Abstract
Aims
The real‐world usage of intra‐aortic balloon pump (IABP) in various cardiogenic shocks (CS) and the association with outcomes are lacking. We aimed to investigate IABP adoption in CS in a nationwide registry in China.
Methods and results
We retrospectively retrieved data of 30 106 CS patients (age 67.1 ± 14.6 years, 37.6% female patients) in the Hospital Quality Monitoring System registry from 2013 to 2016. Ischaemic heart disease was the leading cause of CS (73.9%). Hypertension, cardiomyopathy, myocarditis, valvular, and congenital heart disease were seen in 36.0%, 7.5%, 2.6%, 7.3%, and 2.4% of the population. IABP was employed in 2320 (7.7%) subjects. The association between IABP usage and primary outcome of in‐hospital mortality and secondary outcomes of expenses and lengths of stay were investigated. The patients with IABP support had similar in‐hospital mortality to those without IABP (39.6% vs. 38.3%, P = 0.226), but longer hospital‐stay [8.0 (2.0–16.0) vs. 6.0 (2.0–13.0) days, P < 0.001] and higher expenses [7.1(4.4–11.1) vs. 2.3 (0.8–5.5) 10 000RMB, P < 0.001]. IABP support was not associated with reduced mortality in the overall CS population in multivariate regression analysis [odds ratio (OR) 1.05, 95% confidence interval (CI) 0.95–1.17], except for subgroups with myocarditis (OR 0.61, 95% CI 0.39–0.95, P for interaction = 0.010) and those who did not receive the early percutaneous coronary intervention (PCI) (OR 0.86, 95% CI 0.75–0.97, P for interaction < 0.001). Similar results were further confirmed in the propensity‐score‐matched population.
Conclusions
In this nationwide registry of CS patients, IABP was not noted with improved survival but increased healthcare consumption. However, IABP appears protective in those with myocarditis or who failed to receive early PCI.
Keywords: Intra‐aortic balloon pump, Cardiogenic shock, Mortality
Introduction
Cardiogenic shock (CS), although constituting less than 1% of the total heart failure burden, is a leading cause of cardiovascular mortality. 1 Because most CS is caused by acute myocardial infarction (AMI), the majority of the data are derived from registries of AMI; only 5–8% of cases in these registries, although, presented with CS. 2 , 3 , 4 The clinical profiles and outcomes of patients with whole spectrum aetiologies of CS are infrequently reported. The intra‐aortic balloon pump (IABP) has been the most commonly used mechanical circulatory support for haemodynamic stabilization in patients with CS. However, IABP failed to show benefits in the reduction of infarct size, short‐term and long‐term morbidity or mortality in large randomized control trials in patients with acute coronary syndrome (ACS), and high risk percutaneous coronary intervention (PCI). 5 The disappointing results led to the down‐regulation of the guideline recommendation for IABP. 6 , 7 , 8 , 9 The advent of newer devices such as extracorporeal membrane oxygenation (ECMO) and ventricular assist device also provide more options for circulatory support in modern practice. However, the IABP was still used occasionally in our daily clinical practice in those with CS, as IABP is not only well established for circulatory support but also simplest, easy to implant and explant in the coronary catheterization laboratory. To date, real‐world usage of IABP in patients with CS has been largely unknown in China, especially considering the heterogeneous nature of CS and the imbalanced development of medical care across China. The present study attempted to evaluate the characteristics of patients with CS who were admitted with and without the use of IABP and to assess the efficacy of IABP in CS in a nationwide registry database.
Methods
Study database
The Hospital Quality Monitoring System (HQMS) is a mandatory patient‐level national database for hospital accreditation, authorized by the National Health Commission of the People's Republic of China. Details of the HQMS database have been previously described. 10 Each HQMS record includes demographic information, up to 10 discharge diagnosis coded with International Classification of Diseases (ICD) codes; interventional and surgical treatment information; and in‐hospital outcome information, such as death, length of stay, and total charges. During the study period (1 Jan 2013 to 31 Dec 2016), the database contains over 140 million inpatient discharge records, covering 996 tertiary hospitals. The investigation conforms with the principles outlined in the Declaration of Helsinki. The study was authorized by the HQMS Committee Board and approved by the ethics committee of Peking University First Hospital. As the retrospective nature of the analysis, no informed consent was required.
Study population, variables, and outcomes
Using the HQMS data from 2013 to 2016, a retrospective analysis of admissions including patients > 18 years with the discharge diagnosis of CS (ICD‐10 R57.000) or Killip IV (I50.900 × 016) was included. Cases with duplicate records or no available IABP in the hospitals were excluded. Demographic characteristics, underlying heart disease, comorbidities, mechanical support devices usage, and hospital characteristics were identified from the HQMS database. Specifically, the underlying heart disease and comorbidities were defined and retrieved by ICD‐10 codes as follows: hypertension (I10–11), diabetes (E10–14), chronic kidney disease (N18–19), chronic obstructive pulmonary disease (J44), stroke (I60–63), and anaemia (D46–64). The primary outcome was in‐hospital mortality, and secondary outcomes included lengths of stay and hospitalized expenses, which was also retrieved and confirmed from the HQMS database.
