Abstract
Aims
The prognostic importance of admission systolic blood pressure (SBP) in heart failure with preserved ejection fraction (HFpEF) is elusive. We aimed to clarify the pathophysiological differences between patients categorized with admission SBP among HFpEF patients.
Methods and results
We studied 1008 inpatients from PURSUIT‐HFpEF, a multicentre prospective observational registry. We classified patients as having elevated (>140 mmHg), preserved (90–140 mmHg), or low (<90 mmHg) admission SBP. Most cases had elevated (n = 584) or preserved (n = 420) SBP; the four cases with low SBP were excluded. Univariable Cox regression testing revealed that preserved SBP patients had a higher risk of a composite of cardiac death and heart failure re‐hospitalization [hazard ratio (HR) 1.48, 95% confidence interval (CI) 1.14–1.92, P = 0.0035] than elevated SBP patients. In multivariable Cox regression models, while prior heart failure hospitalization (HR 1.36, 95% CI 1.01–2.84, P = 0.0453), atrial fibrillation (HR 1.82, 95% CI 1.10–2.99, P = 0.0209), and N‐terminal pro‐B‐type natriuretic peptide (HR 1.94, 95% CI 1.10–3.43, P = 0.0229) at discharge were significantly associated with adverse outcomes in elevated SBP patients, N‐terminal pro‐B‐type natriuretic peptide (HR 2.06, 95% CI 1.04–4.07, P = 0.0373) and right ventricular‐pulmonary artery uncoupling reflected by the tricuspid annular plane systolic excursion/pulmonary artery systolic pressure ratio (HR 0.19, 95% CI 0.05–0.65, P = 0.0075) at discharge were significant prognostic factors in preserved SBP patients.
Conclusions
Patients with preserved admission SBP had significant higher risks for adverse outcomes than those with elevated SBP in HFpEF. Tricuspid annular plane systolic excursion/pulmonary artery systolic pressure was the distinctive prognostic factor between the two groups.
Keywords: Heart failure, Blood pressure, Prognosis
Introduction
Acute heart failure (AHF) refers to rapid onset or worsening of symptoms and/or signs of heart failure (HF). Although a large number of overlapping classifications of AHF based on different criteria have been proposed, the latest guidelines for diagnosis and treatment of AHF and chronic HF in the European Society of Cardiology (ESC) note that most cases are classified according to clinical presentation on admission, which enables clinicians to promptly identify its severity and plan the initial strategy. 1 At presentation, systolic blood pressure (SBP) on admission is often used and classified as elevated (>140 mmHg; hypertensive AHF), preserved (90–140 mmHg), or low (<90 mmHg; hypotensive AHF). SBP has been shown to be an important predictor of adverse outcomes in both chronic HF 2 , 3 and AHF, 4 , 5 , 6 and most studies describe an inverse relationship between SBP and mortality. In advanced systolic HF, only 5–8% of all patients are categorized as hypotensive, which is associated with a poor prognosis, especially when accompanied by signs of hypoperfusion. 7 , 8 In patients with heart failure with preserved ejection fraction (HFpEF), Buiciuc et al. reported that lower SBP on admission (SBP < 120 mmHg) was associated with adverse outcomes. 9 In a large HFpEF population from an analysis of the OPTIMIZE‐HF registry, Tsimploulis et al. reported that discharge SBP < 120 mmHg was also associated with a higher risk of 30 day and 1 year all‐cause mortality. 10 However, the specific findings with prognostic differences between preserved (90–140 mmHg) and elevated (>140 mmHg) SBP on admission in HFpEF are elusive.
Because the pathophysiology has been described to differ between AHF patients in general with preserved and elevated SBP on admission, 11 , 12 the differences might also apply to HFpEF patients. In HFpEF patients, factors such as renal dysfunction, 13 N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP), 14 and nutrition status 15 have been reported to be important for prognosis. However, little is known about whether these factors apply equally to HFpEF patients with preserved vs. elevated admission SBP.
Here, in a broad HFpEF population, we investigated the characteristics and prognosis of HFpEF inpatients divided into preserved and elevated SBP on admission and differences in important prognostic factors between the two groups.
Methods
The PURSUIT‐HFpEF registry
This prospective, multicentre, observational cohort study was performed in 1024 consecutive hospitalized HFpEF patients. Details of the PURSUIT‐HFpEF (The Prospective mUlticenteR obServational stUdy of patIenTs with Heart Failure with preserved Ejection Fraction) registry have been described previously (Supporting Information, Data S1 ). 16 Briefly, through the collaboration of 31 hospitals in Japan, this large‐scale registry aimed to collect and record a comprehensive range of clinical data to define the pathophysiology and prognostic factors of HFpEF patients. Inclusion criteria were acute decompensated HFpEF diagnosed by the Framingham criteria 17 for HF, as well as (i) left ventricular ejection fraction (LVEF) ≥ 50% and (ii) NT‐proBNP ≥ 400 ng/L or brain natriuretic peptide (BNP) ≥ 100 ng/L on admission. Major exclusion criteria were age < 20 years, severe valvular diseases, acute coronary syndrome on admission, life expectancy of <6 months due to prognosis of non‐cardiac diseases, and previous heart transplantation. The anonymized data were transferred to the data centre of Osaka University Hospital for analysis via a data capture system connected with electronic health records. 18 Written informed consent was received from each participating patient. This study, including the procedure for enrolment, conformed to the principles of the Declaration of Helsinki and was approved by the institutional review board of each participating facility. It was registered under the Japanese UMIN Clinical Trials Registration (UMIN000021831).
Study population
A total of 1024 inpatients with HFpEF were registered from June 2016 to July 2020. Of these, 16 patients died in the hospital. Admission SBP was measured with a non‐invasive method using arm‐cuff pressure in the period between admission and approximately 48 h after admission. Only four patients had low admission SBP (<90 mmHg; hypotensive AHF) and were excluded for statistical reasons. The remaining 1004 cases consisted of 584 (58.2%) patients with elevated (>140 mmHg) admission SBP and 420 (41.8%) with preserved (90–140 mmHg) admission SBP.
