Abstract
Aims
Continuous‐flow left ventricular assist devices (LVADs) as destination therapy (DT) are a recommended treatment by National Institute for Health and Care Excellence England for end‐stage heart failure patients ineligible for cardiac transplantation. Despite the fact that DT is frequently used as an LVAD indication across other major European countries and the United States, with consistent improvements in quality‐of‐life and longevity, National Health Service (NHS) England does not currently fund DT, mainly due to concerns over cost‐effectiveness. On the basis of the recently published ENDURANCE Supplemental Trial studying DT patients, we assessed for the first time the cost‐effectiveness of DT LVADs compared with medical management (MM) in the NHS England.
Methods and results
We developed a Markov multiple‐state economic model using NHS cost data. LVAD survival and adverse event rates were derived from the ENDURANCE Supplemental Trial. MM survival was based on Seattle Heart Failure Model estimates in the absence of contemporary clinical trials for this population. Incremental cost‐effectiveness ratios (ICERs) were calculated over a lifetime horizon. A discount rate of 3.5% per year was applied to costs and benefits. Deterministic ICER was £46 207 per quality‐adjusted life year (QALY). Costs and utilities were £204 022 and 3.27 QALYs for the LVAD arm vs. £77 790 and 0.54 QALYs for the MM arm. Sensitivity analyses confirmed robustness of the primary analysis.
Conclusions
The implantation of the HeartWare™ HVAD™ System in patients ineligible for cardiac transplantation as DT is a cost‐effective therapy in the NHS England healthcare system under the end‐of‐life willingness‐to‐pay threshold of £50 000/QALY, which applies for VAD patients.
Keywords: Mechanical circulatory support, Ventricular assist device, Destination therapy, Cost‐effectiveness
Introduction
The sole cardiovascular disease with rising prevalence in the United Kingdom (UK) is heart failure (HF). 1 HF currently affects approximately 750 000 individuals in the UK with an increasing burden 2 , 3 mainly due to aging population demographics, earlier recognition and increasing rates of diagnosis, and greater longevity with the disease. 4 In the 2020 report of the National Heart Failure Audit, 78% of patients requiring admission with HF were associated with symptoms at New York Heart Association (NYHA) class III or IV and 35% with NYHA class IV. 5 UK primary and secondary health records from 2002 to 2014 stated an absolute increase in the yearly number of new diagnoses of HF by 12% while the absolute prevalence of HF cases increased by 23%. 2 Patients with advanced or end‐stage HF with symptoms refractory to guideline‐based medical therapy are potential candidates for heart transplantation or left ventricular assist device (LVAD) therapy. 6
Superiority of LVAD therapy over optimal medical management (MM) in patients with end‐stage HF (NYHA class IV) and contraindications to transplant has been well established since the era of the REMATCH trial. 7 LVADs were originally developed for use as bridge to transplantation (BTT) for short‐term support but are now increasingly used for longer periods of time either in BTT patients facing a long extended time on the waiting list for heart transplantation or by patients ineligible for cardiac transplantation who remain on device support permanently as destination therapy (DT). 6 The number of patients on LVAD support as DT has increased dramatically the last few years, representing 73% of implants in the United States (US) 8 and 41% in the International Society for Heart and Lung Transplantation Mechanically Assisted Circulatory Support (IMACS) Registry. 9 In October of 2018, a new heart allocation system was introduced in the US, affording stable LVAD patients a lower priority than the previous one. 10 Data 1 year after these changes show an increased share of DT patients when compared with BTT, reaching 73%. 8 DT patients already presented a high share of LVAD patients before the new donor criteria with 49.5% in 2017 and 56.6% in 2018. 8 LVADs as DT are available in the basic healthcare package in many countries, including US, 11 Germany, 12 and France. 13
In the UK, LVAD implantation is recommended by the National Institute for Health and Care Excellence (NICE) as being safe and effective both for patients awaiting heart transplantation as BTT and for patients ineligible for heart transplantation as DT. 14 However, LVAD therapy is currently only reimbursed for the BTT indication, and not for DT. 3 , 15 , 16 The National Health Service (NHS) Specialised Commissioning consultation reported as one of the key reasons for not commissioning LVADs for DT a lack of UK cost‐effectiveness evidence on LVAD DT patients. 15 On the same consultation report, it was mentioned that the NICE threshold that applies for LVAD patients is the one used for ‘end of life’ care of £50 000/quality‐adjusted life year (QALY). 15 The criteria for end of life interventions are mainly: (a) that the indicated population has a short life expectancy and (b) that treatment will prolong survival by at least three extra months. 17 LVAD DT eligible patients have HF severe enough to be considered under end of life criteria. 15 There is also a third LVAD strategy, called bridge‐to‐candidacy, in which LVADs are used for the purpose of alleviating comorbidities and making patients currently ineligible for heart transplantation well enough to be eligible. 6 In UK, bridge‐to‐candidacy is widely adopted with a comparable survival and heart transplant rate to BTT patients. 18
The National Health Service has performed a Health Technology Assessment (HTA) for LVADs in the BTT indication. 3 The lifetime incremental cost‐effectiveness ratio (ICER) was £53 527/QALY. 3 Previous European cost‐effectiveness LVAD studies on DT patients in the Netherlands and Belgium have estimated ICERs between €82 000 and €94 100. 19 , 20 Both studies used clinical data from a previous generation LVAD that is not widely used anymore in most European countries nor in the US. A recent US study using the latest clinical evidence found substantial improvements in cost‐effectiveness reflecting the improvements in the DT LVAD therapy in terms of better clinical management of patients and latest generation devices used. 21
However, there is no cost‐effectiveness study using the more contemporaneous LVAD DT clinical data in a European healthcare setting, and there is no DT cost‐effectiveness study assessing the associated costs in the framework of the UK healthcare system. It is important to update cost‐effectiveness with recent clinical data; in order to evaluate how the latest LVAD innovation and patient management improvements translate into clinical and economic value for the healthcare system and impact patient therapy access.
