Abstract
Aims
Heart failure (HF) guidelines place patients into 3 discrete groups according to left ventricular ejection fraction (LVEF): reduced (<40%), mid‐range (40–49%), and preserved LVEF (≥50%). We assessed whether clinical phenogroups offer better prognostication than LVEF.
Methods and results
This was a sub‐study of the Patient‐Centered Care Transitions in HF trial. We analysed baseline characteristics of hospitalized patients in whom LVEF was recorded. We used unsupervised machine learning to identify clinical phenogroups and, thereafter, determined associations between phenogroups and outcomes. Primary outcome was the composite of all‐cause death or rehospitalization at 6 and 12 months. Secondary outcome was the composite cardiovascular death or HF rehospitalization at 6 and 12 months. Cluster analysis of 1693 patients revealed six discrete phenogroups, each characterized by a predominant comorbidity: coronary heart disease, valvular heart disease, atrial fibrillation (AF), sleep apnoea, chronic obstructive pulmonary disease (COPD), or few comorbidities. Phenogroups were LVEF independent, with each phenogroup encompassing a wide range of LVEFs. For the primary composite outcome at 6 months, the hazard ratios (HRs) for phenogroups ranged from 1.25 [95% confidence interval (CI) 1.00–1.58 for AF] to 2.04 (95% CI 1.62–2.57 for COPD) (log‐rank P < 0.001); and at 12 months, the HRs for phenogroups ranged from 1.15 (95% CI 0.94–1.41 for AF) to 1.87 (95% 1.52–3.20 for COPD) (P < 0.002). LVEF‐based classifications did not separate patients into different risk categories for the primary outcomes at 6 months (P = 0.69) and 12 months (P = 0.30). Phenogroups also stratified risk of the secondary composite outcome at 6 and 12 months more effectively than LVEF.
Conclusion
Among patients hospitalized for HF, clinical phenotypes generated by unsupervised machine learning provided greater prognostic information for a composite of clinical endpoints at 6 and 12 months compared with LVEF‐based categories.
Trial Registration: ClinicalTrials.gov Identifier: NCT02112227
Keywords: Heart failure, Ejection fraction, Comorbidities, Characteristics, HFpEF, HFmrEF, Phenogroups, Prognosis, Mortality/Survival, Aetiology, Machine Learning, Clinical Studies, Risk Factors
Introduction
Current guidelines for the diagnosis and treatment of heart failure (HF) classify patients into three groups according to left ventricular (LV) ejection fraction (LVEF). 1 , 2 An LVEF of 40–49% (mid‐range LVEF [mrEF]) is used to separate patients with HF and reduced ejection fraction (HFrEF; LVEF <40%) from those with HF and preserved ejection fraction (HFpEF; LVEF ≥50%), although the HFmrEF category is much debated, with some arguing for HFrEF and HFpEF to meet at an LVEF of 45%. 3 Regardless, these cut‐offs were established because patients with a lower LVEF have higher mortality and risk of hospitalization. 4 Most large randomized trials in HF restricted their population to patients with an LVEF below 40% to maximize event rates, an approach which has shown important clinical benefit for several drugs and devices. 5 In society guidelines, these therapies have accordingly been recommended for HFrEF patients, 1 , 2 while current evidence indicates that many treatments are effective in HF patients with LVEF well above 40%. 6 , 7 , 8 , 9
While LVEF remains an informative characteristic for therapeutic decisions in HF, emerging data suggest that classifying patients with HF according to LVEF categories has several disadvantages. There are shared epidemiological features between patients with HFrEF, HFmrEF, and HFpEF, and pathophysiologic mechanisms may not be mutually exclusive. 5 In addition, LVEF measurement varies greatly among methods and observers, 10 and LVEF alone is not a good predictor of adverse outcomes. 11 Alternatively, HF can be considered a heterogeneous syndrome in which disease progression is associated with a dynamic evolution of functional and structural changes, leading to unique disease trajectories, and creating a spectrum of phenogroups with overlapping and distinct characteristics. 12 However, clear evidence that characteristics of HF patients form a continuum across LVEF is still lacking. Also, the role of comorbidities in classification of HF is uncertain as comorbidities are underreported in HF trials. 13
Novel data‐driven methods have been developed for classification or phenotyping of complex chronic medical disorders, incorporating recent advances in artificial intelligence. 14 These alternative classification methods have been applied to patients with HF and were found to be superior to LVEF‐based classification in characterizing patient subgroups. 15 , 16 , 17 , 18 , 19
In this study, we aimed to identify distinct clinical phenogroups among patients with HF using unsupervised machine learning analysis. Unsupervised machine learning looks for historically undetected patterns in a dataset without human‐labelled data or supervision. We hypothesized that a phenotype‐based classification system would be more effective than guideline‐recommended LVEF categories in predicting outcomes following hospital discharge.
Methods
Population
This was a sub‐study of the Patient‐Centered Care Transitions in HF (PACT‐HF) multicentre stepped wedge cluster randomized clinical trial, 20 , 21 which enrolled 2494 adults hospitalized for HF across 10 tertiary or quaternary care urban hospitals in Ontario, Canada. Patients were included if HF was considered the primary reason for hospitalization, and if rule‐out criteria for HF were not met. Rule‐out criteria were as follows: Boston criteria <5, and/or B type natriuretic peptide <50 pg/mL or N‐terminal pro brain natriuretic peptide (NT‐proBNP) <300 pg/mL. 1 , 22 Hospitals were randomized to sequentially deliver an intensive patient‐centred transitional care program or usual care. 20 The primary and secondary clinical outcomes have been reported. 21
This study complies with the Declaration of Helsinki. Because services were evidence‐informed and considered quality improvement, the PACT‐HF study protocol was approved by all institutional research ethics boards with waiver of written consent. Patients provided verbal informed consent for study participation. 19 The use of data in this project was authorized under section 45 of Ontario's Personal Health Information Protection Act, which does not require review by a Research Ethics Board.
Measurements
Only baseline values were included to create a cross‐sectional sample. Trained nurse clinicians extracted laboratory values and LVEF from charts and transmitted them to the study centre via electronic case report forms. LVEF values were obtained from the most recent echocardiogram or radionuclide ventriculogram during the preceding 12 months. Baseline demographics and clinical characteristics were extracted from administrative databases using a 5 year lookback (supporting information, Table S1). Comorbidities were extracted using International Classification of Diseases‐10 codes. Using unique encoded identifiers, clinical trial data were linked to the administrative databases (e.g. Canadian Institute for Health Information) accessed at ICES (formerly known as the Institute for Clinical Evaluative Sciences). 20 ICES is an independent, non‐profit research institute whose legal status under Ontario's health information privacy law allows it to collect and analyse health care and demographic data, without consent, for health system evaluation and improvement. These datasets (supporting information, Table S1) were linked using unique encoded identifiers and analysed at ICES.
Outcomes
Primary outcome was the first occurrence of all‐cause death or all‐cause rehospitalization at 6 and 12 months. Secondary outcome was the first occurrence of cardiovascular death or HF rehospitalization at 6 and 12 months. All outcomes were measured relative to the discharge date of the index HF hospitalization, defined as the first unplanned hospitalization for HF in a participating hospital during the study period. 20
Statistical analysis
Baseline characteristics were summarized using mean and standard deviation (SD) or median and interquartile range (IQR) for continuous variables, and count and percentages for categorical variables. Baseline characteristics were compared using one‐way analysis of variance, Kruskal–Wallis test or χ 2 test where appropriate.