Frailty, nutrition status, and plasma volume estimation
Activities of daily living were assessed using Clinical Frailty Scale. 19 Nutritional status was estimated with the geriatric nutritional risk index (GNRI), calculated as 15
| (1) |
Systemic plasma volume was estimated with plasma volume status (PVS). 20
Actual plasma volume (aPV) was calculated as
| (2) |
where a = 1530 in men and 864 in women and b = 41 in men and 47.9 in women. Ideal plasma volume (iPV) was calculated as
| (3) |
where c = 39 in men and 40 in women. PVS was calculated as
| (4) |
Echocardiography
Comprehensive echocardiographic examinations were performed by trained cardiac sonographers according to the American Society of Echocardiography guidelines. 21 In patients with atrial fibrillation (AF), recordings of consecutive five to seven beats were recommended. Single beat measurement of systolic or diastolic parameters for one beat occurring after two serial beats with average R–R interval or one beat with an average Doppler‐wave contour with an average velocity was also permitted, in accordance with previous studies. 22 LVEF and stroke volume (SV) were calculated by the biplane Simpson's method using apical two‐chamber and four‐chamber views. Left atrial volume index (LAVI) was also calculated by the biplane Simpson's method. The ratio of mitral peak velocity of early filling E to the velocity of mitral annulus early diastolic motion e′ (E/e′) was calculated with the mean e′ velocity obtained from the septal and lateral sides of the mitral annulus. It has been reported that right ventricular (RV) dysfunction and RV‐pulmonary arterial (PA) uncoupling, reflected with tricuspid annular plane systolic excursion (TAPSE) to pulmonary artery systolic pressure (PASP) ratio, are highly prevalent and associated with poor prognosis even in HFpEF patients. 23 , 24 , 25 TAPSE was quantified in the RV‐focused apical four‐chamber view using M‐mode echocardiography. PASP (mmHg) was calculated as
| (5) |
with the pressure measurement based on the inferior vena cava (IVC) diameter and collapsibility. Patients without a noticeable TR signal or lacking either inspiratory/expiratory phases of IVC diameters were excluded.
Follow‐up and endpoints
Among 1004 cases, 226 (22.5%) reached the primary endpoint of cardiac death or re‐hospitalization for HF, with a mean ± standard deviation follow‐up of 374 ± 360 days. A total of 66 cases with a mean follow‐up of 451 ± 379 days and 212 cases with a mean of 374 ± 360 days suffered the secondary endpoints of cardiac death and re‐hospitalization for HF, respectively. A total of 113 (19.3%) elevated SBP cases (out of 584) reached the primary endpoint with a mean of 385 ± 362 days, while 113 (26.9%) preserved SBP cases (out of 420) reached it with a mean of 359 ± 357 days. The duration of the follow‐up period was calculated from the day of discharge until an endpoint or at the time of the last patient contact (including teleconferencing or mailing).
Statistical analysis
Data are presented as medians and interquartile range of 25–75% for continuous variables and frequency/percentage for categorical variables. Continuous variables were compared using the Student's t‐test or Kruskal–Wallis test as appropriate, and categorical variables were compared using Pearson's χ 2 test. The clinical endpoint was assessed by the Kaplan–Meier method and compared with the log‐rank test. Cox proportional hazards regression models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations between clinical factors and each endpoint. Based on our clinical expertise and previous literature, multivariable Cox regression for the primary endpoint was performed using covariates as follows: age, gender, prior HF hospitalization, history of hypertension, diabetes mellitus, AF, estimated glomerular filtration rates (eGFRs), GNRI, NT‐proBNP, E/e′, and TAPSE/PASP at discharge. All statistical tests were two‐sided, and P < 0.05 was regarded as statistically significant. Statistical analysis was performed using JMP® Pro 13.2.1. (SAS Institute Inc., Chicago IL, USA).
Results
Characteristics of the elevated and preserved systolic blood pressure populations
Only four patients had low admission SBP (<90 mmHg), suggesting that hypotensive AHF is uncommon in HFpEF. These patients were excluded for statistical reasons. Demographic and clinical characteristics of the 1004 patients are summarized in Tables 1 and 2 and Supporting Information, Table S1 . The study population had a median age of 83 years; 55% were female. Hypertension (85%) was the most prevalent co‐morbidity, and AF was present in 38% at discharge. The median SBP on admission was 147 mmHg, which was controlled to 118 mmHg at discharge. Compared with elevated SBP patients, preserved SBP patients were significantly older, despite served lower degree of frailty, were predominantly female, had frequent previous HF admissions, had a higher rate of AF, elevated NT‐proBNP at discharge, and exercise intolerance reflected by a shorter 6 min walking distance. In echocardiographic findings at discharge, while LVEF and RV size were comparable, left ventricular (LV) SV and TAPSE were significantly higher in elevated SBP patients, and PASP was higher and TAPSE/PASP was lower in preserved SBP patients. Focusing on the acute phase treatments, elevated SBP patients were characterized by a frequent need for non‐invasive positive pressure ventilation (NIPPV), intravenous nitrates, and calcium channel blockers. In chronic phase treatments, oral angiotensin receptor blockers (ARBs) and calcium channel blockers were more frequently used in elevated SBP patients, while loop diuretics were more frequently used in preserved SBP patients.