Therefore, the aim of this study is to evaluate the cost‐effectiveness of the HeartWare™ HVAD™ System in the DT indication under the NHS perspective.
Methods
Model structure
In line with NICE guidelines, we performed a cost‐utility analysis, using the NHS payer perspective, measuring health effects in terms of QALYs and applying a 3.5% discount rate for both benefits and costs. 22 We developed a Markov model to estimate the cost‐effectiveness of LVADs in the DT indication for end‐stage HF patients ineligible for heart transplantation. We compared costs and QALYs of patients implanted with the HeartWare™ HVAD™ System to patients on optimal MM. The model included two basic health states for the LVAD and MM arms, ‘Alive’ and ‘Dead’ (Figure 1 ). It applied variable mortality rates in every cycle up to 10 years post‐implantation. Each cycle period was 1 month. In each cycle, patients alive were exposed to therapy related adverse events (AEs) and death. Stroke had additional health states defined for when it occurred; every stroke‐related state had a different level of stroke severity. We constructed the model and calculated the results in Excel Office 365 (Microsoft®, California).
Figure 1.
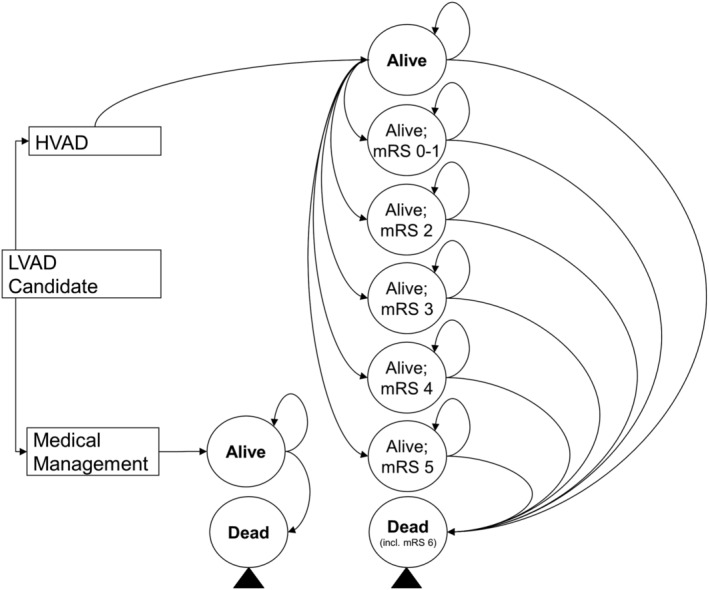
Model schematic. LVAD, left ventricular assist device; mRS, modified Rankin scale.
Mortality and adverse events
In the absence of a randomized control trial comparing continuous flow LVADs to MM patients, the following data were selected as clinical inputs to the model. The last published randomized control trial was REMATCH in 2001, where patients were implanted with a much older device than what is currently used. 7 Individual patient data (IPD) from the most contemporary DT data available, the ENDURANCE Supplemental Trial, 23 were used for the LVAD arm survival. Mean age was 63.3 (±11.4) years, 18.2% were female, 71.8% white, 80% were INTERMACS levels 1–3 and 67% were on inotropic support (Supporting Information, Table S1 ). 23 A Weibull statistical model was fitted to the trial IPD data and informed the predicted survival in the model. Using a Weibull model to extrapolate survival data beyond the available study follow‐up period is a common method adopted in the HF economic evaluation literature and was applied for example in the cardiac resynchronization therapy and implantable cardioverter defibrillator NICE technology appraisals. 24 The Weibull model used the least squares methodology with the sum of the squared residuals below 1−10. Other non‐parametric models tested (e.g. exponential) failed to produce a similarly good fit. For the MM arm survival, the Seattle Heart Failure Model (SHFM) was used by employing a hazard ratio (HR) derived from its medically managed cohort of end‐stage HF patients. 25 Survival curves for both LVAD and MM are shown in Figure 2 .
Figure 2.
