Abstract
Aims
Lung ultrasound B‐lines are the sonographic sign of pulmonary congestion and can be used in the differential diagnosis of dyspnoea to rule in or rule out acute heart failure (AHF). Our aim was to assess the prognostic value of B‐lines, integrated with echocardiography, in patients admitted to a cardiology department, independently of the initial clinical presentation, thus in patients with and without AHF, and in AHF with reduced and preserved ejection fraction (HFrEF and HFpEF).
Methods and results
We enrolled consecutive patients admitted for various cardiac conditions. Patients were classified into three groups: (i) acute HFrEF; (ii) acute HFpEF; and (iii) non‐AHF. All patients underwent an echocardiogram coupled with lung ultrasound at admission, according to standardized protocols. We followed up 1021 consecutive inpatients (69 ± 12 years) for a median of 14.4 months (interquartile range 4.6–24.3) for death and rehospitalization for AHF. During the follow‐up, 126 events occurred. Admission B‐lines > 30, ejection fraction < 50%, tricuspid regurgitation velocity > 2.8 m/s, and tricuspid annular plane systolic excursion < 17 mm were independent predictors at multivariable analysis. B‐lines > 30 had a strong predictive value in HFpEF and non‐AHF, but not in HFrEF.
Conclusions
Ultrasound B‐lines can detect subclinical pulmonary interstitial oedema in patients thought to be free of congestion and provide useful information not only for the diagnosis but also for the prognosis in different cardiac conditions. Their added prognostic value among standard echocardiographic parameters is more robust in patients with HFpEF compared with HFrEF.
Keywords: Lung ultrasound, B‐lines, Pulmonary congestion, Prognosis, HFpEF
Introduction
Pulmonary congestion (PC) is one of the main features of both acute and chronic heart failure (HF). The importance of PC evaluation in HF is confirmed by many clinical trials showing that it is related to a significant increase in mortality and HF hospitalization. 1 Unfortunately, the physical examination has a low sensitivity and poor predictive value, while a negative chest X‐ray (CXR) does not exclude the presence of PC. 1 Lung ultrasound (LUS) has been proposed as a reliable operator‐friendly and patient‐friendly assessment of PC, by the evaluation of sonographic B‐lines. 2 B‐lines are related to lung water score on CXR, to extravascular lung water (EVLW) measured invasively, 3 to natriuretic peptides, 4 , 5 and the severity of diastolic dysfunction, regardless of the level of systolic dysfunction. 5 , 6 , 7 B‐lines can be readily assessed with a pocket‐size hand‐held device and do not require the expertise necessary for echocardiographic examination and interpretation. 8 All these features allowed the dissemination of B‐lines in different relevant settings, mainly for the differential diagnosis of acute dyspnoea, to rule in or rule out acute HF (AHF). 4 , 5 , 9 , 10 , 11
Several studies have shown that B‐lines provide useful information for the prognostic stratification of patients with dyspnoea in the outpatient 12 , 13 , 14 , 15 and inpatient setting. 16 , 17 , 18 , 19 , 20 , 21 Most of the studies focused on patients with HF, while limited information is available about patients with heart disease but without overt AHF. Moreover, little is known about the different potential role of B‐lines in patients with HF with reduced ejection fraction (HFrEF) compared with HF with preserved ejection fraction (HFpEF), notwithstanding the differences in terms of fluid redistribution between the two HF phenotypes, with patients with HFpEF usually displaying predominantly pulmonary rather than systemic congestion, compared with HFrEF. Our study aimed to assess the prognostic value of B‐lines in a broad population of patients with various cardiac conditions—with and without AHF—admitted to a cardiology department, compared with other standardized echocardiographic parameters, and to evaluate the differences in the predictive role of B‐lines in HFrEF and HFpEF.
Methods
Patient population
This was a prospective, observational study in adults with various cardiac conditions who were consecutively admitted to a cardiology department of a tertiary hospital. We enrolled in 4 years 1030 consecutive patients, which were classified into (i) patients with acute HFrEF; (ii) patients with acute HFpEF; and (iii) patients without AHF (irrespective of the ejection fraction). The diagnosis of AHF was based, according to 2016 European Society of Cardiology Guidelines, 22 on the presence of a rapid onset or worsening of symptoms and/or signs of HF requiring urgent evaluation and treatment, with corroborative information including natriuretic peptides values, clinical diary, response to diuretics, and CXR. A definite diagnosis of HFpEF required elevated levels of N‐terminal prohormone of brain natriuretic peptide (>300 pg/mL) and the presence of relevant structural heart disease [left ventricular (LV) hypertrophy or left atrial enlargement] or diastolic dysfunction. 22 The non‐AHF group consisted of patients admitted for chest pain, syncope, supraventricular and ventricular arrhythmias, elective coronary angiography, elective electrophysiological study, elective pacemaker or implantable cardioverter defibrillator (ICD) implantation/replacement, but without signs and symptoms suggestive of acute HF. Two cardiologists, who were blinded to the number of B‐lines and other echocardiographic data, reviewed all the medical records pertaining to each patient and made an independent initial assessment of the probability of having acute HF. In case of disagreement, a third expert was involved in the evaluation. To avoid pulmonary conditions that could interfere with LUS assessment, we carefully excluded all patients with a history of moderate‐to‐severe lung disease or when these conditions emerged during the hospitalization, defined by pulmonary function tests and computed tomography scans; nine patients were thus excluded (five patients for fibrothorax, two patients for pulmonary fibrosis, one patient for acute pneumonia, and one patient for pulmonary malignancy). None of the patients was on dialysis nor received mechanical ventilation. The local ethics committee approved the study. All subjects gave informed consent, and the study was performed by the ethical standards of the 1964 Helsinki Declaration and its later amendments and with local guidelines for good clinical practice.
Echocardiographic study
All patients underwent a comprehensive transthoracic echocardiography examination at rest within 24 h from admission. We used commercially available ultrasound machines (Sonos 7500 and IE33, Philips Medical Systems, Andover, MA, USA; Vivid System 7, GE/Vingmed, Milwaukee, WI, USA). LV volumes were measured, and ejection fraction was obtained by two‐chamber and four‐chamber view using the biplane discs' summation method (modified Simpson's rule). LV mass was calculated by the Devereux formula and then indexed to body surface area. Tricuspid annular plane systolic excursion (TAPSE) was measured with the M‐mode cursor oriented to the junction of the tricuspid valve plane with the right ventricular (RV) free wall, and a value < 17 mm was used to define RV systolic dysfunction. RV–right atrial pressure gradient was derived using the simplified Bernoulli equation from the peak tricuspid regurgitation (TR) velocity. Inferior vena cava (IVC) was reported, and a dilated IVC (diameter > 21 mm) that collapsed <50% with a sniff was considered abnormal. All measurements were performed in a European Association of Cardiovascular Imaging accredited echo laboratory, and all measurements were taken according to the recommendations of the European Association of Cardiovascular Imaging/American Society of Echocardiography. The examinations were performed by sonographers (non‐physicians) and then reported by cardiologists who were not involved in the patients' management.