Table 1.
Baseline clinical characteristics on admission
| All patients (n = 1004) | Elevated SBP (n = 584) | Preserved SBP (n = 420) | P‐value | |
|---|---|---|---|---|
| Age, years | 83 (77–87) | 82 (76–87) | 83 (78–87) | 0.0191* |
| Female | 548 (55) | 302 (52) | 246 (59) | 0.0313 |
| Prior HF hospitalization | 246 (25) | 116 (20) | 130 (31) | <0.0001 |
| Co‐morbidities | ||||
| Hypertension | 846 (85) | 522 (90) | 324 (78) | <0.0001 |
| Diabetes | 329 (33) | 202 (35) | 127 (31) | 0.1549 |
| Dyslipidaemia | 407 (41) | 250 (43) | 157 (38) | 0.0840 |
| Hyperuricaemia | 329 (33) | 194 (34) | 135 (33) | 0.7471 |
| CKD | 396 (40) | 218 (38) | 178 (43) | 0.0980 |
| COPD | 72 (8) | 40 (7) | 32 (8) | 0.6302 |
| Malignancy | 117 (12) | 59 (10) | 58 (14) | 0.0717 |
| General condition | ||||
| SBP, mmHg | 147 (128–170) | 164 (153–185) | 125 (114–133) | <0.0001* |
| DBP, mmHg | 80 (66–93) | 86 (74–102) | 70 (60–82) | <0.0001* |
| Heart rate | 82 (67–100) | 83 (68–101) | 81 (65–100) | 0.2616* |
| AF | 461 (46) | 236 (40) | 225 (54) | <0.0001 |
| GNRI | 98 (90–105) | 99 (91–106) | 97 (89–104) | 0.0113 |
| Laboratory examination | ||||
| Haemoglobin, g/dL | 11.2 (9.8–12.5) | 11.3 (9.8–12.7) | 11.0 (9.7–12.3) | 0.0210 |
| Haematocrit, % | 34.3 (30.2–38.1) | 34.4 (30.5–38.4) | 33.9 (29.9–37.8) | 0.0371 |
| eGFR, mL/min/1.73 m2 | 44.6 (29.8–58.8) | 45.4 (31.2–60.1) | 41.0 (28.4–55.5) | 0.0694* |
| NT‐proBNP, ng/L | 3236 (1722–6426) | 3183 (1633–6420) | 3257 (1878–6633) | 0.0831* |
| CRP, mg/dL | 0.54 (0.19–1.93) | 0.53 (0.20–1.73) | 0.56 (0.19–2.29) | 0.0048* |
| aPV, mL | 2434 (2107–2793) | 2467 (2130–2799) | 2390 (2069–2774) | 0.2640* |
| iPV, mL | 2240 (1912–2640) | 2282 (1960–2673) | 2190 (1883–2574) | 0.0148* |
| PVS, % | +8.6 (−0.3 to +16.8) | +7.9 (−1.0 to +16.2) | +9.2 (+0.9 to +17.4) | 0.0401 |
| Medication before admission | ||||
| Antiplatelet | 305 (30) | 184 (32) | 121 (29) | 0.3593 |
| ACE inhibitor | 96 (10) | 59 (10) | 37 (9) | 0.4918 |
| ARB | 428 (43) | 275 (47) | 153 (36) | 0.0008 |
| Calcium channel blocker | 512 (51) | 309 (53) | 203 (48) | 0.1524 |
| Beta‐blocker | 467 (47) | 259 (44) | 208 (50) | 0.0975 |
| Loops diuretics | 504 (50) | 244 (42) | 260 (62) | <0.0001 |
| Thiazide | 77 (8) | 43 (7) | 34 (8) | 0.6671 |
| Tolvaptan | 54 (5) | 25 (4) | 29 (7) | 0.0691 |
| Aldosterone antagonist | 210 (21) | 96 (16) | 114 (27) | <0.0001 |
| Initial treatment | ||||
| NIPPV usage | 125 (12) | 94 (16) | 31 (7) | <0.0001 |
| Intubation | 16 (2) | 14 (2) | 2 (0.5) | 0.0167 |
| Dobutamine | 17 (2) | 8 (1.4) | 9 (2) | 0.3464 |
| Intravenous PDE3I | 3 (0.3) | 1 (0.2) | 2 (0.5) | 0.3813 |
| Carperitide | 213 (21) | 125 (21) | 88 (21) | 0.8629 |
| Intravenous nitrates | 283 (28) | 233 (40) | 50 (12) | <0.0001 |
| Intravenous calcium channel blocker | 83 (8) | 73 (13) | 10 (2) | <0.0001 |
| Intravenous nicorandil | 7 (0.7) | 4 (0.7) | 3 (0.7) | 0.9535 |
| Diuretics (continuous injection) | 327 (33) | 188 (32) | 139 (33) | 0.7434 |
| Diuretics (bolus injection) | 566 (56) | 331 (57) | 235 (56) | 0.8520 |
ACE, angiotensin‐converting enzyme; AF, atrial fibrillation; aPV, actual plasma volume; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GNRI, geriatric nutritional risk index; HF, heart failure; iPV, ideal plasma volume; NIPPV, non‐invasive positive pressure ventilation; PDE3I, phosphodiesterase‐3 inhibitor; PVS, plasma volume status; SBP, systolic blood pressure.
Values are given as median (interquartile range) or n (%). Between‐group comparisons were performed using the Student's t‐test* (when the continuous variables of both groups were judged as normally distributed using the Shapiro–Wilk W test), Kruskal–Wallis test, or Pearson's χ 2 test.
Table 2.
Clinical characteristics at discharge
| All patients (n = 1004) | Elevated SBP (n = 584) | Preserved SBP (n = 420) | P‐value | |
|---|---|---|---|---|
| Hospital stay, days | 16 (12–22) | 16 (12–22) | 17 (13–24) | 0.1943* |
| BMI, kg/m2 | 21.4 (18.9–24.2) | 21.6 (19.1–24.4) | 21.1 (18.6–23.9) | 0.0150* |
| SBP, mmHg | 118 (106–131) | 123 (110–135) | 112 (102–123) | <0.0001* |
| DBP, mmHg | 65 (58–74) | 66 (58–76) | 64 (57–71) | 0.0022* |
| Heart rate | 70 (61–80) | 69 (60–77) | 72 (63–81) | 0.0001* |
| AF | 378 (38) | 201 (34) | 177 (42) | 0.0134 |
| GNRI | 92 (85–99) | 92 (85–100) | 92 (84–98) | 0.2318 |
| 6MWD, m | 260 (160–340) | 270 (180–352) | 237 (135–327) | 0.0098* |
| NYHA classification | 0.1633 | |||
| NYHA I | 361 (36) | 223 (39) | 138 (34) | |
| NYHA II | 557 (56) | 321 (55) | 236 (57) | |
| NYHA III | 69 (7) | 33 (6) | 36 (9) | |
| NYHA IV | 4 (0.4) | 2 (0.4) | 2 (0.5) | |
| Laboratory examination | ||||
| Haemoglobin, g/dL | 11.3 (10.1–12.7) | 11.4 (10.2–12.8) | 11.2 (10.0–12.7) | 0.5908* |
| Haematocrit, % | 34.5 (31.0–38.5) | 34.6 (31.1–38.3) | 34.4 (31.0–38.8) | 0.7639* |
| Serum total protein, g/dL | 6.6 (6.2–7.1) | 6.6 (6.2–7.1) | 6.7 (6.2–7.2) | 0.0796* |
