Abstract
Aims
Patients with heart failure (HF) suffer from reduced quality‐of‐life (QoL). We aimed to compare QoL, depression, and anxiety scores among outpatients with preserved (HFpEF) and reduced (HFrEF) ejection fraction and non‐HF controls and its relationship to coordination capacity.
Methods and results
Fifty‐five participants were recruited prospectively at the University Hospital Jena, Germany (17 HFpEF, 18 HFrEF, and 20 non‐HF controls). All participants underwent echocardiography, cardiopulmonary exercise testing (CPET), 10 m walking test (10‐MWT), isokinetic muscle function and coordination tests, and QoL assessments using the short form of health survey (SF‐36), and hospital anxiety and depression scale (HADS). Furthermore, inflammatory biomarkers such as growth differentiation factor‐15 (GDF‐15) were assessed. Patients with HFpEF showed compared with HFrEF and non‐HF controls reduced QoL [mental component score (MCS): 43.6 ± 7.1 vs. 50.2 ± 10.0 vs. 50.5 ± 5.0, P = 0.03), vitality (VT): 47.5 ± 8.4 vs. 53.6 ± 8.6 vs. 57.1 ± 5.2, P = 0.004), and elevated anxiety (6.5 ± 3.2 vs. 3.3 ± 2.8 vs. 3.8 ± 2. 8, P = 0.02) and depression scores (6.5 [3.5–10.0] vs. 3.0 [1.0–6.5] vs. 2.0 [0.75–3.0], P = 0.01)]. After adjusting to multiple comparisons, anxiety remained higher in HFpEF patients compared with HFrEF (ppost‐hoc = 0.009). HFpEF and HFrEF patients showed reduced coordination capacity compared with non‐HF controls (P < 0.05). In a logistic regression, the presence of depression score ≥8 remained an independent factor for predicting reduced coordination capacity after adjusting for peak VO2, GDF‐15, 10‐MWT, physical component score (PCS), and peak torque of the leg [odds ratio (OR): 0.1, 95% confidence interval (CI): 0.004–0.626, P = 0.02].
Conclusion
Outpatients with HFpEF had worse QoL and higher anxiety and depression scores compared with HFrEF and non‐HF controls. Depression is associated with reduced QoL and is an independent predictor for reduced coordination capacity.
Keywords: Heart failure, Quality of life, Depression and anxiety, Coordination capacity
Introduction
Heart failure (HF) is a major public health issue with steadily increasing incidence and prevalence. Hospitalization and mortality due to HF remain high in spite of advancements in the management of HF. 1 , 2 While advances in medical and device therapies have improved morbidity and mortality in patients with HFrEF, no benefits have been demonstrated in patients with HFpEF. 3 , 4 , 5 , 6 The only proven therapy so far to improve exercise capacity, dyspnoea, and QoL in patients with HFpEF is exercise training. 7 Patients with HF especially those with HFpEF suffer from exercise intolerance that not only impairs physical activity but also mental, psychological, and social life aspects in these patients. 8 , 9 , 10 Psychological and mental disorders such as depression and anxiety are common in both HFpEF and HFrEF 11 , 12 and have been proved to be independently associated with higher mortality and readmission rates. 10 , 13 Depression prevalence for example in patients with HF is 15–40%, and it increases the risk for morbidity and mortality. 14
Recent studies focused on peripheral factors such as skeletal muscle in explaining the reduced exercise capacity, dyspnoea, and QoL. 15 , 16 One study in animal experiments showed a link between skeletal muscle dysfunction and depression. 17 Another group demonstrated that exercise training in HFpEF improves physical, psychological, and social components of QoL. 10 A further study in acute decompensated HF showed an association between physical function, cognitive dysfunction, and QoL. 18
However, a systematic comprehensive comparison among clinically stable outpatients with HFpEF, HFrEF, and age‐matched non‐HF controls regarding QoL, depression, and anxiety and the relationship to coordination capacity and inflammatory biomarkers is still missing. We hypothesized that patients with HFpEF have worse QoL and increased prevalence of anxiety and depression compared with those with HFrEF and non‐HF controls. Additionally, we investigated the link between QoL, depression, coordination capacity, inflammatory process, and muscle function in these patients.
Methods
Study population
Patients with HF were recruited from the HF outpatient clinic at the University Hospital Jena between September 2016 and June 2017. Non‐HF controls were recruited from the general population in Jena and the neighboured cities. Altogether 55 subjects fulfilled our inclusion and exclusion criteria (17 HFpEF patients, 18 HFrEF patients, and 20 non‐HF controls).
All subjects provided written informed consent at enrolment, and the protocol was approved by the responsible ethical review boards and fulfilled all principles of the Declaration of Helsinki.
Heart failure inclusion criteria
Clinically stable outpatients, men and women with age >55 years both with HFpEF and HFrEF and NYHA class II or III were recruited. HFpEF was defined as recommended by the European society of Cardiology‐HF guidelines (ESC‐HF). 19 Patients were on standard and stable HF medication for the last 3 months. Patients with HFpEF were further divided into groups according to phenotypes as suggested by Cohen et al. 20 The majority of our patients fulfilled the criteria of phenotype 2 and 3. According to Cohen et al., phenotype 2 was characterized by older age, highest proportion of women, a high prevalence of atrial fibrillation and chronic kidney disease, small concentric left ventricles with lowest left ventricle mass among the groups, as well as with the largest left atria, the lowest mitral annular tissue velocities, and the highest levels of inflammatory biomarkers related to the innate immune response (interleukin‐8). On the other hand, phenotype 3 exhibited intermediate age, with a very high prevalence of obesity, diabetes mellitus, and remarkably impaired functional class, and the highest levels of biomarkers of tumour necrosis factor‐mediated inflammation. These patients showed a distinct pattern of concentric left ventricular hypertrophy, with the highest values of left wall thickness, left ventricular mass, and left ventricular mass.
Heart failure exclusion criteria
Patients with major cardiovascular events or procedures in the last 6 weeks or patients with HF secondary to significant uncorrected valvular disease as well as patients with uncontrolled diabetes mellitus, progressive renal dysfunction (GFR < 60 mL/min) and those with primary muscle disorder such as muscular dystrophies were excluded.
