Abstract
Aims
Improved cancer survivorship has led to a higher number of anthracycline‐induced cardiomyopathy patients with end‐stage heart failure. We hypothesize that outcomes following continuous‐flow LVAD (CF‐LVAD) implantation in those with anthracycline‐induced cardiomyopathy are comparable with other aetiologies of cardiomyopathy.
Methods and results
Using the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) from 2008 to 2017, we identified patients with anthracycline‐induced cardiomyopathy who received a CF‐LVAD and compared them with those with idiopathic dilated (IDM) and ischaemic cardiomyopathies (ICM). Mortality was studied using the Cox proportional hazards model. Other adverse events were evaluated using competing risk models. Overall, 248 anthracycline‐induced cardiomyopathy patients underwent CF‐LVAD implantation, with a median survival of 48 months, an improvement compared with those before 2012 [adjusted hazards ratio (aHR): 0.53; confidence interval (CI): 0.33–0.86]. At 12 months, 85.1% of anthracycline‐induced cardiomyopathy, 86.0% of IDM, and 80.2% of ICM patients were alive (anthracycline‐induced cardiomyopathy vs. IDM: aHR: 1.12; CI: 0.88–1.43 and anthracycline‐induced cardiomyopathy vs. ICM: aHR: 0.98; CI: 0.76–1.28). Anthracycline‐induced cardiomyopathy patients had a higher major bleeding risk compared with IDM patients (aHR: 1.23; CI: 1.01–1.50), and a lower risk of stroke and prolonged respiratory support compared to ICM patients (aHR: 0.31 and 0.67 respectively; both P < 0.05). There was no difference in the risk of major infection, acute kidney injury, and venous thromboembolism.
Conclusions
After receiving a CF‐LVAD, survival in patients with anthracycline‐induced cardiomyopathy is similar to those with ICM or IDM. Further research into differential secondary endpoints‐related disparities is warranted.
Keywords: INTERMACS, Anthracycline, Continuous‐flow LVAD, Bleeding, Stroke, Mortality
Introduction
Anthracyclines are a class of antineoplastic agents that are the most common cause of chemotherapy‐induced cardiomyopathy. When used as a part of treatment regimens, anthracyclines pose a dose‐dependent risk of cardiomyopathy. 1 Left ventricular dysfunction and heart failure (HF) develop in about 10% of those who receive anthracycline therapy, and 0.5–2.5% of these patients develop end‐stage systolic HF. 2 In a landmark study by Felker et al., 3 anthracycline‐induced cardiomyopathy patients had a two to three times higher risk of mortality than those with non‐ischaemic cardiomyopathy. More recently, the 5 year survival rate of anthracycline‐induced cardiomyopathy has significantly improved compared with that of non‐ischaemic cardiomyopathies at around 91%, 4 which may be due to the increasing use of goal‐directed medical therapy (GDMT) for systolic HF. 5 However, the use of advanced HF therapies for anthracycline‐induced cardiomyopathy patients has been limited. Historically, anthracycline‐induced cardiomyopathy patients were considered too high‐risk for a heart transplant or left ventricular assist device (LVAD) placement due to concerns for cancer recurrence. 3 Additionally, anthracycline‐induced cardiomyopathy patients were more prone to adverse events like infection and overall poor survival. 3
Notably, the prevalence of cancer survivors is expected to increase to 20 million by 2026 6 ; thus, it stands to reason that significantly more of these patients are likely going to require advanced HF therapies. In a study using the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) spanning from 2006 to 2011 by Oliveira et al., 7 anthracycline‐induced cardiomyopathy was associated with a higher incidence of right ventricular failure when compared with idiopathic dilated cardiomyopathy (IDM) undergoing LVAD placement. The small sample size and the inclusion of pulsatile flow LVADs limited this study's application to current practice. 8 Additionally, GDMT use and its effect 9 were not quantified, and the small sample size did not allow for subgroup analysis, thus missing disparities.
Given these limitations, contemporary analysis of the INTERMACS registry spanning from 2008 to 2017 was conducted. We hypothesize that outcomes following continuous‐flow LVAD implantation in those with anthracycline‐induced cardiomyopathy are comparable with other aetiologies of cardiomyopathy.
Methods
Data source
We used INTERMACS, a prospective registry that includes baseline and follow‐up information on individuals who receive Food and Drug Administration approved LVADs in the USA and Canada. We obtained the publicly available data from the Biological Specimen and Data Repository Information Coordinating Center. This study was approved by the Yale University Institutional Review Board.
Study population and definitions
We included all ≥18 years old who received their first continuous‐flow LVAD from January 2008 to June 2017. To evaluate a homogenous cohort of devices, we excluded patients who received non‐continuous‐flow types of devices or those who concomitantly received right ventricular support for right ventricular dysfunction or had more than one listed predominant aetiology for cardiomyopathy. We classified individuals based on their primary diagnosis into three groups: anthracycline‐induced cardiomyopathy, IDM, and ICM. The dataset design is detailed in Figure 1 .
Figure 1.
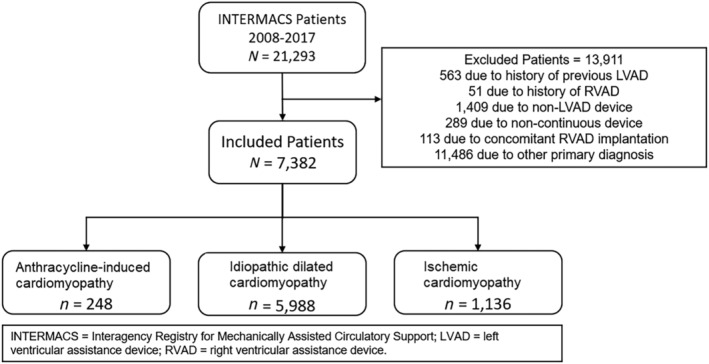
Consort diagram showing the derivation of final INTERMACS dataset.