| Serum albumin, g/dL | 3.4 (3.1–3.7) | 3.4 (3.1–3.7) | 3.4 (3.1–3.7) | 0.4692* |
| eGFR, mL/min/1.73 m2 | 41.7 (29.9–55.0) | 41.7 (29.0–55.2) | 41.7 (30.9–54.5) | 0.4120* |
| NT‐proBNP, ng/L | 1111 (481–2486) | 1036 (445–2352) | 1250 (556–2677) | 0.1295* |
| CRP, mg/dL | 0.29 (0.11–0.89) | 0.30 (0.11–0.80) | 0.28 (0.11–1.03) | 0.6192* |
| aPV, mL | 2246 (1953–2575) | 2293 (1984–2607) | 2203 (1908–2489) | 0.0022* |
| iPV, mL | 2020 (1712–2376) | 2054 (1772–2407) | 1952 (1688–2309) | 0.0007* |
| PVS, % | +11.1 (+1.8 to +19.3) | +10.9 (+1.1 to +18.9) | +11.2 (+2.8 to +19.6) | 0.3811 |
| ΔaPV, mL | −181 (−322 to −42) | −171 (−311 to −25) | −197 (−333 to −77) | 0.0023* |
| ΔiPV, mL | −200 (−306 to −120) | −196 (−296 to −121) | −208 (−316 to −117) | 0.2549* |
| ΔPVS, % | +2.7 (−2.1 to +7.4) | +3.2 (−1.9 to +7.9) | +1.8 (−2.3 to +6.6) | 0.0164 |
| Echocardiographic variables | ||||
| LVDD, mm | 45 (41–50) | 46 (42–51) | 45 (40–49) | 0.0003 |
| LVEF (m‐Simpson), % | 61 (55–66) | 61 (56–66) | 61 (55–66) | 0.1631* |
| LVEDV, mL | 78 (58–102) | 81 (60–104) | 75 (57–98) | 0.0019* |
| LVESV, mL | 30 (21–42) | 31 (22–43) | 29 (21–40) | 0.0665* |
| SV, mL | 47 (36–62) | 49 (37–63) | 44 (33–58) | 0.0002* |
| LAD, mm | 44 (39–49) | 44 (39–49) | 44 (39–50) | 0.4487* |
| LAVI, mL/m2 | 50 (37–65) | 49 (36–65) | 51 (37–65) | 0.1597* |
| E/e′ | 12.5 (9.8–16.8) | 13.0 (10.1–17.3) | 12.0 (9.0–16.3) | 0.1302* |
| RVD, mm | 32 (28–36) | 32 (28–36) | 32 (28–37) | 0.3448* |
| TAPSE, mm | 17.3 (14.6–20.2) | 17.9 (15.0–20.9) | 17.0 (13.9–19.5) | <0.0001* |
| TRPG, mmHg | 27 (22–33) | 26 (21–32) | 27 (22–33) | 0.0648* |
| PASP, mmHg | 31 (26–38) | 31 (25–38) | 33 (27–39) | 0.0268* |
| TAPSE/PASP, mm/mmHg | 0.54 (0.42–0.71) | 0.57 (0.43–0.74) | 0.50 (0.38–0.68) | 0.0001* |
| Medication | ||||
| Antiplatelet | 290 (29) | 181 (31) | 109 (26) | 0.0863 |
| ACE inhibitor | 179 (18) | 112 (19) | 67 (16) | 0.1935 |
| ARB | 368 (37) | 256 (44) | 112 (27) | <0.0001 |
| Calcium channel blocker | 483 (48) | 346 (59) | 137 (33) | <0.0001 |
| Beta‐blocker | 551 (55) | 313 (54) | 238 (57) | 0.3142 |
| Loops diuretics | 788 (78) | 430 (74) | 358 (85) | <0.0001 |
| Thiazide | 66 (7) | 37 (6) | 29 (7) | 0.7196 |
| Tolvaptan | 159 (16) | 83 (14) | 76 (18) | 0.0964 |
| Aldosterone antagonist | 393 (39) | 200 (34) | 193 (46) | 0.0002 |
| Statin | 331 (33) | 198 (34) | 133 (32) | 0.4727 |
| DPP4 inhibitor | 167 (17) | 100 (17) | 67 (16) | 0.6231 |
| SGLT2 inhibitor | 52 (5) | 35 (6) | 17 (4) | 0.1753 |
| Anticoagulant | 594 (59) | 301 (52) | 293 (70) | <0.0001 |
6MWD, 6 min walking distance; ACE, angiotensin‐converting enzyme; AF, atrial fibrillation; aPV, actual plasma volume; ARB, angiotensin receptor blocker; BMI, body mass index; CRP, C‐reactive protein; DBP, diastolic blood pressure; DPP4, dipeptidyl peptidase‐4; E/e', the ratio of mitral peak velocity of early filling E to the velocity of mitral annulus early diastolic motion e'; eGFR, estimated glomerular filtration rate; GNRI, geriatric nutritional risk index; iPV, ideal plasma volume; LAD, left atrial dimension; LAVI, left atrial dimension index; LVDD, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association functional class; PASP, pulmonary artery systolic pressure; PVS, plasma volume status; RVD, basal right ventricular linear dimension; SBP, systolic blood pressure; SGLT2, sodium glucose cotransporter‐2; SV, stroke volume; TAPSE, tricuspid annular plane systolic excursion; TRPG, tricuspid regurgitation pressure gradient, ΔaPV, aPV at discharge − aPV on admission; ΔiPV, iPV at discharge − iPV on admission; ΔPVS, PVS at discharge − PVS on admission.
Values are given as median (interquartile range) or n (%). Between‐group comparisons were performed using the Student's t‐test* (when the continuous variables of both groups were judged as normally distributed using the Shapiro–Wilk W test), Kruskal–Wallis test, or Pearson's χ 2 test.
Systolic blood pressure on admission and prognosis
During a mean follow‐up of 374 days, 226 patients suffered from a primary composite endpoint. The optimal cut‐off criterion of admission SBP for predicting the primary endpoint was 143 mmHg (area under the curve = 0.554, P = 0.0293, Supporting Information, Figure S1 ). Kaplan–Meier curve analyses (Figure 1 ) and univariable Cox regression testing revealed that preserved SBP patients had significantly higher rate of primary composite endpoints (HR 1.48, 95% CI 1.14–1.92, P = 0.0035), whereas the two secondary endpoints of cardiac death and HF re‐admission were comparable (HR 1.45, 95% CI 0.89–2.35, P = 0.1350, and HR 1.29, 95% CI 0.98–1.69, P = 0.0652, respectively). The causes of cardiac death are illustrated in the Supporting Information, Figure S2 . The major cause of cardiac death was HF (55% of whole population, Supporting Information, Figure S2 A), and the causes were comparable between elevated SBP population and preserved SBP population (P = 0.2615, Supporting Information, Figure S2 B and S2 C).
Figure 1.
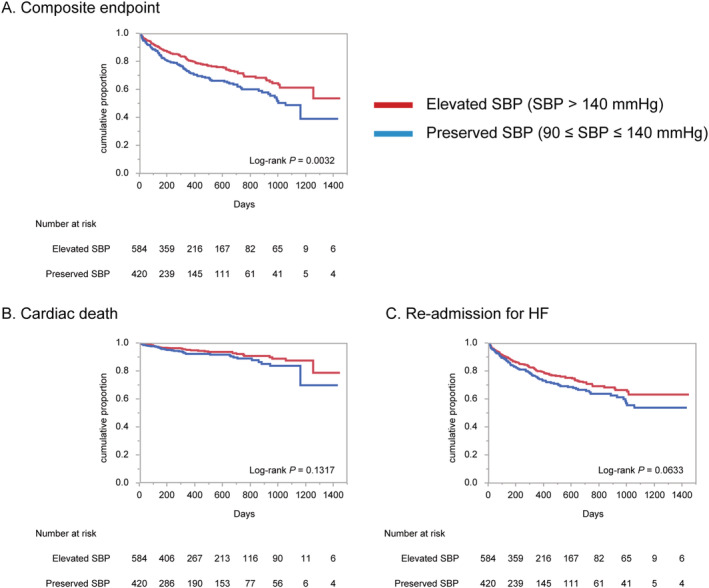
Kaplan–Meier survival curves for adverse outcomes in the elevated and preserved SBP populations. Kaplan–Meier survival curves for composite endpoint (A), cardiac death (B), and re‐admission for heart failure (C). HF, heart failure; SBP, systolic blood pressure.