Lung ultrasound
Lung ultrasound examinations were performed at the end of the standard two‐dimensional echocardiogram with the patient in the supine position. 2 , 23 , 24 The ultrasound scanning of the anterior and lateral chest on the right and left hemithorax was obtained with the same probe used for the echocardiographic study, with the transducer orientation parallel to the ribs. We identified 28 scanning sites from the second to fourth intercostal spaces on the left hemithorax and from the second to fifth intercostal spaces on the right hemithorax, along the parasternal, mid‐clavicular, anterior axillary, and midaxillary line. In each intercostal space, the number of B‐lines was quantified real time: when B‐lines were distinguishable, they were counted one by one; when they were confluent, the percentage of the white screen compared with the black screen below the pleural line was considered and then divided by 10. 2 , 23 , 24 The sum of B‐lines from the 28 scanning sites yielded a score denoting the extent of the EVLW. Zero was defined as a complete absence of B‐lines, while >30 B‐lines were considered as severe sonographic PC, according to previous literatures. 16 , 18 The intra‐observer and inter‐observer variability for the B‐lines score were previously assessed by two independent observers in a set of 20 consecutive cases and were 5% and 7%, respectively. No change in therapy was decided because of B‐lines evaluation.
Follow‐up data
Follow‐up data were obtained in all enrolled patients. In patients who died in a hospital or at home, the cause of death was elucidated from the medical records or the local physician who signed the death certificate. The definition of cardiac death required documentation of significant arrhythmias or cardiac arrest, or both, or death attributable to congestive HF or myocardial infarction in the absence of any other precipitating factor. We defined a composite endpoint, which consisted of cardiac death and rehospitalization for AHF. When both events occurred, patients were censored at the time of the first event.
Statistical analysis
Data were analysed with SPSS Version 25.0 (IBM Corp., Armonk, NY, USA). Continuous measures will be expressed as the mean value ± standard deviation or median and interquartile range (IQR). Categorical variables were presented as percentages and were compared using the χ 2 test or the Fisher exact test. ANOVA or Kruskal–Wallis test was used to test the differential distribution of data among groups, with appropriate post hoc corrections for interactions (Tukey–Kramer or Conover test, respectively). Cox proportional‐hazard regression analysis was used to identify predictors of outcome. Multivariate analysis was performed in a stepwise fashion, including the echo‐derived variables with the highest statistical significance at univariate analysis, considering P‐value and χ 2 goodness of fit. We excluded collinearity using variance inflation factor > 5 and privileged categorical variables in the final model to favour a more practical, faster, and easier clinical decision making. 25 Kaplan–Meier curves were constructed, and log‐rank tests were used to test for differences between curves. A P‐value of 0.05 was considered statistically significant.
Results
We divided the overall final population (n = 1021) into patients with acute HFrEF (n = 199), with acute HFpEF (n = 97), and without AHF (n = 725). The main characteristics of the study population are reported in Table 1 , showing that the majority of demographic and ultrasound parameters had a significantly different distribution in the three subgroups. LUS images were interpretable in all patients with a feasibility of 100%, and the time needed for LUS examination was <5 min in all patients. About one‐third of the HF population (n = 94) had pleural effusion at admission, which precluded B‐lines assessment in those scanning sites where pleural effusion was visible; the number of scanning sites that were unavailable to B‐lines assessment in these patients ranged from 1 to 4 on the total of 28 scanning sites, usually at the right and left lung bases. Patients with HFpEF were older and had the highest prevalence of female subjects with arterial hypertension and atrial fibrillation, while HFrEF showed the highest frequency of atherosclerosis risk factor (smoking, diabetes mellitus, and dyslipidaemia) and history of coronary artery disease, including previous revascularization. HFrEF had the worst blood test profile and some worse ultrasound parameters, including dilated IVC without collapse. In contrast, diastolic function parameters and B‐lines number did not show significant differences in both HFrEF and HFpEF: patients with HFrEF had a similar number of B‐lines compared with HFpEF: median 14 (IQR 3–37) vs. 12 (IQR 0–28), P = 0.18; the percentage of patients with >30 B‐lines, although slightly higher in HFrEF than HFpEF, was also not significantly different between the two groups: median 40 (20.1%) vs. 21 (21.6%), P = 0.054. The non‐AHF group showed the most favourable profile: 46 patients (6.3%) had a severe degree of PC (>30 B‐lines), and 56 (7.7%) had a moderate degree of B‐lines (>15 B‐lines). Differences between the subgroup of patients with moderate–severe B‐lines and those with mild or no B‐lines—within the non‐AHF group—are presented in Supporting Information, Table S2 .
Table 1.
