Abstract
Aims
Myocarditis may lead to malignant arrhythmias and sudden cardiac death. As of today, there are no reliable predictors to identify individuals at risk for these catastrophic events. The aim of this study was to evaluate if a wearable cardioverter defibrillator (WCD) may detect and treat such arrhythmias adequately in the peracute setting of myocarditis.
Methods and results
In this observational, retrospective, single centre study, we reviewed patients presenting to the Charité Hospital from 2009 to 2017, who were provided with a WCD for the diagnosis of myocarditis with reduced ejection fraction (<50%) and/or arrhythmias. Amongst 259 patients receiving a WCD, 59 patients (23%) were diagnosed with myocarditis by histology. The mean age was 46 ± 14 years, and 11 patients were women (19%). The mean WCD wearing time was 86 ± 63 days, and the mean daily use was 20 ± 5 h. During that time, two patients (3%) had episodes of sustained ventricular tachycardia (VT; four total) corresponding to a rate of 28 sustained VT episodes per 100 patient‐years. Consequently, one of these patients underwent rhythm stabilization through intravenous amiodarone, while the other patient received an implantable cardioverter defibrillator. Two patients (3.4%) were found to have non‐sustained VT.
Conclusions
Using a WCD after acute myocarditis led to the detection of sustained VT in 2/59 patients (3%). While a WCD may prevent sudden cardiac death after myocarditis, our data suggest that WCD may have impact on clinical management through monitoring and arrhythmia detection.
Keywords: Wearable cardioverter defibrillator, Myocarditis, HFrEF, HFmrEF
Introduction
Myocarditis is estimated to be one of the most common causes of sudden cardiac death (SCD) and has been found in up to 30% of autopsies. 1 , 2 , 3 Major efforts have been underway to predict major adverse cardiovascular events (MACE) over the last decade. Recent studies using cardiac magnetic resonance imaging (CMR) in patients with myocarditis suggested predictors of MACE based on late gadolinium enhancement. 4 , 5 Furthermore, case series have shown that QRS prolongation ≥ 120 ms on electrocardiogram (ECG) is an independent predictor of death in myocarditis. 6
However, individual predictions of MACE are still not accurate enough, and wearable cardioverter defibrillators (WCDs) have been increasingly introduced to protect patients during the most vulnerable time after diagnosis of myocarditis. 7 , 8 , 9 The value of WCD has been demonstrated in ischaemic or non‐ischaemic cardiomyopathy, 10 , 11 in the early post‐myocardial infarction period, 12 , 13 , 14 and in patients with peripartum cardiomyopathy. 15
In myocarditis, the potential benefit of a WCD has been solely evaluated in subgroup analyses of larger trials as of today. 16 , 17 , 18 , 19 Therefore, descriptions of clinical patient characteristics, methods of diagnosis, and a clinical trajectory of patients with myocarditis have been limited in those studies.
We sought to address this issue by performing a retrospective study in patients who were diagnosed with myocarditis [based on clinical presentation and endomyocardial biopsy (EMB)] and who were prescribed a WCD. Comprehensive clinical data were collected on all patients, including device wearing time and clinical trajectory.
Methods
Study population
In this retrospective analysis, we reviewed all patients, who had been prescribed a WCD for SCD prevention (LifeVest; ZOLL, Pittsburgh, PA) from January 2009 to December 2017 at the Division of Cardiology of the Charité University Hospital Berlin, Campus Benjamin Franklin. Amongst those patients, we selected individuals with a diagnosis of myocarditis. Common indications for WCD use were severely reduced left ventricular ejection fraction (LVEF), episodes of sustained ventricular tachyarrhythmia, and histological confirmation of giant cell myocarditis or cardiac sarcoidosis. An overview of specific indications for the prescription of a WCD in our cohort is listed in Table 1 . The diagnosis of myocarditis was based on EMB with subsequent histological, immunohistological, and molecular biological analysis following the indications outlined by the European Society of Cardiology (ESC) position statement 8 in patients with classic clinical symptoms, abnormalities on ECG, and/or elevated high‐sensitivity troponin‐T.
Table 1.
Indications for wearable cardioverter defibrillator prescription in patients with myocarditis
| WCD indication | n (%) |
|---|---|
| LVEF ≤ 35 ± prior VF/VT ± cardiac sarcoidosis/giant cell myocarditis | 41 (69.5) |
| VT/VF during hospitalization and LVEF > 35% | 6 (10.2) |
| Sarcoidosis/giant cell and LVEF > 35% | 2 (3.4) |
| LVEF 36–40% | 4 (6.8) |
| Based on the physician's discretion | 6 (10.2) |
LVEF, left ventricular ejection fraction; VF, ventricular fibrillation; VT, ventricular tachycardia; WCD, wearable cardioverter defibrillator.
