Abstract
Aims
Therapy with phosphodiesterase‐5 inhibitors (PDE5Is) after left ventricular assist device (LVAD) implantation has been associated with lower mortality and device thrombosis but increased risk for post‐operative and gastrointestinal bleeding. We aimed to evaluate the impact of long‐term PDE5Is on the overall bleeding risk after LVAD implantation.
Methods and results
We retrospectively included patients who received a continuous‐flow LVAD at our site and were prescribed with long‐term oral PDE5Is after discharge from the index hospitalization. The primary endpoint was the occurrence of bleeding at 12 month follow‐up. Secondary endpoints were all‐cause death and the combination of bleeding and all‐cause death. Our analysis included 109 patients of whom 75 (69%) received long‐term PDE5Is. Mean age was 56 years, and 85% were male. At 12 months, 19 (17%) patients experienced at least one bleeding event. Patients on PDE5Is had higher bleeding rates (23% vs. 6%, P = 0.03) and more bleeding events per patient‐year (0.32 vs. 0.06, P = 0.03) compared with patients not on PDE5Is. While overall bleeding incidence was excessively higher in the PDE5I group, there were no significant differences in the incidence of major bleeding (19% vs. 6%, P = 0.08) and gastrointestinal bleeding (11% vs. 3%, P = 0.18). Kaplan–Meier analysis revealed higher cumulative incidence of bleeding for the PDE5I group (log rank = 0.04) with no difference on all‐cause death (log rank = 0.67) and the combination of bleeding and all‐cause death (log rank = 0.13). Hospitalizations for bleeding and their duration were numerically higher in the PDE5I group (0.28 vs. 0.03, P = 0.07 and 2.4 vs. 0.2, P = 0.07, respectively).
Conclusions
Phosphodiesterase‐5 inhibitor treatment after LVAD implantation is associated with increased bleeding risk after LVAD implantation. The safety of long‐term PDE5Is in LVAD patients remains unclear and needs to be further clarified in prospective studies with randomized study design.
Keywords: Left ventricular assist devices, Sildenafil, Tadalafil, Phosphodiesterase‐5 inhibitors
Introduction
Left ventricular assist device (LVAD) therapy continues to evolve towards a standard of care for a growing population with advanced heart failure and refractory symptoms on optimal medical and device therapy. With over 50% of all patients being assigned to destination therapy and older patients becoming eligible for LVAD, there is a growing need to counteract device‐related adverse events. 1 While the incidence of pump thrombosis declines with the last generation devices, infections and bleeding continue to pose significant treatment challenges. 2 , 3 Oral phosphodiesterase‐5 inhibitors (PDE5Is) are frequently implemented in the perioperative setting of LVAD implantation in case of imminent or manifested post‐operative right ventricular (RV) failure to achieve afterload reduction and facilitate weaning from inotropes and short‐acting vasodilators. The 2013 International Society for Heart and Lung Transplantation guidelines for mechanical circulatory support recommends the use of PDE5Is additionally to pharmacological or device interventions for RV failure in the setting of persistent pulmonary hypertension after LVAD implantation (Class IIb, Level of Evidence C). 4 This recommendation is based on expert opinions supported by exploratory studies that demonstrated improvements in right heart haemodynamics weeks to several months after LVAD implantation. No randomized controlled studies have been published so far with PDE5Is in long‐term supported circulation. 5 , 6 , 7 Despite the lack of robust evidence, it is common practice among institutions to prescribe off‐label oral PDE5Is as long‐term therapy in LVAD recipients aiming to optimize right heart haemodynamics, prevent late‐onset RV failure, or enable candidacy for transplantation. 8 , 9 Retrospective studies on adult patients report 16–21% use of PDE5Is at 3–6 months after implantation. 10 , 11 The safety and efficacy of this practice have recently been called into question, as a retrospective Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) analysis of 12 144 patients showed an increased risk of early post‐operative RV failure in patients pretreated with PDE5Is as well as a higher incidence of major bleeding within the first week after implantation. 12
Phosphodiesterase‐5 is abundant in platelets and is a major regulator of cellular cyclic guanosine monophosphate (cGMP) level by catalyzing its hydrolysis. The major target of cGMP is cGMP‐dependent protein kinase I, which phosphorylates multiple substrates involved in different inhibitory pathways (adhesion, shape change, aggregation, and secretion) in platelets. 13 PDE5Is increase cGMP level and have been shown to attenuate platelet aggregation in previous studies. 14 , 15 , 16 , 17 Higher plasma cGMP concentration was also associated with gastrointestinal bleeding in patients on continuous‐flow LVAD support. 18 Based on this knowledge, we hypothesized that long‐term oral PDE5I therapy may be associated with an overall higher bleeding risk in LVAD recipients.
Methods
Study population and data collection
We retrospectively reviewed the database of our interdisciplinary heart failure unit to identify consecutive adult patients who received a durable LVAD at our institution from December 2010 through May 2019. Clinical data regarding patient medical history and disease status in the recent preoperative period were prospectively collected in a digitalized database dedicated to clinical surveys. Post‐operative and follow‐up data were extracted retrospectively from the individual electronic health records. Patients were included in the analysis if they received long‐term follow‐up at our site after LVAD implantation. Patients were excluded from analysis if they were on biventricular support, in case of post‐operative death during the index hospitalization, and in case of follow‐up interruption or follow‐up treatment in an affiliated hospital. Patients who received PDE5Is due to early post‐operative RV failure according to the International Society for Heart and Lung Transplantation recommendations 4 and were thereafter discharged on oral PDE5Is after the index hospitalization comprised the PDE5I group. The decision to continue PDE5Is in the long‐term aimed to one of three goals, as reported elsewhere: (i) reduction of persistently elevated pulmonary artery pressures, (ii) reduction of pulmonary vascular resistance to enable listing for transplantation, or (iii) prevention of deterioration or recurrence of RV failure after initial stabilization. 9 Follow‐up visits in the outpatient clinic were prospectively scheduled at 3 month intervals after LVAD implantation according to a standardized institutional protocol (Supporting Information, Figure S1 ). Additional visits or inpatient treatments were arranged depending on the clinical course and the occurrence of adverse events. All patients received pre‐implant haemodynamic assessment by right heart catheterization not longer than 1 month before LVAD placement. Patients who were thereafter eligible for transplant received regular haemodynamic follow‐up by right heart catheterization every 6 months, whereas patients in the destination therapy group underwent haemodynamic studies depending on the clinical course, that is, in case of haemodynamic deterioration due to overt RV failure or signs of pulmonary hypertension.