Characteristics of the overall population and of different subgroups
| Total population (n = 1021) | Acute HFrEF (n = 199) | Acute HFpEF (n = 97) | Non‐AHF (n = 725) | P‐value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 70 (61–78) | 70 (62–76) # | 73 (66–80) | 70 (60–78) # | 0.01 |
| Female gender | 329 (32%) | 40 (20%) | 56 (54%) | 233 (32%) | <0.0001 |
| Family history of CVD | 440 (42%) | 84 (42%) | 32 (31%) | 324 (74%) | 0.04 |
| Smoking | 417 (40%) | 94 (47%) | 33 (32%) | 290 (40%) | 0.04 |
| Diabetes mellitus | 295 (28%) | 81 (40%) | 35 (34%) | 179 (24%) | <0.0001 |
| Arterial hypertension | 655 (63%) | 115 (57%) | 75 (73%) | 465 (63%) | 0.03 |
| Dyslipidaemia a | 614 (59%) | 136 (67%) | 40 (39%) | 438 (60%) | <0.0001 |
| CAD | 553 (53%) | 120 (59%) | 28 (27%) | 405 (55%) | <0.0001 |
| Previous MI | 97 (9%) | 40 (20%) | 0 (0%) | 57 (8%) | <0.0001 |
| Previous PCI | 219 (21%) | 53 (26%) | 11 (11%) | 155 (21%) | 0.02 |
| Previous CABG | 154 (15%) | 46 (23%) | 8 (8%) | 100 (14%) | 0.001 |
| Atrial fibrillation | 181 (17%) | 50 (25%) | 34 (33%) | 97 (13%) | <0.0001 |
| Creatinine (mg/dL) | 1.04 (0.99–1.27) | 1.23 (0.99–1.54) # | 1.04 (0.85–1.29) | 1.01 (0.89–1.18) § | <0.0001 |
| NT‐proBNP (pg/mL) | 821 (50–1662) | 2191 (1066–5249) # | 1345 (416–2660) | 423 (131–931) # , § | <0.0001 |
| CRP (mg/L) | 0.28 (0.10–0.89) | 0.38 (0.15–1.11) | 0.37 (0.15–1.01) | 0.22 (0.08–0.6) § | 0.003 |
| NYHA Class III–IV | 159 (15%) | 103 (52%) | 38 (39%) | 0 (0%) | <0.0001 |
| Ultrasound parameters | |||||
| EDV (mL/m2) | 124 (97–160) | 195 (162–233) # | 124 (97–154) | 107 (92–130) § | <0.0001 |
| ESV (mL/m2) | 54 (35–92) | 131 (97–174) # | 54 (35–83) | 40 (32–58) § | <0.0001 |
| LV ejection fraction (%) | 55 (39–60) | 30 (23–35) # | 55 (50–60) | 55 (52–60) # , § | <0.0001 |
| WMSI | 1.0 (1.0–1.9) | 2.1 (2.0–2.4) # | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) # , § | <0.0001 |
| LVMi (g/m2) | 109 (90–137) | 149 (123–172) # | 124 (101–146) | 97 (82–115) # , § | <0.0001 |
| E wave (cm/s) | 80 (62.5–101) | 84 (62–109) # | 104 (81–130) | 75 (61–90) # , § | <0.0001 |
| E‐wave deceleration time (ms) | 213 (171–260) | 174 (150–228) # | 206 (167–254) | 225 (190–270) § | <0.0001 |
| E/A ratio | 0.9 (0.7–1.8) | 1.0 (0.6–2.0) | 1.0 (0.7–1.5) | 0.9 (0.7–1.1) # , § | <0.0001 |
| E/e′ ratio | 12.1 (11.3–12.8) | 14.4 (11.2–19.8) | 14.7 (10.7–21.2) | 9.2 (7.1–11.8) # , § | <0.0001 |
| LA anteroposterior diameter (mm) | 40 (37–45) | 45 (40–50) | 44 (40–48) | 39 (35–43) # , § | <0.0001 |
| Mitral regurgitation b | 247 (24%) | 115 (57%) | 40 (39%) | 92 (13%) | <0.0001 |
| Aortic regurgitation b | 55 (5%) | 16 (8%) | 14 (14%) | 25 (3%) | <0.0001 |
| Aortic stenosis b | 57 (6%) | 12 (6%) | 17 (17%) | 28 (4%) | <0.0001 |
| TAPSE (mm) | 19 (16–23) | 17 (14–20) # | 18 (15–22) | 21 (17–24) # , § | <0.0001 |
| TAPSE < 17 mm | 181 (17%) | 72 (36%) | 15 (15%) | 94 (13%) | <0.0001 |
| TR velocity (m/s) | 2.83 (2.50–3.24) | 3.04 (2.50–3.46) | 3.16 (2.69–3.53) | 2.73 (2.50–3.04) # , § | <0.0001 |
| TR velocity > 2.8 m/s | 516 (50%) | 137 (68%) | 71 (69%) | 308 (42%) | <0.0001 |
| IVC expiratory diameter (mm) | 16 (13–21) | 21 (17–24) # | 18 (15–22) | 16 (13–18) # , § | <0.0001 |
| Dilated IVC without collapse c | 153 (15%) | 57 (28%) | 23 (22%) | 73 (10%) | <0.0001 |
| B‐lines at admission | 3 (0–25) | 14 (3–37) | 12 (0–28) | 0 (0–19) # , § | <0.0001 |
| B‐lines < 5 | 541 (53.0%) | 48 (24.1%) | 31 (32.0%) | 462 (63.7%) # , § | <0.0001 |
| B‐lines 5–15 | 234 (22.9%) | 48 (24.1%) | 25 (25.8%) | 161 (22.3%) | 0.665 |
| B‐lines 16–30 | 117 (11.5%) | 40 (20.1%) | 21 (21.6%) | 56 (7.7%) # , § | <0.0001 |
| B‐lines ≥ 30 | 129 (12.6%) | 63 (31.7%) | 20 (20.6%) | 46 (6.3%) # , § | <0.0001 |
| Clinical follow‐up | |||||
| Cardiac death | 69 (6.7%) | 32 (16.1%) | 8 (8.2%) | 29 (4.0%) § | <0.0001 |
| Rehospitalization for AHF | 57 (5.6%) | 30 (15.1%) | 12 (12.4%) | 16 (2.2%) # , § | 0.001 |
| Composite endpoint | 126 (12.3%) | 62 (31.2%) # | 20 (20.6%) | 45 (6.2%) # , § | <0.0001 |
AHF, acute heart failure; CABG, coronary artery bypass graft; CAD, coronary artery disease; CRP, C‐reactive protein; CVD, cardiovascular disease; EDV, end‐diastolic volume; ESV, end‐systolic volume; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IVC, inferior vena cava; LA, left atrial; LV, left ventricular; LVMi, LV mass index; MI, myocardial infarction; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation; WMSI, wall motion score index.
Data are presented as number and %, mean and 95% confidence interval if normally distributed, or median and first and third quartile if not normally distributed.
Total cholesterol ≥ 200 mg/dL or LDL‐C ≥ 130 mg/dL or on lipid‐lowering therapy.
At least moderate severity.
IVC expiratory diameter > 21 mm that collapses <50% with a sniff.
P < 0.01 vs. acute HFpEF.
P < 0.01 vs. acute HFrEF.
A CXR at admission was available in 976/1021 patients. We divided the patients according to the CXR report, between CXR with signs of interstitial oedema and CXR without signs of interstitial oedema. The distribution of B‐lines in patients with and without radiological signs of interstitial oedema is shown in Table 2 . Patients with HFrEF had radiological signs of interstitial oedema in 60.8% of cases, patients with HFpEF in 66% (P = 0.861 vs. HFrEF), and patients without AHF in 14.9% (P < 0.0001 vs. HFrEF, P < 0.0001 vs. HFpEF, P < 0.0001 among groups).