Histology of EMBs was performed by light microscopy for evidence of myocardial necrosis and interstitial fibrosis and for the presence of lymphocytic infiltrates. Antibodies directed against surface antigens of human lymphocytes (CD3, CD4, and CD8) were used for detection and quantification of lymphocytic infiltrates in myocardial tissue.
Polymerase chain reaction/reverse transcription polymerase chain reaction was performed for the detection of enteroviruses (including coxsackievirus and echovirus), adenovirus, erythrovirus, human herpesvirus type 6, human cytomegalovirus, and Epstein–Barr virus.
Furthermore, all patients underwent cardiac catheterization to exclude relevant coronary artery disease (stenosis > 50%).
Clinical data were retrieved from the electronic medical record system. Compliance with wearing the WCD and arrhythmic events were collected via the ZOLL database.
Recordings of all baseline ECGs and arrhythmic events were individually examined by a specialist trained in electrophysiology.
All patients received guideline‐directed medical therapy and comprehensive training in the use of the WCD to optimize compliance.
This study was approved by the local ethics committee (EA4/117/17) and conforms to the updated guiding principles of the Declaration of Helsinki of 2013. 20
All authors had full access to the data and have read and agreed to the manuscript as written.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation and, if appropriate, as median and first and third quartile, and categorical variables as absolute and relative frequencies. Due to the skewed distribution of most of the quantitative measurements, Mann–Whitney U‐test was used for comparisons between independent groups. Spearman's correlation coefficient was used for associations between two quantitative variables. Categorical data were compared using the χ 2 test.
A P‐value of ≤0.05 was considered statistically significant. No Bonferroni correction has been performed due to the explorative character of the study. For simple and multiple logistic regression analyses, odds ratio (OR) and confidence interval (CI) were used. Statistical analysis was performed using SPSS statistics (Version 23).
Results
Study population
Between 2009 and 2017, a total of 259 patients received a WCD at the Charité University Hospital Berlin. Patients with cognitive impairment preventing the safe use of a WCD or patients who refused this device were excluded.
Baseline characteristics are illustrated in Table 2 . Myocarditis was diagnosed based on histology in a total of 59 (65%) patients (Figure 1 ). Immunohistology of EMBs revealed lymphocytic myocarditis in most cases (n = 45; 76%). The most frequently detected virus was Parvovirus B19 (n = 42; 71%). Further subtypes of myocarditis detected based on EMB are listed in Tables 3a and 3b .
Table 2.
Baseline characteristics of the study cohort (n = 59)
| Parameter | Value ± SD (mean) |
|---|---|
| Age, years, mean ± SD | 46 ± 14 |
| Female, n (%) | 11 (18.6) |
| Renal disease (KDIGO 2012), n (%) | 15 (25.4) |
| Diabetes mellitus type II, n (%) | 11 (18.6) |
| Arterial hypertension, n (%) | 20 (33.9) |
| BMI, kg/m2, mean ± SD | 27 ± 4 |
| ECG on admission | |
| Sinus rhythm, n (%) | 47 (79.7) |
| Atrial fibrillation, n (%) | 12 (20.3) |
| Left bundle brunch block, n (%) | 11 (18.6) |
| Atrial fibrillation during hospital stay, n (%) | 15 (25.4) |
| Paroxysmal, n (%) | 7 (11.9) |
| Persistent, n (%) | 7 (11.9) |
| Permanent, n (%) | 1 (1.7) |
| Ventricular arrhythmias during hospital stay, n (%) | 11 (18.6) |
| Sustained VT, n (%) | 9 (15.3) |
| VF, n (%) | 2 (3.4) |
| Heart rate at diagnosis, b.p.m., mean ± SD | 88 ± 24 |
| LVEF at diagnosis, %, mean ± SD | 32 ± 15 |
| LVEDD at diagnosis, mm, mean ± SD | 62 ± 9 |
| TAPSE, mean ± SD, mm | 19 ± 5 |
| NYHA at diagnosis | |
| I, n (%) | 11 (18.6) |
| II, n (%) | 22 (37.3) |
| III, n (%) | 20 (33.9) |
| IV, n (%) | 4 (6.8) |
| TnT‐hs on admission, ng/L, mean ± SD | 193 ± 493 |
| NT‐proBNP at diagnosis, ng/L, mean ± SD | 7102 ± 13 168 |
| Medication at discharge | |
| Beta‐blockers, n (%) | 56 (94.9) |
| ACE inhibitors, n (%) | 54 (91.5) |
| Angiotensin receptor blockers, n (%) | 3 (5.1) |
| Aldosterone antagonists, n (%) | 47 (79.7) |
| Nephrolysin inhibitors, n (%) | 1 (1.7) |
| Antiviral therapy, n (%) | 5 (8.5) |
| Immunosuppressive therapy, n (%) | 15 (25.4) |
ACE, angiotensin‐converting enzyme; BMI, body mass index; ECG, electrocardiography; KDIGO, Kidney Disease Improving Global Outcomes; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation; TAPSE, tricuspid annular plane systolic excursion; TnT‐hs, high‐sensitivity cardiac troponin‐T; VF, ventricular fibrillation; VT, ventricular tachycardia.