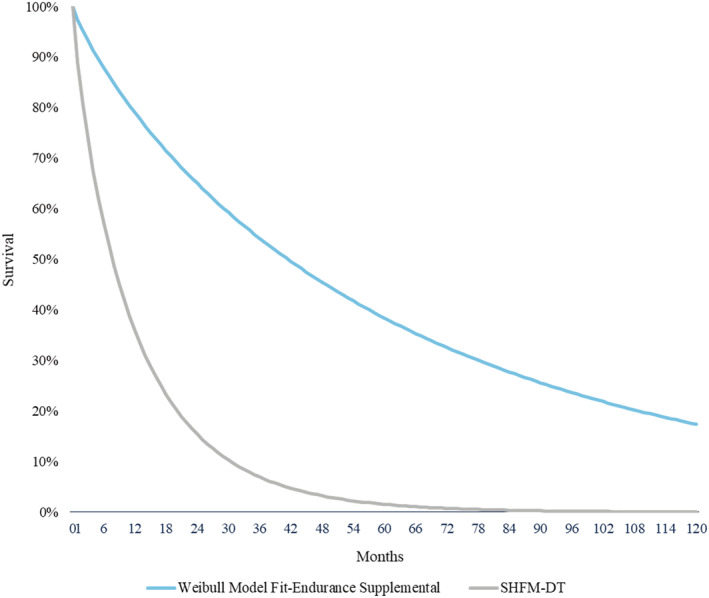
Survival curves in the model. DT, destination therapy; SHFM: Seattle Heart Failure.
Adverse event rates were obtained from the ENDURANCE Supplemental Trial. 23 AEs included in the model were pump exchange for thrombus or VAD failure, ischemic and haemorrhagic stroke, gastrointestinal (GI) bleeding, driveline infection, right‐sided HF with and without the need for a right ventricular assist device, and other AEs that require hospitalization. Stroke data were further stratified by functional outcomes using the modified Rankin scale score reported at 24 weeks post‐event. Whenever an AE occurred, the related cost and utility decrement was applied. A summary of the AE transition probabilities is presented in Table 1 .
Table 1.
Transition probabilities
| Event | Event rate b | Transformed monthly event rate DT as used in the model |
|---|---|---|
| Stroke | ||
| Ischemic | 0.17 EPPY | 0.014 |
| Haemorrhagic | 0.06 EPPY | 0.005 |
| Pump exchange | ||
| VAD thrombus | 0.06 EPPY | 0.005 |
| VAD failure | 0.01 EPPY | 0.001 |
| Driveline infection | 0.24 EPPY | 0.020 |
| GI bleed | 0.57 EPPY | 0.048 |
| RHF | 0.28 EPPY | 0.025 |
| RVAD | 0.02 EPPY | 7% a |
| Other AEs | 0.45 EPPY | 0.038 |
AE, adverse event; DT, destination therapy; EPPY, events per patient year; GI, gastrointestinal; IPD, individual patient data; MM: medical management; RHF: right heart failure; RVAD: right ventricular assist device; VAD: ventricular assist device.
Percentage of the RHF population.
ENDURANCE Supplemental data, Medtronic data on file.
Costs
We employed inpatient costs for the LVAD cohort for the index hospitalization, including the device cost and all major AEs from the NHS national tariffs and reference costs. 26 , 27 Monthly outpatient expenses incurred by ‘living with an LVAD’ were taken from a real‐world UK database of BTT patients (Table 2 ). For the MM arm, monthly inpatient and outpatient costs comprehensively were taken from the NHS BTT HTA study. 3 All costs were inflated to March 2019. All costs are listed in Table 2 .
Table 2.
NHS costs and utilities
| Parameter | NHS cost a | Utility | Cost reference |
|---|---|---|---|
| Medical management | |||
| MM inpatient & outpatient | £6430 | 0.54 | Clarke et al. 16 |
| Living with MM > 10 years | £6547 | 0.54 | b |
| LVAD | |||
| LVAD Implantation (incl. device cost) | £91 162 | — | National Health Service (NHS) England and Improvement 26 |
| Living with LVAD | £1069 | 0.72 | c |
| Living with LVAD > 10 years | £13 362 | 0.72 | b |
| Stroke (ischemic or haemorrhagic) index hospitalization | |||
| Stroke mRS 0 | £1965 | 0.68 | National Health Service (NHS) England and Improvement 28 |
| Stroke mRS 1 | £1965 | 0.68 | National Health Service (NHS) England and Improvement 28 |
| Stroke mRS 2 | £2757 | 0.59 | National Health Service (NHS) England and Improvement 28 |
| Stroke mRS 3 | £3945 | 0.59 | National Health Service (NHS) England and Improvement 28 |
| Stroke mRS 4 | £5594 | 0.27 | National Health Service (NHS) England and Improvement 28 |
| Stroke mRS 5 | £7681 | 0.27 | National Health Service (NHS) England and Improvement 28 |
| Stroke mRS 6 | £12 656 | 0.00 | National Health Service (NHS) England and Improvement 28 |
| Stroke (ischemic or haemorrhagic) follow up | |||
| Stroke mRS 0 | £200 | 0.68 | Luengo‐Fernandez et al. 29 |
| Stroke mRS 1 | £200 | 0.68 | Luengo‐Fernandez et al. 29 |
| Stroke mRS 2 | £200 | 0.59 | Luengo‐Fernandez et al. 29 |
| Stroke mRS 3 | £390 | 0.59 | Luengo‐Fernandez et al. 29 |
| Stroke mRS 4 | £390 | 0.27 | Luengo‐Fernandez et al. 29 |
| Stroke mRS 5 | £592 | 0.27 | Luengo‐Fernandez et al. 29 |
| Pump exchange | |||
| Pump thrombus | £91 162 | 0.69 | National Health Service (NHS) England and Improvement 26 |
| VAD failure | £91 162 | 0.57 | National Health Service (NHS) England and Improvement 26 |
| Driveline infection | £7662 | 0.71 | National Health Service (NHS) England and Improvement 28 |
| Gastrointestinal bleed | £6899 | 0.69 | National Health Service (NHS) England and Improvement 28 |
| Right heart failure | £5976 | 0.69 | National Health Service (NHS) England and Improvement 28 |
| RVAD (short‐term device) | £60 975 | 0.69 | National Health Service (NHS) England and Improvement 28 |
| Other important AEs LVAD | £5976 | 0.72 | National Health Service (NHS) England and Improvement 28 |
AE, adverse events; LVAD, left ventricular assisted device; MM, medical management; mRS, modified Rankin scale; NHS, National Health Service; RVAD, right ventricular assist device.
