; grants, personal fees, and other from GSK; personal fees, non‐financial support, and other from Novartis, Chiesi Farmaceutici, and Actelion; personal fees and other from Teva and Regeneron; other from Gilead; and personal fees and non‐financial support from Roche, outside the submitted work. F.R. reports grants from Novartis, during the conduct of the study; grants, personal fees, and non‐financial support from Air Liquide; grants and personal fees from Abbott, Novartis, and AstraZeneca; and personal fees from Vifor, Servier, Abiomed, ZOLL, Medtronic, ResMed, LVL, Eole Santé, Pfizer, Novo Nordisk, Amgen, and Boehringer Ingelheim, outside the submitted work. E.N., M.D., V.P., F.G., and N.P. report no conflicts of interest in relation to the present work.
Funding
This work was supported by an unrestricted grant from Novartis. Novartis has no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supporting information
Appendix S1. Definitions of respiratory events, definition of population‐groups
Figure S1. Correlation matrix.
Table S1. Univariate linear regressions analyses with the AHI relative difference (%) as the variable‐of‐interest in G1 and G2 patients (restricted to patients without positive‐airway‐pressure‐treatment).
Table S2. Group 1, initial and final (3 months) polygraphy data.
Table S3. Group 2, initial and final (3 months) polygraphy data.
Table S4. Final (3 months) PAP‐data.
Table S5. Changes in cardiological data, baseline and final (3 months).
Table S6. Cardiological treatment (initial polygraphy and final polygraphy).
Table S7. Baseline and final (3 months) quality of life data.
Acknowledgements
The authors would like to thank Dr Jean Christian Borel [Grenoble Alpes University, INSERM U1042, HP2 (Hypoxia Physiopathology) Laboratory, Centre Hospitalier Universitaire Grenoble Alpes, Grenoble, France] and Dr Fabrice Thoin (Sleep Centre, Bouchard Clinic, Marseille, France), for their reviews of the present manuscript.
Jaffuel, D. , Nogue, E. , Berdague, P. , Galinier, M. , Fournier, P. , Dupuis, M. , Georger, F. , Cadars, M.‐P. , Ricci, J.‐E. , Plouvier, N. , Picard, F. , Puel, V. , Mallet, J.‐P. , Suehs, C. M. , Molinari, N. , Bourdin, A. , and Roubille, F. (2021) Sacubitril‐valsartan initiation in chronic heart failure patients impacts sleep apnea: the ENTRESTO‐SAS study. ESC Heart Failure, 8: 2513–2526. 10.1002/ehf2.13455.
Data availability statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force Members, Document Reviewers . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 2. Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015; 175: 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stewart S, Ekman I, Ekman T, Odén A, Rosengren A. Population impact of heart failure and the most common forms of cancer: a study of 1 162 309 hospital cases in Sweden (1988 to 2004). Circ Cardiovasc Qual Outcomes 2010; 3: 573–580. [DOI] [PubMed] [Google Scholar]
- 4. Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 2016; 13: 368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Javaheri S, Barbe F, Campos‐Rodriguez F, Dempsey JA, Khayat R, Javaheri S, Malhotra A, Martinez‐Garcia MA, Mehra R, Pack AI, Polotsky VY, Redline S, Somers VK. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69: 841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pearse SG, Cowie MR. Sleep‐disordered breathing in heart failure. Eur J Heart Fail 2016; 18: 353–361. [DOI] [PubMed] [Google Scholar]
- 7. Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Töpfer V. Sleep‐disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail 2007; 9: 251–257. [DOI] [PubMed] [Google Scholar]