Outcomes
The primary outcome of this study evaluated mortality rates between anthracycline‐induced cardiomyopathy, IDM, and ICM. The secondary endpoints included infection, ischaemic stroke, major bleeding, a requirement for prolonged respiratory support, acute kidney injury, venous thromboembolism, and delayed right ventricular (RV) failure after implant, which were defined using prior contemporary INTERMACS analyses. 10 , 11 , 12 , 13 , 14 , 15 , 16
Statistical analysis
Categorical variables were compared using the χ 2 test and described using frequency and percentage. Continuous variables were compared using the Wilcoxon rank‐sum test and described using a median and interquartile range. To compare survival probability by HF aetiology, we used Kaplan–Meier survival estimates and log‐rank test. We used the unadjusted and adjusted Cox proportional hazards model to evaluate each aetiology's association with mortality. Consistent with previous INTERMAC studies, 9 , 16 our adjusted models included early hazards of mortality identified by the eighth annual INTERMACS report [age, sex, body mass index, implantable cardioverter‐defibrillator (ICD), INTERMACS profile 1 or 2, albumin, dialysis, blood urea nitrogen, total bilirubin, history of cardiac surgery, and concomitant cardiac surgery]. 15 Proportional hazards assumption was checked and met in the primary analysis. The secondary outcomes were analysed using adjusted Fine–Gray competing risk analysis accounting for competing risk of death, transplant, or explant, whichever happened first.
Pre‐specified subgroup analyses were performed on the male gender, female gender, white race, black race, Hispanic ethnicity, and pre‐transplant GDMT. Proportional hazards assumption for all variables were checked and met in all subgroups except the black race, where the extended Cox model was utilized for all outcomes. Pre‐LVAD GDMT was divided into triple therapy, dual therapy, monotherapy, and no therapy groups. Triple therapy included a beta‐blocker, either an angiotensin receptor blocker/angiotensin‐converting enzyme inhibitor and an aldosterone receptor antagonist pre‐LVAD placement. Dual therapy required any two, and monotherapy required any of these agents to be present in the patient's medication list.
Sensitivity analysis for primary and secondary endpoint was performed using bridge‐to‐transplant (BTT) cohort, destination therapy (DT) cohort, and those who received continuous flow LVAD's before 2012 to simulate the time period of Oliveira et al. 7 analysis.
For all analyses, P < 0.05 was considered statistically significant. All statistical analyses were performed in Stata 16.1 SE (StataCorp, College Station, TX) and SAS 9.4 (Cary, NC).
Results
From 2008 to 2017, we identified 248 patients with dilated non‐ischaemic cardiomyopathy due to anthracycline therapy who had continuous flow LVADs implantation, as well as 5998 LVADs implanted in those with IDM and 1136 in those with ICM.
Regarding patient demographics, anthracycline‐induced cardiomyopathy patients were much more likely to be female (71.8%) compared with those with IDM (23.5%; P < 0.001) and ICM (15.3%; P < 0.001). They were also less likely to have chronic kidney disease (10.3%; both P < 0.001) compared with those with IDM (18.7%) and ICM patients (19.7%). Index hospitalization, co‐morbidities, lab, haemodynamic profile, and procedures by aetiology are listed in Table 1 . Based on therapy intent, anthracycline‐induced cardiomyopathy patients had lower use of LVAD for BTT therapy (52.0%) compared with IDM (62.9%; P = 0.03). The proportion of patients with NYHA class IV functional status was similar across HF subtypes [anthracycline‐induced cardiomyopathy 80.6%, IDM 78.0%, and ICM 74.1%, cohorts (all comparison P > 0.05)]. Considering those with INTERMACS 1 profiles, the anthracycline‐induced cardiomyopathy arm (14.9%) had a similar proportion of patients compared with the IDM arm (13.4%, P = 0.26) and a lower proportion of patients compared to the ICM arm (25.1%, P < 0.001) of the study.
Table 1.
Baseline characteristics
| Dilated, anthracycline | Dilated, idiopathic | P‐value | ICM | P‐value | |
|---|---|---|---|---|---|
| N = 248 | N = 5998 | N = 1136 | |||
| Age (years), median (IQR) | 54 (44–63) | 55 (45–64) | 0.21 | 62 (55–68) | <0.001 |
| Female sex, n (%) | 178 (71.8) | 1407 (23.5) | <0.001 | 174 (15.3) | <0.001 |
| White race, n (%) | 158 (63.7) | 3325 (55.4) | 0.01 | 917 (80.7) | <0.001 |
| Hispanic ethnicity, n (%) | 17 (6.9) | 382 (6.4) | 0.76 | 83 (7.3) | 0.8 |