To further clarify the association between IABP usage and the in‐hospital outcomes in the CS population, CS subjects with no other shock state (e.g. haemorrhagic shock, hypovolemic shock, septic shock, and allergic shock) coexistent were analysed. In addition, a subanalysis of CS complicated AMI (with Killip IV diagnosis) was conducted.
Statistical analysis
Baseline characteristics were described for the overall population and each treatment group. Categorical variables were described with numbers or percentages. Continuous variables were described with means, standard deviations, medians, and interquartile ranges as appropriate. χ 2 and Student's t‐test or Mann–Whitney U‐tests were used to compare categorical and continuous variables, respectively. Mixed‐effect models were used to control for hospital‐related random effects.
Logistic regression was constructed to evaluate the associations between IABP treatment and in‐hospital mortality; adjustments were made for age, sex, underlying cardiovascular disease (myocardial infarction, hypertension, cardiomyopathy, myocarditis, valvular heart disease, and congenital heart disease) and comorbidities (diabetes, chronic kidney disease, chronic obstructive pulmonary disease, stroke, and anaemia), mechanical support [early (within 24 h) PCI, ECMO, mechanical ventilation, and continuous renal replacement therapy], hospital type and level, and medical insurance type. We further performed stratified analyses to assess the relationship between IABP usage and in‐hospital mortality in various subgroups. P values for interaction were analysed based on a logistic regression model that included IABP treatment together as an interaction variable by two‐way interaction tests.
The propensity score for IABP usage was calculated for each patient by a logistic regression model incorporating clinically relevant covariates, such as age, sex, underlying cardiovascular disease and comorbidities, mechanical support, hospital types and levels, and medical insurance type. IABP recipients were matched 1:2 to non‐IABP recipients by their propensity scores, using the nearest neighbour method with a calliper of 0.05 and no replacement. The propensity‐matched sample had standardized differences < 10% for all baseline characteristics, which was considered insignificant. Mixed‐effect models were then used in the matched data, and a logistic regression model was used for in‐hospital mortality as in the overall population.
Two‐tailed P < 0.05 was considered statistically significant. All statistical analyses were performed using R, Version 3.4.1 (http://www.R‐project.org).
Results
Baseline characteristics of patients with cardiogenic shock and stratified by intra‐aortic balloon pump usage
In the 4 year period from 2013 to 2016, 30 106 patients diagnosed with CS were screened from the total admissions of the HQMS registry and enrolled into the analysis (Figure 1 ). Baseline characteristics of the overall subjects, patients with and without the use of IABP, are detailed in Table 1 . In the whole study population, the average age was 67.1 ± 14.6 years, and 37.6% were female patients. Ischaemic heart disease was the leading underlying heart disease (73.9%), and AMI accounted for 56.6% of the patients. Hypertension was seen in 36.0% of the cases. Other potential cardiac aetiologies, such as cardiomyopathy (7.5%), valvular heart disease (7.3%), myocarditis (2.6%), and congenital heart disease (2.4%), were less. The most common comorbidity was diabetes (21.0%), then chronic kidney disease (13.3%), stroke (8.3%), anaemia (6.5%), and chronic obstructive pulmonary disease (6.3%). IABP was used in 2320 (7.7%) of the CS admissions. Patients receiving IABP were more likely to be young, male gender; have an AMI, hypertension, acute myocarditis, and congenital heart disease as underlying heart disease; and had less comorbidity burden except for diabetes. The procedures were more conducted in tertiary A and specialized hospitals than in other levels or general hospitals (all P < 0.05) (Table 1 ).
Figure 1.
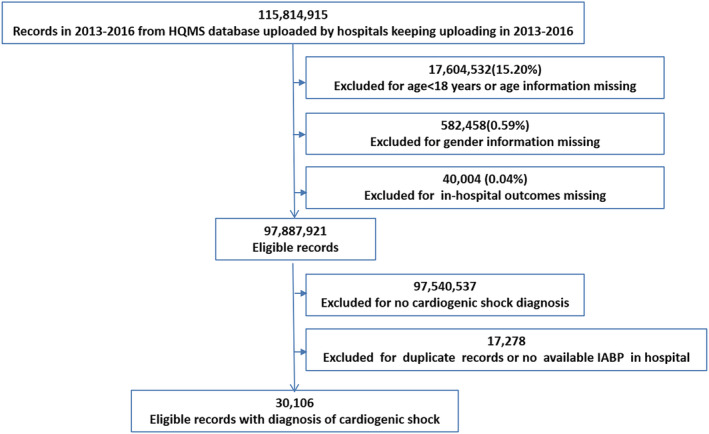
Flow diagram of selection of the study population. Abbreviations: HQMS, the Hospital Quality Monitoring System; IABP, intra‐aortic balloon pump.