Control subjects
Non‐HF controls with a history of cardiovascular disease or other diseases except arterial hypertension and diabetes mellitus were excluded.
All subjects underwent a standardized series of assessments over two visits. Visit 1: Informed consent, DEXA scan, cardiopulmonary exercise test, echocardiography, and 6‐min walk test. Visit 2: Muscle function/fatigability test, questionnaires, and blood tests.
Cardiopulmonary exercise testing
Exercise testing in association with air–gas exchange is considered to be an optimal gauge of functional capacity. We performed cardiopulmonary exercise testing (CPET) in all participants using incremental biking exercise on an electronically braked cycle ergometer. 21 Maximal O2‐uptake was abbreviated as PVO2.
Questionnaire tools to complement functional assessment measurements
To assess measures of daily activity of patients with HF, we utilized several questionnaires that assess physical limitation, symptoms, and quality‐of‐life (QoL).
Visual analogue scale
Visual analogue scale is part of the European quality of life–5 dimensions (EQ‐5D) questionnaire. 22 This questionnaire captures a self‐rating of health status on a 20‐cm vertical VAS, anchored at 100 (best imaginable health state) at the top and 0 (worst imaginable health state) at the bottom of the score. EQ‐5D (VAS) ratings are a quantitative measure, and differences in this scale can be used as a measure of outcome, as judged by the individual respondents. 23 , 24
The short form of health survey (SF‐36)‐assessment
SF‐36 was performed as part of the QoL evaluations in all participants. This assessment consists of 36 items assigned to eight dimensions (physical functioning (PF), role limitation because of physical problems (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role limitation because of emotional health (RE), and mental health (MH). The eight dimensions were summarized in one score for mental (MCS) and physical (PCS) quality of life, respectively. We used a German translation of SF‐36 with adapted norm values on German population. 25 , 26
Hospital anxiety and depression scale
In order to perform a psychometric analysis and evaluate co‐existing anxiety and depression, we asked the patients and the non‐HF controls to fill in the HADS questionnaire. 27 , 28
Dual‐energy X‐ray absorptiometry (DEXA) scan
A whole body DEXA scan was performed in all subjects to characterize the different compartments of soft tissue in the body. DEXA scanning is the most established method for the characterization of patients with advanced HF with a low radiation dosage and very low associated risks. 29 Appendicular lean mass was defined as the sum of muscle mass of arms and legs.
Muscle function by isokinetic dynamometry
The muscle function of the upper and lower extremities was assessed by the isokinetic dynamometry (CSMi Cybex HumacNorm®). A standardized measuring protocol was used to detect the parameters: (i) maximum muscle strength, (ii) muscle strength endurance, and (iii) muscular fatigue in the knee extension and knee flexion. The test protocol of the lower extremities included three different angular velocities in the concentric and eccentric mode was used. All values of the isokinetic measurement of the lower extremities were related to the muscle mass of legs unless mentioned otherwise.
-
i
Maximum muscle strength
The participants were asked to perform five repetitions with the maximum force with the velocity of 60°/s (concentric knee extension and flexion), and 30°/s (eccentric knee extension and flexion). The best single attempt was defined as peak torque muscle strength. The higher the value, the better is the muscle strength.
-
ii
Muscle strength endurance
The participants were asked to perform 15 repetitions with the maximum speed and to maintain it across the required performance. The velocity of the dynamometer was defined as 180°/s (knee extension and flexion). To detect the muscular endurance, the areas under the curves of every single attempt were summed. This outcome is equal to the physical work. The higher the value, the higher was the endurance and the higher the force level, which the participant was able to perform and maintain across the 15 repetitions. 30 , 31
6 min walk test
Using standard methodology, 32 patients were asked to walk as fast as possible on a 25 m course for 6 min. The test was scored in rounded meters walked in 6 min.
Gait performance—10 m walk test (10‐MWT)
A 10 m Walk Test was conducted to assess the gait speed of the individuals. The participants were asked to walk with a high velocity over 10 m in a straight line on a flat ground. The test was performed with a static start with a timed 10 m distance. 33 , 34
Coordination capacity by dynamic balance
A straight‐line walk test was used to detect the dynamic balance of the individuals. The participants were asked to walk forward and backward along three different lines (2 m length, 25 cm, 20 and 15 cm line wide). This is a modified test from the previously published functional dynamic walking test by Lark et al. 35 In total, participants performed six single walks. As a result, the number of missteps (i.e. stepping with the entire foot beside the line = 1 point) was counted and compared among the groups. 36
Serum analyses
Serum levels of GDF‐15 and soluble urokinase‐type plasminogen activator receptor (suPAR) were measured by using commercially available enzyme‐linked immunosorbent assay (ELISA) kits (GDF‐15: DY957, suPAR: DY807, R&D Systems, USA). Intra‐assay variability and inter‐assay variability were as follows: for GDF‐15, 1.8–2.8%, 5.1–5.9% and for suPAR, 2.1–7.5%, 4.7–6%. Preparation of patient samples, assay reagents, and measurements was performed according to manufacturer's instructions based on previously published work. In short, patient samples and standard protein were added to the appropriate wells of the ELISA plates (Nunc MaxiSorp flat‐bottom 96 well plates, VWR International GmbH, Austria), and plates were incubated for 2 h. ELISA plates were then washed using a Tween 20/PBS mix solution (Sigma Aldrich, USA). Afterwards, a biotin‐labelled antibody was added and incubated for another 2 h. Plates were washed once more, and a streptavidin‐horseradish‐peroxidase solution was added to the wells. After another washing step and adding tetramethylbenzidine (TMB; Sigma Aldrich, USA), a colour reaction was achieved. This reaction was stopped by adding sulphuric acid. Values of optical density (OD) were determined at 450 nm on an ELISA plate‐reader (iMark Microplate Absorbance Reader, Bio‐Rad Laboratories, Austria).