Prognostic factors in elevated and preserved systolic blood pressure patients
Univariable and multivariable Cox regression tests for elevated and preserved SBP patients were analysed. The interactions for primary endpoint between elevated/preserved SBP on admission and each prognostic covariate are described in the Supporting Information, Table S2 . Of all the covariates, only TAPSE/PASP had significant prognostic interaction with categorized SBP on admission (P for interaction = 0.0456). For elevated SBP patients, in the multivariable analysis, prior HF hospitalization, AF, and NT‐proBNP at discharge were significantly associated with the primary endpoint (HR 1.36, 95% CI 1.01–2.84, P = 0.0453; HR 1.82, 95% CI 1.10–2.99, P = 0.0209; and HR 1.94, 95% CI 1.10–3.43, P = 0.0229, respectively) (upper column of Table 3 ). In preserved SBP patients, in addition to elevated NT‐proBNP (HR 2.06, 95% CI 1.04–4.07, P = 0.0373), RV‐PA uncoupling reflected by the TAPSE/PASP ratio (HR 0.19, 95% CI 0.05–0.65, P = 0.0075) was also revealed to be a strong independent negative prognostic factor (lower column of Table 3 ).
Table 3.
Cox regression models for prognostic prediction of primary endpoint
| Elevated SBP (>140 mmHg) on admission population | ||||
|---|---|---|---|---|
| Unadjusted HR (95% CI) | P‐value | Adjusted HR (95% CI) | P‐value | |
| Age | 1.03 (1.01–1.06) | 0.0070 | 1.02 (0.99–1.06) | 0.1720 |
| Female | 1.28 (0.88–1.86) | 0.1983 | 1.09 (0.69–1.73) | 0.7287 |
| Prior HF hospitalization | 2.28 (1.51–3.39) | 0.0001 | 1.36 (1.01–2.84) | 0.0453 |
| Hypertension | 2.06 (0.99–5.26) | 0.0552 | 1.18 (0.51–3.43) | 0.7295 |
| Diabetes mellitus | 1.27 (0.86–1.84) | 0.2297 | 1.05 (0.64–1.69) | 0.8488 |
| AF | 1.50 (1.03–2.18) | 0.0360 | 1.82 (1.10–2.99) | 0.0209 |
| eGFR | 0.98 (0.97–0.99) | 0.0001 | 0.99 (0.97–1.00) | 0.1008 |
| Log NT‐proBNP | 2.38 (1.70–3.32) | <0.0001 | 1.94 (1.10–3.43) | 0.0229 |
| E/e′ | 1.02 (0.99–1.05) | 0.1340 | 1.01 (0.98–1.04) | 0.5015 |
| GNRI | 0.98 (0.97–1.00) | 0.0193 | 0.99 (0.97–1.01) | 0.4235 |
| TAPSE/PASP | 0.58 (0.23–1.39) | 0.2248 | 1.63 (0.55–4.49) | 0.3686 |
| Preserved SBP (90–140 mmHg) on admission population | ||||
|---|---|---|---|---|
| Unadjusted HR (95% CI) | P‐value | Adjusted HR (95% CI) | P‐value | |
| Age | 1.02 (1.00–1.04) | 0.1150 | 1.00 (0.97–1.04) | 0.8545 |
| Female | 1.16 (0.80–1.72) | 0.4396 | 1.14 (0.68–1.94) | 0.6271 |
| Prior HF hospitalization | 1.88 (1.29–2.73) | 0.0012 | 0.98 (0.58–1.63) | 0.9388 |
| Hypertension | 1.01 (0.66–1.62) | 0.9509 | 1.09 (0.58–2.21) | 0.7942 |
| Diabetes mellitus | 1.04 (0.69–1.53) | 0.8522 | 1.30 (0.76–2.16) | 0.3332 |
| AF | 1.16 (0.80–1.68) | 0.4382 | 0.67 (0.39–1.14) | 0.1437 |
| eGFR | 0.98 (0.97–1.00) | 0.0045 | 1.00 (0.99–1.02) | 0.6940 |
| Log NT‐proBNP | 2.67 (1.71–4.15) | <0.0001 | 2.06 (1.04–4.07) | 0.0373 |
| E/e′ | 1.02 (1.00–1.04) | 0.0785 | 1.02 (0.99–1.04) | 0.2518 |
| GNRI | 0.99 (0.98–1.01) | 0.5378 | 0.99 (0.97–1.02) | 0.6308 |
| TAPSE/PASP | 0.07 (0.02–0.22) | <0.0001 | 0.19 (0.05–0.65) | 0.0075 |
AF, atrial fibrillation; CI, confidence interval; E/e', the ratio of mitral peak velocity of early filling E to the velocity of mitral annulus early diastolic motion e'; eGFR, estimated glomerular filtration rate; GNRI, geriatric nutritional risk index; HF, heart failure; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PASP, pulmonary artery systolic pressure; SBP, systolic blood pressure; TAPSE, tricuspid annular plane systolic excursion.
Discussion
In a large‐scale prospective multicentre study of HFpEF inpatients, we obtained the following findings: (i) admission of HFpEF patients with SBP < 90 mmHg was extremely rare; (ii) patients with preserved SBP on admission had a poorer prognosis than those with elevated SBP; and (iii) prognostic factors for elevated and preserved SBP patients differed, except for NT‐proBNP.
Complicated pathophysiology makes it difficult to improve poor outcomes of HFpEF patients, and phenotype‐specific therapeutic strategies are wanting. 26 As conceptualized in clinical scenarios, 27 one way to classify the pathophysiological types of AHF is admission SBP. We show here the clinical outcomes for HFpEF patients with elevated and preserved admission SBP. Among them, the unique negative prognostic factor of RV‐PA uncoupling in the preserved SBP HFpEF patients may open the possibility of a treatment strategy tailored to address this finding.