Table 1.
Baseline characteristics of the overall cardiogenic shock patients and propensity‐score‐matched patients stratified by IABP usage
| Variable | Whole population | Propensity‐score‐matched population | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | IABP | No IABP | P value | Overall | IABP | No IABP | P value | Absolute standardized difference | |
| (N = 30 106) | (N = 2320) | (N = 27 786) | (N = 5737) | (N = 2027) | (N = 3710) | ||||
| Age, years (mean ± SD) | 67.1 ± 14.6 | 63.3 ± 14.0 | 67.4 ± 14.6 | <0.001 | 64.2 ± 14.2 | 63.6 ± 13.9 | 64.5 ± 14.3 | 0.020 | 0.065 |
| Female gender, n (%) | 11 314 (37.6%) | 702 (30.3%) | 10 612 (38.2%) | <0.001 | 1794 (31.3%) | 623 (30.7%) | 1171 (31.6%) | 0.518 | 0.018 |
| Underlying cardiovascular disease, n (%) | |||||||||
| IHD | 22 244 (73.9%) | 2095 (90.3%) | 20 149 (72.5%) | <0.001 | 5204 (90.7%) | 1838 (90.7%) | 3366 (90.7%) | 0.948 | 0.002 |
| AMI | 17 051 (56.6%) | 1895 (81.7%) | 15 156 (54.5%) | <0.001 | 4387 (76.5%) | 1661 (81.9%) | 2726 (73.5%) | <0.001 | |
| Hypertension | 10 850 (36.0%) | 911 (39.3%) | 9939 (35.8%) | 0.001 | 2276 (39.7%) | 800 (39.5%) | 1476 (39.8%) | 0.814 | 0.006 |
| Cardiomyopathy | 2251 (7.5%) | 71 (3.1%) | 2180 (7.8%) | <0.001 | 179 (3.1%) | 60 (3.0%) | 119 (3.2%) | 0.606 | 0.014 |
| Myocarditis | 797 (2.6%) | 152 (6.6%) | 645 (2.3%) | <0.001 | 338 (5.9%) | 118 (5.8%) | 220 (5.9%) | 0.867 | 0.005 |
| VHD | 2193 (7.3%) | 107 (4.6%) | 2086 (7.5%) | <0.001 | 274 (4.8%) | 97 (4.8%) | 177 (4.8%) | 0.980 | 0.001 |
| CHD | 730 (2.4%) | 73 (3.1%) | 657 (2.4%) | 0.019 | 170 (3.0%) | 61 (3.0%) | 109 (2.9%) | 0.879 | 0.004 |
| Comorbidities, n (%) | |||||||||
| CKD | 4000 (13.3%) | 251 (10.8%) | 3749 (13.5%) | <0.001 | 651 (11.3%) | 230 (11.3%) | 421 (11.3%) | 0.999 | 0.000 |
| Diabetes | 6315 (21.0%) | 568 (24.5%) | 5747 (20.7%) | <0.001 | 1426 (24.9%) | 498 (24.6%) | 928 (25.0%) | 0.709 | 0.010 |
| COPD | 1907 (6.3%) | 62 (2.7%) | 1845 (6.6%) | <0.001 | 164 (2.9%) | 58 (2.9%) | 106 (2.9%) | 0.993 | 0.000 |
| Stroke | 2491 (8.3%) | 115 (5.0%) | 2376 (8.6%) | <0.001 | 324 (5.6%) | 110 (5.4%) | 214 (5.8%) | 0.592 | 0.015 |
| Anaemia | 1949 (6.5%) | 103 (4.4%) | 1846 (6.6%) | <0.001 | 264 (4.6%) | 94 (4.6%) | 170 (4.6%) | 0.924 | 0.003 |
| Hospital type, n (%) | <0.001 | 0.351 | 0.026 | ||||||
| Specialized hospital | 1122 (3.7%) | 173 (7.5%) | 949 (3.4%) | 425 (7.4%) | 159 (7.8%) | 266 (7.2%) | |||
| General hospital | 28 984 (96.3%) | 2147 (92.5%) | 26 837 (96.6%) | 5312 (92.6%) | 1868 (92.2%) | 3444 (92.8%) | |||
| Hospital level, n (%) | <0.001 | 0.782 | 0.019 | ||||||
| Tertiary level A | 25 571 (84.9%) | 1998 (86.1%) | 23 573 (84.8%) | 4935 (86.0%) | 1747 (86.2%) | 3188 (85.9%) | |||
| Tertiary level B | 3025 (10.0%) | 118 (5.1%) | 2907 (10.5%) | 287 (5.0%) | 96 (4.7%) | 191 (5.1%) | |||
| Other | 1510 (5.0%) | 204 (8.8%) | 1306 (4.7%) | 515 (9.0%) | 184 (9.1%) | 331 (8.9%) | |||
| Treatment modality, n (%) | |||||||||
| Early PCI | 4014 (13.3%) | 905 (39.0%) | 3109 (11.2%) | <0.001 | 2036 (35.5%) | 778 (38.4%) | 1258 (33.9%) | 0.001 | 0.093 |
| ECMO | 102 (0.3%) | 46 (2.0%) | 56 (0.2%) | <0.001 | 63 (1.1%) | 30 (1.5%) | 33 (0.9%) | 0.040 | 0.055 |
| MV | 1948 (6.5%) | 231 (10.0%) | 1717 (6.2%) | <0.001 | 590 (10.3%) | 216 (10.7%) | 374 (10.1%) | 0.493 | 0.019 |
| CRRT | 636 (2.1%) | 84 (3.6%) | 552 (2.0%) | <0.001 | 197 (3.4%) | 70 (3.5%) | 127 (3.4%) | 0.952 | 0.002 |
AMI, acute myocardial infarction; CHD, congenital heart disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; IHD, ischemic heart disease; IABP, intra‐aortic balloon pump; MV, mechanical ventilation; PCI, percutaneous coronary intervention; SD, standard deviation; VHD, valvular heart disease.