Table 2.
Comparison between lung ultrasound B‐lines and chest X‐ray (CXR) signs of interstitial oedema
| CXR without signs of interstitial oedema (n = 690) | CXR with signs of interstitial oedema (n = 286) | P | |
|---|---|---|---|
| B‐lines at admission | 0 (0–7) | 23 (5–51) | <0.0001 |
| B‐lines < 5 | 447 (64.8%) | 65 (22.7%) | <0.0001 |
| B‐lines 5–15 | 178 (25.8%) | 44 (15.4%) | <0.0001 |
| B‐lines 16–30 | 53 (7.7) | 60 (21.0%) | <0.0001 |
| B‐lines > 30 | 12 (1.7%) | 117 (41.3%) | <0.0001 |
Data are presented as median and first and third quartile, and number and %.
During a median follow‐up of 14.4 months (IQR 4.6–24.3), a total of 126 events (12.3%) occurred: 69 (6.7%) cardiac deaths and 57 (5.6%) rehospitalizations for AHF. Adverse events were more frequent in patients with HFrEF and HFpEF vs. non‐AHF group (Table 1 ). The event‐free survival curves showed a significantly better outcome for patients with ≤30 B‐lines at admission, compared with patients with >30 B‐lines, in both the overall population and each subgroup (Figure 1 ). We included cardiopulmonary ultrasound independent predictors of the composite endpoint at univariate analysis (Supporting Information, Table S1 ) in a multivariate model (Table 3 ). A small number of variables along with B‐lines were selected to avoid over‐fitting, after collinearity analysis: TR velocity > 2.8 m/s, LV ejection fraction < 50%, and TAPSE < 17 mm. B‐lines at admission showed an independent prognostic value in the overall population [hazard ratio (HR) 2.45, confidence interval (CI) 1.49–4.02, P < 0.0001], in acute HFpEF (HR 5.54, CI 1.35–22.73, P = 0.017), and non‐AHF group (HR 3.04, CI 1.12–8.26, P = 0.014), while the presence of TAPSE < 17 mm was the only independent predictor of the composite endpoint in acute HFrEF (HR 2.14, CI 1.12–4.07, P = 0.021). To assess whether B‐lines have additional value over CXR, we built a regression model including CXR signs of interstitial oedema; B‐lines > 30 remain an independent predictor of events even when analysed together with CXR: B‐lines > 30 HR 2.55, CI 1.62–4.00, P < 0.0001; CXR HR 2.15, CI 1.38–3.36, P < 0.001. When adding CXR signs of interstitial oedema at the multivariate model that included also echocardiographic parameters, CXR was no more an independent predictor of events (HR 1.27, CI 0.70–2.31, P = 0.430), whereas B‐lines > 30 were still an independent predictor of events (HR 2.17, CI 1.20–3.91, P = 0.010).
Figure 1.
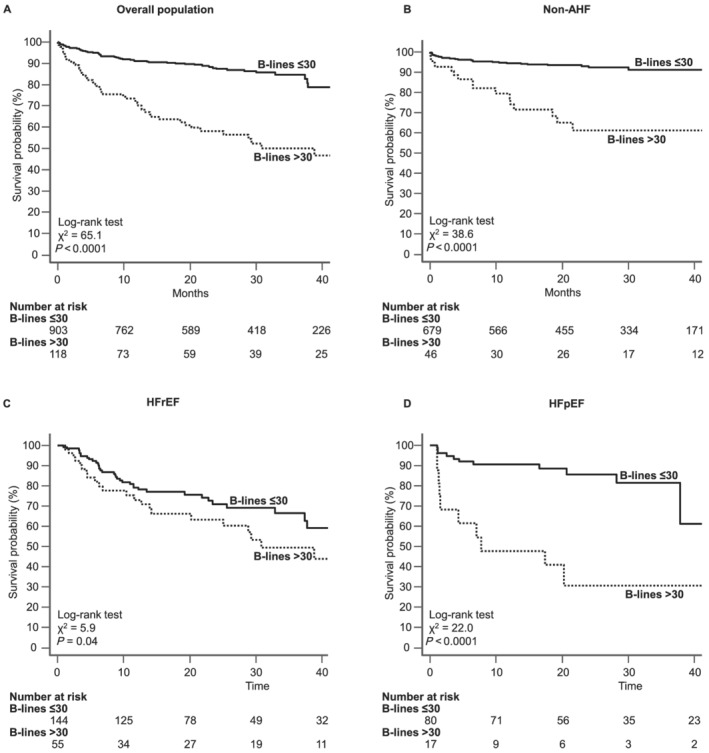
Kaplan–Meier survival curves for the primary endpoint (cardiac death and hospitalization for AHF) in the overall population (A), non‐AHF group (B), HFrEF (C), and HFpEF (D). The presence of B‐lines > 30 (dotted line) significantly increased the incidence of adverse events compared with patients with B‐lines ≤ 30 (solid line) in the overall population and the three subgroups. AHF, acute heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Table 3.
Multivariable analysis of cardiopulmonary ultrasound parameters to predict the composite endpoint (cardiac mortality and rehospitalization for AHF) in the overall population, non‐AHF, HFrEF, and HFpEF
| Overall population (n = 1021) | Acute HFrEF (n = 199) | Acute HFpEF (n = 97) | Non‐AHF (n = 725) | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| B‐lines > 30 | 2.45 (1.49–4.02) | <0.0001 | 1.63 (0.86–3.09) | 0.12 | 5.54 (1.35–22.73) | 0.017 | 3.044 (1.12–8.26) | 0.029 |
| TR velocity > 2.8 m/s | 1.89 (1.15–3.02) | 0.011 | 1.05 (0.53–2.05) | 0.89 | 2.41 (0.30–19.45) | 0.41 | 2.05 (0.94–4.44) | 0.070 |
| LVEF < 50% | 2.30 (1.38–3.84) | 0.001 | — | — | — | — | 0.37 (0.17–0.82) | 0.014 |
| TAPSE < 17 mm | 1.61 (1.01–2.56) | 0.044 | 2.14 (1.12–4.07) | 0.021 | 2.79 (0.68–11.46) | 0.16 | 0.96 (0.40–2.26) | 0.918 |
AHF, acute heart failure; CI, confidence interval; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; LVEF, left ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Note: Numbers marked in bold indicate numbers that are significant.