Statistical analysis
All data and statistics are reported as mean ± standard deviation (n ± SD) for continuous normally distributed data or as median and interquartile range [25–75%] for variables that were not normally distributed, respectively. Categorical data were summarized by percentages. The χ 2 test was used to look for trend for categorical variables and Kruskal–Wallis test was applied for not normally distributed data, respectively. Analysis of variance (ANOVA), Pearson's, or Spearman simple regression were used as appropriate. Variables perceived as clinically important and those with P < 0.1 in univariate analyses were included in the multivariate model. A two‐tailed P‐value <0.05 or 0.0167 for post hoc comparisons indicates statistical significance. The Statistical Package for Social Sciences software (SPSS 26, IBM, Armonk, USA) was used for statistical analysis.
Results
Quality‐of‐life and mental health in heart failure
Basic characteristics are presented in Table 1 .
Table 1.
Basic characteristics, co‐morbidities and medications in patients with HFpEF, HFrEF, and non‐HF controls
| Characteristic | Non‐HF controls | HFrEF | HFpEF | P‐value |
|---|---|---|---|---|
| N = 20 | N = 18 | N = 17 | ||
| Age (years) | 66 ± 7 | 68 ± 9 | 71 ± 6 | 0.17 |
| Sex (m/f) f% | 7/13 (65) | 15/3 (17)** | 8/9 (53) | 0.009 |
| BMI (kg/m2) | 26.4 ± 4.2 | 27.9 ± 5.3 | 28.7 ± 4.6 | 0.18 |
| NYHA (II/III) % | (0/0) | (83.3/16.7)** | (76.5/23.5)* | <0.001 |
| Left ventricle mass index (kg/m3) | 97.3 ± 22.7 | 165 ± 53.2** | 152 ± 30.8* | <0.001 |
| Left ventricle end‐diastolic volume index (mL/m2) | 23.3 ± 2.6 | 28.6 ± 7.0** | 24.9 ± 3.2*** | 0.004 |
| Left ventricle ejection fraction (%) | 61.0 [57.3–66.3] | 30.0 [23.5–32.5]** | 62.0 [53.0–66.0]*** | <0.001 |
| Left atrial volume index (mL/m2) | 17.2 ± 8.3 | 44.9 ± 19.0** | 34.1 ± 7.1* | <0.001 |
| E/e′ | 9.8 [8.1–11.8] | 15.9 [13.9–24.5]** | 13.1 [10.6–15.3]* | 0.001 |
| BNP (pg/mL) | 35.5 [25.3–56.5] | 317 [181–430]** | 128 [73–218]*** , * | <0.001 |
| GFR (mL/min) | 85.3 [70.9–94.3] | 84.5 [60.1–94.4] | 72.4 [67.5–82.2] | 0.11 |
| Acute myocardial infarct % | 0 (0) | 6 (33)** | 5 (29)* | 0.019 |
| Hypertension % | 10 (50) | 15 (83) | 15 (88)* | 0.016 |
| Diabetes mellitus % | 2 (10) | 7 (39) | 6 (35) | 0.091 |
| Atrial fibrillation % | 1 (5) | 5 (28) | 9 (53)* | 0.005 |
| ASS % | 2 (10) | 5 (39)** | 7 (29) | 0.004 |
| Oral anticoagulation% | 2 (10) | 7 (39) | 9 (53)* | 0.017 |
| Beta‐blocker % | 4 (20) | 17 (94)** | 13 (77)* | <0.001 |
| ACEI/ARB/neprilysin inhibitor % | 8 (40) | 18 (100)** | 12 (71) *** | <0.001 |
| Aldosterone antagonist % | 0 (0) | 12 (67)** | 3 (18) *** | <0.001 |
| Diuretics % | 6 (30) | 16 (89)** | 9 (53) | <0.001 |
| Statins % | 2 (10) | 13 (72)** | 11 (65)* | <0.001 |
| Oral antidiabetic therapy % | 0 (0) | 4 (22) | 5 (29)* | 0.039 |
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker; ASS, aspirin, BNP, brain natriuretic peptide; GFR, glomerular filtrating rate; NYHA, New York Heart Association.
P < 0.0167 comparison between HFpEF and non‐HF controls.
P < 0.0167 comparison between HFrEF and non‐HF controls.
P < 0.0167 comparison between HFpEF and HFrEF.
To address different clinical phenogroups of patients with HFpEF, we provide here a summary of the clinical status of these patients. 20 There was only 2 (11.8%) patients <60 years old, 5 (29.4%) patients were obese (BMI > 30 kg/m2), and only 2 (11.8%) patients had GFR < 60 mL/min. In general, similar to the suggested phenogroups by Cohen et al., 20 the majority of our patients (12 patients) would fit the phenogroup 2: Age 72 ± 6 years old, 7 (58.3%) were women, and 7 (58.3%) had atrial fibrillation, E/e′ 13.2 ± 3.2, LAVI: 32.4 ± 6.5 mL/m2. Further, 3 patients would match the phenogroups 3: [BMI: 36.7 ± 2.7 kg/m2, 2 patients (66.6%) had NYHA III and 2 (66.6%) had diabetes mellitus].
Compared with HFrEF and non‐HF controls, patients with HFpEF showed reduced mental component (MCS) and vitality (VT)‐scores in the SF‐36 questionnaire as well as elevated anxiety and depression scores in the HADS questionnaires (Table 2 and Figure 1 A–D ). After adjusting to multiple comparisons and adjusting to sex and atrial fibrillation, anxiety remained higher in HFpEF patients compared with HFrEF (ppost‐hoc 0.009). As a result to the significantly different distributed gender among the three groups, we applied the same analysis on men only from the three groups and found that anxiety remains higher in male patients with HFpEF compared with HFrEF and non‐HF controls (7.0 [5.0–9.0] vs. 2.0 [1.3–5.0] vs. 2.0 [1.5–5.5], P = 0.03). Compared with HFrEF and non‐HF controls, female patients with HFpEF showed reduced vitality score (49.53 ± 3.52 vs. 62.30 ± 5.63 vs. 57.47 ± 5.21, P = 0.002). In a direct comparison to females with non‐HF controls, women with HFpEF showed higher depression scores [(6.25 ± 3.33 vs. 2.64 ± 2.38, P = 0.02).
Table 2.