Classification of heart failure with preserved ejection fraction patients by systolic blood pressure in acute heart failure
The 2016 ESC guidelines described the definition and classification of AHF based on admission SBP; however, it also noted that several reports described the classification of AHF based on admission SBP regardless of LVEF and that only 5–8% of all patients presented with SBP < 90 mmHg. 1 Few prospective studies specifically targeted at HFpEF patients have appeared. In a large AHF study of the European Society of Cardiology Heart Failure Long‐Term (ESC‐HF‐LT) registry (16 012 patients), Chioncel et al. reported that only 2.9% of AHF patients presented with cardiogenic shock on admission and that 1.9% presented with SBP < 85 mmHg on admission, followed by 24.9% with SBP 85–110 mmHg, 42.9% with SBP 110–140 mmHg, and 30.3% with SBP > 140 mmHg. 28 From the Acute Decompensated Heart Failure Syndromes (ATTEND) registry, one of the largest nationwide hospital‐based prospective cohort studies for AHF patients in Japan (4831 patients), Kajimoto et al. reported that the lowest SBP quartile on admission (<120 mmHg) was more likely to have a reduced EF than a preserved EF, whereas other quartiles contained preserved or reduced EF equally. 29 These findings suggest that hypotensive AHF is quite limited regardless with LVEF and that HFpEF seems to be even less common in AHF patients with low SBP on admission. These findings are in agreement with our finding of only four cases of hypotensive HFpEF.
From the Acute Decompensated Heart Failure National Registry (ADHERE) database, Yancy et al. reported that the most powerful predictor of in‐hospital mortality in patients with preserved systolic function was SBP ≤ 125 mmHg. 30 The ideal cut‐off of admission SBP 143 mmHg for predicting adverse outcomes in our cohort was different as described in the Supporting Information, Figure S1 ; however, the differences were derived from the patient background (e.g. ages: ours vs. Yancy et al., 83 vs. 73.9 years) and the target outcomes (post‐discharge cardiac death and re‐hospitalization for HF vs. in‐hospital mortality).
We classified the patients with the threshold of admission SBP 140 mmHg, which could divide high blood pressure and normal blood pressure to investigate the pathophysiology of early phase of AHF syndromes 11 in our cohort. AHF can also be classified as either ‘vascular’ or ‘cardiac’ failure, in which the former typically demonstrates elevated SBP, preserved LVEF, and lung congestion, while the latter often shows low or preserved SBP, impaired LVEF, and systemic congestion. 11 Compared with the elevated SBP cases, preserved SBP cases had more prior HF admissions (preserved vs. elevated SBP: 31% vs. 20%, P < 0.0001), more AF (42% vs. 34%, P = 0.0134), more elevated PVS on admission (+9.2% vs. +7.9%, P = 0.0401), a greater decrease in aPV during hospitalization (−197 vs. −171 mL, P = 0.0023), and a smaller LV SV (44 vs. 49 mL, P = 0.0002) in spite of comparable LVEFs (61% vs. 61%, P = 0.1631) (Tables 1 and 2 ). Although PVS increased during hospitalization in both groups because the decrease in iPV exceeded the decrease in aPV, the more elevated PVS on admission and greater aPV decrease during hospitalization in the preserved SBP cases suggested that they represented greater fluid accumulation in the preserved SBP cases than in the elevated SBP cases. Focusing on the initial treatment of the elevated SBP HFpEF cases, the higher proportion requiring NIPPV (elevated vs. preserved SBP: 16% vs. 7%, P < 0.0001) and intubation (2% vs. 0.5%, P = 0.0167) might reflect their higher frequency of lung congestion (Table 1 ). These findings suggested that in this HFpEF cohort, cases with elevated SBP had major aspects of vascular failure, while cases with preserved SBP had those of cardiac failure. As a consequence, preserved SBP patients had frequent prior HF admissions (10% vs. 31%, P < 0.0001), elevated NT‐proBNP at discharge (1036 vs. 1250 ng/L, P = 0.1295), and exercise intolerance reflected by a shorter 6 min walking distance (270 vs. 237 m, P = 0.0098), which could suggest that preserved SBP patients were more advanced phase of HFpEF and therefore with worse outcomes.
Pathophysiological and prognostic factors behind admission systolic blood pressure
In a report from the Japanese Heart Failure Syndrome With Preserved Ejection Fraction (JASPER) registry, 31 patients were divided by SBP on the following day after admission (<100, 100–140, and >140 mmHg), which should be noted to differ from our current investigation, and assessed in detail for admission echocardiographic parameters among groups. The LVEF, left ventricular diastolic diameter, left atrial dimension, IVC diameter, and TR pressure gradient were comparable among groups. The LV outflow tract velocity time integral (LVOT‐VTI), which roughly reflects SV, positively correlated with the SBP (16.4, 19.4, and 23.3 cm, P = 0.001). It was also shown that heart rate was negatively correlated with the SBP (74.0, 70.0, and 64.5 mmHg, P = 0.009), suggesting that the compensatory mechanism works to maintain cardiac output across a range of SBPs. The authors speculated that patients in the low SBP group had impaired contractile reserve, which is regulated by the Frank–Starling mechanism, force–frequency relationship, and sympathetic nerve stimulation, and therefore demonstrated a positive correlation with LVOT‐VTI. 31 We also showed that preserved SBP was accompanied by lower SV (elevated vs. preserved SBP: 49 vs. 44 mL, P = 0.0002) and higher heart rate (69 vs. 72 b.p.m., P = 0.0001), consistent with the findings from the JASPER registry. RV‐PA uncoupling reflected by TAPSE/PASP clearly differed between elevated and preserved SBP cases (0.57 vs. 0.50 mm/mmHg, P = 0.0001).
It should be noted that prognostic factors were quite different between elevated and preserved SBP patients. A multivariable Cox hazard model revealed that NT‐proBNP and TAPSE/PASP were significantly associated with the primary endpoint in preserved SBP patients, whereas prior HF hospitalization, NT‐proBNP, and AF were important in elevated SBP patients (Table 3 ). As was shown in the result, NT‐proBNP, which is a well‐established prognostic marker in HFpEF patients, 32 was significantly associated with prognosis in both populations. Although the prognostic factors for elevated SBP patients coincided with well‐known prognostic factors for HFpEF patients in general, 13 , 33 RV‐PA uncoupling was a prominent negative prognostic factor in the preserved SBP patients. Ghio et al. reported that RV‐PA uncoupling was a reliable prognostic factor in all HF patients with reduced, preserved, and mid‐range LVEF. 34 Recently, we reported that the TAPSE/PASP ratio was an independent predictor of adverse outcomes in the whole cohort of the PURSUIT‐HFpEF registry. 35 In the present study, it should be noted that RV‐PA uncoupling was a strong prognostic factor, particularly in HFpEF patients with preserved admission SBP, and was not a strong prognostic factor in those with elevated SBP. Based on these findings, further investigation is needed to assess phenotype‐specific therapeutic strategies such as intervention in AF in HFpEF patients with elevated admission SBP and in RV‐PA uncoupling in patients with preserved admission SBP.