Invasive procedure or mechanical support usage in CS patients was not common. Although AMI patients constituted more than half of the CS population, early PCI (within 24 h after admission) was conducted in only 13.3% of the CS subjects (23.5% of AMI patients). Patients with IABP received more early PCI procedures than those without IABP (39.0% vs. 11.2%). Mechanical support measures other than IABP were generally less used. Greater rates of ECMO (2.0% vs. 0.2%), mechanical ventilation (10.0% vs. 6.2%), and continuous renal replacement therapy (3.6% vs. 2.0%) were noted in the patients who received IABP than those who did not (Table 1 ).
The propensity score matching identified 5737 patients. The performance of the propensity score model is shown in Table 1 , depicting baseline characteristics that entered the propensity matching. The post‐matching standardized difference < 10% indicates excellent covariate balance.
The in‐hospital outcome of cardiogenic shock and the association with intra‐aortic balloon pump usage
All‐cause death occurred in 11 572 (38.4%) of patients with CS during hospitalization. The subjects with IABP support had similar in‐hospital mortality to those without IABP (39.6% vs. 38.3%, P = 0.226) but longer hospital‐stay [8.0 (2.0–16.0) vs. 6.0 (2.0–13.0) days, P < 0.001] and higher expenses [7.1 (4.4–11.1) vs. 2.3 (0.8–5.5) 10 000¥, P < 0.001] (Table 2 ). In a multivariate model adjusting for age, sex, underlying cardiovascular disease and comorbidities, mechanical support, hospital type and level, and medical insurance type, the IABP usage showed no significant association with improved in‐hospital mortality [odds ratio (OR) 1.05, 95% confidence interval (CI) 0.95–1.17].
Table 2.
In‐hospital outcomes of the cardiogenic shock patients and stratified by IABP usage
| Variable | Whole population | Propensity‐score‐matched population | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | IABP | No IABP | P value | Overall | IABP | No IABP | P value | |
| (N = 30 106) | (N = 2320) | (N = 27 786) | (N = 5737) | (N = 2027) | (N = 3710) | |||
| In‐hospital outcome | ||||||||
| All‐cause death, n (%) | 11 572 (38.4%) | 919 (39.6%) | 10 653 (38.3%) | 0.226 | 2262 (39.4%) | 811 (40.0%) | 1451 (39.1%) | 0.505 |
| Length of stay, days, median (IQR) | 6.0 (2.0–14.0) | 8.0 (2.0–16.0) | 6.0 (2.0–13.0) | <0.001 | 7.0 (2.0–15.0) | 8.0 (2.0–16.0) | 7.0 (2.0–14.0) | 0.002 |
| Expenses, 10 000¥, median (IQR) | 2.6 (0.9–6.1) | 7.1 (4.4–11.1) | 2.3 (0.8–5.5) | <0.001 | 5.3 (2.5–9.1) | 7.1 (4.4–11.1) | 4.4 (1.7–7.7) | <0.001 |
IABP, intra‐aortic balloon pump; IQR, interquartile range; SD, standard deviation.
After propensity matching for baseline variables, the results observed in the overall population was further confirmed in this propensity score‐matched sample. The in‐hospital mortality was 40.0% in the IABP group vs. 39.1% in the control group (P = 0.505). The hospital‐stay and expenses were [8.0 (2.0–16.0) vs. 7.0 (2.0–14.0) days, P = 0.002] and [7.1 (4.4–11.1) vs. 4.4 (1.7–7.7) 10 000¥, P < 0.001] for patients with and without IABP, respectively (Table 2 ). In the multivariate model, the use of IABP was still not suggestive for improved in‐hospital mortality (propensity‐adjusted OR 1.09, 95% CI 0.96–1.23).