All costs were inflated to March 2019; recovery and stroke follow‐up costs are per month; >10 years costs are to be intended as residual annual costs.
NHS heart failure hospitalization cost for cost estimation; event rate post 18 month resource use. 30
Wong W, Bottle A. Healthcare utilisation, outcomes and patient pathways for heart failure patients with or without cardiac implantable electronic devices in England. Imperial College London, Medtronic data on file. 2019.
Utilities
We used LVAD‐specific IPD data from the quality of life (QoL) questionnaires collected during contemporary HeartWare HVAD (Medtronic, Minneapolis, USA) trials to calculate the utilities (Table 2 ). For the LVAD arm, the ‘Living with LVAD’ utility was calculated as the mean value across all available time points from patients with no major AEs from the ENDURANCE and the ENDURANCE Supplemental EQ‐5D‐3L and 5L questionnaires. 23 , 31 We estimated the utility decrements for the AEs included in the model using the ADVANCE BTT + CAP (EQ‐5D‐3L), 32 the ENDURANCE (EQ‐5D‐3L), and the ENDURANCE Supplemental (EQ‐5D‐5L) questionnaires. 23 , 31 We used the average of the before–after score difference by patient as decrements. We estimated the ‘Living on MM’ utility based on the pre‐implant measurement of the ENDURANCE and ENDURANCE Supplemental Trials. 23 , 31 All the utilities were converted to reflect UK QoL values using the Dolan 1997 algorithm. 33
Sensitivity and scenario analyses
In addition to the base‐case analysis, we performed several sensitivity and scenario analyses to test the robustness of our results. First, we performed a deterministic (one‐way) sensitivity analysis (DSA) for each of the major LVAD AEs, including stroke, driveline infection, GI bleeding, right HF, and pump exchange (minimum was zero, and maximum was +100% increase from the base case values). The results from the one‐way sensitivity analyses are shown in the Tornado diagram (Figure 3 ) which sequentially graphs the variables with the largest impact on the ICER outcomes. Second, we ran probabilistic sensitivity analyses where default inputs were varied ±25% and 1000 simulations were performed. We also ran two scenario analyses. First, we substituted LVAD and MM utilities with values from the NHS HTA of LVADs for patients eligible for heart transplantation in the BTT indication, 3 and second, we used two different HRs for the MM survival ‐ a lower (0.105) 7 , 33 and a higher (0.52) 7 value than the base case (0.23).
Figure 3.
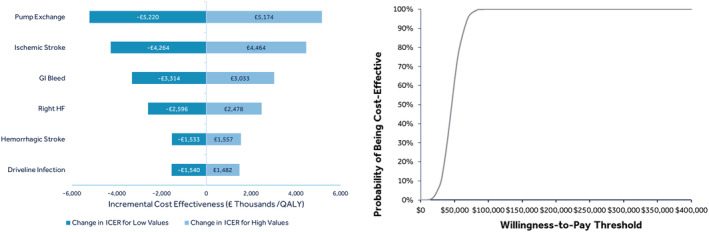
Sensitivity analyses—Tornado diagram & cost‐effectiveness acceptability curve. GI, gastrointestinal; ICER, incremental cost‐effectiveness ratio; QALY, quality adjusted life years.
Results
Base case
We found a deterministic ICER of £46 207 per QALY and £34 907 per life‐year (LY) (Table 3 ). The total cost for patients on LVAD support was £204 022, and for patients on MM therapy £77 790. LVAD patients accrued more QALYs and LYs than MM patients (3.27 vs. 0.54 QALYs and 4.63 vs. 1.01 LYs).
Table 3.
Base case results
| Variable | QALYs | Life years | ||
|---|---|---|---|---|
| LVAD | MM | LVAD | MM | |
| QALYs/LYs | 3.27 | 0.54 | 4.63 | 1.01 |
| Medical costs (£) | 204 022 | 77 790 | 204 022 | 77 790 |
| ICER (£/QALY/LY) | 46 207 | 34 907 | ||
ICER, incremental cost‐effectiveness ratio; LVAD, left ventricular assist device; LY, life year; MM, medical management; QALY, quality‐adjusted life year.
Sensitivity and scenario analyses—results
One‐way DSA for the major LVAD AEs overall confirmed the robustness of our model. Variation in ICER for pump exchange and ischemic stroke rates were higher than changes for right HF or haemorrhagic stroke rates; however, for all scenarios, the change in the ICER was small with the lowest estimated ICER being £40 987/QALY and highest being £51 381/QALY (Figure 3 ). Changes in the ICER for the worse‐case values of the DSA analyses remained around the £50 000/QALY threshold.