Each baseline characteristic was assessed for an association with LVEF using Spearman's rank correlation coefficient (r) and univariable linear regression analysis reporting mean difference, 95% confidence intervals (CIs), and R 2 value. Given that LVEF was not normally distributed, the analysis was repeated with LVEF values categorized per 10% using interval regression analysis, obtaining a pseudo R 2 value. A multivariable linear regression model was constructed with clinically relevant, independent baseline variables. Mean differences were reported with 95% CIs, and a two‐sided P value of 0.05 was considered to be significant. Associations were defined as strong, moderate, or weak based on a Spearman's r value of >0.5, 0.5–0.3, or <0.3 respectively.
Cluster analysis
We included all independent variables in the dataset for further analysis: age, sex, weight, systolic blood pressure, glucose, creatinine, sodium, NT‐proBNP, troponin I, and history of hypertension, atrial fibrillation (AF), chronic obstructive pulmonary disease (COPD), severe valvular disease, and coronary revascularization. We did not consider the Charlson comorbidity index in the clustering analysis, as it encapsulates comorbidities already included in our analysis. We did not include classes of medication as these are typically prescribed based on LVEF. R v3.6.3 (R Foundation for Statistical Computing) and SAS v9.4 (SAS Institute) were used for machine learning and statistical analyses, respectively. Clustering of baseline characteristics was performed (i) independent of LVEF, (ii) according to guideline‐recommended categories of LVEF (<40, 40–49, and ≥50), and (iii) dividing LVEF in categories per 10% (<20, 20–29, 30–39, 40–49, 50–59, and ≥60). Gower's distance metric, which can handle categorical variables and missing values, was used to construct a dissimilarity matrix that served as an input to our clustering analysis, providing a pairwise comparison of observations. For missing values, two cases were compared if there was at least one variable where both had correct values. A majority of variables had <3% missing values. We performed agglomerative hierarchical clustering with Ward linkage (cluster R package). We used the elbow method, Dunn statistic, silhouette coefficient, and Hubert index (factoextra R package) to determine the optimal number of clusters. We constructed heatmaps using the ComplexHeatmap R package. We compared the performance of each of the 3 clustering approaches using six metrics to evaluate cluster stability, cohesion, separation, and information gain between analyses (details in Table S4 legend).
Survival analysis
We generated Kaplan–Meier survival curves for each clinical outcome and grouping and compared curves using the log‐rank test. We estimated hazard ratios (HRs) and CI, adjusted for age and sex, using Cox proportional hazards models. Violation of the proportional hazards assumption was determined through the analysis of Martingale residuals.
Results
Population characteristics
We included 1693 patients with available LVEF data in the analysis. LVEF was recorded at a median of 1 day post‐admission to hospital (IQR 45 days pre‐admission, to 3 days post‐admission). Baseline characteristics of the study population are displayed in Table 1 . Mean [SD] age was 77 [12] years, and 49% of the patients were female. Hypertension (76%), AF (57%), and diabetes (52%) were the most common comorbidities. Median (IQR) LVEF was 49 (33–58) percent. LVEF was abnormally distributed, with the histogram showing a peak at LVEF 50–54% (Figure S1).
Table 1.
Baseline characteristics of the 1693 patients, stratified according to six phenogroups identified in cluster analysis
| Characteristics | Overall | Phenogroup 1 (CAD) | Phenogroup 2 (VHD) | Phenogroup 3 (None) | Phenogroup 4 (AF) | Phenogroup 5 (COPD) | Phenogroup 6 (OSA) | P value |
|---|---|---|---|---|---|---|---|---|
| N | 1693 | 433 | 173 | 298 | 371 | 251 | 167 | |
| LVEF, median (IQR), % | 49 (33–58) | 45 (30–55) | 48 (30–55) | 47 (30–57) | 55 (40–60) | 49 (33–58) | 51 (33–59) | <0.001 |
| Clinical characteristics | ||||||||
| Age, mean (SD), years | 77 (12) | 77 (12) | 80 (12) | 75 (15) | 82 (10) | 77 (10) | 71 (12) | <0.001 |
| Female sex, no. (%) | 828 (48.9) | 91 (21.0) | 60 (34.7) | 159 (53.4) | 364 (98.1) | 80 (31.9) | 74 (44.3) | <0.001 |
| Weight, mean (SD), kg | 95.9 (44.9) | 101.0 (46.5) | 83.1 (31.0) | 94.8 (46.1) | 86.1 (40.0) | 96.6 (44.3) | 118.1 (51.5) | <0.001 |
| Systolic blood pressure, mean (SD), mmHg | 137 (26) | 137 (27) | 128 (24) | 144 (29) | 136 (25) | 135 (23) | 135 (25) | <0.001 |
| Heart rate, mean (SD), min−1 | 87 (23) | 87 (24) | 85 (22) | 87 (20) | 91 (26) | 86 (24) | 83 (21) | 0.003 |
| Respiratory rate, mean (SD), min−1 | 22 (5) | 22 (5) | 22 (6) | 22 (5) | 22 (5) | 22 (6) | 22 (5) | 0.597 |
| Comorbidities | ||||||||
| Atrial fibrillation, no. (%) | 965 (57.0) | 271 (62.6) | 105 (60.7) | 0 | 348 (93.8) | 148 (59.0) | 93 (55.7) | <0.001 |
| Cerebrovascular disease, no. (%) | 173 (10.2) | 102 (23.6) | 13 (7.5) | 0 | ≤10 | 46 (18.3) | ≤10 | <0.001 |
| COPD, no. (%) | 415 (24.5) | 0 | 0 | 0 | 108 (29.1) | 251 (100.0) | 56 (33.5) | <0.001 |
| Coronary revascularization, no. (%) | 510 (30.1) | 232 (53.6) | 50 (28.9) | 0 | 73 (19.7) | 93 (37.1) | 62 (37.1) | <0.001 |
| Diabetes, no. (%) | 879 (51.9) | 212 (48.9) | 50 (28.9) | 149 (50.0) | 197 (53.1) | 137 (54.6) | 124 (74.3) | <0.001 |
| Hypertension, no. (%) | 1292 (76.3) | 324 (74.8) | 124 (71.7) | 198 (66.4) | 293 (79.0) | 205 (81.7) | 148 (88.6) | <0.001 |
| Severe valvular heart disease, no. (%) | 325 (19.2) | 15 (3.5) | 173 (100.0) | 0 | 96 (25.9) | 18 (7.2) | 23 (13.8) | <0.001 |
| Sleep apnoea, no. (%) | 198 (11.7) | ≤10 | ≤10 | 0 | 0 | 29 (11.6) | 167 (100.0) | <0.001 |
| Charlson comorbidity score, mean (SD), points | 2.6 (1.4) | 2.54 (1.41) | 2.27 (1.34) | 2.43 (1.55) | 2.50 (1.35) | 2.92 (1.43) | 2.87 (1.28) | <0.001 |
| Laboratory analysis | ||||||||
| Creatinine, mean (SD), μmol/L | 137 (93) | 150 (109) | 150 (98) | 139 (117) | 114 (64) | 133 (71) | 140 (72) | <0.001 |
| Blood urea nitrogen, mean (SD), mg/dL | 12.1 (8.1) | 12.3 (8.2) | 14.1 (10.1) | 11.1 (6.9) | 11.2 (7.5) | 12.0 (7.4) | 13.5 (8.9) | <0.001 |
| Sodium, mean (SD), mmol/L | 137 (5) | 137 (5) | 137 (5) | 138 (5) | 137 (5) | 138 (5.0) | 138 (4) | 0.173 |
