Abstract
A positive nuclear scintigraphy with hydroxy bisphosphonate bone tracer (99mTc‐HPD) is believed to have high sensitivity (>99%) and specificity (91%) for the diagnosis of transthyretin amyloid cardiomyopathy. We report the case of an 85‐year‐old man with increased thickness of ventricular walls and a positive bone scintigraphy, who was unexpectedly found to have sarcomeric hypertrophic cardiomyopathy at left ventricular endomyocardial biopsy. Congo Red staining, immunohistochemistry, and transmission electronmicroscopy on six left ventricular samples scored negative for amyloidosis but were suggestive for sarcomeric hypertrophic cardiomyopathy. Genetic study did not show TTR and most commonly involved sarcomeric genes mutations. In hypertrophic cardiomyopathy focal cell necrosis related to demand/supply oxygen mismatch, small vessels disease or inflammation could be responsible of a false‐positive bone scintigraphy signal for transthyretin amyloidosis. Because of this, especially in view of a possible specific treatment, endomyocardial biopsy is highly recommended for the correct diagnosis of cardiomyopathies with hypertrophic phenotype.
Keywords: Elderly hypertrophic cardiomyopathy, ATTR cardiac amyloidosis, Bone scintigraphy
Introduction
Transthyretin (TTR) cardiac amyloidosis has been detected in as many as 25% of adults older than 85 years on autopsy, 1 with an estimated prevalence of about 190 cases per million persons. Improved cardiac imaging modalities, particularly bone scintigraphy scan, have contributed to improve non‐invasive detection of the disease, confining the use of endomyocardial biopsy to cases with equivocal imaging findings. 2
Case Report
An 85‐year‐old man with systemic arterial hypertension was admitted because of pre‐syncope associated to evidence at Holter monitoring of both bradiarrhythmias (1st degree atrioventricular block with PR interval of 300 ms and several episodes of 2nd degree Mobitz type 1 atrioventricular block with electrical pause greater than 3 s) and tachyarrhythmias (non‐sustained ventricular tachycardia). He suffered from a bilateral carpal tunnel syndrome that underwent surgery 2 years earlier. Cardiac examination was unremarkable. Routine laboratory tests were normal except for elevated cardiac troponin HS (0.295 mcg/L, nv < 0.014 mcg/L) and N‐terminal pro‐B‐type natriuretic peptide (384.3 pg/mL, nv = 0–254 pg/mL). Electrocardiogram showed sinus rhythm (53 bpm), 1st degree AV block, left anterior fascicular block, and normal left ventricular QRS voltages (Figure 1 A ). Two‐dimensional echocardiography showed a severe hypertrophy of the left ventricle (LV), and strain imaging was not detected because of the suboptimal echocardiographic window. Cardiac magnetic resonance (CMR) confirmed left ventricular hypertrophy with maximal wall thickness of 20 mm (Figure 1 B and Supporting Information, Video S1 ) in the middle‐basal septum. Native T1 map (Figure 1 C ) indicated increase of myocardial T1 value at the inferior septum and at the inferior wall. The area of high nT1 corresponded to increased ECV on extracellular volume fraction (ECV) map (Figure 1 D ) and to the extension of late‐gadolinium enhancement (Figure 1 E,F ), that was more prominent in the mid‐ventricular and subepicardial layer of the middle‐basal septum.
Figure 1.