- 8. Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener‐West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation 2010; 122: 352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khayat R, Jarjoura D, Porter K, Sow A, Wannemacher J, Dohar R, Pleister A, Abraham WT. Sleep disordered breathing and post‐discharge mortality in patients with acute heart failure. Eur Heart J 2015; 36: 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naughton MT. Heart failure and sleep‐disordered breathing. The chicken or the egg? Am J Respir Crit Care Med 2016; 193: 482–483. [DOI] [PubMed] [Google Scholar]
- 11. Randerath W, Verbraecken J, Andreas S, Arzt M, Bloch KE, Brack T, Buyse B, De Backer W, Eckert DJ, Grote L, Hagmeyer L, Hedner J, Jennum P, La Rovere MT, Miltz C, McNicholas WT, Montserrat J, Naughton M, Pepin J‐L, Pevernagie D, Sanner B, Testelmans D, Tonia T, Vrijsen B, Wijkstra P, Levy P. Definition, discrimination, diagnosis and treatment of central breathing disturbances during sleep. Eur Respir J 2017; 49: 1600959. [DOI] [PubMed] [Google Scholar]
- 12. Javaheri S, Caref EB, Chen E, Tong KB, Abraham WT. Sleep apnea testing and outcomes in a large cohort of Medicare beneficiaries with newly diagnosed heart failure. Am J Respir Crit Care Med 2011; 183: 539–546. [DOI] [PubMed] [Google Scholar]
- 13. Kasai T, Narui K, Dohi T, Yanagisawa N, Ishiwata S, Ohno M, Yamaguchi T, Momomura S‐I. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest 2008; 133: 690–696. [DOI] [PubMed] [Google Scholar]
- 14. Damy T, Margarit L, Noroc A, Bodez D, Guendouz S, Boyer L, Drouot X, Lamine A, Paulino A, Rappeneau S, Stoica M‐H, Dubois‐Randé J‐L, Adnot S, Hittinger L, d'Ortho MP. Prognostic impact of sleep‐disordered breathing and its treatment with nocturnal ventilation for chronic heart failure. Eur J Heart Fail 2012; 14: 1009–1019. [DOI] [PubMed] [Google Scholar]
- 15. Aurora RN, Chowdhuri S, Ramar K, Bista SR, Casey KR, Lamm CI, Kristo DA, Mallea JM, Rowley JA, Zak RS, Tracy SL. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence‐based literature review and meta‐analyses. Sleep 2012; 35: 17–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aurora RN, Bista SR, Casey KR, Chowdhuri S, Kristo DA, Mallea JM, Ramar K, Rowley JA, Zak RS, Heald JL. Updated adaptive servo‐ventilation recommendations for the 2012 AASM guideline: ‘The Treatment of Central Sleep Apnea Syndromes in Adults: Practice Parameters with an Evidence‐Based Literature Review and Meta‐Analyses’. J Clin Sleep Med 2016; 12: 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cowie MR, Woehrle H, Wegscheider K, Angermann C, d'Ortho M‐P, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H. Adaptive servo‐ventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015; 373: 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lyons OD, Floras JS, Logan AG, Beanlands R, Cantolla JD, Fitzpatrick M, Fleetham J, John Kimoff R, Leung RST, Lorenzi Filho G, Mayer P, Mielniczuk L, Morrison DL, Ryan CM, Series F, Tomlinson GA, Woo A, Arzt M, Parthasarathy S, Redolfi S, Kasai T, Parati G, Delgado DH, Bradley TD, ADVENT‐HF Investigators . Design of the effect of adaptive servo‐ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT‐HF trial. Eur J Heart Fail 2017; 19: 579–587. [DOI] [PubMed] [Google Scholar]
- 19. Jaffuel D, Molinari N, Berdague P, Pathak A, Galinier M, Dupuis M, Ricci J‐E, Mallet J‐P, Bourdin A, Roubille F. Impact of sacubitril–valsartan combination in patients with chronic heart failure and sleep apnoea syndrome: the ENTRESTO‐SAS study design. ESC Heart Fail 2018; 5: 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Burnier M. Angiotensin II type 1 receptor blockers. Circulation 2001; 103: 904–912. [DOI] [PubMed] [Google Scholar]
- 21. Chen C‐H. Critical questions about PARADIGM‐HF and the future. Acta Cardiol Sin 2016; 32: 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM‐HF Investigators and Committees . Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 23. Mentz RJ, Xu H, O'Brien EC, Thomas L, Alexy T, Gupta B, Vilaro J, Lala A, DeVore AD, Dhingra R, Briasoulis A, Simon MA, Stehlik J, Rodgers JE, Dunlay SM, Abshire M, Wells QS, Barringhaus KG, Eckman PM, Lowes BD, Espinoza J, Blanco R, Shen X, Duffy CI, Hernandez AF. PROVIDE‐HF primary results: Patient‐Reported Outcomes inVestigation following Initiation of Drug therapy with Entresto (sacubitril/valsartan) in heart failure. Am Heart J 2020; 230: 35–43. [DOI] [PubMed] [Google Scholar]