| Body mass index (kg/m2), median (IQR) | 25.2 (22.25–29.8) | 28.3 (24.2–33.3) | <0.001 | 27.7 (24.2–32.1) | <0.001 |
| BMI ≥ 30 kg/m2, n (%) | 58 (23.4) | 2463 (41.1) | <0.001 | 404 (35.6) | <0.001 |
| Co‐morbidities, n (%) | |||||
| Previous cardiac surgery | 27 (10.9) | 761 (12.7) | 0.4 | 541 (47.6) | <0.001 |
| Peripheral vascular disease | 3 (1.2) | 104 (1.8) | 0.54 | 93 (8.4) | <0.001 |
| Atrial arrhythmia | 23 (14.1) | 957 (22.0) | 0.017 | 145 (20.7) | 0.056 |
| Ischaemic heart disease | 4 (1.6) | 188 (3.1) | 0.17 | 1136 (100.0) | <0.001 |
| Chronic lung disease | 8 (3.3) | 368 (6.2) | 0.061 | 93 (8.3) | 0.007 |
| Pulmonary hypertension | 39 (16.5) | 1164 (19.9) | 0.21 | 203 (18.7) | 0.44 |
| Current smoker | 6 (2.5) | 236 (4.0) | 0.23 | 96 (8.6) | 0.001 |
| Chronic kidney disease | 25 (10.3) | 1109 (18.7) | <0.001 | 220 (19.7) | <0.001 |
| Prior stroke | 4 (1.7) | 166 (2.8) | 0.28 | 28 (2.5) | 0.42 |
| NYHA classification, n (%) | |||||
| III | 33 (13.3) | 1025 (17.1) | 0.47 | 179 (15.8) | 0.23 |
| IV | 200 (80.6) | 4676 (78.0) | 842 (74.1) | ||
| INTERMACS profile, n (%) | |||||
| 1 | 37 (14.9) | 802 (13.4) | 0.26 | 285 (25.1) | <0.001 |
| 2 | 104 (41.9) | 2329 (38.8) | 335 (29.5) | ||
| 3 | 83 (33.5) | 2037 (34.0) | 336 (29.6) | ||
| Index hospitalization events prior to implant, n (%) | |||||
| ECMO | 4 (1.6) | 110 (1.8) | 0.8 | 80 (7.0) | 0.001 |
| IABP | 41 (16.5) | 1147 (19.1) | 0.31 | 228 (20.1) | 0.2 |
| Cardiac arrest | 4 (1.6) | 176 (2.9) | 0.22 | 139 (12.2) | <0.001 |
| Dialysis | 3 (1.2) | 132 (2.2) | 0.29 | 38 (3.3) | 0.072 |
| Major infection | 17 (6.9) | 306 (5.1) | 0.22 | 102 (9.0) | 0.28 |
| Major myocardial infarction | 0 (0.0) | 17 (0.3) | 0.4 | 175 (15.4) | <0.001 |
| Ultrafiltration | 1 (0.4) | 39 (0.7) | 0.63 | 8 (0.7) | 0.59 |
| Inotrope support | 221 (89.1) | 5110 (85.2) | 0.087 | 883 (77.7) | <0.001 |
| Device strategy, n (%) | |||||
| Bridge to recovery | 3 (1.2) | 16 (0.3) | 0.008 | 5 (0.4) | 0.15 |
| Bridge to transplant | 139 (56.0) | 3771 (62.9) | 0.03 | 597 (52.6) | 0.32 |
| Destination therapy | 105 (42.3) | 2205 (36.8) | 0.075 | 520 (45.8) | 0.32 |
| Laboratory values | |||||
| Sodium (meq/L), median (IQR) | 134 (132–137) | 135 (132–138) | 0.068 | 136 (133–138) | <0.001 |
| Potassium (meq/L), median (IQR) | 4.1 (3.7–4.4) | 4 (3.7–4.4) | 0.64 | 4.1 (3.8–4.3) | 0.81 |
| Creatinine (mg/dL), median (IQR) | 1.1 (.865–1.5) | 1.29 (1–1.6) | <0.001 | 1.26 (1–1.6) | <0.001 |
| BUN (mg/dL), median (IQR) | 22 (15–32) | 24 (17–35) | 0.007 | 26.5 (19–37) | <0.001 |
| BNP (ng/L), median (IQR) | 1180 (543–1857) | 867.5 (413–1662) | 0.032 | 843 (441.5–1495.5) | 0.026 |
| Bilirubin (mg/dL), median (IQR) | 1 (.6–1.5) | 1.1 (.7–1.7) | 0.055 | .9 (.6–1.5) | 0.48 |
| Platelet count (×103/μL), median (IQR) | 201 (152–264.5) | 190 (147–242) | 0.028 | 179.5 (132–236) | <0.001 |
| INR, median (IQR) | 1.2 (1.1–1.3) | 1.2 (1.1–1.4) | 0.002 | 1.2 (1.1–1.4) | 0.026 |
| Haemodynamic variables | |||||
| SBP (mmHg), median (IQR) | 101 (90–113) | 103 (94–113) | 0.044 | 104 (94–116) | 0.008 |
| DBP (mmHg), median (IQR) | 64 (57–72) | 65 (58–72) | 0.15 | 64 (56–71) | 0.7 |
| CVP (mmHg), median (IQR) | 11 (7–16) | 10 (6–15) | 0.21 | 10 (7–14) | 0.3 |
| PWP (mmHg), median (IQR) | 24 (17–28) | 25 (19–31) | 0.01 | 24 (18–30) | 0.12 |
| CO (L/min), median (IQR) | 3.5 (2.8–4.5) | 4 (3.2–5) | <0.001 | 4.3 (3.45–5.3) | <0.001 |
| LVEF, n (%) | |||||
| 20–29% | 53 (22.5) | 1091 (18.8) | 0.15 | 357 (32.7) | 0.002 |
| <20% | 164 (69.5) | 4314 (74.5) | 590 (54.1) | ||
| Unknown | 7 (3.0) | 240 (4.2) | 50 (4.5) | ||
ICM, ischaemic cardiomyopathy.
Mortality
At 12 months, 85.1% of anthracycline‐induced cardiomyopathy, 86.0% of IDM and 80.2% of ICM patients were alive (Figure 2 A , log‐rank P‐value <0.001). A total of 77 (31.1%) anthracycline‐induced cardiomyopathy patients died with a median time to death of 12.0 months (Figure 2 A ), compared with 1638 (27.3%) in IDM and 443 (39.0%) ICM arm during the study period. The most common cause of death was a neurological event among anthracycline‐induced cardiomyopathy patients (N = 15, 19.5%).
Figure 2.
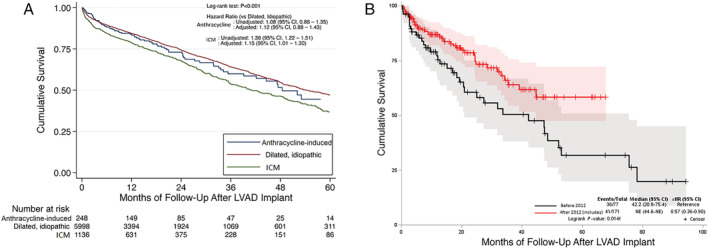
(A) Kaplan–Meier curves showing survival among those with anthracycline induced cardiomyopathy, dilated idiopathic cardiomyopathy, and ischaemic cardiomyopathy. (B) Kaplan–Meier curves showing survival among those with anthracycline induced cardiomyopathy implanted before 2012 and after 2012. aHR, adjusted hazards ratio; ICM, ischaemic cardiomyopathy.