The probabilistic sensitivity analyses found a probabilistic ICER of £46 258/QALY (95% confidence interval £20 009–£72 255/QALY). ICERs were 63.1% below £50 000/QALY (Figures 3 and S1 ).
For the scenario analysis which substituted LVAD and MM utilities with values from the NHS HTA of LVADs for patients eligible for heart transplantation in the BTT indication, the ICER remained similar to our base case value (£44 879/QALY vs. £46 207/QALY, Table S1 ).
In the second scenario analysis, when the HR for the MM survival was substituted with a lower value than the base case, the ICER increased but remained very close to the £50 000/QALY threshold (£56 357/QALY), and when the HR was substituted with a higher value than the base case, the ICER decreased (£11 017/QALY) (Table S2 ).
Discussion
We used individual‐level patient data from a contemporary DT LVAD trial to assess cost‐effectiveness of LVADs in the DT indication compared with the MM strategy in the NHS England healthcare setting. Our analysis found that compared with the MM cohort, DT patients implanted with an LVAD incurred higher mean costs but benefited with higher survival and QoL resulting in a favourable deterministic ICER of £46 207/QALY and a slightly higher probabilistic ICER of £46 258/QALY (95% confidence interval £20 009–£72 255/QALY) for a lifetime horizon.
Compared with our findings, previous cost‐effectiveness studies showed higher ICER values for a DT population, in both Europe 19 , 20 and the US. 34 Until recently, there was a large difference between US and European ICER outcomes in the LVAD cost‐effectiveness literature. 19 , 20 , 34 In contemporary US studies, a significant improvement in cost‐effectiveness was observed in all LVAD indications as evidenced by publications of Silvestry et al. 21 and Mahr et al. 35 Both publications used more current LVAD data than historic US studies and accounted for MM survival using the SHFM. 21 , 35 Our results are in line with these improved ICER outcomes.
Our observed improvement in ICER value can be explained by several reasons:
Cost‐effectiveness assessments vary from country to country, as shown in a Western European economic evaluation review for pharmaceuticals, where the estimated ICER varied substantial in more than half of the studies in the analysis. 36
Costs may vary substantially in different healthcare systems. US cost‐effectiveness studies such as Rogers et al. 34 and Silvestry et al. 21 found ICERs of $198 184/QALY and $102 587/QALY for DT LVAD population. These studies used US health care costs, which are substantially higher than in England. For example hospitalizations for driveline infection and GI bleed cost £7662 and £6899 in the UK vs. $13 681 and $9990 in the US. 35
Our study uses the most contemporary DT clinical trial data for LVADs. Similar to Rogers et al., Neyt et al., and the KCE HTA study used clinical data from Slaughter et al., studying a DT population with the HeartMate II device in 2009. 19 , 20 , 34 , 37 The use of contemporary LVAD data was a key reason for lower ICERs in recently US published studies. 21 , 35 Indeed, survival with DT LVAD has improved in the contemporary era. For example, 1 and 2 year survival rates were 68% and 58%, respectively, in Slaughter et al.; while comparably, 1 and 2 year survival in ENDURANCE Supplemental—the trial used for this analysis—was 82% and 70%. 23 , 37 Improvements in survival can be explained by the use of latest generation LVADs, increasing user experience, and advances in patient management with DT LVAD therapy. For example, results from the ENDURANCE Supplemental Trial demonstrated that improved blood pressure management reduces stroke rates in LVAD subjects, highlighting the importance of tight blood pressure control. 23
Existing cost‐effectiveness analyses regarding the use of LVADs in the UK refer only to patients eligible for heart transplantation in the BTT indication. 3 Our ICER value for DT LVAD is in the range of the seminal cost‐effectiveness evaluation of the NHS HTA and Clarke et al. that studied the cost‐effectiveness of BTT patients vs. an MM cohort for the UK in 2013. 3 , 16 The main difference between the two indications, BTT and DT, is whether patients are eligible for heart transplantation. The difference in survival rates between the LVAD and MM cohort is much higher in patients being considered for DT without the option of heart transplantation as this magnifies the benefit of LVAD over MM for survival.
Our study has several strengths as follows: (i) To our knowledge, this is the first analysis studying the cost‐effectiveness of LVADs in the DT indication in the UK. (ii) Further, to our knowledge, this is the first analysis in Europe utilizing DT clinical data from third generation LVAD technology. (iii) We used the most contemporary DT clinical trial data as inputs for the LVAD cohort in our model. 23 (iv) We used IPD for mortality, AEs, and QoL inputs. While most of the previous cost‐effectiveness studies used secondary sources from previous generation LVAD devices or from general HF populations, 16 , 19 , 20 , 34 IPD data allowed us to develop and use continuous‐flow LVAD specific utilities for the first time. In addition, our model calculates utility decrements specific to each major adverse event of the therapy. This results in a more realistic estimation of utility levels and decreases uncertainty around QoL. (v) Additionally, our analysis models all LVAD related major AEs, including pump exchanges, strokes, driveline infections, GI bleeding and right ventricular assist device implantation due to right HF and assigns NHS costs, respectively. This allows for a detailed and more accurate cost estimation for the LVAD cohort. (vi) Our model was robust to deterministic and probabilistic sensitivity analyses. A change of 100% in major AEs only slightly changed the ICER value.