All study participants provided written consent to anonymized data analysis for scientific purposes at admission but no individual informed consent. The study was conducted in accordance with the Declaration of Helsinki on ethical principles for medical research and received approval by the ethics committee of our institution (20‐9282‐BO).
Outcomes
The primary outcome of the study was the occurrence of bleeding during ongoing LVAD support and documented oral intake of PDE5Is at 12 months after implantation. Secondary outcomes were all‐cause death and the composite of bleeding or all‐cause death during the same period. Major bleeding was defined as fatal bleeding, and/or symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra‐articular or pericardial, or intramuscular with compartment syndrome, and/or bleeding causing a fall in haemoglobin level of 2 g/dL (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells. All non‐major bleedings were considered as minor bleedings. 19 Post‐operative bleeding or bleeding before discharge from the index hospitalization was not included in our analysis. Gastrointestinal bleeding was defined as documented episode of bleeding in the medical record confirmed by either a positive faecal occult blood test or endoscopic study. 20
The Bleeding Academic Research Consortium (BARC) classification was used to categorize the bleeding complications 21 after excluding Type 4 bleeding category for non‐applicability in the LVAD population (bleeding after aortocoronary bypass grafting). In summary, the following BARC categories of bleeding were derived: Type 0: no bleeding; Type 1: bleeding that is not actionable and does not cause the patient to seek unscheduled performance of studies, hospitalization, or treatment by a healthcare professional; Type 2: any overt, actionable sign of haemorrhage that does not fit the criteria for Type 3, 4, or 5 but does meet at least one of the following criteria: (i) requiring non‐surgical, medical intervention by a healthcare professional, (ii) leading to hospitalization or increased level of care, or (iii) prompting evaluation; Type 3: Type 3a: overt bleeding plus haemoglobin drop of 3 to <5 g/dL related to bleeding and any transfusion with overt bleeding; Type 3b: overt bleeding plus haemoglobin drop ≥5 g/dL related to bleeding, cardiac tamponade, bleeding requiring surgical intervention for control, and bleeding requiring intravenous vasoactive agents; and Type 3c: intracranial haemorrhage, subcategories confirmed by autopsy or imaging or lumbar puncture, and intraocular bleed compromising vision; and Type 5: fatal bleeding.
Late RV failure was defined by the presence of symptoms of RV dysfunction (e.g. peripheral oedema and jugular venous distention) requiring readmission more than 30 days after discharge from the index hospitalization (LVAD implantation). 22
Statistical analysis
Continuous variables are summarized as means (standard deviations) unless indicated otherwise and categorical variables as counts (percentages). Continuous data were evaluated for normality of distribution with the Shapiro–Wilk test. The two‐sided t‐test was used for comparison of continuous, normally distributed data, otherwise the non‐parametric Mann–Whitney U test. The χ 2 test was used for testing the association between categorical variables. Kaplan–Meier analysis was performed to estimate differences in bleeding events among patients with and without PDE5I therapy. For the primary endpoint analysis, patients were censored in case of heart transplantation, device explantation, death, or discontinuation of PDE5Is. The log‐rank test was utilized to determine differences between groups. The level of significance was set at 0.05. All analyses were performed using SPSS (IBM Corp., SPSS Statistics, Version 23.0, Armonk, NY).
Results
During the study period, 193 consecutive patients received univentricular support with a durable continuous‐flow LVAD at our institution. We excluded 19 patients who received follow‐up in an affiliated hospital and 65 patients who died post‐operatively before hospital discharge. Our analysis included 109 patients of whom 75 (69%) were on long‐term PDE5I therapy and 34 (31%) who did not receive PDE5Is or any other pulmonary vasodilator at the time of discharge from the index hospitalization (Figure 1 ). Sildenafil was prescribed in 7 (6.4%) patients and tadalafil in 68 (62.4%) patients. Two (1.8%) patients were on 20 mg sildenafil, 2 (1.8%) patients on 40 mg sildenafil, and 3 (2.8%) patients on 60 mg sildenafil daily. Regarding tadalafil, 20 (18.3%) patients received 20 mg and 48 (44%) patients 40 mg daily. The mean duration of PDE5I therapy up to June 2020 was 794 days (inter‐quartile range: 775 days), and all patients received PDE5Is for at least 12 months. Baseline characteristics of the study patients are summarized in Table 1 . Mean age was 56 years, and 85% were male. The vast majority were on New York Heart Association IV functional class. No significant differences in INTERMACS profile categories were observed among patients with PDE5Is vs. without PDE5Is. Nearly half of the patients were assigned to destination therapy. In general, the two groups were balanced regarding pre‐implant haemodynamic parameters and co‐morbidities.
Figure 1.