Subgroup analysis
Stratification and interaction analyses were performed based on important baseline information in the whole study population (Figure 2 ). IABP usage was not associated with a reduced risk of mortality in most of the subgroups. Interactions were found between subgroups of patients who did and did not receive early PCI and between patients with and without myocarditis. Subjects who did not undergo early PCI benefited from IABP (OR 0.86, 95% CI 0.75–0.97), while those who received early PCI did not (OR 1.60, 95% CI 1.34–1.90, P < 0.001 for interaction). The protective effect of IABP was also observed in patients with myocarditis (OR 0.61, 95% CI 0.39–0.95) but not for patients without myocarditis (OR 1.09, 95% CI 0.98–1.21, P = 0.010 for interaction) (Figure 2 ).
Figure 2.
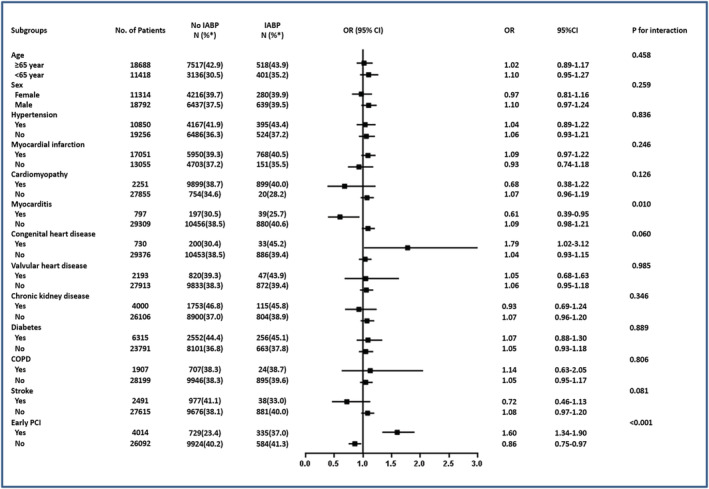
Interaction analyses for in‐hospital mortality of the whole study population. Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; IABP, intra‐aortic balloon pump; OR, odds ratio; PCI, percutaneous coronary intervention. *in‐hospital mortality.
Supplementary analysis
In further analysis of our study population, less than 5% of patients [n = 1465 (4.9%)] in our study population had a shock state other than CS. We have also supplemented the analysis excluding those with mixed shocks. The main results were consistent with the original research ( Data S1 , 1).
Further analysis for subgroups of CS patients with AMI was conducted. The conclusion drawn in the subgroup of CS patients complicated with AMI was consistent with the general CS population, as well as earlier research reports. No survival benefit was obtained from IABP support, yet early PCI showed a favourite effect for CS patients with AMI ( Data S1 , 2).
CS Patients with myocarditis consumed the most interventional support modalities while achieved the lowest in‐hospital mortality among CS patients with various underlying heart disease ( Data S1 , 3).
Discussion
By access to a large real‐world population with CS, we demonstrated that the usage of IABP was not associated with increased survival but with a longer hospital stay and higher costs. However, the survival benefit was observed in some subgroups of patients.
Cardiogenic shock is a severe state of cardiac dysfunction, often resulting in high in‐hospital mortality. AMI with ventricular dysfunction and/or mechanical complications are the most common cause of CS. Non‐AMI‐related CS may be caused by decompensated heart failure with heterogeneous underlying heart disease. In prior studies, IABP‐SHOCK II is the largest randomized trial in CS, yet only patients with MI were included. 11 Contemporary data on CS patients with varied aetiologies are lacking. 12 The CardShock study 12 is the largest European prospective, multicentre observational study including the whole spectrum of aetiologies of CS. ST‐segment elevation myocardial infarction and other forms of ACS are still the leading causes of CS, and ST‐segment elevation myocardial infarction patients accounted for 68% of all patients. 12 Furthermore, four‐fifth of those CS subjects had ACS. 12 In our study population by access to national register database in China, despite AMI accounted for more than half of the patients, the proportions of ischemic heart disease were much lower than in the aforementioned cohorts, representing comprehensive aetiology of CS in our real‐world clinical settings.
Our findings were mostly in line with earlier reports, showing the usage of IABP generally could not lead to a better in‐hospital prognosis in CS patients. The primary outcome was consistent across most subgroups with diverse demographic and clinical characteristics, with only two notable exceptions. First of all, there was a significant interaction between IABP support and early PCI usage. This was supported by our data from the whole population and the subgroup of CS complicated with AMI. Patients who had not received early PCI had significantly lower mortality with IABP support than those without, whereas patients who had undergone early PCI had even further elevated mortality with IABP support. In fact, one of the controversies about the usefulness of IABP in most of the prior investigations has arisen from the failure of taking into account the revascularization manners for AMI. 13 For example, a significant benefit of IABP was seen in patients who did not receive revascularization or thrombolysis as reperfusion treatment in the IABP‐Shock II trial, 11 in which a neutral result of the primary outcome was found in the whole population. Multiple observatory studies and adjusted meta‐analysis, on the contrary, demonstrated the effect of IABP on in‐hospital mortality was significantly in disfavour in the PCI subgroup. 13 , 14 The lack of efficacy of IABP might well result from the minimal add‐on effect after salvage of the ischemic myocardium by successful reperfusion. Indeed, because the landmark SHOCK trial established the role of early revascularization in AMI patients with shock, 15 primary PCI was recommended as the first‐line management of AMI complicated with CS 7 , 16 owing to its significant survival benefit. Moreover, data from the studies focusing on the time course of CS indicate that percutaneous mechanical cardiac support has a limited ability to change the outcome if initiated when overt multi‐organ dysfunction has already occurred. The prognosis could only be improved when cardiac support was initiated early in the disease course (e.g. before reperfusion). 17 Therefore, IABP could act as a bridge while waiting for PCI, or supplementary support when CS patients could not receive reperfusion or are not fit for timely or sufficient reperfusion. If the early revascularization could be achieved, IABP should not be routinely used, especially considering the possible delay to reperfusion for IABP implantation.