Limitations
Several limitations of this study should be noted. First, although we put some emphasis on the importance of RV‐PA uncoupling, we analysed this in cases whose TAPSE/PASP ratio was calculated with obtained echocardiographic parameters. Among 584 elevated SBP patients, 137 (23.5%) were excluded due to missing echocardiographic data (TAPSE missing, 80; PASP missing, 111; both missing, 54). Among 420 preserved SBP cases, 108 (25.7%) were excluded (TAPSE missing, 83; PASP missing, 83; both missing, 58). Second, we assessed RV function only by echocardiography, although cardiac magnetic resonance imaging is considered the gold standard for RV functional assessment. Moreover, RV function was assessed only by TAPSE and two‐dimensional RV dimension, and other parameters such as three‐dimensional measurement, fractional area change, RV S′, and RV global/free wall systolic strain were not assessed. Third, cardiac sonographers were not blinded to clinical information, which may have caused a degree of measurement bias. Fourth, because admission SBP was measured in a wide range of time between admission and 48 h after admission, we could not ensure that there was no pharmacological intervention prior to the admission SBP measurement. Further investigations are required to confirm the results of this study and to support understanding of the importance of SBP on admission in HFpEF patients.
Conclusions
We showed in a multicentre observational cohort study that preserved SBP on admission was associated with a higher risk of adverse outcomes than elevated SBP in HFpEF patients. NT‐proBNP elevation was important in estimating prognosis in both groups. Previous HF history and AF were important for HFpEF patients with elevated SBP, whereas RV‐PA uncoupling as shown by the TAPSE/PASP ratio was an important prognostic indicator for HFpEF patients with preserved SBP.
Conflict of interest
D.N. has received honoraria from Roche Diagnostics. S.H. has received personal fees from Daiichi Sankyo Company, Bayer, Astellas Pharma, Pfizer Pharmaceuticals, and Boehringer Ingelheim Japan and received grants from Roche Diagnostics, FUJIFILM Toyama Chemical, and Actelion Pharmaceuticals. Y.S. received personal fees from Otsuka Pharmaceutical, Ono Pharmaceutical, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation, and Actelion Pharmaceuticals and received grants form Roche Diagnostic, FUJIFILM Toyama Chemical, Abbott Medical, Japan, Otsuka Pharmaceutical, Daiichi Sankyo Company, Mitsubishi Tanabe Pharma Corporation, and Biotronik. Other authors have no conflicts of interest to disclose.
Funding
This work was supported by Roche Diagnostics K.K. (Minato‐ku, Tokyo, Japan) and Fuji Film Toyama Chemical Co. Ltd. (Chuo‐ku, Tokyo, Japan).
Supporting information
Data S1 Supporting information.
Figure S1 . Receiver operating characteristic (ROC) curve analysis for predicting the primary endpoint with admission systolic blood pressure.
Figure S2. Causes of cardiac deaths in the cohorts.
Table S1. Baseline daily activities of living on admission.
Table S2. Interactions with categorization of elevated/preserved SBP on admission in Cox regression testing for primary endpoint.
Acknowledgements
The authors thank all the investigators, clinical research coordinators, and data managers involved in the PURSUIT‐HFpEF registry for their dedicated contributions. Especially, the authors thank Nagisa Yoshioka, Kyoko Tatsumi, Satomi Kishimoto, Noriko Murakami, and Sugako Mitsuoka for their excellent assistance with data collection. We thank Libby Cone, MD, MA, from DMC Corp. (http://www.dmed.co.jp/) for editing a draft of this manuscript.
Nakagawa, A. , Yasumura, Y. , Yoshida, C. , Okumura, T. , Tateishi, J. , Yoshida, J. , Tamaki, S. , Yano, M. , Hayashi, T. , Nakagawa, Y. , Yamada, T. , Nakatani, D. , Hikoso, S. , Sakata, Y. , and Osaka CardioVascular Conference (OCVC)‐Heart Failure investigators (2021) Distinctive prognostic factor of heart failure with preserved ejection fraction stratified with admission blood pressure. ESC Heart Failure, 8: 3145–3155. 10.1002/ehf2.13420.
Clinical Trial Registration: UMIN000021831 <https://upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000024414>; PURSUIT‐HFpEF.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Lee TT, Chen J, Cohen DJ, Tsao L. The association between blood pressure and mortality in patients with heart failure. Am Heart J 2006; 151: 76–83. [DOI] [PubMed] [Google Scholar]
- 3. Raphael CE, Whinnett ZI, Davies JE, Fontana M, Ferenczi EA, Manisty CH, Mayet J, Francis DP. Quantifying the paradoxical effect of higher systolic blood pressure on mortality in chronic heart failure. Heart (British Cardiac Society) 2009; 95: 56–62. [DOI] [PubMed] [Google Scholar]
- 4. Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006; 296: 2217–2226. [DOI] [PubMed] [Google Scholar]
- 5. Fonarow GC, Adams KF Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA 2005; 293: 572–580. [DOI] [PubMed] [Google Scholar]
- 6. Siirilä‐Waris K, Lassus J, Melin J, Peuhkurinen K, Nieminen MS, Harjola VP. Characteristics, outcomes, and predictors of 1‐year mortality in patients hospitalized for acute heart failure. Eur Heart J 2006; 27: 3011–3017. [DOI] [PubMed] [Google Scholar]
- 7. Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, Stevenson LW. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003; 41: 1797–1804. [DOI] [PubMed] [Google Scholar]
- 8. Stevenson LW. Design of therapy for advanced heart failure. Eur J Heart Fail 2005; 7: 323–331. [DOI] [PubMed] [Google Scholar]
- 9. Buiciuc O, Rusinaru D, Lévy F, Peltier M, Slama M, Leborgne L, Tribouilloy C. Low systolic blood pressure at admission predicts long‐term mortality in heart failure with preserved ejection fraction. J Card Fail 2011; 17: 907–915. [DOI] [PubMed] [Google Scholar]
- 10. Tsimploulis A, Lam PH, Arundel C, Singh SN, Morgan CJ, Faselis C, Deedwania P, Butler J, Aronow WS, Yancy CW, Fonarow GC, Ahmed A. Systolic blood pressure and outcomes in patients with heart failure with preserved ejection fraction. JAMA Cardiol 2018; 3: 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gheorghiade M, De Luca L, Fonarow GC, Filippatos G, Metra M, Francis GS. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol 2005; 96: 11g–17g. [DOI] [PubMed] [Google Scholar]
- 12. Mebazaa A, Gheorghiade M, Piña IL, Harjola VP, Hollenberg SM, Follath F, Rhodes A, Plaisance P, Roland E, Nieminen M, Komajda M, Parkhomenko A, Masip J, Zannad F, Filippatos G. Practical recommendations for prehospital and early in‐hospital management of patients presenting with acute heart failure syndromes. Crit Care Med 2008; 36: S129–S139. [DOI] [PubMed] [Google Scholar]