Quality of life in patients with HFpEF, HFrEF and non‐HF controls
| Parameters of quality of life | Non‐HF controls | HFrEF | HFpEF | P‐value |
|---|---|---|---|---|
| N = 20 | N = 18 | N = 17 | ||
| Physical functioning (PF) | 58.2 [56.2–60.4] | 50.9 [46.1–54.2]** | 51.8 [43.7–55.3]* | <0.0001 |
| Role limitation because of physical problems (RP) | 56.8 [53.5–59.0] | 48.0 [41.0–55.8]** | 47.4 [42.4–51.6]* | 0.002 |
| Bodily pain (bp) | 61.7 [54.3–63.3] | 51.3 [39.6–62.1] | 49.5 [38.7–60.1]* | 0.01 |
| General health (GH) | 55.4 ± 9.8 | 46.1 ± 6.0** | 47.2 ± 8.6* | 0.006 |
| Vitality (VT) | 57.1 ± 5.2 | 53.6 ± 8.6 | 47.5 ± 8.4* , † | 0.004 |
| Social functioning (SF) | 57.6 [55.7–58.3] | 57.2 [43.8–57.8] | 46.7 [44.8–50.2]* | 0.002 |
| Role limitation because of emotional health (RE) | 55.5 [50.6–56.3] | 53.9 [41.4–56.2] | 48.9 [44.1–55.7] | 0.08 |
| Mental health (MH) | 52.5 ± 7.0 | 50.2 ± 11. 7 | 45.2 ± 8.5 | 0.09 |
| Physical score component (PCS) | 56.3 ± 3.8 | 45.6 ± 5.9** | 47.5 ± 8.3* | <0.0001 |
| Mental score component (mcs) | 50.5 ± 5.0 | 50.2 ± 10.0 | 43.6 ± 7.1* , † | 0.03 |
| EQ‐5D (VAS) | 85.0 [80–95.0] | 65.0 [50.0–77.5]** | 60.0 [50.0–72.5]* | <0.0001 |
| HADS‐Anxiety | 3.8 ± 2. 8 | 3.3 ± 2.8 | 6.5 ± 3.2*,*** | 0.02 |
| HADS‐Depression | 2.0 [0.75–3.0] | 3.0 [1.0–6.5] | 6.5 [3.5–10.0]* , † | 0.01 |
Data from SF‐36, EQ‐5D, and HADS.
EQ‐5D, European quality of life–5 dimensions; HADS, hospital anxiety and depression scale; VAS, visual analogue scale.
P < 0.0167 comparison between HFpEF and non‐HF controls.
P < 0.0167 comparison between HFrEF and non‐HF controls.
P < 0.0167 comparison between HFpEF and HFrEF.
P < 0.05 comparison between HFpEF and HFrEF.
Figure 1.
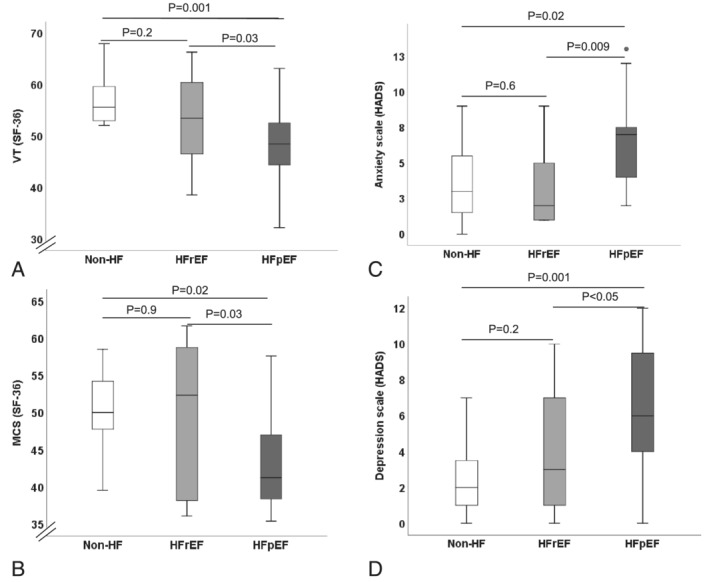
Comparison of quality of life between HFpEF, HFrEF patients and non‐HF controls. (A) Vitality (VT) as part of the SF‐36‐questionnaire. (B) Mental health component summery (MCS) as part of the SF‐36‐questionnaire. (C) Anxiety scale as part of the HADS‐questionnaire. (D) Depression scale as part of the HADS‐questionnaire. HADS, hospital anxiety and depression scale; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; MCS, mental health component summery; non‐HF, non‐heart failure; SF‐36, short form of health survey; VT, vitality.
Coordination capacity and gait performance in patients with heart failure
Patients with HFpEF versus non‐HF controls and HFrEF versus non‐HF controls showed reduced balance and coordination capacities in the dynamic balance tests and in gait performance during 10‐MWT (Table 3 ). There was no difference between HFpEF and HFrEF. Male patients with HFpEF compared with non‐HF controls were slower in the 10‐MWT (5.3 [5.0–7.0] vs. 4.9 [4.3–5.0] s, P = 0.007) and had reduced balance and coordination capacity in walking forward (FW‐15: 2.0 [0.0–2.0] vs. 0.0 [0.0–0.0] misstep, P = 0.04). No difference was noted in this regard between HFpEF and HFrEF.
Table 3.
Coordination capacity in patients with HFpEF, HFrEF, and age‐matched non‐HF controls
| Measurements of coordination capacity | Non‐HF controls N = 20 | HFrEF N = 18 | HFpEF N = 17 | P‐value |
|---|---|---|---|---|
| 10‐MWT (s) | 4.9 [4.5–5.3] | 5.3 [4.8–7.3]** | 5.8 [5.0–8.3]* | 0.003 |
| Walking forward 20 cm | 0.0 [0.0–0.0] | 0.0 [0.0–0.0] | 0.0 [0.0–0.3] | 0.11 |
| Walking forward 15 cm | 0.0 [0.0–0.0] | 0.0 [0.0–1.0]** | 1.5 [0.0–3.0]* | 0.01 |
| Walking backward 20 cm | 0.0 [0.0–1.0] | 2.0 [1.0–3.0]** | 2.0 [0.0–2.3]* | 0.002 |
| Walking backward 15 cm | 1.5 [1.0–3.0] | 3.0 [1.0–5.0] | 4.0 [2.8–6.0]* | 0.006 |
10‐MWT, 10 m Walk Test.