Figure 1.
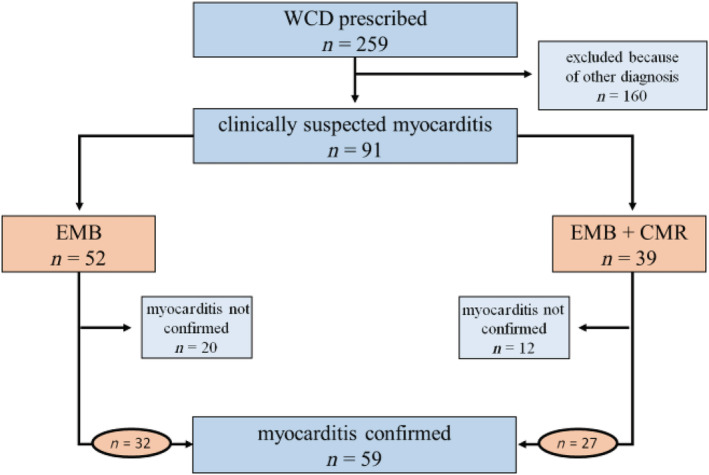
Study design: 259 patients were prescribed a WCD at our clinic from 2009 to 2017. In 32 cases, myocarditis was histologically proven. CMR, cardiac magnetic resonance imaging; EMB, endomyocardial biopsy; WCD, wearable cardioverter defibrillator.
Table 3a.
Subtypes of myocarditis based on histology and detection of viral genome (n = 59)
| Diagnosis | Number of patients, n (%) | Number of patients with detection of viral genome, n (%) | Number of patients with borderline myocarditis, n (%) |
|---|---|---|---|
| Lymphocytic myocarditis | 45 (76) | 32 (54) | 30 (51) |
| Eosinophilic myocarditis | 2 (3) | 2 (3) | 0 (0) |
| Polymorphic myocarditis | 7 (12) | 6 (10) | 3 (5) |
| Giant cell myocarditis | 4 (7) | 2 (3) | 0 (0) |
| Cardiac sarcoidosis | 1 (2) | 0 (0) | 0 (0) |
Table 3b.
Virus types by diagnosis (n = 59)
| Diagnosis | PVB19, n (%) | EBV, n (%) | HHV6, n (%) | Coxsackie B3, n (%) |
|---|---|---|---|---|
| Lymphocytic myocarditis | 32 a (53) | 3 a (5) | 3 a (5) | 1 a (2) |
| Eosinophilic myocarditis | 2 (3) | 0 (0) | 0 (0) | 0 (0) |
| Polymorphic myocarditis | 6 a (10) | 0 (0) | 1 a (2) | 0 (0) |
| Giant cell myocarditis | 2 a (3) | 1 a (2) | 0 (0) | 0 (0) |
| Cardiac sarcoidosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
EBV, Epstein–Barr virus; HHV6, human herpesvirus type 6; PVB19, Parvovirus B19.
Coinfection.
Wearable cardioverter defibrillator use data
The mean WCD use was 86 ± 63 days, and the mean daily use was 20 ± 5 h. There was a trend of a positive correlation between average daily wearing time and age (R = 0.193, P = 0.144) (Supporting Information, Figure S1 ) and a trend of a negative correlation between cumulative WCD prescription time and age (R = −0.063, P = 0.636) (Supporting Information, Figure S2 ).
Cardiovascular outcomes
Two patients (3%) had sustained haemodynamically stable ventricular tachycardia (VT) (total of four events), corresponding to a rate of 28 episodes of sustained VT per 100 patients‐years. No shocks were delivered during WCD wearing time.