Notably, one of the significant differences between earlier studies and ours lies in the percentage of patients undergoing early PCI. Even in the CARDShock study that enrolled all‐cause CS, nearly 90% of the CS patients with ACS underwent PCI. 12 In contrast, early PCI was conducted in only 13.3% of our CS population (23.5% of AMI patients). Total PCI were conducted in less than 30% of our CS patients complicated with MI. The results were consistent with contemporary data on MI patients 18 , 19 and might reflect the more conservative strategies for CS patients in our population or delayed reperfusion to some extent. Further national efforts are needed to improve the care for CS patients with MI considering the gaps between guidelines and our practice. Meanwhile, early PCI was more often undergone in patients with IABP (39.0%) than in patients without IABP (11.2%). The combinational usages of IABP and early PCI might be because of the beliefs of the doctors performing the PCI. This is, however, not supported by our findings as a combination could not result in better outcomes rather than contributing to the higher total expenses and longer hospital stay.
Intra‐aortic balloon pump usage was also observed to have different impacts on subgroups of patients with and without myocarditis as underlying heart disease. CS patients with myocarditis got protection from in‐hospital death with IABP support. The findings are noteworthy as prior studies regarding CS with causes other than AMI were scarce. IABP therapy has been suggested leading to clinical stabilization and improved tissue perfusion in CS in some small, single‐centre studies and registries, 20 , 21 , 22 , 23 serving as a bridge to ventricular assist device implantation or heart transplantation. Compared with other decompensated heart failure, patients with fulminant myocarditis might be more likely to experience acute deterioration processes with the overall self‐limited course. Therefore, temporary aggressive mechanical cardiac support might be more effective in providing help to the patients to live through the critical stage. In line with this, the CS patients with myocarditis in our study population consumed the most mechanical support devices while showed the lowest in‐hospital mortality in comparison to CS patients with other causes ( Data S1 , 3). The various supportive measures demonstrated the efficacy in this subgroup of subjects.
Finally, despite advances in the management of CS worldwide, the mortality in this population has remained high at 30% to 40%. 24 , 25 Although mortality as well as other outcomes between our study and the prior studies cannot be directly compared due to the different study populations and definition of endpoint events, the overall in‐hospital mortality of 38.4% in our study population was consistent with earlier studies. And these data were achieved with fewer early PCI procedures employed in MI patients while PCI was demonstrated with significant survival benefits. As critical salvage for MI patients complicated with CS, early PCI should be more stressed and be put effort into our future practice. And as the subgroup analysis has shown, further stratification on subjects with different aetiologies and intervention manners, and planning for optimal timing in the usage of IABP might also provide valuable help for CS patients.
Limitations
The present study was a retrospective registry study, and the identification of the diagnosis was based on the ICD‐10 code, in which inherent ascertain bias exists. Due to the data extraction process and the heterogeneous nature of heart failure, there might be multiple diagnoses of underlying heart disease in one subject identified, leading to overlapped diagnoses, which might be assumed both as potential aetiologies of CS. Meanwhile, the diagnosis of AMI, as the most common cause of CS, was definite. A more precise evaluation of the severity of CS and the impacts of medical treatment (e.g. vasoactive agents) were difficult because the detailed information on laboratory results and medications were unavailable. However, the report of the invasive interventions, as well as the endpoint events (including in‐hospital mortality, hospital stay and expenses), were accurate.
Conclusions
Intra‐aortic balloon pump is currently used in less than 10% of patients with CS in this nationwide registry. Patients receiving IABP had no reduction for in‐hospital mortality but longer length‐of‐stay and higher hospital expenses in general. However, in selected subgroups such as AMI patients who did not receive early PCI and patients with myocarditis, IABP showed protective results. Further research is warranted to determine the optimal patient populations, clinical characteristics, and timing for IABP as the simplest circulatory support to improve clinical outcomes of this high‐risk population.
Conflict of interest
None.
Funding
This study was supported by China‐World Health Organization Biennial Collaborative Projects 2016–2017 (design and collection parts).