- 13. Komajda M, Carson PE, Hetzel S, McKelvie R, McMurray J, Ptaszynska A, Zile MR, Demets D, Massie BM. Factors associated with outcome in heart failure with preserved ejection fraction: findings from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I‐PRESERVE). Circ Heart Fail 2011; 4: 27–35. [DOI] [PubMed] [Google Scholar]
- 14. Hamatani Y, Nagai T, Shiraishi Y, Kohsaka S, Nakai M, Nishimura K, Kohno T, Nagatomo Y, Asaumi Y, Goda A, Mizuno A, Yasuda S, Ogawa H, Yoshikawa T, Anzai T. Long‐term prognostic significance of plasma B‐type natriuretic peptide level in patients with acute heart failure with reduced, mid‐range, and preserved ejection fractions. Am J Cardiol 2018; 121: 731–738. [DOI] [PubMed] [Google Scholar]
- 15. Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, Yamamoto K. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circulation journal: official journal of the Japanese Circulation Society 2013; 77: 705–711. [DOI] [PubMed] [Google Scholar]
- 16. Suna S, Hikoso S, Yamada T, Uematsu M, Yasumura Y, Nakagawa A, Takeda T, Kojima T, Kida H, Oeun B, Sunaga A, Kitamura T, Dohi T, Okada K, Mizuno H, Nakatani D, Iso H, Matsumura Y, Sakata Y. Study protocol for the PURSUIT‐HFpEF study: a Prospective, Multicenter, Observational Study of Patients with Heart Failure with Preserved Ejection Fraction. BMJ Open 2020; 10: e038294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vasan RS, Levy D. Defining diastolic heart failure: a call for standardized diagnostic criteria. Circulation 2000; 101: 2118–2121. [DOI] [PubMed] [Google Scholar]
- 18. Matsumura Y, Hattori A, Manabe S, Takahashi D, Yamamoto Y, Murata T, Nakagawa A, Mihara N, Takeda T. Case report form reporter: a key component for the integration of electronic medical records and the electronic data capture system. Stud Health Technol Inform 2017; 245: 516–520. [PubMed] [Google Scholar]
- 19. Shimura T, Yamamoto M, Kano S, Kagase A, Kodama A, Koyama Y, Tsuchikane E, Suzuki T, Otsuka T, Kohsaka S, Tada N, Yamanaka F, Naganuma T, Araki M, Shirai S, Watanabe Y, Hayashida K. Impact of the clinical frailty scale on outcomes after transcatheter aortic valve replacement. Circulation 2017; 135: 2013–2024. [DOI] [PubMed] [Google Scholar]
- 20. Ling HZ, Flint J, Damgaard M, Bonfils PK, Cheng AS, Aggarwal S, Velmurugan S, Mendonca M, Rashid M, Kang S, Papalia F, Weissert S, Coats CJ, Thomas M, Kuskowski M, Cohn JN, Woldman S, Anand IS, Okonko DO. Calculated plasma volume status and prognosis in chronic heart failure. Eur J Heart Fail 2015; 17: 35–43. [DOI] [PubMed] [Google Scholar]
- 21. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 22. Torii Y, Kusunose K, Yamada H, Nishio S, Hirata Y, Amano R, Yamao M, Zheng R, Saijo Y, Yamada N, Ise T, Yamaguchi K, Yagi S, Soeki T, Wakatsuki T, Sata M. Updated left ventricular diastolic function recommendations and cardiovascular events in patients with heart failure hospitalization. J Am Soc Echocardiogr 2019; 32: 1286–1297.e1282. [DOI] [PubMed] [Google Scholar]
- 23. Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014; 35: 3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mohammed SF, Hussain I, AbouEzzeddine OF , Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM. Right ventricular function in heart failure with preserved ejection fraction: a community‐based study. Circulation 2014; 130: 2310–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gorter TM, Hoendermis ES, van Veldhuisen DJ, Voors AA, Lam CS, Geelhoed B, Willems TP, van Melle JP. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Eur J Heart Fail 2016; 18: 1472–1487. [DOI] [PubMed] [Google Scholar]
- 26. Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ. Phenotype‐specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 2016; 134: 73–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Luca L, Fonarow GC, Adams KF Jr, Mebazaa A, Tavazzi L, Swedberg K, Gheorghiade M. Acute heart failure syndromes: clinical scenarios and pathophysiologic targets for therapy. Heart Fail Rev 2007; 12: 97–104. [DOI] [PubMed] [Google Scholar]
- 28. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo‐Leiro MG, Laroche C, Seferovic PM, Anker SD, Ferrari R, Ruschitzka F, Lopez‐Fernandez S, Miani D, Filippatos G, Maggioni AP. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 1242–1254. [DOI] [PubMed] [Google Scholar]
- 29. Kajimoto K, Sato N, Sakata Y, Takano T. Relationship between systolic blood pressure and preserved or reduced ejection fraction at admission in patients hospitalized for acute heart failure syndromes. Int J Cardiol 2013; 168: 4790–4795. [DOI] [PubMed] [Google Scholar]
- 30. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol 2006; 47: 76–84. [DOI] [PubMed] [Google Scholar]
- 31. Sato Y, Yoshihisa A, Oikawa M, Nagai T, Yoshikawa T, Saito Y, Yamamoto K, Takeishi Y, Anzai T. Relation of systolic blood pressure on the following day with post‐discharge mortality in hospitalized heart failure patients with preserved ejection fraction. Int Heart J 2019; 60: 876–885. [DOI] [PubMed] [Google Scholar]
- 32. Jhund PS, Anand IS, Komajda M, Claggett BL, McKelvie RS, Zile MR, Carson PE, McMurray JJ. Changes in N‐terminal pro‐B‐type natriuretic peptide levels and outcomes in heart failure with preserved ejection fraction: an analysis of the I‐Preserve study. Eur J Heart Fail 2015; 17: 809–817. [DOI] [PubMed] [Google Scholar]
- 33. Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community‐based study. Circulation 2013; 128: 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, Temporelli PL, Rossi A, Faggiano P, Traversi E, Vriz O, Dini FL. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail 2017; 19: 873–879. [DOI] [PubMed] [Google Scholar]
- 35. Nakagawa A, Yasumura Y, Yoshida C, Okumura T, Tateishi J, Yoshida J, Abe H, Tamaki S, Yano M, Hayashi T, Nakagawa Y, Yamada T, Nakatani D, Hikoso S, Sakata Y. Prognostic importance of right ventricular‐vascular uncoupling in acute decompensated heart failure with preserved ejection fraction. Circ Cardiovasc Imaging 2020; 13: e011430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting information.
Figure S1 . Receiver operating characteristic (ROC) curve analysis for predicting the primary endpoint with admission systolic blood pressure.
Figure S2. Causes of cardiac deaths in the cohorts.
Table S1. Baseline daily activities of living on admission.
Table S2. Interactions with categorization of elevated/preserved SBP on admission in Cox regression testing for primary endpoint.