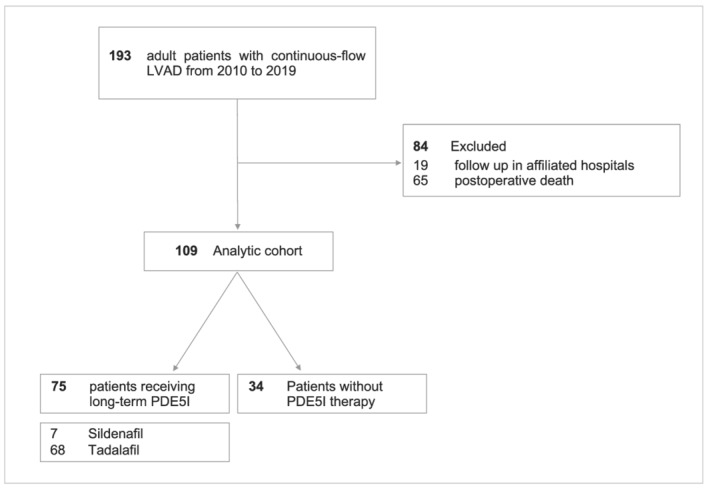
Derivation of the analytic cohort of the study. LVAD, left ventricular assist device; PDE5I, phosphodiesterase‐5 inhibitor.
Table 1.
Baseline characteristics of the study patients
| Variable | Overall (n = 109) | PDE5I (n = 75) | No PDE5I (n = 34) | P value |
|---|---|---|---|---|
| Age (years) | 56 ± 11 | 53 ± 13 | 57 ± 10 | 0.08 |
| Male, n (%) | 93 (85) | 66 (88) | 27 (79) | 0.24 |
| NYHA class | ||||
| III, n (%) | 19 (17) | 13 (17) | 6 (18) | 0.97 |
| IV, n (%) | 90 (83) | 62 (83) | 28 (82) | |
| INTERMACS profile category | ||||
| 1, n (%) | 23 (21) | 14 (19) | 9 (27) | 0.36 |
| 2, n (%) | 12 (11) | 7 (9) | 5 (15) | 0.41 |
| 3, n (%) | 22 (20) | 16 (21) | 6 (18) | 0.66 |
| 4, n (%) | 33 (30) | 24 (22) | 9 (27) | 0.56 |
| ≥5, n (%) | 19 (17) | 14 (19) | 5 (15) | 0.70 |
| Mean INTERMACS profile | 3.2 ± 1.5 | 3.3 ± 1.5 | 2.9 ± 1.5 | 0.18 |
| Indication | ||||
| BTT/BTR, n (%) | 57 (52) | 39 (52) | 18 (53) | 0.93 |
| DT, n (%) | 52 (48) | 36 (48) | 16 (47) | |
| HF aetiology | ||||
| IHD, n (%) | 55 (50) | 42 (56) | 13 (38) | 0.09 |
| Cardiomyopathy, n (%) | 54 (50) | 33 (44) | 21 (62) | |
| Device type | ||||
| HeartWare HVAD | 98 (90) | 67 (89) | 31 (91) | 0.77 |
| HeartMate 3 | 11 (10) | 8 (11) | 3 (9) | |
| LVEF (%) | 17.1 ± 6.2 | 17.5 ± 6.1 | 16 ± 6.5 | 0.24 |
| BMI (kg/m 2 ) | 26.5 ± 5.6 | 27.2 ± 5.8 | 25.0 ± 4.9 | 0.08 |
| BSA (m 2 ) | 2.0 ± 0.2 | 2.0 ± 0.2 | 1.9 ± 0.2 | 0.09 |
| CI (L/min/m 2 ) | 1.9 ± 0.4 | 1.9 ± 0.4 | 1.9 ± 0.5 | 0.87 |
| mPAP (mmHg) | 31 ± 11 | 33 ± 11 | 29 ± 13 | 0.24 |
| PVR (WU) a | 3.3 (2.7) | 3.4 (2.9) | 3.2 (2.0) | 0.67 |
| ACEI/ARB, n (%) | 65 (60) | 46 (61) | 19 (56) | 0.59 |
| Post‐implant RVF, n (%) | 74 (68) | 53 (71) | 21 (62) | 0.36 |
| Co‐morbidities | ||||
| CKD, n (%) | 38 (35) | 24 (32) | 14 (41) | 0.35 |
| DM, n (%) | 29 (27) | 18 (24) | 11 (32) | 0.36 |
| PAD, n (%) | 9 (8) | 6 (8) | 3 (9) | 0.89 |
| COPD, n (%) | 22 (20) | 14 (19) | 8 (24) | 0.56 |
| HTN, n (%) | 65 (60) | 45 (60) | 20 (59) | 0.91 |
| AF, n (%) | 44 (40) | 31 (41) | 13 (38) | 0.76 |
| Stroke, n (%) | 23 (21) | 17 (23) | 6 (18) | 0.55 |
| Prior bleeding, n (%) | 37 (34) | 30 (40) | 7 (21) | 0.05 |
ACEI, angiotensin‐converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; BMI, body mass index; BSA, body surface area; BTR, bridge to recovery; BTT, bridge to transplant; CI, cardiac index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; DT, destination therapy; HF, heart failure; HTN, hypertension; IHD, ischaemic heart disease; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVEF, left ventricular ejection fraction; mPAP, mean pulmonary artery pressure; NYHA, New York Heart Association; PAD, peripheral arterial disease; PDE5I, phosphodiesterase‐5 inhibitor; PVR, pulmonary vascular resistance; RVF, right ventricular failure; WU, Wood units.
The values presented indicate mean (inter‐quartile range).