Supporting information
Data S1. Supplementary files.
1. Analysis for CS patients excluding those with other shocks.
Table S1. Baseline characteristics of the cardiogenic shock patients with no other shock co‐existed and stratified by IABP usage.
Table S2. In‐hospital outcomes of the CS patients with no other shock co‐existed and stratified by IABP usage.
Table S3. The association between IABP usage and in‐hospital mortality of CS patients with no other shock co‐existed assessed by multi‐variate regression.
Figure S1. Interaction analyses for in‐hospital mortality of the CS patients with no other shock co‐existed.
2. Analysis for subgroups of CS patients with AMI.
Table S1. Baseline characteristics of the CS patients with AMI and stratified by IABP usage.
Table S2. In‐hospital outcomes of the CS patients with AMI and stratified by IABP usage.
Table S3. The association between IABP usage and in‐hospital mortality assessed by multi‐variate regression.
Table S4. Interaction analysis for In‐hospital mortality of the CS with AMI patients stratified by early PCI.
3. Comparison of mechanical support modalities and in‐hospital outcomes for CS patients with myocarditis and other underlying heart diseases.
Table S1. Mechanical support usage of CS patients with different underlying heart disease.
Table S2. In‐hospital outcomes of CS patients with different underlying heart disease.
Acknowledgements
The authors thank the China Standard Medical Information Research Centre for their help and support throughout the study.
Chu, S. , Sun, P. , Zhang, Y. , Li, J. , Liu, L. , Shi, Y. , Wang, H. , Chen, H. , Fu, M. , and Huo, Y. (2021) Intra‐aortic balloon pump on in‐hospital outcomes of cardiogenic shock: findings from a nationwide registry, China. ESC Heart Failure, 8: 3286–3294. 10.1002/ehf2.13479.
References
- 1. Fang JC, Ewald GA, Allen LA, Butler J, Westlake Canary CA, Colvin‐Adams M, Dickinson MG, Levy P, Stough WG, Sweitzer NK, Teerlink JR, Heart Failure Society of America Guidelines C . Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail 2015; 21: 519–534. [DOI] [PubMed] [Google Scholar]
- 2. Jeger RV, Radovanovic D, Hunziker PR, Pfisterer ME, Stauffer JC, Erne P, Urban P, Investigators APR. Ten‐year trends in the incidence and treatment of cardiogenic shock. Ann Intern Med 2008; 149: 618–626. [DOI] [PubMed] [Google Scholar]
- 3. Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty‐year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population‐based perspective. Circulation 2009; 119: 1211–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aissaoui N, Puymirat E, Tabone X, Charbonnier B, Schiele F, Lefèvre T, Durand E, Blanchard D, Simon T, Cambou JP, Danchin N. Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST‐MI French nationwide registries. Eur Heart J 2012; 33: 2535–2543. [DOI] [PubMed] [Google Scholar]
- 5. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, de Waha A, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Lauer B, Böhm M, Ebelt H, Schneider S, Werdan K, Schuler G. Intraaortic Balloon Pump in cardiogenic shock II (IABP‐SHOCK II) trial investigators . Intra‐aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP‐SHOCK II): final 12 month results of a randomised, open‐label trial. The Lancet. 2013;382: 1638–1645. [DOI] [PubMed] [Google Scholar]
- 6. O'gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127: 529–555. [DOI] [PubMed] [Google Scholar]
- 7. Task Force on the management of ST‐segment elevation acute myocardial infarction of the European Society of Cardiology (ESC) , Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, di Mario C, Dickstein K, Ducrocq G, Fernandez‐Aviles F. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J 2012; 33: 2569–2619. [DOI] [PubMed] [Google Scholar]
- 8. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis PT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 9. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 267–315. [DOI] [PubMed] [Google Scholar]
- 10. Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, Li Y, Yeung RO, Wang J, Matsushita K, Coresh J, Zhao M‐H, Wang H. Trends in chronic kidney disease in China. N Engl J Med 2016; 375: 905–906. [DOI] [PubMed] [Google Scholar]
- 11. Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012; 367: 1287–1296. [DOI] [PubMed] [Google Scholar]
- 12. Harjola VP, Lassus J, Sionis A, Køber L, Tarvasmäki T, Spinar J, Parissis J, Banaszewski M, Silva‐Cardoso J, Carubelli V, Di Somma S. Clinical picture and risk prediction of short‐term mortality in cardiogenic shock. Eur J Heart Fail 2015; 17: 501–509. [DOI] [PubMed] [Google Scholar]
- 13. Acconcia MC, Caretta Q, Romeo F, Borzi M, Perrone MA, Sergi D, Chiarotti F, Calabrese CM, Sili Scavalli A, Gaudio C. Meta‐analyses on intra‐aortic balloon pump in cardiogenic shock complicating acute myocardial infarction may provide biased results. Eur Rev Med Pharmacol Sci 2018; 22: 2405–2414. [DOI] [PubMed] [Google Scholar]