| Glucose, mean (SD), mmol/L | 8.4 (4.2) | 8.4 (4.0) | 7.7 (4.3) | 8.6 (4.2) | 8.4 (3.9) | 8.4 (3.7) | 9.2 (5.3) | 0.071 |
| Troponin I, median (IQR), ng/L | 0.04 (0.02–0.11) | 0.05 (0.03–0.13) | 0.05 (0.03–0.11) | 0.05 (0.03–0.13) | 0.03 (0.02–0.08) | 0.04 (0.02–0.11) | 0.04 (0.02–0.07) | <0.001 |
| NT‐proBNP, median (IQR), ng/L | 5090 (2453–9000) | 6572 (2655–9000) | 7668 (3430–9000) | 4211 (2106–9000) | 4720 (2475–9000) | 4905 (2120–9000) | 4260 (1902–8202) | <0.001 |
| Current medication | ||||||||
| ACE inhibitor, ARB or ARNI, no. (%) | 936 (55.8) | 243 (56.1) | 85 (49.1) | 182 (61.1) | 183 (49.3) | 136 (54.2) | 94 (56.3) | 0.013 |
| Beta blocker, no. (%) | 1,266 (74.8) | 346 (79.9) | 129 (74.6) | 220 (73.8) | 278 (74.9) | 173 (68.9) | 120 (71.9) | 0.260 |
| Mineralocorticoid receptor antagonist, no. (%) | 351 (20.7) | 95 (21.9) | 40 (23.1) | 67 (22.5) | 67 (18.1) | 37 (14.7) | 45 (26.9) | 0.222 |
| Diuretic, no. (%) | 1541 (91.0) | 394 (91.0) | 160 (92.5) | 260 (87.2) | 346 (93.3) | 227 (90.4) | 154 (92.2) | 0.360 |
| Acetylsalicylic acid, no. (%) | 691 (40.8) | 196 (45.3) | 65 (37.6) | 165 (55.4) | 103 (27.8) | 101 (40.2) | 61 (36.5) | <0.001 |
ACE, angiotensin converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro brain natriuretic peptide; OSA, obstructive sleep apnoea; SD, standard deviation; VHD, valvular heart disease. P value derived from one‐way analysis of variance (normal distribution) or Kruskal–Wallis test (skewed distribution).
Establishing phenogroups through clustering analysis
Clustering analysis of individual patients in an LVEF‐independent analysis identified six clusters as the optimal number of groupings (Figure S2). Patient characteristics stratified according to these six phenogroups are displayed in Figure 1 A and Table 1 . Most phenogroups showed a high prevalence of a specific comorbidity: coronary artery disease (CAD, 54%, Phenogroup 1), valvular heart disease (VHD, 100%, Phenogroup 2), AF (94%, Phenogroup 4), COPD (100%, Phenogroup 5), and obstructive sleep apnoea (OSA, 100%, Phenogroup 6). Phenogroup 3 only had hypertension and diabetes as comorbidities (66% and 50%, respectively), labelled ‘few comorbidities’. Although LVEF significantly differed between groups, there was no clear between‐group clinical separation according to traditional LVEF categories: LVEF of most phenogroups ranged between 30% and 55%, except the AF phenogroup, which had a significantly higher LVEF than Phenogroups 1 through 5 (all P < 0.001, Figure S3) and the CAD phenogroup, which had a significantly lower LVEF than the AF, OSA, and COPD phenogroups (P < 0.001, P = 0.016, and P = 0.004, respectively, Figure S3).
Figure 1.

Heatmaps of characteristics (rows) and LVEF categories (columns) of 1693 patients. (A) Patient characteristics stratified according to clusters identified by an unbiased, LVEF‐independent approach. (B) Patient characteristics stratified according to the three guideline‐recommended LVEF categories, reduced, mid‐range, and preserved EF. (C) Patient characteristics stratified according to six LVEF categories (per 10%). Darker means higher value, lighter means lower value. AF, atrial fibrillation; CAD, coronary artery disease, COPD, chronic obstructive pulmonary disease; EF, ejection fraction; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro brain natriuretic peptide; OSA, obstructive sleep apnoea; PCI/CABG, percutaneous coronary intervention or coronary artery bypass grafting; VHD, valvular heart disease.
Associations between clinical characteristics and left ventricular ejection fraction
Clustering analysis of characteristics and their association with the 3 guideline‐recommended LVEF categories (<40%, 40–49%, >50%) and 6 LVEF categories in per 10% increments is shown in Figure 1 B‐C and Table S2. Relative to LVEF > 50, LVEF <50% was associated with higher concentrations of NT‐proBNP (P < 0.001) and troponin (P < 0.001), and more prevalent history of coronary revascularization (P < 0.001, Table S2). LVEF >50% was associated with older age (P < .001), higher percentage of women (P < 0.001), and higher prevalence of AF (P < 0.001) relative to those with LVEF <50% (Table S2).
Accordingly, in univariable linear regressions LVEF had a significant positive association with sex, age, systolic blood pressure, presence of AF, and presence of hypertension, and a significant negative association with NT‐proBNP and history of coronary revascularization (Table 2 ). However, the associations were weak, with r values <0.3 (Table 2 ). In multivariable linear regression analysis, age, systolic blood pressure, glucose, sodium, NT‐proBNP, and female sex had significant associations with LVEF (Table S3).
Table 2.
Univariable associations of baseline characteristics and left ventricular ejection fraction
| Clinical characteristics | Mean difference (95% CI)* | P value* | r a | R 2 * | Pseudo R 2 lower bound b | Pseudo R 2 upper bound b |
|---|---|---|---|---|---|---|
| Age, years | 0.31 (0.25–0.37) | <0.001 | 0.222 | 0.063 | 0.067 | 0.055 |
| Female | 6.89 (5.51–8.28) | <0.001 | 0.229 | 0.005 | 0.050 | 0.046 |
| Weight, kg c | −0.01 (−0.16 to 0.17) | 0.923 | −0.017 | <0.001 | <0.001 | <0.001 |
| Systolic blood pressure, mmHg c | 1.23 (0.96–1.49) | <0.001 | 0.214 | 0.046 | 0.042 | 0.038 |
| Comorbidities | ||||||
| Atrial fibrillation | 2.37 (0.94–3.80) | 0.001 | 0.072 | 0.006 | 0.007 | 0.004 |
| Cerebrovascular disease | 1.89 (−0.46 to 4.23) | 0.115 | 0.038 | 0.002 | 0.002 | 0.002 |
| COPD | 3.09 (1.45–4.74) | <0.001 | 0.088 | 0.008 | 0.007 | 0.007 |
| Diabetes | 1.05 (−0.37 to 2.47) | 0.148 | 0.038 | 0.001 | 0.001 | 0.002 |
| PCI/CABG | −2.63 (−4.17 to −1.08) | 0.001 | −0.089 | 0.007 | 0.005 | 0.005 |
| Severe valvular heart disease | 0.41 (−1.39 to 2.22) | 0.655 | 0.008 | <0.001 | <0.001 | <0.001 |
| Sleep apnoea | 0.60 (−1.61 to 2.82) | 0.592 | 0.017 | <0.001 | 0.001 | <0.001 |
| Laboratory analysis | ||||||
| Creatinine, μmol/L c | −0.02 (−0.09 to 0.06) | 0.680 | −0.004 | <0.001 | <0.001 | <0.001 |
| Sodium, mmol/L | −0.03 (−0.18 to 0.11) | 0.652 | 0.014 | <0.001 | <0.001 | <0.001 |
| Troponin I, ng/L | 0.38 (−0.75 to −0.01) | 0.050 | −0.257 | 0.004 | 0.005 | 0.005 |
| NT‐proBNP, ng/L d | −0.04 (−0.05 to −0.03) | <0.001 | −0.282 | 0.003 | 0.028 | 0.029 |
CI, confidence interval; COPD, chronic obstructive pulmonary disease; NT‐proBNP, N‐terminal pro brain natriuretic peptide.