P < 0.0167 comparison between HFpEF and non‐HF controls.
P < 0.0167 comparison between HFrEF and non‐HF controls.
P < 0.0167 comparison between HFpEF and HFrEF.
Muscle function, mental health, and gait performance
The balance between knee concentric and eccentric movements is important to stabilize the gait and prevent against falls especially in elderly. We found that peak torque of knee in eccentric extension and after adjusting to sex was significantly lower in patients with HFpEF and HFrEF than in non‐HF controls (151 ± 50.8 vs. 187 ± 39.7 vs. 220 ± 42.1 Nm/kg, P = 0.02). Furthermore, peak torque of right knee in eccentric flexion was associated with peak torque of right knee in concentric flexion was associated with (r = 0.7, P < 0.0001) and inversely with balance coordination capacity (walking backward on 15 cm wide line: r = −0.4, P = 0.04). These correlations remained unchanged after adjusting to sex.
Patients with at least borderline anxiety (Anxiety in the HADS score ≥8) showed reduced QoL [(GH: 42.0 ± 6.5 vs. 49.7 ± 5.1), (VT: 43.8 ± 8.4 vs. 53.2 ± 7.2), (MH: 41.1 ± 9.1 vs. 49.2 ± 9.4), (VAS‐score: 54.5 ± 12.1 vs. 72.8 ± 12.2, all P < 0.05)] (Figure 2 A,B ). Similarly, patients with at least borderline depression score in the HADS questionnaire (Depression in the HADS score ≥8) showed reduced coordination capacity (FW‐15: 2.8 ± 3.0 vs. 0.9 ± 1.3 missteps, P = 0.03), and reduced several aspects of QoL [(MCS: 41.2 ± 4.8 vs. 49.2 ± 9.6, P = 0.04), (VT: 44.8 ± 7.5 vs. 52.2 ± 8.5, P = 0.04), (SF: 43.8 ± 7.8 vs. 51.0 ± 7.7, P = 0.02)] (Figure 2 C,D ). Similar results were shown by adjusting to sex among HFpEF, HFrEF, and non‐HF controls.
Figure 2.
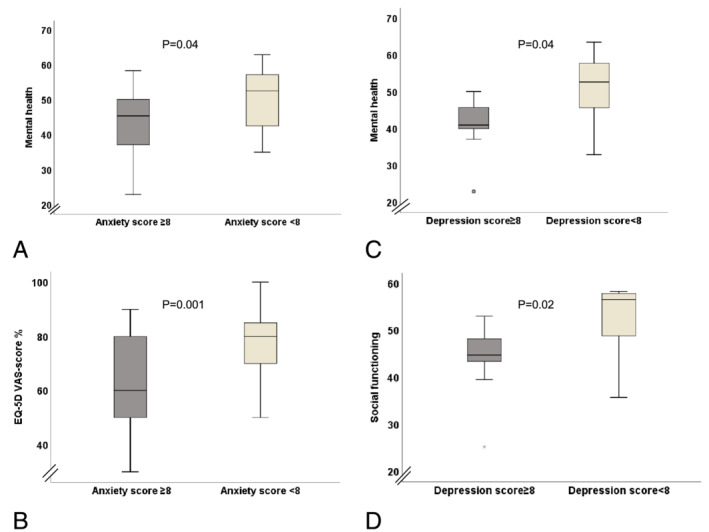
The influence of depression and anxiety in patients with HF on QoL. (A) Reduced mental health (SF‐36) patients with at least borderline anxiety score. (B) Reduced QoL measured by VAS‐score in patients with at least borderline anxiety score. (C) Reduced mental health (SF‐36) in patients with at least borderline depression score. (D) Reduced social functioning (SF‐36) in patients with at least borderline depression score. EQ‐5D, European quality of life–5 dimensions; VAS‐score, visual analogue scale.
In a logistic regression, the presence of at least borderline depression (≥8 points in the HADS questionnaire) remained an independent factor for predicting reduced coordination capacity in the dynamic balance tests (defined as number of missteps in walking forward on the 15‐cm wide line ≥ mean value) after adjusting for peak VO2, GDF‐15, 10‐MWT, PCS, and peak torque of the right leg in extension [odds ratio (OR): 0.1, 95% confidence interval (CI): 0.004–0.626, P = 0.02] (Table 4 ).
Table 4.
Logistic regression model with reduced coordination capacity estimated in walking forward on 15‐cm‐wide line (< mean value of the cohort) serving as the dependent variable
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P‐value | OR | 95% CI | P‐value | |
| Age (per year increase) | 1.1 | 0.97–1.13 | 0.2 | |||
| Sex (male/female) | 2.3 | 0.72–7.11 | 0.2 | |||
| Peak VO2 (per 1 mL/kg/min increase) | 0.9 | 0.74–0.97 | 0.02 | 1.2 | 0.86–1.71 | 0.3 |
| Peak torque of right leg in extension (per 1 Nm/kg increase) | 0.8 | 0.65–0.99 | 0.04 | 0.7 | 0.44–1.17 | 0.2 |
| GDF‐15 (per 1 pg/mL increase) | 1.0 | 1.00–1.01 | 0.01 | 1.0 | 1.00–1.01 | 0.2 |
| Depression score ≥8 (present) | 0.1 | 0.01–0.41 | 0.006 | 0.1 | 0.004–0.63 | 0.02 |
| PCS (per 1‐point increase) | 0.9 | 0.83–0.99 | 0.04 | 0.9 | 0.76–1.04 | 0.1 |
| 10‐MWT (per 1 s increase) | 1.4 | 0.96–1.96 | 0.08 | 0.9 | 0.42–1.98 | 0.2 |
GDF‐15, growth differentiation factor‐15; 10‐MWT, 10 m walking test; PCS, physical component score of the SF‐36 questionnaire; Peak VO2, maximal oxygen consumption.