- 24. Khariton Y, Fonarow GC, Arnold SV, Hellkamp A, Nassif ME, Sharma PP, Butler J, Thomas L, Duffy CI, DeVore AD, Albert NM, Patterson JH, Williams FB, McCague K, Spertus JA. Association between sacubitril/valsartan initiation and health status outcomes in heart failure with reduced ejection fraction. JACC Heart Fail 2019; 7: 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, Marcus CL, Mehra R, Parthasarathy S, Quan SF, Redline S, Strohl KP, Davidson Ward SL, Tangredi MM, American Academy of Sleep Medicine . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012; 8: 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garin O, Herdman M, Vilagut G, Ferrer M, Ribera A, Rajmil L, Valderas JM, Guillemin F, Revicki D, Alonso J. Assessing health‐related quality of life in patients with heart failure: a systematic, standardized comparison of available measures. Heart Fail Rev 2014; 19: 359–367. [DOI] [PubMed] [Google Scholar]
- 27. EuroQol Group . EuroQol—a new facility for the measurement of health‐related quality of life. Health Policy Amst Neth 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 28. Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 1991; 14: 540–545. [DOI] [PubMed] [Google Scholar]
- 29. Correale M, Mallardi A, Mazzeo P, Tricarico L, Diella C, Romano V, Ferraretti A, Leopizzi A, Merolla G, Di Biase M, Brunetti ND. Sacubitril/valsartan improves right ventricular function in a real‐life population of patients with chronic heart failure: the Daunia Heart Failure Registry. Int J Cardiol Heart Vasc 2020; 27: 100486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ganesananthan S, Shah N, Shah P, Elsayed H, Phillips J, Parkes A, Morgan A, Yousef Z. Real‐world treatment switching to sacubitril/valsartan in patients with heart failure with reduced ejection fraction: a cohort study. Open Heart 2020; 7: e001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haddad H, Bergeron S, Ignaszewski A, Searles G, Rochdi D, Dhage P, Bastien N. Canadian real‐world experience of using sacubitril/valsartan in patients with heart failure with reduced ejection fraction: insight from the PARASAIL study. CJC Open 2020; 2: 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. López‐Azor JC, Vicent L, Valero‐Masa MJ, Esteban‐Fernández A, Gómez‐Bueno M, Pérez Á, Díez‐Villanueva P, De‐Juan J, Manuel‐Iniesta Á, Bover R, del Prado S, Martínez‐Sellés M. Safety of sacubitril/valsartan initiated during hospitalization: data from a non‐selected cohort. ESC Heart Fail 2019; 6: 1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Masarone D, Errigo V, Melillo E, Valente F, Gravino R, Verrengia M, Ammendola E, Vastarella R, Pacileo G. Effects of sacubitril/valsartan on the right ventricular arterial coupling in patients with heart failure with reduced ejection fraction. J Clin Med 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O'Hanlon R, O'Shea P, Ledwidge M, O'Loughlin C, Lange S, Conlon C, Phelan D, Cunningham S, McDonald K. The biologic variability of B‐type natriuretic peptide and N‐terminal pro‐B‐type natriuretic peptide in stable heart failure patients. J Card Fail 2007; 13: 50–55. [DOI] [PubMed] [Google Scholar]