In the subgroup of patients without AHF, 462 patients had non‐significant B‐lines (<5 B‐lines, among which 374 had 0 B‐lines), and 263 patients had some degree of sonographic PC: mild (5–15 B‐lines) in 161 (22.2%) patients, moderate (16–30 B‐lines) in 56 (7.7%) patients, and severe in only 46 (6.3%) patients (>30 B‐lines), with 14% of patients with moderate–severe degree of sonographic congestion. Similarly, 103/691 patients (14.9%) had signs of interstitial oedema on CXR. When we evaluated the prognostic value of LUS and CXR in this subgroup, we found that at univariate analysis, both LUS and CXR are significantly associated with events (B‐lines > 30 HR 6.30, CI 3.30–12.01, P < 0.0001; CXR HR 3.58, CI 1.93–6.64, P < 0.0001), but at multivariate analysis, radiological signs of interstitial oedema are no longer an independent prognosticator (B‐lines > 30 HR 4.39, CI 1.77–10.84, P < 0.0001; CXR HR 1.67, CI 0.71–3.95, P = 0.24).
B‐lines presented a significant direct correlation with TR velocity and indirect correlation with LV ejection fraction and TAPSE (all P < 0.0001; Table 4 ). Figure 2 summarizes the results of the integrated cardiopulmonary evaluation in three patients belonging to the three different groups (one admitted for ICD replacement, one for acute HFrEF, and one for acute HFpEF).
Table 4.
Correlation between B‐lines and the ultrasound parameters that were found to be independent predictors of the composite endpoint
| Ultrasound parameter | Correlation coefficient r | P‐value |
|---|---|---|
| TR velocity (m/s) | 0.42 | <0.0001 |
| LV ejection fraction (%) | −0.32 | <0.0001 |
| TAPSE | −0.23 | <0.0001 |
LV, left ventricular; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Figure 2.
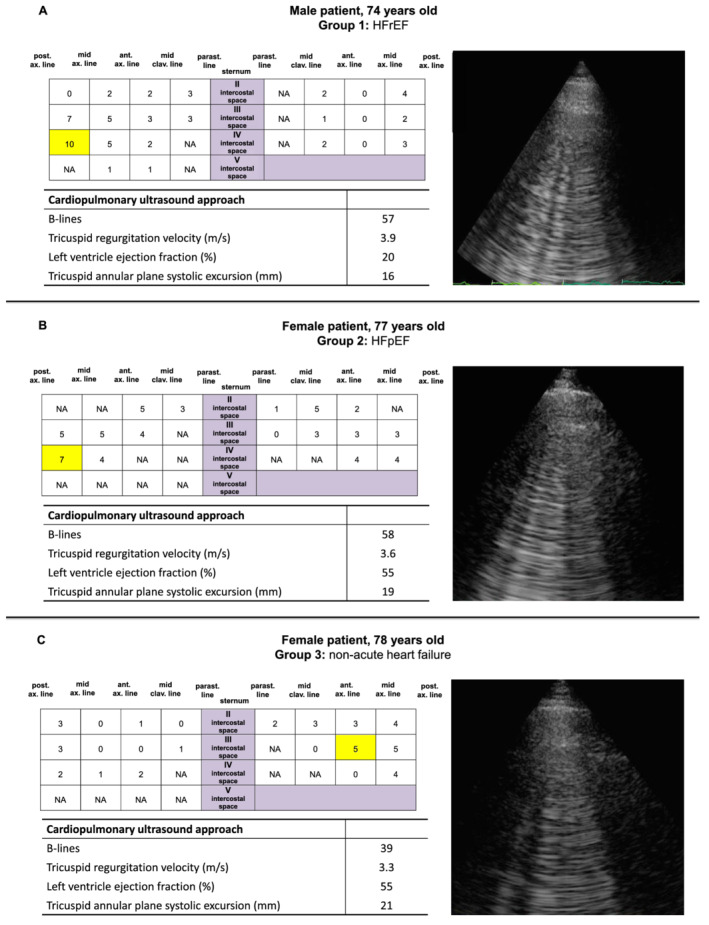
The integrated cardiopulmonary evaluation in three different patients from each group of the study: (A) Group 1: HFrEF; (B) Group 2: HFpEF; and (C) Group 3: subject with dilated cardiomyopathy and reduced ejection fraction admitted for implanted cardioverter defibrillator replacement, without signs and symptoms of acute heart failure. The scheme with 28 scanning sites shows the distribution of B‐lines. The image displayed for each example refers to the highlighted area in the scheme. HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NA, not available because images were not interpretable.
Discussion
In patients admitted to a cardiology department for various cardiac conditions, the presence of a severe PC at admission evaluated by LUS (B‐lines > 30) identifies a subgroup at higher risk of events, independently of the presence of overt AHF and the aetiology. The independent prognostic value of B‐lines is especially significant in HFpEF compared with HFrEF and is valid also in patients without apparent overt AHF.