At 12 month follow‐up, 19 (17%) patients experienced cumulatively 28 bleeding events (26 on PDE5Is vs. 2 not on PDE5Is) leading to 27 red blood cell (RBC) unit transfusions (27 on PDE5Is vs. 0 RBC units in the no PDE5I group). There was a profound preponderance of patients on PDE5Is over the occurrence of bleeding (23% vs. 6%, P = 0.03) and the frequency of bleeding events per patient (0.32 vs. 0.06, P = 0.03). Overall, four patients of the PDE5I group received a total of 27 RBC units during the first year after implantation, whereas no patient in the group without PDE5Is received transfusion for bleeding. BARC Types 2, 3, and 5 of bleeding were observed during the first year after LVAD implantation, the majority being Type 2 bleedings. Patients on PDE5Is had higher rates of BARC Type 2 bleedings (19% vs. 3%, P = 0.03), and bleeding Types 3 and 5 were observed similarly among the two groups. While bleeding incidence was excessively higher in the PDE5I group, there were no significant differences in the incidence of major bleeding (19% vs. 6%, P = 0.08) and gastrointestinal bleeding (11% vs. 3%, P = 0.18). At least 95% of all patients were on antiplatelet therapy, this being acetylsalicylic acid in approximately 75% of all cases. There were no differences in frequency and pharmacological category of antiplatelet therapy between the two groups. All patients were on long‐term oral vitamin K antagonists with a target international normalized ratio (INR) of 2.2–2.8. Bleeding in the context of over‐therapeutic anticoagulation occurred rarely (4% vs. 3%, P = 0.79), and the INR absolute values during bleeding episodes did not differ between the two groups. Cumulative hospitalizations for bleeding per individual and the cumulative duration of hospital stay for bleeding complications were numerically higher in the PDE5I group, but this difference did not reach statistical significance (0.28 vs. 0.03, P = 0.07 and 2.4 vs. 0.2, P = 0.07, respectively).
At the end of the first year, 23% of patients in the PDE5I group and 6% of patients in the group without PDE5Is met the primary endpoint of bleeding yielding a four‐fold higher risk for the PDE5I group. Kaplan–Meier analysis demonstrates the higher bleeding rates with PDE5Is, as shown in Figure 2 A (log rank = 0.04). During the same period, 9% of patients in the PDE5I group and 12% of those in the no PDE5I group experienced death (log rank = 0.67), while 28% of patients in the PDE5I group and 15% of the no PDE5I group met the secondary endpoint of bleeding or all‐cause death (log rank = 0.13). Figure 2 B and 2 C illustrates the differences in the cumulative incidence of death and the composite of all‐cause death or bleeding, respectively, through 12 months after LVAD implantation. The incidence of late‐onset RV failure did not differ between patients with and without PDE5Is (8% vs. 12%, respectively, P = 0.26) (Table 2 ).
Figure 2.
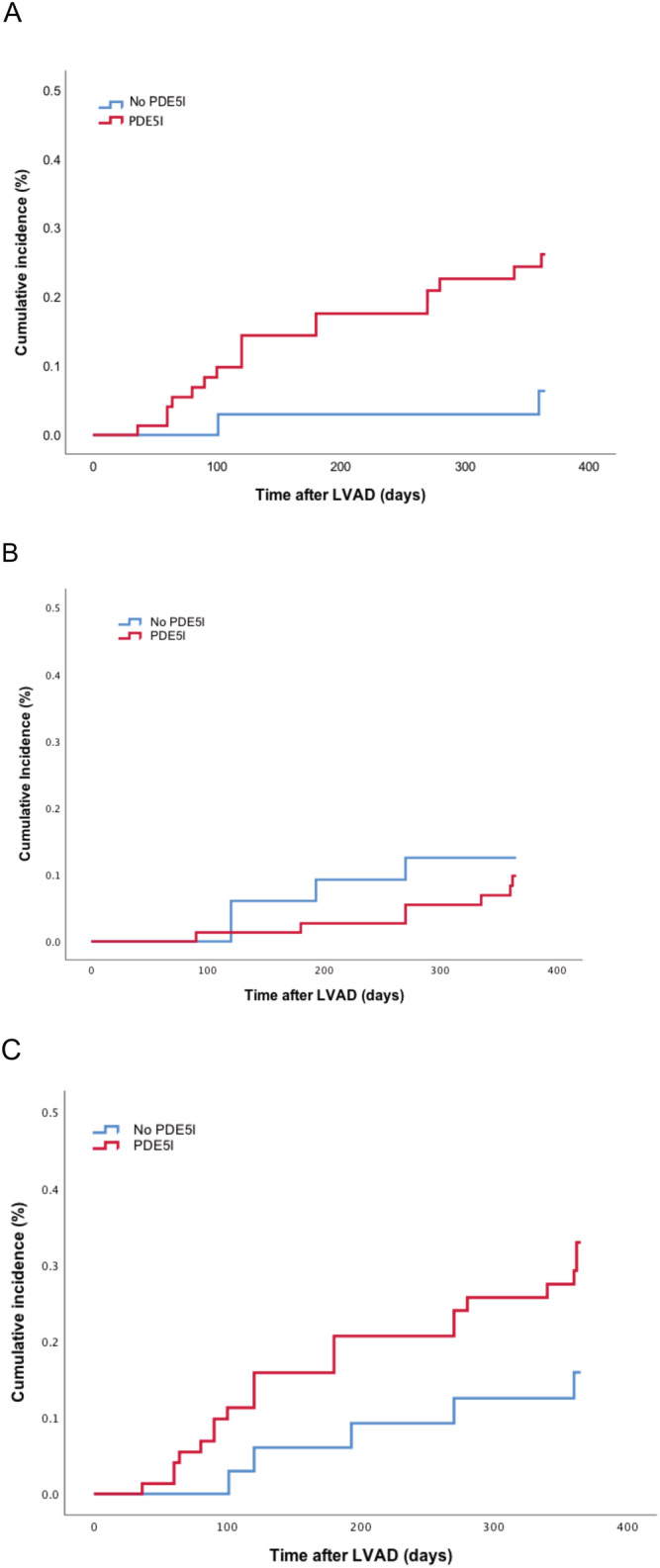
Kaplan–Meier analysis depicting cumulative incidence of (A) bleeding (log rank = 0.04), (B) all‐cause death (log rank = 0.67), and (C) the combination of bleeding and all‐cause death (log rank = 0.13) through the first year after left ventricular assist device (LVAD) implantation. PDE5I, phosphodiesterase‐5 inhibitor.