Mean difference and confidence intervals, R 2 value, and P value derived from univariable linear regression analysis.
r value derived from correlation analysis.
Pseudo R 2 value derived from interval regression analysis (six left ventricular ejection fraction categories using per 10% cut‐offs).
Per a unit of 10.
Per a unit of 100.
Cluster separation and stability
Comparative statistics for the clustering analyses demonstrated that the LVEF‐independent analysis provided the most separated clusters, but at the cost of cluster stability (Table S4). The LVEF‐independent analysis demonstrated the highest information gain when compared with three and six LVEF categories (Table S4), which means that the LVEF‐independent categorization has less ‘uncertainty’ or ‘noise’. The use of three LVEF categories resulted in an intermediate separation of clusters, with some overlap between categories, but the most ‘stable’ categorization of HF patients. This stability means that in a slightly different population (e.g. missing 1% of subjects), likely the same categorization would be obtained for the remaining subjects. The use of six LVEF categories had the lowest phenogroup cohesion and separation between categories and intermediate stability (Table S4).
Left ventricular ejection fraction and phenogroup‐based classifications and outcomes
The phenogroup‐based HF classification successfully stratified patients according to clinical outcomes (Table 3 , Figures 2 , 3 , S4, and S5). For the primary composite outcome of all‐cause death or rehospitalization at 6 months, the HRs for the phenogroups that ranged from 1.25 (95% CI 1.00–1.58 for phenogroup AF) to 2.04 (95% CI 1.62–2.57 for phenogroup COPD) (log‐rank P < 0.001, Figure 2 A ). For the primary composite outcome at 12 months, the HRs for phenogroups ranged from 1.15 (95% CI 0.94–1.41 for phenogroup AF) to 1.87 (95% CI 1.52–2.30 for phenogroup COPD) (P < 0.002, Figure 3 A ). For the secondary composite outcome of cardiovascular death or HF rehospitalization at 6 months, the HRs for phenogroups ranged from 1.15 (95% CI 0.85–1.56 for phenogroup CAD) to 1.68 (95% CI 1.19–2.36 for phenogroup VHD) (P = 0.002, Figure S4A); and at 12 months, the HRs ranged from 1.15 (95% CI 0.88–1.51 for phenogroup CAD) to 1.84 (95% CI 1.35–2.50 for phenogroup OSA) (P < 0.001, Figure S5A). Patients with COPD (Phenogroup 5) had the worst outcomes: 69% of patients died or were rehospitalized at 6 months, 79% at 12 months (Table 3 , Figure 2 A ). Patients with no major comorbidity (Phenogroup 3) had the best outcomes: 44% of patients died or were rehospitalized at 6 months, 59% at 12 months. Results were similar for the secondary outcomes (Table 3 , Figures S4 and S5), demonstrating early identification of risk using phenogroup classification.
Table 3.
Primary and secondary clinical outcomes according to LVEF categories or LVEF‐independent clinical phenogroups
| Grouping | Events (% of group with event) | Hazard ratio (95% CI)* | P value | Log‐rank P value | Events (% of group with event) | Hazard ratio (95% CI)* | P value | Log‐rank P value |
|---|---|---|---|---|---|---|---|---|
| Phenogroups | Composite of all‐cause death or rehospitalization at 6 months | Composite of all‐cause death or rehospitalization at 12 months | ||||||
| 1 CAD | 232 (54) | 1.32 (1.05–1.64) | 0.015 | <0.001 | 282 (65) | 1.22 (1.00–1.48) | 0.045 | <0.001 |
| 2 VHD | 116 (67) | 1.88 (1.46–2.42) | <0.001 | 134 (78) | 1.70 (1.35–2.14) | <0.001 | ||
| 3 None | 130 (44) | Reference | 175 (59) | Reference | ||||
| 4 AF | 206 (56) | 1.25 (1.00–1.58) | 0.054 | 249 (67) | 1.15 (0.94–1.41) | 0.174 | ||
| 5 COPD | 173 (69) | 2.04 (1.62–2.57) | <0.001 | 199 (79) | 1.87 (1.52–2.30) | <0.001 | ||
| 6 OSA | 99 (59) | 1.63 (1.25–2.12) | <0.001 | 127 (76) | 1.66 (1.32–2.09) | <0.001 | ||
| LVEF, 3 categories | ||||||||
| <40% | 314 (55) | Reference | 0.687 | 377 (66) | Reference | 0.300 | ||
| 40–49% | 157 (56) | 0.96 (0.79–1.17) | 0.711 | 194 (69) | 1.01 (0.85–1.20) | 0.906 | ||
| ≥50% | 485 (58) | 1.01 (0.87–1.17) | 0.938 | 595 (71) | 1.06 (0.93–1.21) | 0.408 | ||
| LVEF, 6 categories | ||||||||
| <20% | 22 (44) | Reference | 0.401 | 28 (56) | Reference | 0.338 | ||
| 20–29% | 145 (57) | 1.46 (0.93–2.29) | 0.096 | 172 (68) | 1.39 (0.93–2.08) | 0.105 | ||
| 30–39% | 147 (54) | 1.27 (0.81–2.00) | 0.291 | 177 (65) | 1.23 (0.82–1.83) | 0.316 | ||
| 40–49% | 157 (56) | 1.28 (0.82–2.01) | 0.281 | 194 (69) | 1.29 (0.86–1.92) | 0.213 | ||
| 50–59% | 274 (57) | 1.28 (0.82–1.98) | 0.275 | 344 (71) | 1.32 (0.90–1.95) | .160 | ||
| ≥60% | 211 (60) | 1.42 (0.91–2.22) | 0.121 | 251 (72) | 1.39 (0.93–2.06) | 0.104 | ||
| Phenogroups | Composite of cardiovascular death or HF rehospitalization at 6 months | Composite of cardiovascular death or HF rehospitalization at 12 months | ||||||
| 1 CAD | 121 (28) | 1.15 (0.85–1.56) | 0.360 | 0.002 | 146 (34) | 1.15 (0.88–1.51) | 0.304 | <0.001 |
| 2 VHD | 65 (38) | 1.68 (1.19–2.36) | 0.003 | 80 (46) | 1.75 (1.29–2.38) | <0.001 | ||
| 3 None | 71 (24) | Reference | 89 (30) | Reference | ||||
| 4 AF | 106 (29) | 1.17 (0.85–1.61) | 0.328 | 143 (39) | 1.27 (0.96–1.68) | 0.091 | ||
| 5 COPD | 89 (36) | 1.63 (1.19–2.24) | 0.002 | 115 (46) | 1.77 (1.34–2.35) | <0.001 | ||
| 6 OSA | 55 (33) | 1.58 (1.11–2.25) | 0.012 | 78 (47) | 1.84 (1.35–2.50) | <0.001 | ||
| LVEF, 3 categories | ||||||||
| <40% | 192 (33) | Reference | 0.075 | 238 (41) | Reference | 0.180 | ||
| 40–49% | 80 (28) | 0.78 (0.60–1.01) | 0.063 | 101 (36) | 0.80 (0.63–1.01) | 0.056 | ||
| ≥50% | 235 (28) | 0.75 (0.62–0.92) | 0.005 | 312 (37) | 0.81 (0.68–0.97) | 0.019 | ||
| LVEF, 6 categories | ||||||||
| <20% | 17 (34) | Reference | 0.096 | 23 (46) | Reference | 0.193 | ||
| 20–29% | 93 (37) | 1.08 (0.64–1.81) | 0.779 | 111 (44) | 0.95 (0.61–1.49) | 0.829 | ||
| 30–39% | 82 (30) | 0.79 (0.46–1.33) | 0.367 | 104 (38) | 0.73 (0.46–1.15) | 0.170 | ||
| 40–49% | 80 (28) | 0.72 (0.42–1.22) | 0.217 | 101 (36) | 0.67 (0.42–1.05) | 0.083 | ||
| 50–59% | 141 (29) | 0.71 (0.42–1.18) | 0.184 | 187 (39) | 0.70 (0.45–1.08) | 0.110 | ||
| ≥60% | 94 (27) | 0.67 (0.40–1.14) | 0.139 | 125 (36) | 0.65 (0.41–1.03) | 0.065 | ||
AF, atrial fibrillation; CAD, coronary artery disease; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnoea; LVEF, left ventricular ejection fraction; VHD, valvular heart disease.