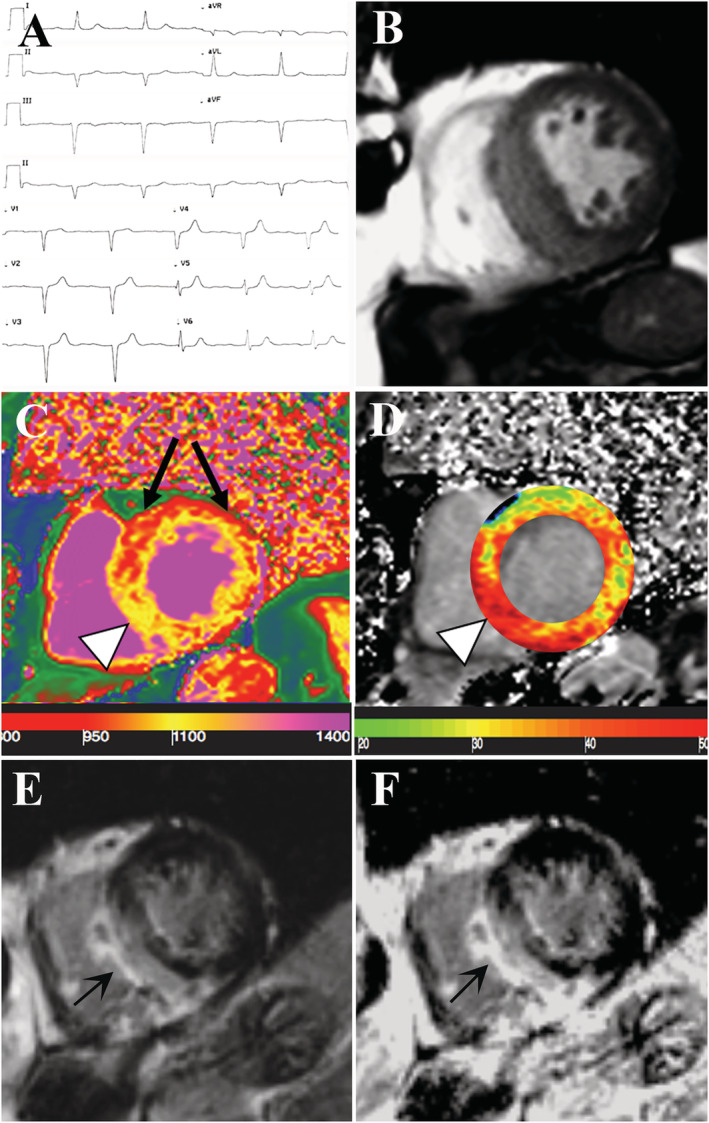
Non‐invasive evaluation of the 85‐year‐old patient with suspected TTR cardiac amyloidosis. (A) Electrocardiogram showing a 1st degree atrioventricular block, a left anterior fascicular block and normal voltages. (B–F) CMR images in short axis view. CineMR image (B) demonstrates severe left ventricular (LV) hypertrophy. Native T1 (nT1) map (C) showing an increase in the myocardial T1 value at the inferior septum (arrowhead, nT1 = 1130 ± 25 ms, high value relative to the scanner normal range = 970–1030 ms) and at the inferior wall (nT1: 1080 ± 34 ms, slightly increased) while the anterior and antero‐lateral wall (black arrows) remained within normal limits (nT1 between 980 and 1005). On extracellular volume fraction (ECV) map (D) the area of high nT1 corresponded to increased ECV (arrowhead). Late gadolinium enhanced (LGE) image with magnitude reconstruction (E) and with phase‐sensitive inversion recovery reconstruction (PSIR, F) showing extensive area of non‐ischemic LGE involving the area of LV hypertrophy and also the inferior right ventricle wall. There is concordance between myocardial T1 (nT1 and T1 map) and both LGE images.
Because of clinical suspicion of senile amyloidosis, nuclear scintigraphy with hydroxy bisphosphonate bone tracer (99mTc‐HPD) was performed, that revealed a grade 3 myocardial uptake (Figure 2 A,B ). Serum‐urine immunofixation and immunoglobulin free light‐chain was negative for monoclonal gammopathy. Neurological examination was unremarkable. Genetic test did not show TTR mutation. Thus, a diagnosis of wild‐type TTR cardiac amyloidosis was suggested. The patient, in view of Tafamidis treatment, after informed consent, underwent a LV endomyocardial biopsy (EMB). Six specimens were drawn from the LV (3–5 mm2 each), cut and processed for histology, immunohistochemistry, and transmission electron microscopy studies. Unexpectedly, histology failed to confirm amyloid deposition but revealed the presence of severely hypertrophied disarrayed cardiomyocytes (Figure 2 C ) fragmented in short runs by interstitial and replacement fibrosis that was increased (10% vs. 2–3% of age‐matched normal controls) and associated to narrowing of intramural small arteries (Figure 2 D ), denoting a sarcomeric hypertrophic cardiomyopathy. Congo red staining at polarized light scored negative for areas of apple‐green florescence (Figure 2 E ). Immunohistochemistry for transthyretin and kappa/lambda light chain was negative (Figure 2 F ). Transmission electron microscopy failed to demonstrate the presence of rigid unbranched amyloid fibrils and confirmed the presence of larger cardiomyocytes with increase number and disarrayed myofibrils and intermyofibrillar mitochondria. Thus, a final diagnosis of hypertrophic cardiomyopathy was reached. Genetic studies failed to demonstrate mutation in the most commonly involved sarcomeric genes (i.e. MYH7, MYBPC3, TNNT2, and TNNI3). Because of electrical instability and clinical pre‐syncopal episodes, an electrophysiological study was performed that documented an HV interval of 70 ms and infra‐Hisian block at Wenckebach point. Therefore, a bicameral pacemaker was implanted, and beta‐blocker was added to therapy with resolution of ventricular tachycardia.