- 14. Romeo F, Acconcia MC, Sergi D, Romeo A, Muscoli S, Valente S, Gensini GF, Chiarotti F, Caretta Q. The outcome of intra‐aortic balloon pump support in acute myocardial infarction complicated by cardiogenic shock according to the type of revascularization: a comprehensive meta‐analysis. Am Heart J 2013; 165: 679–692. [DOI] [PubMed] [Google Scholar]
- 15. Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med 1999; 341: 625–634. [DOI] [PubMed] [Google Scholar]
- 16. Authors/Task Force members , Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P. 2014 ESC/EACTS Guidelines on myocardial revascularization: the task force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 2014. Oct 1; 35: 2541–2619. [DOI] [PubMed] [Google Scholar]
- 17. O'Neill WW, Schreiber T, Wohns DH, Rihal C, Naidu SS, Civitello AB, Dixon SR, Massaro JM, Maini B, Ohman EM. The current use of Impella 2.5 in acute myocardial infarction complicated by cardiogenic shock: results from the USpella Registry. J Interv Cardiol 2014; 27: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li J, Li X, Wang Q, Hu S, Wang Y, Masoudi FA, Spertus JA, Krumholz HM, Jiang L, China PCG. ST‐segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE‐Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet 2015; 385: 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun P, Li J, Fang W, Su X, Yu B, Wang Y, Li C, Chen H, Wang X, Zhang B, Li Y, Momin M, Shi Y, Wang H, Zhang Y, Xiang D, Huo Y. Effectiveness of chest pain centre accreditation on the management of acute coronary syndrome: a retrospective study using a national database. BMJ Qual Saf 2020. :bmjqs‐2020‐011491 Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 20. Norkiene I, Ringaitiene D, Rucinskas K, Samalavicius R, Baublys A, Miniauskas S, Sirvydis V. Intra‐aortic balloon counterpulsation in decompensated cardiomyopathy patients: bridge to transplantation or assist device. Interact Cardiovasc Thorac Surg 2007; 6: 66–70. [DOI] [PubMed] [Google Scholar]
- 21. Estep JD, Cordero‐Reyes AM, Bhimaraj A, Trachtenberg B, Khalil N, Loebe M, Bruckner B, Orrego CM, Bismuth J, Kleiman NS, Torre‐Amione G. Percutaneous placement of an intra‐aortic balloon pump in the left axillary/subclavian position provides safe, ambulatory long‐term support as bridge to heart transplantation. JACC Heart Fail 2013; 1: 382–388. [DOI] [PubMed] [Google Scholar]
- 22. Bezerra CG, Adam EL, Baptista ML, Ciambelli GS, Kopel L, Bernoche C, Lopes LN, Macatrao‐Costa MF, Falcao Bde A, Lage SG. Aortic counterpulsation therapy in patients with advanced heart failure: analysis of the TBRIDGE Registry. Arq Bras Cardiol 2016; 106: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sintek MA, Gdowski M, Lindman BR, Nassif M, Lavine KJ, Novak E, Bach RG, Silvestry SC, Mann DL, Joseph SM. Intra‐aortic balloon counterpulsation in patients with chronic heart failure and cardiogenic shock: clinical response and predictors of stabilization. J Card Fail 2015; 21: 868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H. Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation 2017; 136: e232–e268. [DOI] [PubMed] [Google Scholar]
- 25. Kolte D, Khera S, Dabhadkar KC, Agarwal S, Aronow WS, Timmermans R, Jain D, Cooper HA, Frishman WH, Menon V, Bhatt DL. Trends in coronary angiography, revascularization, and outcomes of cardiogenic shock complicating non‐ST‐elevation myocardial infarction. Am J Cardiol 2016; 117: 1–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplementary files.
1. Analysis for CS patients excluding those with other shocks.
Table S1. Baseline characteristics of the cardiogenic shock patients with no other shock co‐existed and stratified by IABP usage.
Table S2. In‐hospital outcomes of the CS patients with no other shock co‐existed and stratified by IABP usage.
Table S3. The association between IABP usage and in‐hospital mortality of CS patients with no other shock co‐existed assessed by multi‐variate regression.
Figure S1. Interaction analyses for in‐hospital mortality of the CS patients with no other shock co‐existed.
2. Analysis for subgroups of CS patients with AMI.
Table S1. Baseline characteristics of the CS patients with AMI and stratified by IABP usage.
Table S2. In‐hospital outcomes of the CS patients with AMI and stratified by IABP usage.
Table S3. The association between IABP usage and in‐hospital mortality assessed by multi‐variate regression.
Table S4. Interaction analysis for In‐hospital mortality of the CS with AMI patients stratified by early PCI.
3. Comparison of mechanical support modalities and in‐hospital outcomes for CS patients with myocarditis and other underlying heart diseases.
Table S1. Mechanical support usage of CS patients with different underlying heart disease.
Table S2. In‐hospital outcomes of CS patients with different underlying heart disease.