The adjusted mortality risk for those with anthracycline‐induced cardiomyopathy was similar compared to those with IDM [adjusted hazards ratio (aHR): 1.12; 95% confidence interval (CI): 0.88–1.43] and ICM (aHR: 0.98; CI: 0.76–1.28). Similar mortality outcomes were noted in the BTT subgroup across the three difference cardiomyopathy types (Supporting Information, Figure S1 A). However, there was no difference in mortality between the three types of cardiomyopathies in the DT subgroup (Supporting Information, Figure S1 B). Additionally, there was no difference in survival observed in anthracycline‐induced cardiomyopathy vs. IDM in those who underwent continuous‐flow LVAD placement before 2012 (aHR: 1.38; CI: 0.98–1.94; Supporting Information, Figure S1 C). There has been a reduction in mortality between those implanted before 2012 and those after 2012 for anthracycline‐induced HF patients (aHR: 0.57; CI: 0.36–0.90; Figure 2 B ).
In pre‐specified subgroup analysis (Supporting Information, Table S1 ), no survival difference was noted between gender, race, or Hispanic ethnicity between anthracycline‐induced cardiomyopathy vs. either IDM or ICM.
When only anthracycline‐induced cardiomyopathy patients are considered (Table 2 ), there is improved overall mortality noted in those with GDMT compared with no pre‐LVAD GDMT (aHR: 0.44; CI: 0.19–0.99). In a sensitivity analysis, the survival benefit remains significant in the BTT arm (aHR: 0.39; CI: 0.17–0.93) and the DT arm (aHR: 0.44; CI: 0.19–0.99). Specific comparisons between the number of GDMT medications (one, two, or three drugs or no drug therapy) was not significant. There was no difference in survival between genders or among various races and ethnicity (Table 2 ) in the entire anthracycline‐induced cardiomyopathy cohort as well as the BTT and DT sensitivity analysis.
Table 2.
Adjusted hazards ratio of specific subgroups of only those with left ventricular assist device placement due to anthracycline induced cardiomyopathy from 2008 to 2017
| Variable | All LVADS | BTT | Destination therapy | |||
|---|---|---|---|---|---|---|
| Adjusted hazards ratio | P‐value | Adjusted hazards ratio | P‐value | Adjusted hazards ratio | P‐value | |
| Female vs. male gender | 0.92 (0.55–1.55) | 0.76 | 1.35 (0.54–3.40) | 0.53 | 0.59 (0.28–1.27) | 0.18 |
| Race | 0.67 | 0.70 | 0.31 | |||
| Black vs. White | 0.86 (0.53–1.40) | 0.71 (0.29–1.76) | 1.71 (0.76–3.83) | |||
| Other vs. White | 0.68 (0.24–1.89) | 0.59 (0.08–4.52) | 0.69 (0.19–2.45) | |||
| Hispanic vs. non‐Hispanic | 1.59 (0.62–4.08) | 0.34 | 6.72 (1.34–33.73) | 0.02 | 1.11 (0.29–4.33) | 0.88 |
| Neurohormonal blocker any vs. none prior to LVAD | 0.54 (0.33–0.91) | 0.02 | 0.39 (0.17–0.93) | 0.03 | 0.44 (0.19–0.99) | 0.047 |
| 3 drug vs. none | 0.65 (0.30–1.37) | 0.26 | 0.50 (0.14–1.87) | 0.07 | 0.53 (0.17–1.63) | 0.27 |
| 2 drug vs. none | 0.55 (0.30–1.01) | 0.053 | 0.38 (0.14–1.87) | 0.30 | 0.46 (0.17–1.21) | 0.11 |
| 1 drug vs. none | 0.49 (0.25–0.95) | 0.04 | 0.36 (0.11–1.12) | 0.08 | 0.39 (0.15–0.99) | 0.048 |
Adjusted models included early hazards of mortality identified by the eighth annual INTERMACS report. 15
BTT, bridge to transplant; LVAD, left ventricular assist device.
First infection
Overall, 130 (52.4%) anthracycline‐induced cardiomyopathy patients had an infection during the follow‐up period with a median time to event of 7.6 months. There was no difference in risk of infection among anthracycline‐induced cardiomyopathy patients when compared with IDM (aHR: 1.10; CI: 0.91–1.34) or ICM (aHR: 1.03; CI: 0.84–1.28) patients when accounting for competing risks (Figure 3 A ). Additionally, there was no difference in infection risk among the HF subtypes in the pre‐specified subgroup analysis by gender, race, ethnicity, and GDMT use (Supporting Information, Table S2 ), as well as among the HF subtypes in the sensitivity analysis arms of BTT, DT, or those who received LVADs before 2012.
Figure 3.
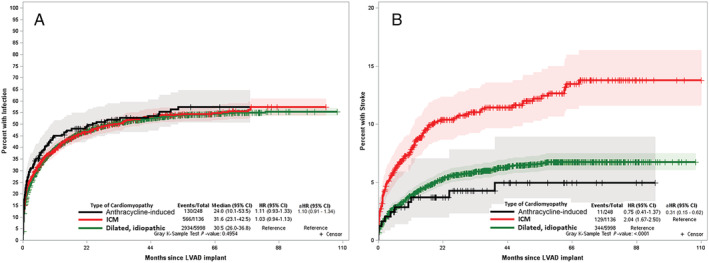
Cumulative incidence function curve using Fine–Grey competing risk model comparing hazards of first infection (A) and ischaemic stroke (B) in anthracycline induced cardiomyopathy, dilated idiopathic cardiomyopathy, and ischaemic cardiomyopathy accounting for competing risk of death. aHR, adjusted hazards ratio; ICM, ischaemic cardiomyopathy.