Adjusted for age and sex.
Figure 2.
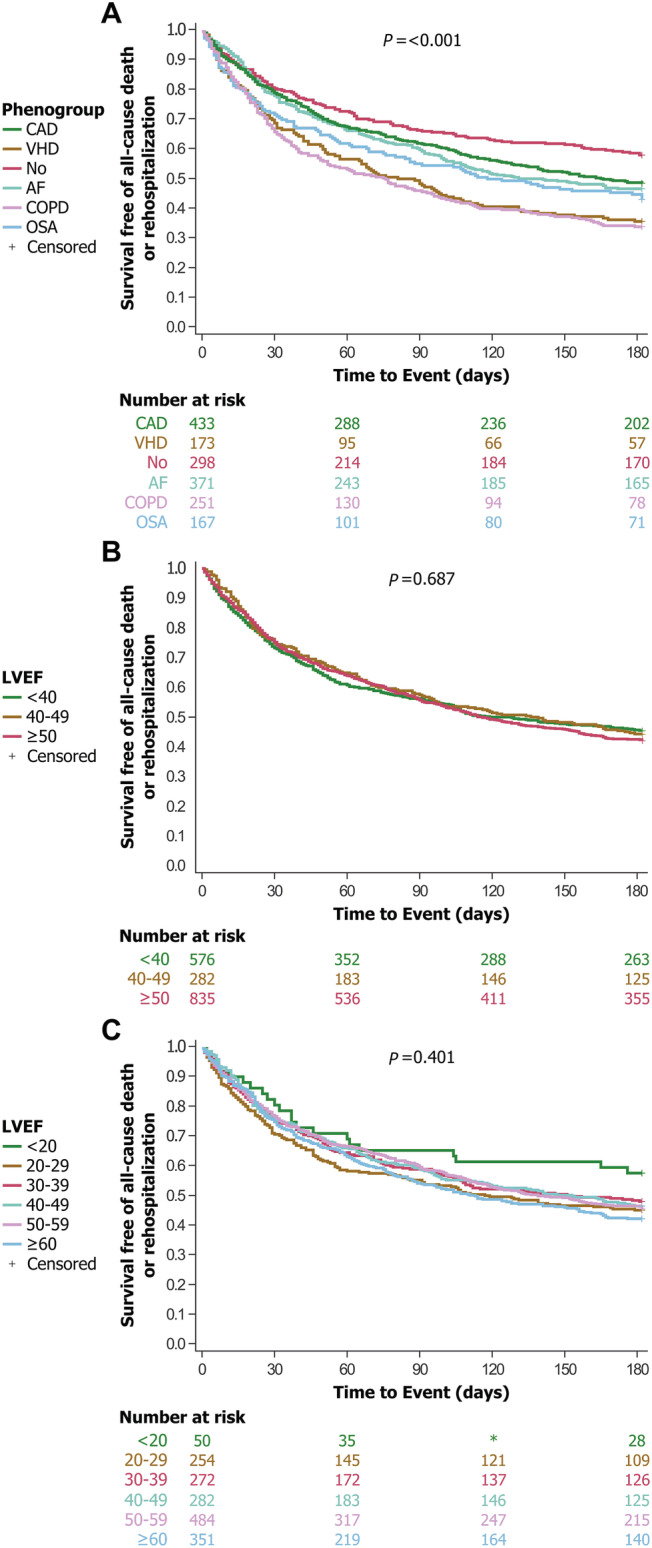
Survival curves of heart failure patients stratified according to LVEF‐based classifications or LVEF‐independent phenogroup‐based classification. Survival curves for all‐cause death or rehospitalization at 6 months, adjusted for age and sex. P value from log‐rank test. Survival differs significantly across groups using the LVEF‐independent phenogroup‐based classification (A) but not in LVEF‐based classifications (B,C). (A) Kaplan–Meier curves stratified according to phenogroups. (B) Kaplan–Meier curves stratified according to the three guideline‐recommended LVEF categories, reduced, mid‐range, and preserved EF. (C) Kaplan–Maier curves according to six LVEF categories (per 10%). AF, atrial fibrillation; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; LVEF, left ventricular ejection fraction; OSA, obstructive sleep apnoea; VHD, valvular heart disease.
Figure 3.

Survival curves of heart failure patients stratified according to LVEF‐based classifications or LVEF‐independent phenogroup‐based classification. Survival curves for all‐cause death or rehospitalization at 12 months, adjusted for age and sex. P value from log‐rank test. Survival differs significantly across groups using the LVEF‐independent phenogroup‐based classification (A) but not in LVEF‐based classifications (B,C). (A) Kaplan–Meier curves stratified according to phenogroups. (B) Kaplan–Meier curves stratified according to the three guideline‐recommended LVEF categories, reduced, mid‐range, and preserved EF. (C) Kaplan–Meier curves according to six LVEF categories (per 10%). AF, atrial fibrillation; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; LVEF, left ventricular ejection fraction; OSA, obstructive sleep apnoea; VHD, valvular heart disease.
LVEF‐based groupings—either three or six categories—did not stratify clinical outcomes well, whether at 6 or 12 month outcomes (all P > 0.05, Table 3 , Figures 2 B,C , 3 B,C , S4B,C, and S5B,C). The exception was LVEF ≥50% in the three‐class grouping, which showed significantly less cardiovascular deaths or HF readmissions (HR 0.75, CI 0.62–0.92, P = 0.005).
Discussion
In this cohort of 1693 patients hospitalized for HF and enrolled in the Patient‐Centered Care Transitions in HF multicentre randomized clinical trial, we found that an unsupervised machine learning approach separated patients according to different comorbidity profiles or phenogroups, each of which included patients across the LVEF spectrum. The classification of patients by clinical phenogroups and independent of LVEF resulted in greater separation of clusters and better association with clinical outcomes at 6 and 12 months than classification based on LVEF categories. This pertained to both all‐cause and HF readmissions, and all‐cause and CV death. These results suggest that classifying HF patients based on clinical characteristics rather than LVEF categories provides relevant prognostic information, and that comorbidities are major drivers of outcomes in HF patients. Figure 4 summarizes our findings.
Figure 4.