Figure 2.
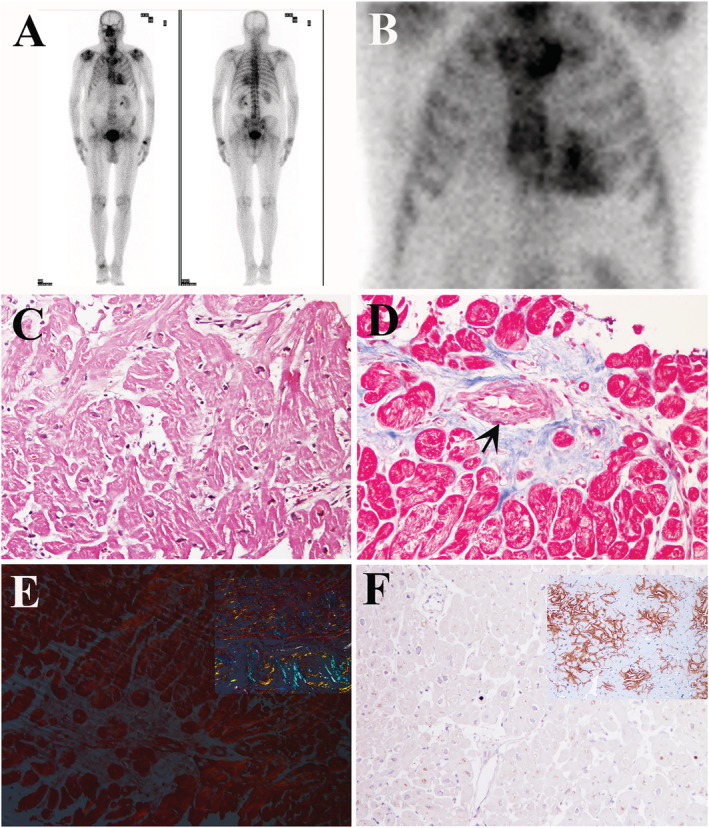
Bone scintigraphy and LV Endomyocardial biopsy findings. (A, B) Whole body (A) and planar (B) images of the chest 90 minutes after 360 MBq of 99mTc diphosphonates i.v. administration, showing an evident accumulation of the radiopharmaceutical in the cardiac region (Perugini score 3). (C–F) LV endomyocardial biopsy revealing the presence of severely hypertrophied and disarrayed cardiomyocytes (C, haematoxylin and eosin, 200×), interrupted in short runs by interstitial and replacement fibrosis, with severe lumen narrowing of a small artery due to hypertrophy and hyperplasia of smooth muscle cells (D) (Masson thrichrome, 200×). LV endomyocardial biopsy did not show positivity either for apple‐green florescence at Congo‐Red staining (E, positive control in the square) or for TTR immunostaining (F, positive control in the square).