Ischaemic stroke
Among anthracycline‐induced cardiomyopathy patients, 11 (4.4%) had an ischaemic stroke event during the follow‐up period, with the median time to event of 2.2 months. There was no difference in the risk of ischaemic stroke among anthracycline‐induced cardiomyopathy patients when compared with IDM (aHR: 0.57; CI: 0.29–1.11); however, the risk was lower when compared with ICM (aHR: 0.31; CI: 0.15–0.62) patients when accounting for competing risks (Figure 3 B ).
There was no difference in stroke risk among anthracycline‐induced cardiomyopathy vs. IDM groups in the pre‐specified subgroup analysis by gender, race, ethnicity, and GDMT use, as well as the three groups in the sensitivity analysis arms of BTT, DT, and those receiving LVADs before 2012 (Supporting Information, Table S2 ). The stroke risk among anthracycline‐induced cardiomyopathy vs. ICM was lower in subgroups of the female gender, both races, and GDMT use, as well as sensitivity analysis considering BTT and DT (P < 0.05). However, there was no difference in stroke risk between anthracycline‐induced cardiomyopathy and ICM in the subgroups of male gender and Hispanic ethnicity, as well as in the sensitivity analysis that considered LVADs placed before 2012.
Major bleeding
Major bleeding was relatively more common (N = 121; 48.8%) in those with anthracycline‐induced cardiomyopathy during the follow‐up with a median time to event of 2.7 months. There was an increased risk of major bleeding among anthracycline‐induced cardiomyopathy patients when compared with IDM (aHR: 1.23; CI: 1.01–1.50) but no difference compared with ICM (aHR: 1.05; CI: 0.84–1.30) patients when accounting for competing risks (Figure 4 A ).
Figure 4.
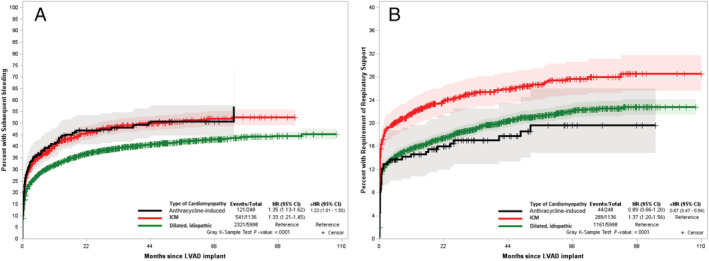
Cumulative incidence function curve using Fine–Grey competing risk model comparing hazards of major bleeding (A) and a requirement for prolonged respiratory support (B) in anthracycline induced cardiomyopathy, dilated idiopathic cardiomyopathy, and ischaemic cardiomyopathy accounting for competing risk of death. aHR, adjusted hazards ratio; ICM, ischaemic cardiomyopathy.
Bleeding risk among anthracycline‐induced cardiomyopathy vs. ICM groups was similar in the pre‐specified subgroups (Supporting Information, Table S2 ). However, the bleeding hazard among anthracycline‐induced cardiomyopathy vs. IDM was higher in the white race subgroup (aHR: 1.49; CI: 1.16–1.90) with no difference in either gender, black race, Hispanic ethnicity, or GDMT use subgroups. Similarly, there was no difference in bleeding risk noted among the three HF subtypes in the sensitivity analysis arms.
Requirement for prolonged respiratory support
Fewer anthracycline‐induced cardiomyopathy patients (17.7%) required prolonged respiratory support (Figure 4 B ), compared with 19.4% IDM and 25.4% ICM patients, respectively. There was no difference in the hazard of the requirement of prolonged respiratory support among anthracycline‐induced cardiomyopathy patients when compared with IDM (aHR: 0.87; CI: 0.63–1.20). All the subgroup analyses reflected this finding (Supporting Information, Table S2 ). However, anthracycline‐induced cardiomyopathy patients had a lower hazard of respiratory support requirement than ICM (aHR: 0.67; CI: 0.47–0.94) patients when accounting for competing risks. This finding prevailed in females and white race subgroups (Supporting Information, Table S2 ). However, no such association was noted in the male, black race, Hispanic ethnicity, or GDMT use subgroups. Similarly, there was no difference in respiratory support noted among the three heart failure subtypes in the sensitivity analysis arms of BTT, DT, and those getting LVADs before 2012.
Acute kidney injury
Overall, 32 (12.9%) anthracycline‐induced cardiomyopathy patients had acute kidney injury (AKI) during the follow‐up period with a median time to event of 3.4 months. There was a similar risk of acute kidney injury among anthracycline‐induced cardiomyopathy patients when compared with IDM (aHR: 1.15; CI: 0.78–1.68) and ICM (aHR: 0.92; CI: 0.61–1.39) patients when accounting for competing risks (Figure 5 A ). Further, there were no differences in either gender, either race, Hispanic ethnicity, or GDMT use subgroups, as well as among the three HF subtypes in the sensitivity analysis arms of BTT, DT, and those getting LVADs before 2012 (Supporting Information, Table S2 ).
Figure 5.
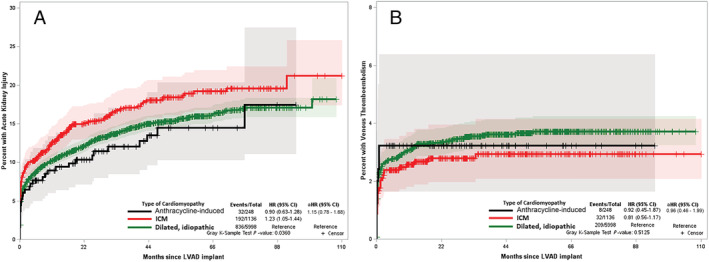
Cumulative incidence function curve using Fine–Grey competing risk model comparing hazards of acute kidney injury (A) and venous thromboembolism (B) in anthracycline induced cardiomyopathy, dilated idiopathic cardiomyopathy, and ischaemic cardiomyopathy accounting for competing risk of death. aHR, adjusted hazards ratio; ICM, ischaemic cardiomyopathy.