Heart failure is the most frequent admitting diagnosis in cardiology departments, and congestion rather than low cardiac output is the primary pathophysiological event leading to hospitalization. 26 However, PC is related to increased EVLW and has an asymptomatic phase that offers the possibility to anticipate the clinical stage and guide the therapy. 27 B‐lines are a marker of pulmonary interstitial oedema, and it is reasonable that their severity is associated with a worse outcome. 12 , 13 , 16 , 17 , 18 , 19 , 20 Previous studies have demonstrated that B‐lines predict adverse events in chronic HF. 12 , 13 Likewise, the value of B‐lines at discharge and their dynamic changes from admission have been linked to adverse outcomes also in AHF. 16 , 17 , 18 , 19 , 20 To the best of our knowledge, this is the first study to address the prognostic value of LUS very early during admission (i.e. the first day of the hospitalization) in a large cohort and is the first study including patients with heart disease, hospitalized for reasons other than AHF, and to analyse HFrEF and HFpEF separately. Our findings confirm the independent prognostic role of LUS in a large population of consecutive patients both with and without AHF, suggesting the added value of LUS independently of the initial diagnosis. The predicted power of LUS also in the non‐AHF group reveals that a non‐negligible number of patients with apparently no features of AHF and seemingly stable haemodynamic status carry a significant degree of pulmonary interstitial oedema indeed, with prognostic relevance. In particular, we observed B‐lines > 30 in 6.3% and B‐lines > 15 in 14.2% of patients, mainly in those with a history of HF but admitted for other cause (e.g. elective coronary angiography, elective electrophysiological study, and pacemaker or ICD implantation/replacement). This implies that a high percentage of patients with HF thought to be free of congestion, i.e. clinically stable, have instead silent ongoing PC, which has an impact on outcomes. Our results are in line with Pellicori et al. who observed that many stable outpatients with chronic HF have subclinical PC, which mediates a worse prognosis, 13 and with Platz et al. who recently demonstrated that a higher number of B‐lines at admission in patients with AHF are linked to adverse events, even adjusting for creatinine and N‐terminal prohormone of brain natriuretic peptide values. 20
The different prognostic relevance of B‐lines, which is more reliable in patients with acute HFpEF compared with HFrEF, had also not been reported in previous literature. In patients with acute HFrEF, the presence of a TAPSE < 17 mm, reflecting the critical role of concomitant RV dysfunction, has a higher impact than admission B‐lines in predicting the composite endpoint. In our study, the number of B‐lines at admission was similar between HFrEF and HFpEF [median 14 (IQR 3–37) vs. median 12 (IQR 0.24), P = 0.12], consistently with other cohorts. However, our patients with HFrEF presented more often an RV dysfunction and a dilated IVC without inspiratory collapse than HFpEF, possibly suggesting a higher burden of systemic congestion. Indeed, systemic congestion is a typical feature of HFrEF more than HFpEF, 28 whereas patients with HFpEF often exhibit more PC with little weight increase. 29 Thus, the discrepancy in pulmonary and systemic haemodynamics could explain the different prognostic value of B‐lines we observed in HFrEF and HFpEF. At the same time, HFrEF is usually more easily recognized and better treated than HFpEF, which represents a more heterogeneous and challenging syndrome. 6 , 22 , 30 Noteworthy, the absence of evidence‐based therapy in patients with HFpEF should encourage further research to determine whether management guided by ultrasonic measures of PC could improve outcome.
Clinical implications
Lung ultrasound is readily available in the clinical routine and can provide a large amount of information in terms of the degree of decompensation and risk stratification. 31 Although counting B‐lines shows some degree of intra‐rater and inter‐rater variability, 32 the number of B‐lines and their distribution are highly informative and help to differentiate between mild degrees of PC (few B‐lines localized in the lateral scanning sites especially at pulmonary bases) and severe degrees of PC (multiple and diffuse B‐lines, fanning out also on the more apical regions of the chest). 2 Prior studies using LUS protocols demonstrated the prognostic importance of a higher number of B‐lines at the time of discharge after admission for AHF. 16 , 17 , 18 , 19 , 20 Recently, the LUS‐HF study has shown that tailored LUS‐guided diuretic treatment of PC reduced the number of decompensations and improved walking capacity in patients with HF. 33 Our study expands on these findings evaluating a more extensive, heterogeneous population with different types of heart disease at admission and for a longer follow‐up. We demonstrated that LUS might provide useful information regardless of the admitting diagnosis because sonographic signs of PC at admission are associated with an increased risk for rehospitalization for HF and cardiac death even in patients without conventional signs and symptoms of AHF at admission. Moreover, the more definite prognostic value of B‐lines in HFpEF compared with HFrEF underlines the relevance of this biomarker in HFpEF, opening promising horizons for the management of this complex condition.
Limitations
This is a single‐centre study with a broad, unselected population with different aetiologies of heart disease. This limitation, however, reflects the potentialities of LUS B‐lines in different clinical scenarios, including patients without signs and symptoms of overt AHF at admission. It should be emphasized that B‐lines are a non‐specific sign of PC and can also be found in interstitial pulmonary fibrosis, interstitial pneumonia, and acute respiratory distress syndrome. 2 This must be reminded, especially when using B‐lines for the differential diagnosis of dyspnoea. Interpreting B‐lines not as an isolated imaging biomarker but in the context of the overall clinical picture and other findings is the key to avoid gross misinterpretation of this sign. LUS was performed as soon as possible after patients' admission, but in all patients with AHF, the first diuretic dose was administered before the echocardiogram and LUS examinations, not to delay life‐saving treatment for the patients. We did not report data on LUS at discharge because they were available only in a minority of the population. The sonographers did the LUS exam at the end of the echocardiogram; therefore, they were not completely blind to the patients' conditions. We used a 28‐zone imaging protocol, which is more time‐consuming than the simplified four‐zone or eight‐zone schemes 12 , 34 ; however, even a more extensive assessment requires only a few minutes in addition to the time needed for a resting echocardiogram and assures interpretable images in all patients. We excluded tissue Doppler imaging‐derived E/e′ ratio from the multivariate analysis due to collinearity with B‐lines at admission (variance inflation factor = 7), and we did not measure more sophisticated methods (e.g. strain imaging). However, this is a ‘real‐world’ analysis, and one of the strengths of the study is the simplicity of the proposed approach, based on very basic echocardiographic parameters that could easily fit in the workflow of a busy clinical arena.
Conclusions
Ultrasound B‐lines can detect subclinical pulmonary interstitial oedema even in patients thought to be free of congestion. A severe degree of PC defined by >30 B‐lines on the anterolateral chest strongly predicts HF rehospitalization and cardiac death in patients with different cardiac conditions, irrespective of the clinical picture at admission. The prognostic value of LUS is especially relevant in HFpEF compared with HFrEF. An integrated cardiopulmonary approach represents an easy, fast, and bedside tool to improve prognostic stratification of patients with different types of heart disease and different degrees of decompensation.
Supporting information
Table S1. Univariable analysis of cardiopulmonary ultrasound parameters to predict the composite endpoint (cardiac mortality and hospitalization for worsening heart failure) in the overall population.
Table S2. Characteristics of non‐AHF patients according to B‐lines degree.
Gargani, L. , Pugliese, N. R. , Frassi, F. , Frumento, P. , Poggianti, E. , Mazzola, M. , De Biase, N. , Landi, P. , Masi, S. , Taddei, S. , Pang, P. S. , and Sicari, R. (2021) Prognostic value of lung ultrasound in patients hospitalized for heart disease irrespective of symptoms and ejection fraction. ESC Heart Failure, 8: 2660–2669. 10.1002/ehf2.13206.