Inflammatory biomarkers and their relation to muscle function, coordination capacity, and quality of life
Patients with elevated GDF‐15 (> mean value) showed reduced coordination [(walking backward 20 cm: 2.6 ± 1.8 vs. 1.3 ± 1.4 misstep), (walking forward 15 cm: 2.4 ± 2.7 vs. 0.9 ± 1.3 misstep, all P < 0.05)]. Additionally, patients with elevated suPAR (> mean value) showed reduced QoL in different aspects of SF‐36 [(GH: 43.5 ± 6.1 vs. 49.3 ± 7.4), (SF: 45.3 ± 9.2 vs. 51.7 ± 6.4), (RE: 45.1 ± 7.7 vs. 52.4 ± 4.2), (MH: 42.9 ± 9.6 vs. 51.5 ± 9.3), (MCS: 41.9 ± 7.2 vs. 50.2 ± 8.8, all P < 0.05)]. The aforementioned results (GDF‐15 and suPAR) remained unchanged after adjusting to sex.
Discussion
We showed for the first time to our knowledge in clinically stable outpatients with HF a profound reduction in QoL in patients with HFpEF assessed by mental health and vitality in the SF‐36 questionnaire and increased anxiety and depression scores in the HADS questionnaire compared with those with HFrEF and non‐HF controls. Both HFpEF and HFrEF showed reduced coordination capacities compared with non‐HF controls. Elevated levels of inflammatory biomarkers were associated with reduced QoL and impaired coordination capacity. In a logistic regression the presence of at least borderline depression (≥8 points in the HADS questionnaire) remained an independent factor for predicting reduced coordination capacity in the dynamic balance tests after adjusting for peak VO2, GDF‐15, 10‐MWT, PCS, and peak torque of the right leg in extension.
The main symptoms in patients with HF are exercise intolerance and fatigue. Peripheral factors such as skeletal muscle dysfunction seem to play an important role in explaining exercise intolerance and reduced QoL in these patients. 15 , 37 In spite of the proven reduced QoL in patients with HF in different aspects such as social, emotional and cognitive fields, 8 , 10 the evaluation and management of QoL in these patients are not included in the daily practice and still considered to be a gap in the management of patients with HF. 38
Quality of life and psychosocial factors
A couple of studies compared QoL (SF‐36) in patients with HFpEF and HFrEF 39 , 40 , 41 and found similar reduction in QoL between these two groups. This is likely due to using different definitions for making the diagnosis of HFpEF than the currently recommended one from the ESC‐HF. 19
Recently, it has been shown in acute decompensated HF patients that patients with HFpEF defined according to the current recommendations of the ESC‐HF guidelines 19 had higher rates of depression than those with HFrEF. 42 Our findings are in line with this showing increased anxiety and depression scores in the HADS questionnaire compared with those with HFrEF and non‐HF controls. In addition, patients with higher depression scores had worse coordination and balance capacity, and reduced QoL demonstrated in the SF‐36‐questionnaire by reduced vitality, social function, and mental component score.
Inflammatory biomarkers and quality of life
An elevation of GDF‐15 in patients with HF is known as well. 43 , 44 However, we described in the current study the relationship between inflammatory biomarkers and coordination capacity and QoL. Patients with elevated GDF‐15 had reduced coordination capacity in the balance tests. Those with elevated levels of the inflammatory biomarker suPAR had reduced QoL in several aspects of SF‐36‐questionnaire such as general health, social function, and mental health. These results should be confirmed in larger studies and the pathophysiology needs to be investigated.
Physical evaluation and dynamic balance
Muscle strength and the balance between knee concentric and eccentric movements as well as the speed of developing peak torque are important factors to stabilize the gait and prevent against falls especially in elderly. 45 , 46 , 47 , 48 , 49 Furthermore, the muscular activation pattern during concentric and eccentric isokinetic movements seems to be different with a higher frequency of motor units during the eccentric muscle performance. 50 This emphasizes the importance of eccentric movements of the knee in creating higher strength and as a result ensuring more gait balance. Our findings are supportive in this regard. We found that coordination capacity and peak torque of knee in eccentric extension were significantly lower in patients with HFpEF and HFrEF than in non‐HF controls. In addition, there was a correlation between ‘the reduced’ muscle strength especially in eccentric movements of the knee and balance dynamic tests. This all makes patients with HF more susceptible to falls and to the following health‐related, social, and economic consequences and emphasizes the importance of normal muscle function in stabilizing gait performance and improving coordination capacity.
In conclusion, clinically stable outpatients with HFpEF have worse QoL and elevated prevalence of anxiety and depression compared with those with HFrEF and non‐HF controls. Depression was associated with reduced QoL and is an independent predictor for reduced coordination capacity.
While our results need to be confirmed with larger cohorts of patients, screening for reduced QoL should be included in our daily practice in the management of patients with HF.
Limitations
Our study was performed on small number of participants. Additional limitation remains the baseline differences (sex distribution, atrial fibrillation, and BNP). Although we addressed this issue by performing an analysis adjusted to these factors, the small sample volume remains a main limitation to this analysis. Therefore, larger studies are required to confirm our results.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Bekfani, T. , Nisser, J. , Derlien, S. , Hamadanchi, A. , Fröb, E. , Dannberg, G. , Lichtenauer, M. , Smolenski, U. C. , Lehmann, G. , Möbius‐Winkler, S. , and Schulze, P. C. (2021) Psychosocial factors, mental health, and coordination capacity in patients with heart failure with preserved ejection fraction compared with heart failure with reduced ejection fraction. ESC Heart Failure, 8: 3268–3278. 10.1002/ehf2.13468.