Venous thromboembolism
There were significantly fewer venous thromboembolism (VTE) events (anthracycline‐induced cardiomyopathy: 8 (3.2%), IDM: 209 (3.5%), and ICM: 32 (2.8%), Figure 5 B ). There was no difference in the hazard of venous thromboembolism among anthracycline‐induced cardiomyopathy patients when compared with IDM (aHR: 0.96; CI: 0.46–1.99) or ICM (aHR: 1.35; CI: 0.60–3.06) patients when accounting for competing risks. No subgroup or sensitivity analysis was performed due to low event rates and no difference among HF subtypes.
Delayed right ventricular failure
Right ventricular failure was seen very early following LVAD implantation in all arms. Only five anthracycline‐induced cardiomyopathy patients (1.6%) required RV support (RVS) in the first week, compared with 103 (1.2%) IDM and 27 (2.0%) ICM patients. There was no difference in the hazard of RVS among anthracycline‐induced cardiomyopathy patients when compared with IDM (aHR: 0.94; CI: 0.37–2.37) or ICM (aHR: 0.71; CI: 0.26–1.98) patients when accounting for competing risks. There was no difference in RVS among the three groups in the pre‐specified subgroup analysis by gender, race, or GDMT use. Similarly, there was no difference in risk noted among the three groups in the sensitivity analysis arms of BTT, DT, and those getting LVADs before 2012.
Discussion
This study, performed using the contemporary INTERMACS dataset from 2008 to 2017 to evaluate outcomes in anthracycline‐induced cardiomyopathy patients who underwent continuous‐flow LVAD placement, showed an overall improvement in survival rate over the study period, now comparable with the rates of ICM and IDM. Not surprisingly, patients with anthracycline‐induced cardiomyopathy who received GDMT before LVAD placement had improved overall survival rates compared with those not receiving medical therapy, which we will discuss further. Besides, we found no difference in the risk of several commonly associated LVAD complications, including first infection, AKI, VTE, and dilated RV dysfunction, in anthracycline‐induced cardiomyopathy patients relative to both control groups. Of note, anthracycline‐induced cardiomyopathy patients did have a lower risk of ischaemic stroke and prolonged respiratory support use compared with those with ICM but increased risk of major bleeding compared with those with IDM. Given these findings, with an increasing number of cancer survivors with HF, the improved survival rates found in this study are profound and support the equitable use of LVADs in patients with anthracycline‐induced cardiomyopathy.
Over the past decade, HF survival has been improving due to the wide acceptance and compliance with GDMT. Our study, conducted over 10 years, found an excellent survival rate of about 70%, with a median survival of 48.4 months, in a homogenous population of anthracycline‐induced cardiomyopathy patients undergoing continuous‐flow LVAD implantation. To our knowledge, this is the first study assessing continuous‐flow LVADs in the anthracycline‐induced cardiomyopathy population in the contemporary era. The current body of literature on anthracycline‐induced cardiomyopathy patients with LVADs is relatively sparse, and most studies cite only small numbers of LVAD implementations. Two single‐centre studies 4 , 17 found similar survival rates between their anthracycline‐induced cardiomyopathy and IDM populations. In the largest study comparing anthracycline‐induced cardiomyopathy patients who underwent mechanical circulatory support implantation, Oliveira et al. 7 utilized the INTERMACS database to evaluate 75 patients with a heterogeneous mixture of MCS devices, including pulsatile flow LVADs, continuous flow LVADs, right ventricular assist devices, and biventricular assist devices. The authors found a survival rate of about 76% and, contrary to this study, did not a priori exclude RV dysfunction, which highlights a crucial pathophysiological issue with the anthracycline‐induced cardiomyopathy population. Even though Oliveira et al. found a higher survival rate, the authors did not report the median survival rate, which is a more robust epidemiological marker.
In this study, we also sought to evaluate survival among a subset of demographics, including gender, race, and ethnicity, and there was no difference in mortality across the three cardiomyopathy types. The only subgroup in which a mortality benefit was noted was the use of any pre‐transplant GDMT in anthracycline‐induced cardiomyopathy patients. Our previous study using the INTERMACS dataset showed a reduction in mortality with GDMT use regardless of the cardiomyopathy type, and these findings persisted in the anthracycline‐induced cardiomyopathy population. 9 Multiple prior studies have demonstrated that the effect of GDMT for primary and secondary HF prevention in those receiving anthracycline has shown equivocal to modest benefit. 18 , 19 , 20 , 21 , 22 The present research indicates that GDMT use in patients with anthracycline‐induced cardiomyopathy is beneficial. It also points towards the increasing awareness of cardio‐oncology as a specialty, organization of cardio‐oncology services, and special care for patients with anthracycline‐induced cardiomyopathy. 23 , 24
It is well established that the post‐LVAD implantation period is associated with specific acute and chronic complications. 15 Given the improving survival of anthracycline‐induced cardiomyopathy patients, it is critical to better understand these unique populations' complications better, as they likely have quite different underlying pathophysiology compared with the other HF subtypes. Our study found a higher risk of bleeding in the anthracycline‐induced cardiomyopathy arm relative to the IDM arm, corroborated by the Olivera et al. 7 study. In the present study, the increased bleeding prevailed only in the white patient subgroup when considering all the subgroup analyses. It has been speculated that the bleeding risk is due to a lower level of protein S 25 or chemotherapy‐induced bone marrow suppression compounded with the known bleeding risk associated with LVADs. 12 Finally, exposure to thoracic radiation therapy for cancer treatment may have potentially contributed to this bleeding risk; however, this association has not been noted in those undergoing coronary artery bypass grafting with prior mediastinal radiation. 26
Also, this study found a lower risk of ischaemic stroke in anthracycline‐induced cardiomyopathy patients relative to ICM patients. This effect persisted in women, both races, DT and BTT arms. Perhaps the lower risk of ischaemic stroke is because ICM patients have a higher risk of diffuse vasculopathy, yet the impact of anthracycline use and its downstream effects on ischaemic stroke still requires further mechanistic exploration and validation. In a multivariable analysis, Acharya et al. 10 also found a higher risk of stroke in patients with LVADs who had a primary diagnosis of ICM compared with other cardiomyopathies (P = 0.04). This suggests that it is likely that ICM has a higher likelihood of post‐CF‐LVAD stroke rather than lower hazards of stroke with anthracycline‐induced cardiomyopathy.