References
- 1. Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JEA, Cleland JG, Dickstein K, Drazner MH, Fonarow GC, Jaarsma T, Jondeau G, Sendon JL, Mebazaa A, Metra M, Nieminen M, Pang PS, Seferovic P, Stevenson LW, Van Veldhuisen DJ, Zannad F, Anker SD, Rhodes A, McMurray JJV, Filippatos G. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European society of cardiology and endorsed by the European society of intensive care medicine. Eur J Heart Fail John Wiley & Sons, Ltd 2010; 12: 423–433. [DOI] [PubMed] [Google Scholar]
- 2. Gargani L. Ultrasound of the lungs. Heart Fail Clin 2019; 15: 297–303. [DOI] [PubMed] [Google Scholar]
- 3. Agricola E, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, Picano E. ‘Ultrasound comet‐tail images’: a marker of pulmonary edema—a comparative study with wedge pressure and extravascular lung water. Chest Elsevier 2005; 127: 1690–1695. [DOI] [PubMed] [Google Scholar]
- 4. Gargani L, Frassi F, Soldati G, Tesorio P, Gheorghiade M, Picano E. Ultrasound lung comets for the differential diagnosis of acute cardiogenic dyspnoea: a comparison with natriuretic peptides. Eur J Heart Fail 2008; 10: 70–77. [DOI] [PubMed] [Google Scholar]
- 5. Miglioranza MH, Gargani L, Sant'Anna RT, Rover MM, Martins VM, Mantovani A, Weber C, Moraes MA, Feldman CJ, Kalil RAK, Sicari R, Picano E, Leiria TLL. Lung ultrasound for the evaluation of pulmonary congestion in outpatients: a comparison with clinical assessment, natriuretic peptides, and echocardiography. JACC Cardiovasc Imaging 2013; 6: 1141–1151. [DOI] [PubMed] [Google Scholar]
- 6. Pugliese NR, Mazzola M, Fabiani I, Gargani L, De Biase N, Pedrinelli R, Natali A, Dini FL. Haemodynamic and metabolic phenotyping of hypertensive patients with and without heart failure by combining cardiopulmonary and echocardiographic stress test. Eur J Heart Fail John Wiley and Sons Ltd 2020; 22: 1–11. [DOI] [PubMed] [Google Scholar]
- 7. Frassi F, Gargani L, Gligorova S, Ciampi Q, Mottola G, Picano E. Clinical and echocardiographic determinants of ultrasound lung comets. Eur J Echocardiogr 2007; 8: 474–479. [DOI] [PubMed] [Google Scholar]
- 8. Bedetti G, Gargani L, Corbisiero A, Frassi F, Poggianti E, Mottola G. Evaluation of ultrasound lung comets by hand‐held echocardiography. Cardiovasc Ultrasound BioMed Central 2006; 4: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, Ristic AD, Lambrinou E, Masip J, Riley JP, McDonagh T, Mueller C, Defilippi C, Harjola VP, Thiele H, Piepoli MF, Metra M, Maggioni A, McMurray J, Dickstein K, Damman K, Seferovic PM, Ruschitzka F, Leite‐Moreira AF, Bellou A, Anker SD, Filippatos G. Recommendations on pre‐hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergenc. Eur J Heart Fail John Wiley & Sons, Ltd 2015; 17: 544–548. [DOI] [PubMed] [Google Scholar]
- 10. Girerd N, Seronde MF, Coiro S, Chouihed T, Bilbault P, Braun F, Kenizou D, Maillier B, Nazeyrollas P, Roul G, Fillieux L, Abraham WT, Januzzi J, Sebbag L, Zannad F, Mebazaa A, Rossignol P. Integrative assessment of congestion in heart failure throughout the patient journey. JACC Hear Fail Elsevier Inc 2018; 6: 273–285. [DOI] [PubMed] [Google Scholar]
- 11. Pivetta E, Goffi A, Nazerian P, Castagno D, Tozzetti C, Tizzani P, Tizzani M, Porrino G, Ferreri E, Busso V, Morello F, Paglieri C, Masoero M, Cassine E, Bovaro F, Grifoni S, Maule MM, Lupia E, Paolo B, Alessia B, Giuseppina B, Andrea C, Andrea C, Ottavio D, Paola DR, Andrea E, Paolo FP, Patrizia F, Daniela F, Francesca G. Lung ultrasound integrated with clinical assessment for the diagnosis of acute decompensated heart failure in the emergency department: a randomized controlled trial. Eur J Heart Fail 2019; 21: 754–766. [DOI] [PubMed] [Google Scholar]
- 12. Platz E, Lewis EF, Uno H, Peck J, Pivetta E, Merz AA, Hempel D, Wilson C, Frasure SE, Jhund PS, Cheng S, Solomon SD. Detection and prognostic value of pulmonary congestion by lung ultrasound in ambulatory heart failure patients. Eur Heart J Narnia 2016; 37: 1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pellicori P, Shah P, Cuthbert J, Urbinati A, Zhang J, Kallvikbacka‐Bennett A, Clark AL, Cleland JGF. Prevalence, pattern and clinical relevance of ultrasound indices of congestion in outpatients with heart failure. Eur J Heart Fail 2019; 21: 904–916. [DOI] [PubMed] [Google Scholar]
- 14. Fabiani I, Pugliese NR, Galeotti GG, D'Agostino A, Mazzola M, Pedrinelli R, Dini FL. The added value of exercise stress echocardiography in patients with heart failure. Am J Cardiol 2019; 123: 1470–1477. [DOI] [PubMed] [Google Scholar]
- 15. Rivas‐Lasarte M, Maestro A, Fernández‐Martínez J, López‐López L, Solé‐González E, Vives‐Borrás M, Montero S, Mesado N, Pirla MJ, Mirabet S, Fluvià P, Brossa V, Sionis A, Roig E, Cinca J, Álvarez‐García J. Prevalence and prognostic impact of subclinical pulmonary congestion at discharge in patients with acute heart failure. ESC Hear Fail John Wiley & Sons, Ltd 2020; 7: 2621–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frassi F, Gargani L, Tesorio P, Raciti M, Mottola G, Picano E. Prognostic value of extravascular lung water assessed with ultrasound lung comets by chest sonography in patients with dyspnea and/or chest pain. J Card Fail 2007; 13: 830–835. [DOI] [PubMed] [Google Scholar]