Summary of the study findings. We analysed baseline characteristics of 1693 patients with heart failure included in the Patient‐Centered Care Transitions in Heart Failure randomized clinical trial. Clustering analysis independent of LVEF, better separated survival curves, provided more information and less overlap of clinical phenogroups compared to guideline‐recommended subdivision in three LVEF‐based categories. Through clustering analysis, we found six clinical phenogroups mainly based on comorbidities. COPD, chronic obstructive pulmonary disease; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; LVEF, left ventricular ejection fraction.
The LVEF has served as an inclusion criterion for HF clinical trials, is recommended in guidelines to classify HF, and is a practical parameter to guide treatment decisions in HF; however, this classification approach has some disadvantages. First, LVEF estimates vary between techniques and repeat measurements, but are often treated as absolute. 10 , 23 Patients with an LVEF just above an established threshold for treatment may be denied HF therapies without recognition of the variation in LVEF estimates. Second, many epidemiological, pathophysiological, and clinical features are shared between patients with HFrEF, HFmrEF, and HFpEF. Similar pathophysiologic mechanisms across the LVEF spectrum include endothelial dysfunction, neurohumoral activation, myocardial fibrosis, and skeletal myopathy, among others. 5 , 24 Abnormalities of diastolic function are universally present in HFrEF, and systolic dysfunction—as evidenced by a reduction of global longitudinal strain and cardiac output reserve—is often present in HFpEF. 25 Activation of the renin–angiotensin–aldosterone system is not restricted to HFrEF, 26 yet therapies targeting the renin–angiotensin–aldosterone system are largely recommended for those with LVEF <40% based on the inclusion criterion used in historic HF trials. 5 Third, the relation of lower LVEF with increased mortality is only maintained until an LVEF of 45%. Above this value, LVEF is an unreliable prognosticator of mortality. 4 This may be due, in part, to increasing heterogeneity and varying pathophysiology among patients at the higher end of the LVEF spectrum. 27
Our LVEF‐independent, phenotype‐driven approach provided the most information about patients, facilitated better separation between groups of patients (Figure 1 A , Table S4), and better identified patients at risk for all‐cause rehospitalization and death as well as HF rehospitalization and CV death than LVEF‐based groupings (Table 3 , Figure 2 , 3 , S4, and S5). Our analysis confirms that comorbidities are a major driver of outcomes, regardless of LVEF. Our findings are consistent with previous studies that demonstrated an association between underlying comorbidities and outcomes in HF. 28 , 29 , 30 , 31 Among patients with HF, those with CAD had a similar mortality risk but increased risk of HF hospitalization as those without CAD 32 ; those with AF had an increased risk of mortality than those without AF 30 ; and those with COPD and OSA had and increased risk of mortality than those without COPD or OSA. 28 , 29 These associations were often independent of LVEF. 33
We recognize that any phenogroup classification system that labels categories on the basis of a single comorbidity is simplistic and that comorbidities overlap between groups. 33 For example, the prevalence of AF in the VHD phenogroup was 61%, and the prevalence of CAD in the COPD and OSA groups was approximately 37%. This reflects the high burden of comorbidities in patients hospitalized with HF. However, the clustering algorithm determined, in an unsupervised manner, that patients with concurrent VHD and AF are more similar to patients with VHD alone than to patients with AF alone. The outcome analysis confirms that outcomes of HF patients with VHD (with or without AF) are significantly worse than outcomes of HF patients with AF (and no VHD).
Our approach differs from prior approaches, which used artificial intelligence to provide a better separation of categories in patients with HF, but results are consistent overall. 15 , 16 , 17 , 18 , 34 Some of the previous models were trained a priori to predict clinical outcomes, thus satisfying a self‐fulfilling prophecy: groupings were based on clinical outcomes, and naturally performed better than LVEF at prediction. 18 The phenogroup approach in prior studies was also limited by the need to input dozens of complex variables to classify a patient. 16 , 18 , 19 Our analysis is different as it only included baseline patient characteristics—and not outcomes—in the clustering model; thus, it was not designed to predict outcomes. However, subsequent linkages to clinical outcomes proved that this unbiased, LVEF‐independent classification was superior to LVEF‐based classification in predicting outcomes. Additionally, our randomized clinical trial database provided granular characteristics, including contemporaneous LVEF and a 5 year lookback of comorbidities. Finally, linkage to provincial databases provided a wealth of clinical outcomes (all‐cause, CV, and HF events), while previous registry‐type datasets were typically limited to all‐cause death.
The goal of our analysis was not to develop a risk prediction model, but to explore HF phenotypes and their associations with outcomes relative to an LVEF based classification system. As such, we did not formally compare our phenotype‐based approach to existing risk scores. The performance of this phenotype‐based classification scheme relative to validated risk models in HF 35 , 36 is unknown and the subject of further investigation. We have demonstrated in a prior work that simple clinical risk prediction indices can predict clinical outcomes with similar or better performance than complex and validated risk prediction scores in HF, 37 , 38 and it is possible that this simple phenotype‐based classification scheme may also compare favourably.
Our findings have important applications. The external validation of our classification scheme is underway, and the results will inform the development of a decision tree to guide the risk assessment in patients hospitalized with HF. The classification scheme may also be useful to select subgroups of patients who are likely to respond to HF therapies in clinical trials, particularly within traditional categories that have been difficult to demonstrate treatment efficacy in. For example, few therapeutic options exist for patients with HFmrEF and HFpEF, and phenotype‐based selection criteria may be an effective way to facilitate homogeneity and response to targeted treatments in clinical trials. 39 , 40 We describe several phenogroups at higher risk of cardiovascular events, across the LVEF spectrum, which could inform design of future trials. Whether certain phenogroups respond differently to guideline‐directed medical therapy remains unknown but possible, and this should be explored in future trials.
This study has some limitations. First, LVEF was sometimes obtained from a recent—rather than current—echocardiogram or nuclear ventriculogram. Second, we included a relatively large amount of categorical variables, which limited the number of combinations that can be produced in a hierarchy. Incorporation of more baseline characteristics, including other echocardiographic variables, could have further improved cluster‐based classification of HF patients. Hierarchical clustering algorithms such as the one used in this study tend to prioritize categorical variables over continuous variables and other clustering algorithms could have been considered to avoid prioritizing categorical variables. 41 We chose Gower's distance and hierarchical clustering because this combination could handle categorical variables and missing values. This approach combined good prognostic capability with ease of application to different HF populations, without the need for difficult algorithms. Third, event rates could have been impacted by factors not captured in our database, such as adherence to medical therapy and quality of care. Finally, we realize that our findings may not be generalizable to other populations. Patients in this pragmatic clinical trial were hospitalized for HF and may have had a comorbidity profile distinct from those in ambulatory care. External validation should be performed before clinical application.
Conclusion
In this sub‐study of the PACT‐HF trial that included patients hospitalized for HF, we found that unbiased classification of baseline characteristics through hierarchical cluster analysis (unsupervised machine learning) resulted in six phenogroups of patients characterized by different comorbidity profiles. While there were LVEF differences between phenogroups, the entire LVEF spectrum was represented in each phenogroup. The phenogroup classification provided superior prognostic information for all‐cause and CV endpoints at 6 months and 1 year compared with classification based on three or six LVEF categories. These findings suggest that comorbidities are major drivers of prognosis in HF patients. While LVEF remains a practical tool for therapeutic decision making in HF, our data suggest that phenogroup‐based classification may better inform the clinician about the risk of future events. In addition to external validation, future studies should focus on identifying treatment‐responsive phenogroups in an effort to more precisely guide treatment decisions.