This study found a lower risk of prolonged respiratory support in those with anthracycline‐induced cardiomyopathy than ICM. In a study by Miller et al., 16 prolonged respiratory support was a sign of an overall sicker patient, essentially represented by the ICM cohort.
For the remainder of the commonly associated complications, there was no difference in hazard of the first infection, AKI, and VTE development within the three subtypes of cardiomyopathy studied, including the subgroup and sensitivity analyses. This lack of difference represents a general improvement in outcomes with a contemporary practice pattern in all patients undergoing CF‐LVAD implantation. This improvement includes similar patterns of use of anticoagulation post‐CF‐LVAD placement despite the aetiology of HF.
In addition to the fact that it is a retrospective analysis, our study has several limitations due to the data available in the INTERMACS dataset. No reliable data on the type or stage of cancer or if other concomitant chemotherapies or cancer treatments were included in the INTERMACS. Conceptually, as noted above, one would expect anthracycline‐induced cardiomyopathy patients to have different outcomes than IDM patients due to underlying genetic predispositions compared with ICM patients, who may have differential ischaemic risk factors. Thus, the most pragmatic approach was to compare anthracycline‐induced cardiomyopathy patients to the large cohorts of IDM and ICM patients undergoing LVAD implantation. Additionally, INTERMACS does not provide information regarding the duration of heart failure and remoteness of anthracycline exposure to the patient. Also, INTERMACS does not contain data concerning medication dosage, compliance, or the reason for medication initiation or discontinuation, and less GDMT might be an indicator of more advanced HF. Sensitivity analyses were limited by a lack of site‐level data, which prevented us from adjusting for differences in prescribing patterns and outcomes unique to the various LVAD centres. INTERMACS registry only includes patients that received a durable device, and there is a possibility that patients who did not survive implantation surgery may have been excluded.
Conclusions
Survival rates are similar in anthracycline‐induced cardiomyopathy, ICM, and IDM patients who have undergone continuous‐flow LVAD placement in the contemporary era. There is a survival benefit in anthracycline‐induced cardiomyopathy patients who receive GDMT before LVAD. These findings are essential, given an increase in cancer survivors with HF. Further research into bleeding, prolonged respiratory support, and stroke‐related disparities is warranted.
Conflict of interest
All authors declare no conflicts of interests in relation to the work presented in this manuscript.
Funding
This work was supported in part by National Cancer Institute grants P30 CA016058 and K12‐CA133250 (D.A.) grants. N.L.W. is supported by National Heart, Lung, and Blood Institute grants HL124097, HL126949, HL134354, AR070029, and AG064895. A.G. and P.J. are funded by the UH R&E. The funding source had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Supporting information
Table S1. Subgroup analysis of mortality hazards among specific classes of patients. Adjusted models included early markers of mortality identified by the eighth annual INTERMACS report (1). IDM = dilated idiopathic cardiomyopathy, ICM = ischemic cardiomyopathy.
Table S2. Adjusted Fine and Grey model for secondary outcomes by pre‐specified subgroups using competing risk of death. Adjusted models included early markers of mortality identified by the eighth annual INTERMACS report (1). IDM = dilated idiopathic cardiomyopathy, ICM = ischemic cardiomyopathy.
Figure S1. Kaplan Meier curves showing survival among those with anthracycline induced cardiomyopathy, dilated idiopathic cardiomyopathy and ischemic cardiomyopathy. CCM = anthracycline induced cardiomyopathy, aHR = adjusted hazards ratio, IDM = idiopathic dilated cardiomyopathy, ICM = ischemic cardiomyopathy.
Guha, A. , Caraballo, C. , Jain, P. , Miller, P. E. , Owusu‐Guha, J. , Clark, K. A. A. , Velazquez, E. J. , Ahmad, T. , Baldassarre, L. A. , Addison, D. , Weintraub, N. L. , and Desai, N. R. (2021) Outcomes in patients with anthracycline‐induced cardiomyopathy undergoing left ventricular assist devices implantation. ESC Heart Failure, 8: 2866–2875. 10.1002/ehf2.13362.