- 17. Gargani L, Pang PS, Frassi F, Miglioranza MH, Dini FL, Landi P, Picano E. Persistent pulmonary congestion before discharge predicts rehospitalisation in heart failure: a lung ultrasound study. Cardiovasc Ultrasound 2015; 13: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coiro S, Porot G, Rossignol P, Ambrosio G, Carluccio E, Tritto I, Huttin O, Lemoine S, Sadoul N, Donal E, Zannad F, Girerd N. Prognostic value of pulmonary congestion assessed by lung ultrasound imaging during heart failure hospitalisation: a two‐centre cohort study. Sci Rep Nature Publishing Group 2016; 6: 39426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Palazzuoli A, Ruocco G, Beltrami M, Nuti R, Cleland JG. Combined use of lung ultrasound, B‐type natriuretic peptide, and echocardiography for outcome prediction in patients with acute HFrEF and HFpEF. Clin Res Cardiol Springer Berlin Heidelberg 2018; 107: 586–596. [DOI] [PubMed] [Google Scholar]
- 20. Platz E, Campbell RT, Claggett B, Lewis EF, Groarke JD, Docherty KF, Lee MMY, Merz AA, Silverman M, Swamy V, Lindner M, Rivero J, Solomon SD, McMurray JJV. Lung ultrasound in acute heart failure: prevalence of pulmonary congestion and short‐ and long‐term outcomes. JACC Hear Fail 2019; 7: 849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cogliati C, Casazza G, Ceriani E, Torzillo D, Furlotti S, Bossi I, Vago T, Costantino G, Montano N. Lung ultrasound and short‐term prognosis in heart failure patients. Int J Cardiol 2016; 218: 104–108. [DOI] [PubMed] [Google Scholar]
- 22. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Filippatos G, McMurray JJV, Aboyans V, Achenbach S, Agewall S, Al‐Attar N, Atherton JJ, Bauersachs J, John Camm A. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J Narnia 2016; 37: 2129–2200.27206819 [Google Scholar]
- 23. Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, Melniker L, Gargani L, Noble VE, Via G, Dean A, Tsung JW, Soldati G, Copetti R, Bouhemad B, Reissig A, Agricola E, Rouby JJ, Arbelot C, Liteplo A, Sargsyan A, Silva F, Hoppmann R, Breitkreutz R, Seibel A, Neri L, Storti E, Petrovic T. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med Springer‐Verlag; 2012; 38: 577–591. [DOI] [PubMed] [Google Scholar]
- 24. Platz E, Jhund PS, Girerd N, Pivetta E, McMurray JJV, Peacock WF, Masip J, Martin‐Sanchez FJ, Miró Ò, Price S, Cullen L, Maisel AS, Vrints C, Cowie MR, DiSomma S, Bueno H, Mebazaa A, Gualandro DM, Tavares M, Metra M, Coats AJS, Ruschitzka F, Seferovic PM, Mueller C. Expert consensus document: reporting checklist for quantification of pulmonary congestion by lung ultrasound in heart failure. Eur J Heart Fail John Wiley and Sons Ltd 2019; 21: 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hsieh FY, Lavori PW, Cohen HJ, Feussner JR. An overview of variance inflation factors for sample‐size calculation. Eval Health Prof 2003; 26: 239–257. [DOI] [PubMed] [Google Scholar]
- 26. Zile MR, Bennett TD, St. John Sutton M, Cho YK, Adamson PB, Aaron MF, Aranda JM, Abraham WT, Smart FW, Stevenson LW, Kueffer FJ, Bourge RC. Transition from chronic compensated to acute d compensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008; 118: 1433–1441. [DOI] [PubMed] [Google Scholar]
- 27. Yu CM, Wang L, Chau E, Chan RHW, Kong SL, Tang MO, Christensen J, Stadler RW, Lau CP. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalisation. Circulation 2005; 112: 841–848. [DOI] [PubMed] [Google Scholar]
- 28. Hsu JJ, Ziaeian B, Fonarow GC. Heart failure with mid‐range (borderline) ejection fraction: clinical implications and future directions. JACC Hear Fail 2017; 5: 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palazzuoli A, Evangelista I, Nuti R. Congestion occurrence and evaluation in acute heart failure scenario: time to reconsider different pathways of volume overload. Heart Fail Rev Springer New York LLC 2019; 25: 119–131. [DOI] [PubMed] [Google Scholar]
- 30. Pugliese NR, Fabiani I, Santini C, Rovai I, Pedrinelli R, Natali A, Dini FL. Value of combined cardiopulmonary and echocardiography stress test to characterise the haemodynamic and metabolic responses of patients with heart failure and mid‐range ejection fraction. Eur Heart J Cardiovasc Imaging 2019; 20: 828–836. [DOI] [PubMed] [Google Scholar]
- 31. Gargani L. Lung ultrasound: a new tool for the cardiologist. Cardiovasc Ultrasound 2011; 9: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gargani L, Sicari R, Raciti M, Serasini L, Passera M, Torino C, Letachowicz K, Ekart R, Fliser D, Covic A, Balafa O, Stavroulopoulos A, Massy ZA, Fiaccadori E, Caiazza A, Bachelet T, Slotki I, Shavit L, Martinez‐Castelao A, Coudert‐Krier MJ, Rossignol P, Kraemer TD, Hannedouche T, Panichi V, Wiecek A, Pontoriero G, Sarafidis P, Klinger M, Hojs R, Seiler‐Mußler S, Lizzi F, Onofriescu M, Zarzoulas F, Tripepi R, Mallamaci F, Tripepi G, Picano E, London GM, Zoccali C. Efficacy of a remote web‐based lung ultrasound training for nephrologists and cardiologists: a lust trial sub‐project. Nephrol Dial Transplant 2016; 31: 1982–1988. [DOI] [PubMed] [Google Scholar]
- 33. Rivas‐Lasarte M, Álvarez‐García J, Fernández‐Martínez J, Maestro A, López‐López L, Solé‐González E, Pirla MJ, Mesado N, Mirabet S, Fluvià P, Brossa V, Sionis A, Roig E, Cinca J. Lung ultrasound‐guided treatment in ambulatory patients with heart failure: a randomized controlled clinical trial (LUS‐HF study). Eur J Heart Fail 2019; 21: 1605–1613. [DOI] [PubMed] [Google Scholar]
- 34. Buessler A, Chouihed T, Duarte K, Bassand A, Huot‐Marchand M, Gottwalles Y, Pénine A, André E, Nace L, Jaeger D, Kobayashi M, Coiro S, Rossignol P, Girerd N. Accuracy of several lung ultrasound methods for the diagnosis of acute heart failure in the ED. Chest 2019; 157: 99–110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariable analysis of cardiopulmonary ultrasound parameters to predict the composite endpoint (cardiac mortality and hospitalization for worsening heart failure) in the overall population.
Table S2. Characteristics of non‐AHF patients according to B‐lines degree.