We have identified the following limitations to our model: (i) In absence of a contemporary randomized clinical trial comparing LVAD with MM in patients not eligible for heart transplantation in the DT indication, we used an indirect comparison from the most recent DT clinical trial for the LVAD cohort and the SHFM for the MM comparator. While this might be considered a ‘modelled control’, the SHFM is well‐regarded in the clinical community for the simulation of MM survival of HF patients and was used in scenario analyses with little varying results in other cost‐effectiveness analyses such as in Clarke et al. 16 An alternate input use for the MM cohort's survival in previous cost‐effectiveness analyses is the REMATCH trial. 7 However, REMATCH does not represent current practice or survival rates. And this can explain the variation and decrease in the ICER value in the scenario analyses. (ii) In the absence of UK DT LVAD data, and similarly to previous European DT cost‐effectiveness studies, 19 , 20 US clinical outcomes were used in the model. Survival and adverse event rates were taken from the latest US LVAD DT trial assessing patients not eligible for heart transplantation. Utility inputs were based on EQ‐5D questionnaires from the US trial but were converted to depict UK QoL values. One‐way sensitivity analyses as well as probabilistic sensitivity analysis were performed for all the key clinical inputs. The results were in line with our base‐case outcomes.
With £46 207/QALY, our deterministic ICER is lower than the NHS willingness‐to‐pay threshold of £50 000/QALY, which is acceptable for end‐of‐life care. NHS considers DT patients for the end‐of‐life criteria. 15 Therefore, our results suggest that the implantation of the HeartWare™ HVAD™ System in patients with the DT indication who are not eligible for heart transplantation is a cost‐effective therapy in the NHS England healthcare system.
Currently, DT is not funded by NHS England mainly due to cost‐effectiveness concerns. We believe our findings, using the latest available clinical DT trial data, coupled with significant improvements in LVAD therapy make a compelling case to revisit DT LVAD as a viable treatment option for end‐stage HF patients ineligible for heart transplantation. LVAD outcomes have vastly improved over the past years due to technological innovations and improved patient management. Recent published data showed 1 and 2 year survival of 89% and 87%, respectively, in BTT patients implanted via thoracotomy 38 ; and long‐term outcomes in patients intended as BTT with 5 and 7 year survival of 54% and 51% alongside with freedom from any stroke and severely disabling stroke at 6 years of 82% and 89%, respectively. 39 Estimating the ‘unmet need’ for the DT population in the UK is difficult. However, there is a substantial number of end‐stage HF patients without current treatment options in the UK despite their suitability as potential DT candidates, while a growing number of DT patients can be observed in other European countries and in the US. 9 , 40
To the best of our knowledge, this is the first cost‐effectiveness analysis for DT specifically designed for the UK healthcare system. Our deterministic ICER of £46 207/QALY makes the implantation the HeartWare™ HVAD™ System in patients with the DT indication a cost‐effective therapy for the NHS England under the willingness‐to‐pay threshold of £50 000/QALY. Policymakers should consider our results in combination with the unmet need for treating end‐stage HF when evaluating funding and patient access to DT LVAD.
Conflict of interest
Professor Schueler is advisor for Medtronic and Leviticus Cardio, and Dr Mahr is a consultant and investigator for Medtronic, Abbott, Abiomed, Syncardia and consultant for Carmat. Dr Silvestry is a consultant for Medtronic, and Abbott. Dr Slaughter is a consultant for Medtronic. Dr Levy is a consultant for Medtronic, and Abbott. Mrs Beckman is a consultant for Medtronic, Abbott, Abiomed and Syncardia. Dr Villinger, Eleni Ismyrloglou, and Stelios Tsintzos are employed by Medtronic. Dr Cheng and Dr Cotts have no relationship with industry.
Funding
ENDURANCE Supplemental, ENDURANCE and ADVANCE are not funding agencies but are all trials which have been funded by Medtronic and have been used as part of the inputs in the manuscript.
Supporting information
Table S1. Baseline Characteristics – ENDURANCE SUPPLEMENTAL.
Table S2. Scenario Analysis ‐ Literature Derived Utilities.
Table S3. Scenario Analysis ‐ Alternate Hazard Ratios.
Figure S1. Incremental Cost‐Effectiveness Ratio Scatterplot.
Acknowledgements
The authors would like to acknowledge Mary V. Jacoski, Simon Eggington of Medtronic for data and statistical support and Alex Bottle, Wun Wong, Martin R Cowie of Imperial College London for provision of NHS patient‐level costing data for this therapy area.
Schueler, S. , Silvestry, S. C. , Cotts, W. G. , Slaughter, M. S. , Levy, W. C. , Cheng, R. K. , Beckman, J. A. , Villinger, J. , Ismyrloglou, E. , Tsintzos, S. I. , and Mahr, C. (2021) Cost‐effectiveness of left ventricular assist devices as destination therapy in the United Kingdom. ESC Heart Failure, 8: 3049–3057. 10.1002/ehf2.13401.