References
- 1. Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well‐defined older population, 1970‐1974 and 1990‐1994. Circulation 2006; 113: 799–805. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd‐Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel‐Smoller S, Wong ND, Wylie‐Rosett J, American Heart Association Statistics C, Stroke Statistics S . Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 2010; 121: 948–954. [DOI] [PubMed] [Google Scholar]
- 3. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med 2006; 355: 260–269. [DOI] [PubMed] [Google Scholar]
- 4. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A, Investigators IP. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008; 359: 2456–2467. [DOI] [PubMed] [Google Scholar]
- 5. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J, Investigators C. Committees. Effects of candesartan in patients with chronic heart failure and preserved left‐ventricular ejection fraction: the CHARM‐Preserved Trial. Lancet 2003; 362: 777–781. [DOI] [PubMed] [Google Scholar]
- 6. Bergstrom A, Andersson B, Edner M, Nylander E, Persson H, Dahlstrom U. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler‐echocardiographic study (SWEDIC). Eur J Heart Fail 2004; 6: 453–461. [DOI] [PubMed] [Google Scholar]
- 7. Pandey A, Parashar A, Kumbhani D, Agarwal S, Garg J, Kitzman D, Levine B, Drazner M, Berry J. Exercise training in patients with heart failure and preserved ejection fraction: meta‐analysis of randomized control trials. Circ Heart Fail 2015; 8: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edelmann F, Stahrenberg R, Polzin F, Kockskamper A, Dungen HD, Duvinage A, Binder L, Kunde J, Scherer M, Gelbrich G, Hasenfuss G, Pieske B, Wachter R, Herrmann‐Lingen C. Impaired physical quality of life in patients with diastolic dysfunction associates more strongly with neurohumoral activation than with echocardiographic parameters: quality of life in diastolic dysfunction. Am Heart J 2011; 161: 797–804. [DOI] [PubMed] [Google Scholar]
- 9. Gary RA, Sueta CA, Dougherty M, Rosenberg B, Cheek D, Preisser J, Neelon V, McMurray R. Home‐based exercise improves functional performance and quality of life in women with diastolic heart failure. Heart Lung 2004; 33: 210–218. [DOI] [PubMed] [Google Scholar]
- 10. Nolte K, Herrmann‐Lingen C, Wachter R, Gelbrich G, Dungen HD, Duvinage A, Hoischen N, von Oehsen K, Schwarz S, Hasenfuss G, Halle M, Pieske B, Edelmann F. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: the Ex‐DHF‐P trial. Eur J Prev Cardiol 2015; 22: 582–593. [DOI] [PubMed] [Google Scholar]
- 11. Easton K, Coventry P, Lovell K, Carter LA, Deaton C. Prevalence and measurement of anxiety in samples of patients with heart failure: meta‐analysis. J Cardiovasc Nurs 2016; 31: 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rutledge T, Reis VA, Linke SE, Greenberg BH, Mills PJ. Depression in heart failure a meta‐analytic review of prevalence, intervention effects, and associations with clinical outcomes. J Am Coll Cardiol 2006; 48: 1527–1537. [DOI] [PubMed] [Google Scholar]
- 13. Lin TK, Hsu BC, Li YD, Chen CH, Lin JW, Chien CY, Weng CY. Prognostic value of anxiety between heart failure with reduced ejection fraction and heart failure with preserved ejection fraction. J Am Heart Assoc 2019; 8: e010739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Angermann CE, Gelbrich G, Stork S, Schowalter M, Deckert J, Ertl G, Faller H, Competence Network Heart F . Somatic correlates of comorbid major depression in patients with systolic heart failure. Int J Cardiol 2011; 147: 66–73. [DOI] [PubMed] [Google Scholar]
- 15. Bekfani T, Pellicori P, Morris DA, Ebner N, Valentova M, Steinbeck L, Wachter R, Elsner S, Sliziuk V, Schefold JC, Sandek A, Doehner W, Cleland JG, Lainscak M, Anker SD, von Haehling S. Sarcopenia in patients with heart failure with preserved ejection fraction: Impact on muscle strength, exercise capacity and quality of life. Int J Cardiol 2016; 222: 41–46. [DOI] [PubMed] [Google Scholar]
- 16. Fulster S, Tacke M, Sandek A, Ebner N, Tschope C, Doehner W, Anker SD, von Haehling S. Muscle wasting in patients with chronic heart failure: results from the studies investigating co‐morbidities aggravating heart failure (SICA‐HF). Eur Heart J 2013; 34: 512–519. [DOI] [PubMed] [Google Scholar]
- 17. Agudelo LZ, Femenia T, Orhan F, Porsmyr‐Palmertz M, Goiny M, Martinez‐Redondo V, Correia JC, Izadi M, Bhat M, Schuppe‐Koistinen I, Pettersson AT, Ferreira DMS, Krook A, Barres R, Zierath JR, Erhardt S, Lindskog M, Ruas JL. Skeletal muscle PGC‐1alpha1 modulates kynurenine metabolism and mediates resilience to stress‐induced depression. Cell 2014; 159: 33–45. [DOI] [PubMed] [Google Scholar]
- 18. Reeves GR, Whellan DJ, Patel MJ, O'Connor CM, Duncan P, Eggebeen JD, Morgan TM, Hewston LA, Pastva AM, Kitzman DW. Comparison of frequency of frailty and severely impaired physical function in patients >/=60 years hospitalized with acute decompensated heart failure versus chronic stable heart failure with reduced and preserved left ventricular ejection fraction. Am J Cardiol 2016; 117: 1953–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M, Document R . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 20. Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, Yarde M, Wang Z, Bhattacharya PT, Chirinos DA, Prenner S, Zamani P, Seiffert DA, Car BD, Gordon DA, Margulies K, Cappola T, Chirinos JA. Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail 2020; 8: 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 Focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2016; 133: e694–e711. [DOI] [PubMed] [Google Scholar]
- 22. Rabin R, de Charro F. EQ‐5D: a measure of health status from the EuroQol Group. Ann Med 2001; 33: 337–343. [DOI] [PubMed] [Google Scholar]