Table 2.
Bleeding, hospitalization, and right ventricular failure at 12 months after implantation
| Overall (n = 109) | PDE5I (n = 75) | No PDE5I (n = 34) | P value | |
|---|---|---|---|---|
| Bleeding, n (%) | 19 (17) | 17 (23) | 2 (6) | 0.03 |
| No. of bleeding events per patient a | 0.24 (0–3) | 0.32 (0–3) | 0.06 (0–1) | 0.03 |
| RBC transfusion, n (%) | 4 (4) | 4 (5) | 0 (0) | 0.31 |
| RBC units per patient a | 0.36 (0–10) | 0.36 (0–10) | 0 (0–0) | 0.22 |
| Major bleeding | 16 (15) | 14 (19) | 2 (6) | 0.08 |
| Gastrointestinal bleeding | 9 (8) | 8 (11) | 1 (3) | 0.18 |
| BARC type, n (%) | ||||
| 1 | 0 (0) | 0 (0) | 0 (0) | — |
| 2 | 15 (14) | 14 (19) | 1 (3) | 0.03 |
| 3 | 4 (4) | 3 (4) | 1 (3) | 0.79 |
| 4 | — | — | — | — |
| 5 | 1 (1) | 1 (1) | 0 (0) | 0.50 |
| ASS, 100 mg daily | 83 (76) | 58 (77) | 25 (74) | 0.67 |
| Clopidogrel, 75 mg daily | 19 (17) | 13 (17) | 6 (18) | 0.97 |
| Prasugrel, 10 mg daily | 2 (2) | 0 (0) | 2 (6) | 0.10 |
| Over‐therapeutic INR, n (%) b | 4 (4) | 3 (4) | 1 (3) | 0.79 |
| INR value | 2.10 ± 0.77 | 2.09 ± 0.78 | 2.21 ± 0.91 | 0.84 |
| Haemoglobin drop (g/dL) a | 0.9 (0–2.8) | 0.7 (0–2.8) | 2.3 (2.2–2.4) | 0.09 |
| No. of hospitalizations/patient, n (%) a | 0.20 (0–7) | 0.28 (0–7) | 0.03 (0–1) | 0.07 |
| Cumulative hospital stay (days) a | 1.7 (0–34) | 2.4 (0–34) | 0.2 (0–7) | 0.07 |
| Late RVF, n (%) | 10 (9) | 6 (8) | 4 (12) | 0.26 |
ASS, acetylsalicylic acid; BARC, Bleeding Academic Research Consortium; INR, international normalized ratio; PDE5I, phosphodiesterase‐5 inhibitor; RBC, red blood cell; RVF, right ventricular failure.
Values in brackets refer to median (range: min–max).
Over‐therapeutic INR refers to INR > 2.8.
Discussion
Non‐surgical bleeding affects 20–40% of LVAD patients annually with the majority of initial cases occurring within the first 3 months of implantation. 23 , 24 Most patients who present with LVAD‐related bleeding have a therapeutic or a subtherapeutic INR and demonstrate even higher bleeding rates than those who require dual or even triple therapy for other cardiovascular conditions. 25 , 26 Bleeding in the setting of LVAD is multifactorial and has been linked to acquired von Willebrand syndrome, angiodysplasia formation, impaired platelet aggregation, and activation of fibrinolytic pathways. 27 The majority of studies have focused on gastrointestinal bleeding as it affects 20–25% of patients at 12 months after implantation. 28 Despite this being the most common bleeding site, sites other than the gastrointestinal tract such as mucosal bleeding, intracranial bleeding, bladder, or muscular bleeding account for considerable morbidity and have a direct impact on the quality of life of LVAD recipients.