References
- 1. Alexander J, Dainiak N, Berger HJ, Goldman L, Johnstone D, Reduto L, Duffy T, Schwartz P, Gottschalk A, Zaret BL. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N Engl J Med 1979; 300: 278–283. [DOI] [PubMed] [Google Scholar]
- 2. Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 2009; 53: 2231–2247. [DOI] [PubMed] [Google Scholar]
- 3. Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long‐term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 2000; 342: 1077–1084. [DOI] [PubMed] [Google Scholar]
- 4. Fornaro A, Olivotto I, Rigacci L, Ciaccheri M, Tomberli B, Ferrantini C, Coppini R, Girolami F, Mazzarotto F, Chiostri M, Milli M, Marchionni N, Castelli G. Comparison of long‐term outcome in anthracycline‐related versus idiopathic dilated cardiomyopathy: a single centre experience. Eur J Heart Fail 2018; 20: 898–906. [DOI] [PubMed] [Google Scholar]
- 5. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 6. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70: 7–30. [DOI] [PubMed] [Google Scholar]
- 7. Oliveira GH, Dupont M, Naftel D, Myers SL, Yuan Y, Tang WW, Gonzalez‐Stawinski G, Young JB, Taylor DO, Starling RC. Increased need for right ventricular support in patients with chemotherapy‐induced cardiomyopathy undergoing mechanical circulatory support: outcomes from the INTERMACS Registry (Interagency Registry for Mechanically Assisted Circulatory Support). J Am Coll Cardiol 2014; 63: 240–248. [DOI] [PubMed] [Google Scholar]
- 8. Moazami N, Dembitsky WP, Adamson R, Steffen RJ, Soltesz EG, Starling RC, Fukamachi K. Does pulsatility matter in the era of continuous‐flow blood pumps? J Heart Lung Transplant 2015; 34: 999–1004. [DOI] [PubMed] [Google Scholar]
- 9. McCullough M, Caraballo C, Ravindra NG, Miller PE, Mezzacappa C, Levin A, Gruen J, Rodwin B, Reinhardt S, van Dijk D, Ali A, Ahmad T, Desai NR. Neurohormonal blockade and clinical outcomes in patients with heart failure supported by left ventricular assist devices. JAMA Cardiol 2019; 5: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Acharya D, Loyaga‐Rendon R, Morgan CJ, Sands KA, Pamboukian SV, Rajapreyar I, Holman WL, Kirklin JK, Tallaj JA. INTERMACS analysis of stroke during support with continuous‐flow left ventricular assist devices: risk factors and outcomes. JACC Heart Fail 2017; 5: 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brisco MA, Kimmel SE, Coca SG, Putt ME, Jessup M, Tang WWH, Parikh CR, Testani JM. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail 2014; 7: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bunte MC, Blackstone EH, Thuita L, Fowler J, Joseph L, Ozaki A, Starling RC, Smedira NG, Mountis MM. Major bleeding during HeartMate II support. J Am Coll Cardiol 2013; 62: 2188–2196. [DOI] [PubMed] [Google Scholar]
- 13. Holman WL, Kirklin JK, Naftel DC, Kormos RL, Desvign‐Nickens P, Camacho MT, Ascheim DD. Infection after implantation of pulsatile mechanical circulatory support devices. J Thorac Cardiovasc Surg 2010; 139: 1632, e1632–1636. [DOI] [PubMed] [Google Scholar]
- 14. Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant 2015; 34: 1495–1504. [DOI] [PubMed] [Google Scholar]
- 15. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant 2017; 36: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 16. Miller PE, Caraballo C, Ravindra NG, Mezzacappa C, McCullough M, Gruen J, Levin A, Reinhardt S, Ali A, Desai NR, Ahmad T. Clinical implications of respiratory failure in patients receiving durable left ventricular assist devices for end‐stage heart failure. Circ Heart Fail 2019; 12: e006369. [DOI] [PubMed] [Google Scholar]
- 17. Araujo‐Gutierrez R, Ibarra‐Cortez SH, Estep JD, Bhimaraj A, Guha A, Hussain I, Park MH, Torre‐Amione G, Trachtenberg BH. Incidence and outcomes of cancer treatment‐related cardiomyopathy among referrals for advanced heart failure. Cardio‐Oncology 2018; 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, Martinelli G, Veglia F, Fiorentini C, Cipolla CM. Prevention of high‐dose chemotherapy‐induced cardiotoxicity in high‐risk patients by angiotensin‐converting enzyme inhibition. Circulation 2006; 114: 2474–2481. [DOI] [PubMed] [Google Scholar]
- 19. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C, Cipolla CM. Anthracycline‐induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010; 55: 213–220. [DOI] [PubMed] [Google Scholar]
- 20. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015; 131: 1981–1988. [DOI] [PubMed] [Google Scholar]
- 21. Avila MS, Ayub‐Ferreira SM, de Barros Wanderley MR Jr, das Dores Cruz F, Goncalves Brandao SM, Rigaud VOC, Higuchi‐Dos‐Santos MH, Hajjar LA, Kalil Filho R, Hoff PM, Sahade M, Ferrari MSM, de Paula Costa RL, Mano MS, Bittencourt Viana Cruz CB, Abduch MC, Lofrano Alves MS, Guimaraes GV, Issa VS, Bittencourt MS, Bocchi EA. Carvedilol for prevention of chemotherapy‐related cardiotoxicity: the CECCY trial. J Am Coll Cardiol 2018; 71: 2281–2290. [DOI] [PubMed] [Google Scholar]
- 22. Cardinale D, Iacopo F, Cipolla CM. Cardiotoxicity of anthracyclines. Front Cardiovasc Med 2020; 7: 26–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mallouppas M, Walker JM, Guha A, Dobson R, Ghosh AK. Cardio‐oncology for the general physician: “old” and “new” cardiovascular toxicities and how to manage them. Br J Hosp Med (Lond) 2020; 81: 1–11. [DOI] [PubMed] [Google Scholar]
- 24. Guha A, Dey AK, Armanious M, Dodd K, Bonsu J, Jneid H, Abraham W, Fradley MG, Addison D. Health care utilization and mortality associated with heart failure‐related admissions among cancer patients. ESC Heart Fail 2019; 6: 733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rojnuckarin P, Uaprasert N, Akkawat B, Settapiboon R, Nanakorn T, Intragumtornchai T. Protein C, protein S and von Willebrand factor levels correlate with bleeding symptoms: a population‐based study. Haemophilia 2012; 18: 457–462. [DOI] [PubMed] [Google Scholar]
- 26. Fender EA, Chandrashekar P, Liang JJ, Dhar PR, Sio TT, Stulak JM, Lennon RJ, Slusser JP, Ashman JB, Miller RC, Herrmann J, Prasad A, Sandhu GS. Coronary artery bypass grafting in patients treated with thoracic radiation: a case–control study. Open Heart 2018; 5: e000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Subgroup analysis of mortality hazards among specific classes of patients. Adjusted models included early markers of mortality identified by the eighth annual INTERMACS report (1). IDM = dilated idiopathic cardiomyopathy, ICM = ischemic cardiomyopathy.
Table S2. Adjusted Fine and Grey model for secondary outcomes by pre‐specified subgroups using competing risk of death. Adjusted models included early markers of mortality identified by the eighth annual INTERMACS report (1). IDM = dilated idiopathic cardiomyopathy, ICM = ischemic cardiomyopathy.
Figure S1. Kaplan Meier curves showing survival among those with anthracycline induced cardiomyopathy, dilated idiopathic cardiomyopathy and ischemic cardiomyopathy. CCM = anthracycline induced cardiomyopathy, aHR = adjusted hazards ratio, IDM = idiopathic dilated cardiomyopathy, ICM = ischemic cardiomyopathy.