- 35. Pharithi RB, Ferre‐Vallverdu M, Maisel AS, O'Connell E, Walshe M, Sweeney C, Barton J, McDonald K, O'Hare D, Watson C, Gallagher J, Ledwidge M, McDonald K. Sacubitril‐Valsartan in a routine community population: attention to volume status critical to achieving target dose. ESC Heart Fail 2020; 7: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arzt M, Floras JS, Logan AG, Kimoff RJ, Series F, Morrison D, Ferguson K, Belenkie I, Pfeifer M, Fleetham J, Hanly P, Smilovitch M, Ryan C, Tomlinson G, Bradley TD, CANPAP Investigators . Suppression of central sleep apnea by continuous positive airway pressure and transplant‐free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation 2007; 115: 3173–3180. [DOI] [PubMed] [Google Scholar]
- 37. Eulenburg C, Wegscheider K, Woehrle H, Angermann C, d'Ortho M‐P, Erdmann E, Levy P, Simonds AK, Somers VK, Zannad F, Teschler H, Cowie MR. Mechanisms underlying increased mortality risk in patients with heart failure and reduced ejection fraction randomly assigned to adaptive servoventilation in the SERVE‐HF study: results of a secondary multistate modelling analysis. Lancet Respir Med 2016; 4: 873–881. [DOI] [PubMed] [Google Scholar]
- 38. Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA 2020; 323: 1389–1400. [DOI] [PubMed] [Google Scholar]
- 39. Bucca CB, Brussino L, Battisti A, Mutani R, Rolla G, Mangiardi L, Cicolin A. Diuretics in obstructive sleep apnea with diastolic heart failure. Chest 2007; 132: 440–446. [DOI] [PubMed] [Google Scholar]
- 40. Yumino D, Redolfi S, Ruttanaumpawan P, Su M‐C, Smith S, Newton GE, Mak S, Bradley TD. Nocturnal rostral fluid shift: a unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation 2010; 121: 1598–1605. [DOI] [PubMed] [Google Scholar]
- 41. Pahus L, Jaffuel D, Vachier I, Bourdin A, Suehs CM, Molinari N, Chanez P. Randomised controlled trials in severe asthma: selection by phenotype or stereotype. Eur Respir J 2019; 53: 1802187. [DOI] [PubMed] [Google Scholar]
- 42. Pahus L, Suehs CM, Halimi L, Bourdin A, Chanez P, Jaffuel D, Marciano J, Gamez A‐S, Vachier I, Molinari N. Patient distrust in pharmaceutical companies: an explanation for women under‐representation in respiratory clinical trials? BMC Med Ethics 2020; 21: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roche N, Anzueto A, Bosnic Anticevich S, Kaplan A, Miravitlles M, Ryan D, Soriano JB, Usmani O, Papadopoulos NG, Canonica GW, Respiratory Effectiveness Group Collaborators . The importance of real‐life research in respiratory medicine: manifesto of the Respiratory Effectiveness Group: endorsed by the International Primary Care Respiratory Group and the World Allergy Organization. Eur Respir J 2019: 54: 1901511. [DOI] [PubMed] [Google Scholar]
- 44. Flemons WW, Douglas NJ, Kuna ST, Rodenstein DO, Wheatley J. Access to diagnosis and treatment of patients with suspected sleep apnea. Am J Respir Crit Care Med 2004; 169: 668–672. [DOI] [PubMed] [Google Scholar]
- 45. Thornton CS, Tsai WH, Santana MJ, Penz ED, Flemons WW, Fraser KL, Hanly PJ, Pendharkar SR. Effects of wait times on treatment adherence and clinical outcomes in patients with severe sleep‐disordered breathing: a secondary analysis of a noninferiority randomized clinical trial. JAMA Netw Open 2020; 3: e203088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rotenberg B, George C, Sullivan K, Wong E. Wait times for sleep apnea care in Ontario: a multidisciplinary assessment. Can Respir J 2010; 17: 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Definitions of respiratory events, definition of population‐groups
Figure S1. Correlation matrix.
Table S1. Univariate linear regressions analyses with the AHI relative difference (%) as the variable‐of‐interest in G1 and G2 patients (restricted to patients without positive‐airway‐pressure‐treatment).
Table S2. Group 1, initial and final (3 months) polygraphy data.
Table S3. Group 2, initial and final (3 months) polygraphy data.
Table S4. Final (3 months) PAP‐data.
Table S5. Changes in cardiological data, baseline and final (3 months).
Table S6. Cardiological treatment (initial polygraphy and final polygraphy).
Table S7. Baseline and final (3 months) quality of life data.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