Our analysis demonstrates a four‐fold increase in bleeding incidence and significantly more bleeding events per patient at 12 months after LVAD implantation in patients treated with the PDE5Is sildenafil or tadalafil. Notably, BARC Type 2 bleeding (actionable bleeding without significant haemoglobin drop >3 g/dL) was observed much more frequently in the PDE5I group and was the most dominant type of bleeding in patients on PDE5Is as well as the overall patient cohort. Fatal bleeding was rare and was observed only once in the PDE5I group. Furthermore, the incidence of major bleeding was three‐fold higher in the PDE5I group with this not reaching statistical significance (P = 0.08). Bleeding was of gastrointestinal origin in nearly half of patients with similar rates observed among patients with or without PDE5I therapy. Only patients on PDE5Is needed RBC transfusions for bleeding. There was only a small percentage of patients with over‐therapeutic anticoagulation during the bleeding events (<5%), which was similar among the two groups. Absolute INR values during the bleedings were similar too. Antiplatelet therapy additional to anticoagulation was implemented in 94% of patients without overt differences regarding the agent used. This is the first study to evaluate the long‐term effect of PDE5Is on the cumulative bleeding incidence and subtype of bleeding. Our analysis encompasses non‐gastrointestinal bleeding, which was shown here to have a similarly high incidence. Our findings corroborate those of another study that analysed the impact of PDE5Is on post‐operative RV failure and bleeding during the first month. 12 This retrospective INTERMACS analysis reported higher rates of post‐operative RV failure in PDE5I‐pretreated patients and major bleeding during the first month after implantation driven by higher bleeding rates during the first week after surgery. The bleeding analysis did not include an adjustment for INR and antiplatelet therapy and no further analysis of the types and sites of bleeding. However, this was the first study to highlight the lack of plausibility with off‐label PDE5Is after LVAD implantation. 29 Another study with perioperative epoprostenol inhalation has also demonstrated higher perioperative blood loss after LVAD implantation, 30 and sildenafil was previously associated with worse clinical outcome in patients with persistent pulmonary hypertension after valve surgery. 31 A recent meta‐analysis of non‐randomized studies demonstrated no significant association between PDE5I and post‐LVAD RV failure. In accordance with this and previous studies, our analysis demonstrated similar rates of post‐operative and late RV failure up to 12 months after implantation among patients with and without PDE5Is. 22 , 32 Also, in this meta‐analysis, there was no significant association between PDE5I and gastrointestinal bleeding, overall stroke, ischaemic stroke, or pump thrombosis. 33
Overall, there is considerable uncertainty regarding the safety of PDE5Is for pulmonary vasodilation in left heart disease and after LVAD implantation. Recently, a large retrospective study examined the impact of PDE5Is on thrombosis and ischaemic stroke after LVAD implantation in >13 000 patients from the INTERMACS. PDE5I therapy was associated with lower rates of LVAD thrombosis or ischaemic stroke at 48 months. 34 Secondary endpoint analysis revealed lower all‐cause mortality with PDE5Is but 14% increased risk for gastrointestinal bleeding compared with patients not on PDE5Is. These findings dictate the need for randomized clinical studies. As in our study, HeartMate 3 was under‐represented in this study, which would have possibly led to lower rates of gastrointestinal bleeding and LVAD thrombosis according to the outcome of the MOMENTUM trial. 2
We observed a high prescription frequency of PDE5Is in our cohort, which is similar to that reported from other single‐centre reports 5 , 35 and much higher than that of the INTERMACS cohorts. 10 , 12 This reflects the lack of international consensus regarding the duration of therapy and is also possibly related to insurance policies in different healthcare systems and the specific treatment goals (destination or bridging). A rational approach is to commence long‐term therapy only in case of persistently elevated pulmonary artery pressures and pulmonary arterial resistance under regular follow‐up haemodynamic assessments and vigilance for PDE5I‐related adverse outcomes. 9
Phosphodiesterase‐5 inhibitor use was not associated with all‐cause death and the composite of bleeding or all‐cause death at 12 months. However, the study has no sufficient power to drive a safe conclusion regarding these endpoints. The incidence and duration of hospitalizations for bleeding were higher in the PDE5I group but not statistically relevant (P = 0.07). It is possible that longer follow‐up would have provided more clarity regarding hospitalization outcomes. On the other hand, with longer follow‐up, potential confounders such as low‐level haemolysis and angiodysplasia formation in the gastrointestinal tract may gain importance, which cannot be easily adjusted outside the context of a prospective study. Additionally, low‐intensity anticoagulation with last generation devices may change the landscape of current haemocompatibility issues. The MAGENTUM 1 study demonstrated the safety of low‐intensity anticoagulation in HeartMate 3 patients. 36 Consequently, it is crucial to estimate the net impact of add‐on PDE5Is on thrombosis and bleeding in the contemporary device era and across the spectrum of background anticoagulation and antiplatelet treatments.
Our study has the inherent limitations of a retrospective unmatched cohort study. Because of the small size of the group without PDE5Is, propensity matching was not implemented for analysis. However, the two groups were balanced regarding demographics, haemodynamics, and co‐morbidities. It is possible that despite a standardized follow‐up protocol, minor bleedings were not consistently reported during follow‐up. Because of the numerically uneven groups and the low event rates in the group of patients without PDE5Is, multivariate and subgroup analysis was not feasible. Event analysis was limited to the first year after LVAD implantation to account for potential confounders with longer device support, which may significantly contribute to increased bleeding risk. Consequently, our results should be considered hypothesis generating and will need confirmation by prospective trials with randomized study design.
Use of long‐term PDE5Is after LVAD implantation was associated with a higher incidence of bleeding at 12 months, which may be attributed to the antiplatelet activity of PDE5Is. Non‐gastrointestinal bleeding has the potential to enlighten the pathophysiological background of haemocompatibility in this growing population and should be included as an endpoint in future studies. As device technology continues to evolve, it is anticipated that established pharmacological therapies will need to be redefined in order to improve outcomes. Considering the questionable role of long‐term PDE5I therapy in LVAD recipients and the safety concerns, off‐label prescription beyond the early post‐operative period should be critically scrutinized.
Conflict of interest
None declared.
Funding
This work was supported by the Universitätsmedizin Essen Clinician Scientist Academy (UMEA) and the German Research Foundation [Deutsche Forschungsgemeinschaft (DFG)] (FU356/12‐1 to M.P., LU2139/2‐1 to P.L., and RA969/12‐1 to T.R.).
Supporting information
Figure S1. Schematic representation of all scheduled in‐person and remote follow‐up visits in case of uncomplicated disease course after LVAD implantation. After 6 months, LVAD recipients who were transplant candidates received an inpatient heart transplant screening including right heart catheterization (RHC), whereas non‐candidates received hemodynamic testing only in case of right ventricular failure or signs of pulmonary hypertension. BTT = Bridge‐to‐transplant, DT = Destination Therapy. HFU = Heart Failure Unit.