Funding
The Patient‐Centered Care Transitions in Heart Failure trial was supported by peer‐reviewed grants from the Canadian Institutes of Health Research (135917) to H.G.C.V.; and Ontario Ministry of Health and Long‐Term Care Health System Research Fund (6686) to H.G.C.V.; as well as in‐kind support from the participating hospitals; Community Care Access Centers; and Roche Diagnostics. H.G.C.V. is supported by the Hamilton Health Sciences Career Award. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). Parts of this material are based on data and information compiled and provided by MOHLTC, Canadian Institute for Health Information, and IQVIA Canada. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Conflicts of interest
None declared.
Supporting information
Table S1. Provincial databases used for baseline characteristics and outcomes.
Table S2. Baseline characteristics stratified according to LVEF categories.
Table S3. Multivariable linear regression model assessing independent associations between baseline characteristics and LVEF.
Table S4. Statistics assessing cluster properties.
Figure S1. Distribution of LVEF across the study population.
Figure S2. Metrics indicating the ideal number of clusters in the LVEF‐independent analysis.
Figure S3. LVEF for each phenogroup.
Figure S4. Survival curves for secondary outcome at 6 months.
Figure S5. Survival curves for secondary outcome at 12 months
Acknowledgements
We thank Richard Perez for analytic oversight of this sub‐study and Kim D. Simek for study coordination of the PACT‐HF clinical trial. We thank the investigators from participating sites: Peter R. Mitoff, (Department of Medicine, St. Joseph's Health Centre, Toronto, Ontario, Canada), Mohamed Panju and Harriette Van Spall (Department of Medicine, McMaster University and Hamilton Health Sciences), Manish Maingi (Cardiac Health Program, Trillium Health Partners, Mississauga, Ontario, Canada), Michael C. Tjandrawidjaja (Department of Medicine, William Osler Health System, Brampton, Ontario, Canada), Michael Heffernan (Department of Medicine, Halton Health Care Services, Oakville, Ontario, Canada), Mohammad I. Zia (Department of Medicine, Michael Garron Hospital, Toronto, Ontario, Canada), Liane Porepa (Department of Medicine, Southlake Regional Health Centre, Newmarket, Ontario, Canada).
Gevaert, A. B. , Tibebu, S. , Mamas, M. A. , Ravindra, N. G. , Lee, S. F. , Ahmad, T. , Ko, D. T. , Januzzi, J. L. Jr , and Van Spall, H. G. C. (2021) Clinical phenogroups are more effective than left ventricular ejection fraction categories in stratifying heart failure outcomes. ESC Heart Failure, 8: 2741–2754. 10.1002/ehf2.13344.
Trial Registration: ClinicalTrials.gov Identifier: NCT02112227
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 3. Lam CSP, Solomon SD. Fussing over the middle child. Circulation 2017; 135: 1279–1280. [DOI] [PubMed] [Google Scholar]
- 4. Solomon SD, Anavekar N, Skali H, McMurray JJV, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA, Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators . Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005; 112: 3738–3744. [DOI] [PubMed] [Google Scholar]
- 5. Triposkiadis F, Butler J, Abboud FM, Armstrong PW, Adamopoulos S, Atherton JJ, Backs J, Bauersachs J, Burkhoff D, Bonow RO, Chopra VK, de Boer RA, de Windt L, Hamdani N, Hasenfuss G, Heymans S, Hulot JS, Konstam M, Lee RT, Linke WA, Lunde IG, Lyon AR, Maack C, Mann DL, Mebazaa A, Mentz RJ, Nihoyannopoulos P, Papp Z, Parissis J, Pedrazzini T, Rosano G, Rouleau J, Seferovic PM, Shah AM, Starling RC, Tocchetti CG, Trochu JN, Thum T, Zannad F, Brutsaert DL, Segers VF, de Keulenaer GW. The continuous heart failure spectrum: moving beyond an ejection fraction classification. Eur Heart J 2019; 40: 2155–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lund LH, Claggett B, Liu J, Lam CS, Jhund PS, Rosano GM, Swedberg K, Yusuf S, Granger CB, Pfeffer MA, McMurray JJV, Solomon SD. Heart failure with mid‐range ejection fraction in CHARM: characteristics, outcomes and effect of candesartan across the entire ejection fraction spectrum. Eur J Heart Fail 2018; 20: 1230–1239. [DOI] [PubMed] [Google Scholar]
- 7. Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O'meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 2016; 37: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solomon SD, Vaduganathan M, Brian LC, Packer M, Zile M, Swedberg K, Rouleau J, Marc AP, Desai A, Lund LH, Kober L. Sacubitril/valsartan across the spectrum of ejection fraction in heart failure. Circulation 2020; 141: 352–361. [DOI] [PubMed] [Google Scholar]
- 9. Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, Manzano L, McMurray JJV, Ruschitzka F, van Veldhuisen DJ, von Lueder TG. Beta‐blockers for heart failure with reduced, mid‐range, and preserved ejection fraction: an individual patient‐level analysis of double‐blind randomized trials. Eur Heart J 2018; 39: 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pellikka PA, She L, Holly TA, Lin G, Varadarajan P, Pai RG, Bonow RO, Pohost GM, Panza JA, Berman DS, Prior DL, Asch FM, Borges‐Neto S, Grayburn P, al‐Khalidi HR, Miszalski‐Jamka K, Desvigne‐Nickens P, Lee KL, Velazquez EJ, Oh JK. Variability in ejection fraction measured by echocardiography, gated single‐photon emission computed tomography, and cardiac magnetic resonance in patients with coronary artery disease and left ventricular dysfunction. JAMA Netw Open 2018; 1: e181456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015; 175: 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brutsaert DL, de Keulenaer GW. Diastolic heart failure: a myth. Curr Opin Cardiol 2006; 21: 240–248. [DOI] [PubMed] [Google Scholar]
- 13. Khan MS, Samman Tahhan A, Vaduganathan M, Greene SJ, Alrohaibani A, Anker SD, Vardeny O, Fonarow GC, Butler J. Trends in prevalence of comorbidities in heart failure clinical trials. Eur J Heart Fail 2020; 22: 1032–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gevaert AB, Adams V, Bahls M, Bowen TS, Cornelissen V, Dörr M, Hansen D, Kemps HMC, Leeson P, van Craenenbroeck EM, Kränkel N. Towards a personalised approach in exercise‐based cardiovascular rehabilitation: how can translational research help? A ‘call to action’ from the Section on Secondary Prevention and Cardiac Rehabilitation of the European Association of Preventive Cardiolo. Eur J Prev Cardiol 2020; 27: 1369–1385. [DOI] [PubMed] [Google Scholar]