- 23. Goldsmith KA, Dyer MT, Buxton MJ, Sharples LD. Mapping of the EQ‐5D index from clinical outcome measures and demographic variables in patients with coronary heart disease. Health Qual Life Outcomes 2010; 8: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dyer MT, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ‐5D in studies of cardiovascular disease. Health Qual Life Outcomes 2010; 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McHorney CA, Ware JE Jr, Raczek AE. The MOS 36‐Item Short‐Form Health Survey (SF‐36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993; 31: 247–263. [DOI] [PubMed] [Google Scholar]
- 26. Morfeld M, Bullinger M, Nantke J, Brahler E. The version 2.0 of the SF‐36 Health Survey: results of a population‐representative study. Soz Praventivmed 2005; 50: 292–300. [DOI] [PubMed] [Google Scholar]
- 27. Hunt‐Shanks T, Blanchard C, Reid R, Fortier M, Cappelli M. A psychometric evaluation of the Hospital Anxiety and Depression Scale in cardiac patients: addressing factor structure and gender invariance. Br J Health Psychol 2010; 15: 97–114. [DOI] [PubMed] [Google Scholar]
- 28. Djukanovic I, Carlsson J, Arestedt K. Is the Hospital Anxiety and Depression Scale (HADS) a valid measure in a general population 65‐80 years old? A psychometric evaluation study. Health Qual Life Outcomes 2017; 15: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual‐energy X‐ray absorptiometry body composition model: review of physical concepts. Am J Physiol 1996; 271: E941–E951. [DOI] [PubMed] [Google Scholar]
- 30. Drouin JM, Valovich‐mcLeod TC, Shultz SJ, Gansneder BM, Perrin DH. Reliability and validity of the Biodex system 3 pro isokinetic dynamometer velocity, torque and position measurements. Eur J Appl Physiol 2004; 91: 22–29. [DOI] [PubMed] [Google Scholar]
- 31. Osternig LR. Isokinetic dynamometry: implications for muscle testing and rehabilitation. Exerc Sport Sci Rev 1986; 14: 45–80. [PubMed] [Google Scholar]
- 32. Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, Kronenberg MW, Kostis JB, Kohn RM, Guillotte M, Greenberg B, Woods PA, Bourassa MG. Prediction of mortality and morbidity with a 6‐minute walk test in patients with left ventricular dysfunction. SOLVD Investigators. JAMA 1993; 270: 1702–1707. [PubMed] [Google Scholar]
- 33. Jorgensen JR, Bech‐Pedersen DT, Zeeman P, Sorensen J, Andersen LL, Schonberger M. Effect of intensive outpatient physical training on gait performance and cardiovascular health in people with hemiparesis after stroke. Phys Ther 2010; 90: 527–537. [DOI] [PubMed] [Google Scholar]
- 34. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006; 54: 743–749. [DOI] [PubMed] [Google Scholar]
- 35. Lark SD, Pasupuleti S. Validity of a functional dynamic walking test for the elderly. Arch Phys Med Rehabil 2009; 90: 470–474. [DOI] [PubMed] [Google Scholar]
- 36. Kirchner G RA, Wittermann G. Seniorensport Meyer & Meyer Sport; 1999.
- 37. Maurer MS, Schulze PC. Exercise intolerance in heart failure with preserved ejection fraction: shifting focus from the heart to peripheral skeletal muscle. J Am Coll Cardiol 2012; 60: 129–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 39. Ohno Y, Okura Y, Ramadan MM, Taneda K, Suzuki K, Tomita M, Hao K, Kimura S, Hoyano M, Mitsuma W, Tanaka K, Kashimura T, Ito M, Hirono S, Hanawa H, Kodama M, Aizawa Y. Health‐related quality of life of outpatients with systolic and isolated diastolic dysfunction: Sado Heart Failure Study. Circ J 2008; 72: 1436–1442. [DOI] [PubMed] [Google Scholar]
- 40. Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 2002; 288: 2144–2150. [DOI] [PubMed] [Google Scholar]
- 41. Lewis EF, Lamas GA, O'Meara E, Granger CB, Dunlap ME, McKelvie RS, Probstfield JL, Young JB, Michelson EL, Halling K, Carlsson J, Olofsson B, McMurray JJ, Yusuf S, Swedberg K, Pfeffer MA, Investigators C. Characterization of health‐related quality of life in heart failure patients with preserved versus low ejection fraction in CHARM. Eur J Heart Fail 2007; 9: 83–91. [DOI] [PubMed] [Google Scholar]
- 42. Warraich HJ, Kitzman DW, Whellan DJ, Duncan PW, Mentz RJ, Pastva AM, Nelson MB, Upadhya B, Reeves GR. Physical function, frailty, cognition, depression, and quality of life in hospitalized adults >/=60 years with acute decompensated heart failure with preserved versus reduced ejection fraction. Circ Heart Fail 2018; 11: e005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stahrenberg R, Edelmann F, Mende M, Kockskamper A, Dungen HD, Luers C, Binder L, Herrmann‐Lingen C, Gelbrich G, Hasenfuss G, Pieske B, Wachter R. The novel biomarker growth differentiation factor 15 in heart failure with normal ejection fraction. Eur J Heart Fail 2010; 12: 1309–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bouabdallaoui N, Claggett B, Zile MR, McMurray JJV, O'Meara E, Packer M, Prescott MF, Swedberg K, Solomon SD, Rouleau JL, Investigators P‐H. Committees. Growth differentiation factor‐15 is not modified by sacubitril/valsartan and is an independent marker of risk in patients with heart failure and reduced ejection fraction: the PARADIGM‐HF trial. Eur J Heart Fail 2018; 20: 1701–1709. [DOI] [PubMed] [Google Scholar]
- 45. Hughes MA, Myers BS, Schenkman ML. The role of strength in rising from a chair in the functionally impaired elderly. J Biomech 1996; 29: 1509–1513. [PubMed] [Google Scholar]
- 46. Macrae PG, Lacourse M, Moldavon R. Physical performance measures that predict faller status in community‐dwelling older adults. J Orthop Sports Phys Ther 1992; 16: 123–128. [DOI] [PubMed] [Google Scholar]
- 47. Reichard LB, Croisier JL, Malnati M, Katz‐Leurer M, Dvir Z. Testing knee extension and flexion strength at different ranges of motion: an isokinetic and electromyographic study. Eur J Appl Physiol 2005; 95: 371–376. [DOI] [PubMed] [Google Scholar]
- 48. Bento PC, Pereira G, Ugrinowitsch C, Rodacki AL. Peak torque and rate of torque development in elderly with and without fall history. Clin Biomech (Bristol, Avon) 2010; 25: 450–454. [DOI] [PubMed] [Google Scholar]
- 49. Lee IH, Park SY. Balance improvement by strength training for the elderly. J Phys Ther Sci 2013; 25: 1591–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McHugh MP, Tyler TF, Greenberg SC, Gleim GW. Differences in activation patterns between eccentric and concentric quadriceps contractions. J Sports Sci 2002; 20: 83–91. [DOI] [PubMed] [Google Scholar]