Acknowledgement
We acknowledge the contribution of Mrs Lucie Hiepen to the collection of data.
Open access funding enabled and organized by Projekt DEAL.
Jakstaite, A.‐M. , Luedike, P. , Schmack, B. , Pizanis, N. , Riebisch, M. , Weymann, A. , Kamler, M. , Ruhparwar, A. , Rassaf, T. , and Papathanasiou, M. (2021) Increased bleeding risk with phosphodiesterase‐5 inhibitors after left ventricular assist device implantation. ESC Heart Failure, 8: 2419–2427. 10.1002/ehf2.13322.
References
- 1. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant 2017; 36: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 2. Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, Ransom J. A fully magnetically levitated left ventricular assist device—final report. N Engl J Med 2019; 380: 1618–1627. [DOI] [PubMed] [Google Scholar]
- 3. Papathanasiou M, Pohl J, Jánosi RA, Pizanis N, Kamler M, Rassaf T, Luedike P. Colonization with multiresistant bacteria: impact on ventricular assist device patients. Ann Thorac Surg 2018; 105: 557–563. [DOI] [PubMed] [Google Scholar]
- 4. Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, Morgan JA, Arabia F, Bauman ME, Buchholz HW, Deng M. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013; 32: 157–187. [DOI] [PubMed] [Google Scholar]
- 5. Hamdan R, Mansour H, Nassar P, Saab M. Prevention of right heart failure after left ventricular assist device implantation by phosphodiesterase 5 inhibitor. Artif Organs 2014; 38: 963–967. [DOI] [PubMed] [Google Scholar]
- 6. Klodell CT Jr, Morey TE, Lobato EB, Aranda JM Jr, Staples ED, Schofield RS, Hess PJ, Martin TD, Beaver TM. Effect of sildenafil on pulmonary artery pressure, systemic pressure, and nitric oxide utilization in patients with left ventricular assist devices. Ann Thorac Surg 2007; 83: 68–71. [DOI] [PubMed] [Google Scholar]
- 7. Tedford RJ, Hemnes AR, Russell SD, Wittstein IS, Mahmud M, Zaiman AL, Mathai SC, Thiemann DR, Hassoun PM, Girgis RE, Orens JB. PDE5A inhibitor treatment of persistent pulmonary hypertension after mechanical circulatory support. Circ Heart Fail 2008; 1: 213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Papathanasiou M, Ruhparwar A, Kamler M, Rassaf T, Luedike P. Off‐label use of pulmonary vasodilators after left ventricular assist device implantation: calling in the evidence. Pharmacol Ther 2020: 107619. [DOI] [PubMed] [Google Scholar]
- 9. Sparrow CT, LaRue SJ, Schilling JD. Intersection of pulmonary hypertension and right ventricular dysfunction in patients on left ventricular assist device support: is there a role for pulmonary vasodilators? Circ Heart Fail 2018; 11: e004255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khazanie P, Hammill BG, Patel CB, Kiernan MS, Cooper LB, Arnold SV, Fendler TJ, Spertus JA, Curtis LH, Hernandez AF. Use of heart failure medical therapies among patients with left ventricular assist devices: insights from INTERMACS. J Card Fail 2016; 22: 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McCullough M, Caraballo C, Ravindra NG, Miller PE, Mezzacappa C, Levin A, Gruen J, Rodwin B, Reinhardt S, van Dijk D, Ali A. Neurohormonal blockade and clinical outcomes in patients with heart failure supported by left ventricular assist devices. JAMA Cardiol 2019; 5: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gulati G, Grandin EW, Kennedy K, Cabezas F, DeNofrio DD, Kociol R, Rame JE, Pagani FD, Kirklin JK, Kormos RL, Teuteberg J. Preimplant phosphodiesterase‐5 inhibitor use is associated with higher rates of severe early right heart failure after left ventricular assist device implantation an INTERMACS analysis. Circ Heart Fail 2019; 12: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walter U, Gambaryan S. cGMP and cGMP‐dependent protein kinase in platelets and blood cells. In cGMP: Generators, Effectors and Therapeutic Implications; 2009. p 533–548. [DOI] [PubMed] [Google Scholar]
- 14. Andersson KE. PDE5 inhibitors—pharmacology and clinical applications 20 years after sildenafil discovery. Br J Pharmacol 2018; 175: 2554–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berkels R, Klotz T, Sticht G, Englemann U, Klaus W. Modulation of human platelet aggregation by the phosphodiesterase type 5 inhibitor sildenafil. J Cardiovasc Pharmacol 2001; 37: 413–421. [DOI] [PubMed] [Google Scholar]
- 16. Halcox JP, Nour KR, Zalos G, Mincemoyer R, Waclawiw MA, Rivera CE, Willie G, Ellahham S, Quyyumi AA. The effect of sildenafil on human vascular function, platelet activation, and myocardial ischemia. J Am Coll Cardiol 2002; 40: 1232–1240. [DOI] [PubMed] [Google Scholar]
- 17. Yang HM, Jin S, Jang H, Kim JY, Lee JE, Kim J, Kim HS. Sildenafil reduces neointimal hyperplasia after angioplasty and inhibits platelet aggregation via activation of cGMP‐dependent protein kinase. Sci Rep 2019; 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grosman‐Rimon L, Tumiati LC, Fuks A, Jacobs I, Lalonde SD, Cherney DZ, Rao V. Increased cyclic guanosine monophosphate levels and continuous‐flow left‐ventricular assist devices: implications for gastrointestinal bleeding. J Thorac Cardiovasc Surg 2016; 151: 219–227. [DOI] [PubMed] [Google Scholar]