- 15. Tromp J, Tay WT, Ouwerkerk W, Teng T‐HK, Yap J, MacDonald MR, Leineweber K, McMurray JJV, Zile MR, Anand IS, Lam CS. Multimorbidity in patients with heart failure from 11 Asian regions: a prospective cohort study using the ASIAN‐HF registry. PLoS Med 2018; 15: e1002541 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang C‐C, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015; 131: 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmad T, Pencina MJ, Schulte PJ, O'Brien E, Whellan DJ, Piña IL, Kitzman DW, Lee KL, O'Connor CM, Felker GM. Clinical implications of chronic heart failure phenotypes defined by cluster analysis. J Am Coll Cardiol 2014; 64: 1765–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahmad T, Lund LH, Rao P, Ghosh R, Warier P, Vaccaro B, Dahlström U, O'Connor CM, Felker GM, Desai NR. Machine learning methods improve prognostication, identify clinically distinct phenotypes, and detect heterogeneity in response to therapy in a large cohort of heart failure patients. J Am Heart Assoc 2018; 7: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kao DP, Lewsey JD, Anand IS, Massie BM, Zile MR, Carson PE, McKelvie RS, Komajda M, McMurray JJ, Lindenfeld J. Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. Eur J Heart Fail 2015; 17: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Spall HGC, Lee SF, Xie F, Ko DT, Thabane L, Ibrahim Q, Mitoff PR, Heffernan M, Maingi M, Tjandrawidjaja MC, Zia MI. Knowledge to action: rationale and design of the Patient‐Centered Care Transitions in Heart Failure (PACT‐HF) stepped wedge cluster randomized trial. Am Heart J 2018; 199: 75–82. [DOI] [PubMed] [Google Scholar]
- 21. van Spall HGC, Lee SF, Xie F, Oz UE, Perez R, Mitoff PR, Maingi M, Tjandrawidjaja MC, Heffernan M, Zia MI, Porepa L. Effect of patient‐centered transitional care services on clinical outcomes in patients hospitalized for heart failure. JAMA 2019; 321: 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. di Bari M, Pozzi C, Cavallini MC, Innocenti F, Baldereschi G, de Alfieri W, Antonini E, Pini R, Masotti G, Marchionni N. The diagnosis of heart failure in the community. J Am Coll Cardiol 2004; 44: 1601–1608. [DOI] [PubMed] [Google Scholar]
- 23. Blondheim DS, Beeri R, Feinberg MS, Vaturi M, Shimoni S, Fehske W, Sagie A, Rosenmann D, Lysyansky P, Deutsch L, Leitman M, Kuperstein R, Hay I, Gilon D, Friedman Z, Agmon Y, Tsadok Y, Liel‐Cohen N. Reliability of visual assessment of global and segmental left ventricular function: a multicenter study by the Israeli Echocardiography Research Group. J Am Soc Echocardiogr 2010; 23: 258–264. [DOI] [PubMed] [Google Scholar]
- 24. Gevaert AB, Boen JRA, Segers VF, van Craenenbroeck EM. Heart failure with preserved ejection fraction: a review of cardiac and noncardiac pathophysiology. Front Physiol 2019; 10: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 2014; 11: 507–515. [DOI] [PubMed] [Google Scholar]
- 26. Bishu K, Deswal A, Chen HH, LeWinter MM, Lewis GD, Semigran MJ, Borlaug BA, McNulty S, Hernandez AF, Braunwald E, Redfield MM. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am Heart J 2012; 164: 763–770.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wehner GJ, Jing L, Haggerty CM, Suever JD, Leader JB, Hartzel DN, Kirchner HL, Manus JNA, James N, Ayar Z, Gladding P. Routinely reported ejection fraction and mortality in clinical practice: where does the nadir of risk lie? Eur Heart J 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jilek C, Krenn M, Sebah D, Obermeier R, Braune A, Kehl V, Schroll S, Montalvan S, Riegger GAJ, Pfeifer M, Arzt M. Prognostic impact of sleep disordered breathing and its treatment in heart failure: an observational study. Eur J Heart Fail 2011; 13: 68–75. [DOI] [PubMed] [Google Scholar]
- 29. Tavazzi L, Swedberg K, Komajda M, Böhm M, Borer JS, Lainscak M, Robertson M, Ford I. Clinical profiles and outcomes in patients with chronic heart failure and chronic obstructive pulmonary disease: an efficacy and safety analysis of SHIFT study. Int J Cardiol 2013; 170: 182–188. [DOI] [PubMed] [Google Scholar]
- 30. Mamas MA, Caldwell JC, Chacko S, Garratt CJ, Fath‐Ordoubadi F, Neyses L. A meta‐analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail 2009; 11: 676–683. [DOI] [PubMed] [Google Scholar]
- 31. Thune JJ, Signorovitch JE, Kober L, McMurray JJV, Swedberg K, Rouleau J, Maggioni A, Velazquez E, Califf R, Pfeffer MA, Solomon SD. Predictors and prognostic impact of recurrent myocardial infarction in patients with left ventricular dysfunction, heart failure, or both following a first myocardial infarction. Eur J Heart Fail 2011; 13: 148–153. [DOI] [PubMed] [Google Scholar]
- 32. Mentz RJ, Allen BD, Kwasny MJ, Konstam MA, Udelson JE, Ambrosy AP, Fought AJ, Vaduganathan M, O'Connor CM, Zannad F, Maggioni AP, Swedberg K, Bonow RO, Gheorghiade M. Influence of documented history of coronary artery disease on outcomes in patients admitted for worsening heart failure with reduced ejection fraction in the EVEREST trial. Eur J Heart Fail 2013; 15: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Triposkiadis F, Giamouzis G, Parissis J, Starling RC, Boudoulas H, Skoularigis J, Butler J, Filippatos G. Reframing the association and significance of co‐morbidities in heart failure. Eur J Heart Fail 2016; 18: 744–758. [DOI] [PubMed] [Google Scholar]
- 34. Hedman ÅK, Hage C, Sharma A, Brosnan MJ, Buckbinder L, Gan L‐M, Shah SJ, Linde CM, Donal E, Daubert J‐C, Mälarstig A, Ziemek D, Lund L. Identification of novel pheno‐groups in heart failure with preserved ejection fraction using machine learning. Heart 2020; 106: 342–349. [DOI] [PubMed] [Google Scholar]
- 35. Pocock SJ, Ariti CA, McMurray JJV, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN, Meta‐Analysis Global Group in Chronic Heart Failure . Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013; 34: 1404–1413. [DOI] [PubMed] [Google Scholar]
- 36. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole‐Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model. Circulation 2006; 113: 1424–1433. [DOI] [PubMed] [Google Scholar]
- 37. van Spall HG, Averbuch T, Lee SF, Oz UE, Mamas MA, Januzzi JL, Ko DT. The LENT index predicts 30 day outcomes following hospitalization for heart failure. ESC Heart Fail 2020: ehf2.13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Averbuch T, Lee S, Mamas M, Oz U, Perez R, Connolly S, Ko D, van Spall H. Derivation and validation of a 2‐variable index to predict 30‐day outcomes following Heart Failure hospitalization. ESC Heart Fail 2021. 10.1002/ehf2.13344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roh J, Houstis N, Rosenzweig A. Why don't we have proven treatments for HFpEF? Circ Res 2017; 120: 1243–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Solomon SD, Adams D, Kristen A, Grogan M, González‐Duarte A, Maurer MS, Merlini G, Damy T, Slama MS, Brannagan TH, Dispenzieri A. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin‐mediated amyloidosis. Circulation 2019; 139: 431–443. [DOI] [PubMed] [Google Scholar]
- 41. Ahmad A, Khan SS. Survey of state‐of‐the‐art mixed data clustering algorithms. IEEE Access 2019; 7: 31883–31902. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Provincial databases used for baseline characteristics and outcomes.
Table S2. Baseline characteristics stratified according to LVEF categories.
Table S3. Multivariable linear regression model assessing independent associations between baseline characteristics and LVEF.
Table S4. Statistics assessing cluster properties.
Figure S1. Distribution of LVEF across the study population.
Figure S2. Metrics indicating the ideal number of clusters in the LVEF‐independent analysis.
Figure S3. LVEF for each phenogroup.
Figure S4. Survival curves for secondary outcome at 6 months.
Figure S5. Survival curves for secondary outcome at 12 months