Discussion
Differential diagnosis of cardiomyopathies with hypertrophic phenotype is crucial because some secondary forms of cardiac hypertrophy can be susceptible of specific treatment aimed to revert and/or stabilize the increase in wall thickness (i.e. Tafamidis in amyloidosis and enzyme replacement therapy in Fabry Disease). In particular, the diagnosis of TTR amyloidosis has improved with the introduction of bone‐avid, phosphate‐based isotopes (99mTc‐PYP (pyrophosphate), and 99mTc‐DPD (3,3‐diphosphono‐1,2‐propanodiacarboxylic acid) scintigraphy, that demonstrated a high sensitivity (>99%) and specificity (91%) because of their avidity for TTR amyloid deposits. 3 In particular, a Perugini score grade 2 or 3, in combination with a negative plasma cell dyscrasia, has a specificity and positive predictive value of 100% for diagnosis of TTR cardiac amyloidosis. 3 In spite of that, a positive cardiac signal of bone scintigraphy can occur in other pathologic settings. Previous studies have demonstrated that in acute myocardial infarction nuclear scintigraphy with bisphosphonate shows a significant uptake in ischemic areas, probably as a consequence of calcium release from necrotic cardiomyocytes. 4 In our patient, the markedly positive bone scintigraphy was likely due to high myocardial calcium content. It can be hypothesized that in hypertrophic cardiomyopathy of elderly, with reduction of coronary network and reserve, focal cell necrosis related to demand/supply oxygen mismatch, small vessels disease, or inflammation could be responsible of a false‐positive bone scintigraphy signal for TTR amyloidosis. It can be argue that endomyocaridial biopsy can be negative in the setting of an highly suspected amyloidosis with positive scintigraphy, because of sampling error 5 . However, in our case, the absence of amyloid deposits was confirmed on six samples from the LV and by electron microscopy study. In uncertain cases is also possible to reach a diagnosis of amyloidosis by amyloid typing by proteomics, that unambiguously identifies all amyloid types in a single assay. 6 This is particularly useful in the early stages of cardiac amyloid, where the imaging techniques can show a less apparent diagnostic pattern. 7
In conclusion, false‐positive bone scintigraphy can be observed in hypertrophic cardiomyopathy. Because of this, especially in view of a possible specific, prolonged, and expensive treatment, EMB is recommended for TTR cardiac amyloidosis diagnosis. Future studies are needed to investigate the incidence of false‐positive TTR cardiac amyloidosis to bone scintigraphy (and the related responsible molecular pathways) compared with endomyocardial biopsy.
Conflict of interest
None declared.
Supporting information
Video S1. Supporting information
Acknowledgements
The study was partially supported by European Project ERA‐CVD ‘Transnational Research Projects on Cardiovascular Diseases’ (JTC 2016 IKDT‐IGCM).
Chimenti, C. , Alfarano, M. , Maestrini, V. , Galea, N. , De Vincentis, G. , Verardo, R. , Fedele, F. , and Frustaci, A. (2021) False‐positive bone scintigraphy denoting transthyretin amyloid in elderly hypertrophic cardiomyopathy. ESC Heart Failure, 8: 3387–3391. 10.1002/ehf2.13339.
References
- 1. Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, Singleton A, Kiuru‐Enari S, Paetau A, Tienari PJ, Myllykangas L. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2‐macroglobulin and tau: a population‐based autopsy study. Ann Med 2008; 40: 232–239. [DOI] [PubMed] [Google Scholar]
- 2. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA 2020; 324: 79–89. [DOI] [PubMed] [Google Scholar]
- 3. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AW, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016; 133: 2404–2412. [DOI] [PubMed] [Google Scholar]
- 4. Wakat MA, Chilton HM, Hackshaw BT, Cowan RJ, Ball JD, Watson NE Jr. Comparison of Tc‐99m pyrosphosphate and Tc‐99m hydroxymethylene diphosphonate in acute myocardial infarction: concise communication. J Nucl Med 1980; 21: 203–206. [PubMed] [Google Scholar]
- 5. Krupa M, Nguyen R, Revels J, Johnson LS. Technetium‐99m pyrophosphate cardiac SPECT in endomyocardial biopsy negative cardiac amyloidosis. Radiol Case Rep 2018; 13: 925–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dasari S, Theis JD, Vrana JA, Rech KL, Dao LN, Howard MT, Dispenzieri A, Gertz MA, Hasadsri L, Highsmith WE, Kurtin PJ, McPhail ED. Amyloid typing by mass spectrometry in clinical practice: a comprehensive review of 16,175 samples. Mayo Clin Proc 2020; 95: 1852–1864. [DOI] [PubMed] [Google Scholar]
- 7. Wang J, Marzolf A, Zhang JC, Owens A, Han Y. Cardiac amyloidosis masked as hypertrophic cardiomyopathy: a case report. Cardiol Res 2016; 7: 178–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Supporting information