- 19. Schulman S, Anger SU, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost 2010; 8: 202–204. [DOI] [PubMed] [Google Scholar]
- 20. Houston BA, Schneider AL, Vaishnav J, Cromwell DM, Miller PE, Faridi KF, Shah A, Sciortino C, Whitman G, Tedford RJ, Stevens GR. Angiotensin II antagonism is associated with reduced risk for gastrointestinal bleeding caused by arteriovenous malformations in patients with left ventricular assist devices. J Heart Lung Transplant 2017; 36: 380–385. [DOI] [PubMed] [Google Scholar]
- 21. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation 2011; 123: 2736–2747. [DOI] [PubMed] [Google Scholar]
- 22. Rich JD, Gosev I, Patel CB, Joseph S, Katz JN, Eckman PM, Lee S, Sundareswaran K, Kilic A, Bethea B, Soleimani B, Lima B, Uriel N, Kiernan M. The incidence, risk factors, and outcomes associated with late right‐sided heart failure in patients supported with an axial‐flow left ventricular assist device. J Heart Lung Transplant 2017; 36: 50–58. [DOI] [PubMed] [Google Scholar]
- 23. Patel SR, Vukelic S, Jorde UP. Bleeding in continuous flow left ventricular assist device recipients: an acquired vasculopathy? J Thorac Dis 2016; 8: E1321–E1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gurvits GE, Fradkov E. Bleeding with the artificial heart: gastrointestinal hemorrhage in CF‐LVAD patients. World J Gastroenterol 2017; 23: 3945–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kataria R, Jorde UP. Gastrointestinal bleeding during continuous‐flow left ventricular assist device support state of the field. Cardiol Rev 2019; 27: 8–13. [DOI] [PubMed] [Google Scholar]
- 26. Koliopoulou A, Selzman CH. Stop the LVAD bleeding. J Thorac Dis 2017; 9: E437–E439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imamura T, Kinugawa K, Uriel N. Therapeutic strategy for gastrointestinal bleeding in patients with left ventricular assist device. Circ J 2018; 82: 2931–2938. [DOI] [PubMed] [Google Scholar]
- 28. Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, Higgins RS, Stevenson LW, Stehlik J, Atluri P, Grady KL. The Society of Thoracic Surgeons Intermacs database annual report: evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant 2019; 38: 114–126. [DOI] [PubMed] [Google Scholar]
- 29. Houston BA. Phosphodiesterase‐5 inhibitor therapy and post‐left ventricular assist device outcomes the significance of uncertainty. Circ Heart Fail 2019; 12: 1–3. [DOI] [PubMed] [Google Scholar]
- 30. Groves DS, Blum FE, Huffmyer JL, Kennedy JL, Ahmad HB, Durieux ME, Kern JA. Effects of early inhaled epoprostenol therapy on pulmonary artery pressure and blood loss during LVAD placement. J Cardiothorac Vasc Anesth 2014; 28: 652–660. [DOI] [PubMed] [Google Scholar]
- 31. Bermejo J, Yotti R, García‐Orta R, Sánchez‐Fernández PL, Castaño M, Segovia‐Cubero J, Escribano‐Subías P, San Román JA, Borrás X, Alonso‐Gómez A, Botas J. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double‐blind, randomized clinical trial. Eur Heart J 2018; 39: 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takeda K, Takayama H, Colombo PC, Jorde UP, Yuzefpolskaya M, Fukuhara S, Mancini DM, Naka Y. Late right heart failure during support with continuous‐flow left ventricular assist devices adversely affects post‐transplant outcome. J Heart Lung Transplant 2015; 34: 667–674. [DOI] [PubMed] [Google Scholar]
- 33. Kittipibul V, Blumer V, Angsubhakorn N, Hernandez GA, Chaparro S, Tedford RJ, Agarwal R. Phosphodiesterase‐5 inhibitors and outcomes during left ventricular assist device support: a systematic review and meta‐analysis. J Card Fail;doi:. Published online ahead of print 29 December 2020; 27: 477–485. [DOI] [PubMed] [Google Scholar]
- 34. Xanthopoulos A, Tryposkiadis K, Triposkiadis F, Fukamachi K, Soltesz EG, Young JB, Wolski K, Blackstone EH, Starling RC. Postimplant phosphodiesterase type 5 inhibitors use is associated with lower rates of thrombotic events after left ventricular assist device implantation. JAHA 2020; 9: e015897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sridhar AM, Mukherjee V, Patel DB, Ruiz G, Chawla R, Boyce SW, Najjar SS. Single center experience in the use of sildenafil for right ventricular dysfunction after LVAD implantation. J Heart Lung Transplant 2011; 30: S209–S210. [Google Scholar]
- 36. Netuka I, Ivák P, Tučanová Z, Gregor S, Szárszoi O, Sood P, Crandall D, Rimsans J, Connors JM, Mehra MR. Evaluation of low‐intensity anti‐coagulation with a fully magnetically levitated centrifugal‐flow circulatory pump—the MAGENTUM 1 study. J Heart Lung Transplant 2018; 37: 579–586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Schematic representation of all scheduled in‐person and remote follow‐up visits in case of uncomplicated disease course after LVAD implantation. After 6 months, LVAD recipients who were transplant candidates received an inpatient heart transplant screening including right heart catheterization (RHC), whereas non‐candidates received hemodynamic testing only in case of right ventricular failure or signs of pulmonary hypertension. BTT = Bridge‐to‐transplant, DT = Destination Therapy. HFU = Heart Failure Unit.