References
- 1. Connolly M, Beattie J, Walker D, Dancy M. End of Life Care in Heart Failure: A Framework for Implementation. London: Quality NICE Improvement; 2014. [Google Scholar]
- 2. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, Rahimi K. Temporal trends and patterns in heart failure incidence: a population‐based study of 4 million individuals. Lancet 2018; 391: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sutcliffe P, Connock M, Pulikottil‐Jacob R, Kandala N, Suri G, Gurung T, Grove A, Shyangdan D, Briscoe S, Maheswaran H. Clinical effectiveness and cost‐effectiveness of second‐and third‐generation left ventricular assist devices as either bridge to transplant or alternative to transplant for adults eligible for heart transplantation: systematic review and cost‐effectiveness model. Health Technol Assess (Winch Eng) 2013; 17: 1–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cleland JG, van Veldhuisen DJ, Ponikowski P. The year in cardiology 2018: heart failure. Eur Heart J 2019; 40: 651–661. [DOI] [PubMed] [Google Scholar]
- 5. McDonagh T, Clark A, Mindham R, de Belder M, Shate A, Ajayi S, Singarayer S. National Heart Failure Audit (NHFA): 2020 Summary Report (2018/19 Data). In: HQIP; 2020.
- 6. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 7. Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG. Long‐term use of a left ventricular assist device for end‐stage heart failure. N Engl J Med 2001; 345: 1435–1443. [DOI] [PubMed] [Google Scholar]
- 8. Molina EJ, Shah P, Kiernan MS, Cornwell WK 3rd, Copeland H, Takeda K, Fernandez FG, Badhwar V, Habib RH, Jacobs JP, Koehl D, Kirklin JK, Pagani FD, Cowger JA. The Society of Thoracic Surgeons Intermacs 2020 Annual Report. Ann Thorac Surg 2021; 111: 778–792. [DOI] [PubMed] [Google Scholar]
- 9. Kirklin JK, Xie R, Cowger J, de By T, Nakatani T, Schueler S, Taylor R, Lannon J, Mohacsi P, Gummert J, Goldstein D, Caliskan K, Hannan MM. Second annual report from the ISHLT Mechanically Assisted Circulatory Support Registry. J Heart Lung Transplant 2018; 37: 685–691. [DOI] [PubMed] [Google Scholar]
- 10. UNOS . https://unos.org/ [Available from: https://unos.org/] (2th April 2021).
- 11. Centers for Medicare & Medicaid Services . National Coverage Determination (NCD) for Ventricular Assist Devices (20.9.1): Medicare Coverage Database. [Available from: https://www.cms.gov/medicare‐coverage‐database/details/ncd‐details.aspx?NCDId=360] (31st of March 2021). [PubMed]
- 12. Institut für Qualitätssicherung und Transparenz im Gesundheitswesen (IQTIG) . Herzunterstützungssysteme/Kunstherzen. In: Gesundheitswesen IQTIG, editor; 2018.
- 13. Haute Autorité de Santé (HAS) . Évaluation de L'Assistance Circulatoire Mécanique: Hors dispositifs légers. In: Haute Autorité de Santé; 2008.
- 14. National Institute for Health and Care Excellence (NICE) . Implantation of a left ventricular assist device for destination therapy in people ineligible for heart transplantation In: National Institute for Health and Care Excellence; 2015.
- 15. National Health Service (NHS) England and Improvement . Consultation on a policy proposition for long term left ventricular assist device therapy for advanced heart failure (all ages): National Health Service England; 2018. [Available from: https://www.engage.england.nhs.uk/consultation/long‐term‐left‐ventricular‐assist‐device‐therapy/] (14th of September 2020).
- 16. Clarke A, Pulikottil‐Jacob R, Connock M, Suri G, Kandala NB, Maheswaran H, Banner NR, Sutcliffe P. Cost‐effectiveness of left ventricular assist devices (LVADs) for patients with advanced heart failure: analysis of the British NHS bridge to transplant (BTT) program. Int J Cardiol 2014; 171: 338–345. [DOI] [PubMed] [Google Scholar]
- 17. Developing NICE guidelines: the manual. National Instit Hea Care Excel 2014: 128–162. [PubMed] [Google Scholar]
- 18. Shaw SM, Venkateswaran R, Hogg R, Rushton S, Al‐Attar N, Schueler S, Lim S, Parameshwar J, Banner NR. Durable left ventricular assist device support as a bridge to heart transplant candidacydagger. Interact Cardiovasc Thorac Surg 2019; 28: 594–601. [DOI] [PubMed] [Google Scholar]
- 19. Neyt M, Leroy R, Devos C, Van Brabandt H. Left ventricular assist devices in the treatment of end‐stage heart failure. KCE; 2016. Report No.: 264.
- 20. Neyt M, Van den Bruel A, Smit Y, De Jonge N, Erasmus M, Van Dijk D, Vlayen J. Cost‐effectiveness of continuous‐flow left ventricular assist devices. Int J Technol Assess Health Care 2013; 29: 254–260. [DOI] [PubMed] [Google Scholar]
- 21. Silvestry SC, Mahr C, Slaughter MS, Levy WC, Cheng RK, May DM, Ismyrloglou E, Tsintzos SI, Tuttle E, Cook K, Birk E, Gomes A, Graham S, Cotts WG. Cost‐effectiveness of a small intrapericardial centrifugal left ventricular assist device. ASAIO J 2020; 66: 862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Institute for Health and Care Excellence (NICE) . Guide to the methods of technology appraisal 2013. In: National Instit Heal Care Excel; 2013. [PubMed]
- 23. Milano CA, Rogers JG, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Mokadam NA, Mahr C, Miller JS, Markham DW. HVAD: the ENDURANCE supplemental trial. JACC: Heart Failure 2018; 6: 792–802. [DOI] [PubMed] [Google Scholar]
- 24. National Institute for Health and Care Excellence (NICE) . Implantable cardioverter defibrillators and cardiac resynchronisation therapy for arrhythmias and heart failure. In: National Instit Heal Care Excell; 2014.
- 25. University of Washington . Seattle Heart Failure Model. Seattle: University of Washington. [Available from: https://depts.washington.edu/shfm/index.php?width=1920&height=1080] (3rd of August 2020). [Google Scholar]
- 26. National Health Service (NHS) England and Improvement . Archived Reference Costs. 2017/2018 reference costs and guidance. [Available from: https://webarchive.nationalarchives.gov.uk/20200501111106/ https://improvement.nhs.uk/resources/reference‐costs/] (7th of July 2020).
- 27. National Health Service (NHS) England and Improvement . Improvement N. National Tariff Payment System. In: Annex_DtA_National_tariff_workbook: NHS Improvement; 2019.
- 28. National Health Service (NHS) England and Improvement . National tariff proposals for 2017/18 and 2018/19. In: NHS England and NHS Impro; 2016.
- 29. Luengo‐Fernandez R, Silver LE, Gutnikov SA, Gray AM, Rothwell PM. Hospitalization resource use and costs before and after TIA and stroke: results from a population‐based cohort study (OXVASC). Value Health 2013; 16: 280–287. [DOI] [PubMed] [Google Scholar]
- 30. Smedira NG, Hoercher KJ, Lima B, Mountis MM, Starling RC, Thuita L, Schmuhl DM, Blackstone EH. Unplanned hospital readmissions after HeartMate II implantation: frequency, risk factors, and impact on resource use and survival. JACC: Heart Fail 2013; 1: 31–39. [DOI] [PubMed] [Google Scholar]
- 31. Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, Gregoric ID, Mallidi H, Leadley K, Aaronson KD, Frazier OH, Milano CA. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med 2017; 376: 451–460. [DOI] [PubMed] [Google Scholar]
- 32. Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA, Jessup ML, Gregoric ID, Loyalka P, Frazier O. Use of an intrapericardial, continuous‐flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012; 125: 3191–3200. [DOI] [PubMed] [Google Scholar]
- 33. Dolan P. Modeling valuations for EuroQol health states. Med Care 1997; 35: 1095–1108. [DOI] [PubMed] [Google Scholar]
- 34. Rogers JG, Bostic RR, Tong KB, Adamson R, Russo M, Slaughter MS. Cost‐effectiveness analysis of continuous‐flow left ventricular assist devices as destination therapy. Circ Heart Fail 2012; 5: 10–16. [DOI] [PubMed] [Google Scholar]
- 35. Mahr C, McGee E Jr, Cheung A, Mokadam NA, Strueber M, Slaughter MS, Danter MR, Levy WC, Cheng RK, Beckman JA, May DM, Ismyrloglou E, Tsintzos SI, Silvestry SC. Cost‐effectiveness of thoracotomy approach for the implantation of a centrifugal left ventricular assist device. ASAIO J 2020; 66: 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barbieri M, Drummond M, Willke R, Chancellor J, Jolain B, Towse A. Variability of cost‐effectiveness estimates for pharmaceuticals in Western Europe: lessons for inferring generalizability. Value Health 2005; 8: 10–23. [DOI] [PubMed] [Google Scholar]
- 37. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH, HeartMate III. Advanced heart failure treated with continuous‐flow left ventricular assist device. N Engl J Med 2009; 361: 2241–2251. [DOI] [PubMed] [Google Scholar]
- 38. McGee E Jr, Danter M, Strueber M, Mahr C, Mokadam NA, Wieselthaler G, Klein L, Lee S, Boeve T, Maltais S, Pretorius GV, Adler E, Vassiliades T, Cheung A. Evaluation of a lateral thoracotomy implant approach for a centrifugal‐flow left ventricular assist device: the LATERAL clinical trial. J Heart Lung Transplant 2019; 38: 344–351. [DOI] [PubMed] [Google Scholar]
- 39. Zimpfer D, Fiane AE, Larbalestier R, Tsui S, Jansz P, Simon A, Schueler S, Strueber M, Schmitto JD. Long‐term survival of patients with advanced heart failure receiving an left ventricular assist device intended as a bridge to transplantation: the registry to evaluate the HeartWare left ventricular assist system. Circ Heart Fail 2020; 13: e006252. [DOI] [PubMed] [Google Scholar]
- 40. Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, Higgins RS, Stevenson LW, Stehlik J, Atluri P, Grady KL, Kirklin JK. The Society of Thoracic Surgeons Intermacs Database Annual Report: evolving indications, outcomes, and scientific partnerships. Ann Thorac Surg 2019; 107: 341–353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics – ENDURANCE SUPPLEMENTAL.
Table S2. Scenario Analysis ‐ Literature Derived Utilities.
Table S3. Scenario Analysis ‐ Alternate Hazard Ratios.
Figure S1. Incremental Cost‐Effectiveness Ratio Scatterplot.