The first patient presenting with sustained VT was a 42‐year‐old man with severely decreased LVEF (30%). He had atrial fibrillation and left bundle branch block (QRS 140 ms). EMB revealed borderline myocarditis, no signs of necrosis, and only low levels of perivascular fibrosis. Parvovirus B19 DNA was below level of detection for quantitative measurement. There was no detectable mRNA, suggesting the absence of viral genome transcription. Therefore, in addition to guideline‐directed medical therapy including beta‐blockers, angiotensin receptor blockers, and mineralocorticoid/aldosterone receptor antagonists, the patient underwent treatment with immunosuppressive therapy for 6 months at which time repeat‐EMB confirmed resolved inflammatory infiltrate. After 222 days, the patient developed sustained haemodynamically stable VT over 32 s with a heart rate of 200/min. At the time of the event, the patient had an estimated LVEF of 38% and New York Heart Association II symptoms. No transient or correctable causes of VT could be identified. He underwent placement of an implantable defibrillator following a class IB indication according to the ESC Guidelines. 9
The second patient was a 30‐year‐old man with three episodes of sustained VT. The patient reported episodes of supraventricular tachycardia, which emerged 7 years prior. There was a severely decreased LVEF (25%). EMB revealed borderline myocarditis without necrosis and only slight perivascular fibrosis. Molecular biological analysis detected Parvovirus B19 DNA with a low viral load (53 DNA copies/μg myocardial DNA) and virus‐specific mRNA, indicating active transcription of viral genome. Telbivudine was initiated for 6 months in the framework of an ongoing study 21 in combination with guideline‐directed medical therapy including beta‐blocking agents, angiotensin‐converting enzyme inhibitors, and mineralocorticoid/aldosterone receptor antagonists. After 5 days, the patient developed VT. All VTs were monomorphic, and heart rates varied from 140 to 160/min. By the time of VT onset, the LVEF was unchanged compared with previous measurements, and the patient referred symptoms equivalent to New York Heart Association II. Amiodarone was used for rhythm stabilization. By the end of WCD wearing time (259 days), LVEF had almost normalized (LVEF 50%) and no cardioverter defibrillator was implanted.
A comparison between patients with documented non‐sustained VT vs. patients without VT during WCD usage showed that patients with VT more frequently had underlying health conditions such as atrial flutter (50.0% vs. 1.7%; P < 0.001) and pulmonary hypertension (50% vs. 3.3%; P = 0.002). However, these predictive data should be interpreted with caution, because sample size was small. Simple and multiple logistic regression analyses evaluating the occurrence of VT were not performed due to the low number of arrhythmic events in this cohort.
Clinical follow‐up
Average clinical follow‐up was 24 ± 22 months up to a maximum of 83 months.
Left ventricular ejection fraction improved from a mean 32 ± 15% at baseline to 43 ± 15% at the end of WCD wearing time (Supporting Information, Figure S3 ). Differences between LVEF values at the time of diagnosis and at the end of WCD wearing time were highly significant (P < 0.01).
Twenty patients (34%) had recovered LVEF > 35% at the end of the WCD wearing time and therefore had no indication for prophylactic implantable cardioverter defibrillator (ICD) placement (Table 4 ). Two patients (3%) were lost to follow‐up.
Table 4.
Follow‐up
| Parameter | Value ± SD (mean) |
|---|---|
| Patients with LVEF2 > 35% at the end of LifeVest wearing time, n (%) | 33 (55.9) |
| LVEF at the end of LifeVest wearing time, %, mean ± SD (median [25th; 75th percentile]) | 43 ± 15 (41 [31; 52]) |
| ΔLVEF at the end of LifeVest wearing time, %, mean ± SD (median [25th; 75th percentile]) | 12 ± 11 (12 [1; 20]) |
| Patients with LVEF > 35% at the longest follow‐up, n (%) | 40 (67.8) |
| LVEF at the longest follow‐up, %, mean ± SD (median [25th; 75th percentile]) | 46 ± 15 (45 [36; 60]) |
LVEF, left ventricular ejection fraction; SD, standard deviation.
Patients with an LVEF > 35% versus LVEF ≤ 35% after WCD wearing time differed in left ventricular end‐diastolic diameter, LVEF, and left atrial volume at baseline. Also, borderline myocarditis was more common in patients who improved. Simple logistic regression analysis suggested that these parameters correlate with LVEF improvement (Table 5 ). Multiple logistic regression including these three parameters revealed baseline LVEF as the only independent predictor for LVEF improvement to >35% (Table 6 ). Initial LVEF was the best predictor for recovery [OR 1.15; 95% CI (1.02–1.30); P = 0.023].
Table 5.
Simple logistic regression for prediction of left ventricular ejection fraction improvement to >35%
| Parameter | Odds ratio | 95% CI | P‐value |
|---|---|---|---|
| LVEDD at diagnosis | 0.91 | 0.83–0.99 | 0.022 |
| LAV at diagnosis | 0.98 | 0.96–1.00 | 0.030 |
| Initial LVEF | 1.14 | 1.04–1.24 | 0.004 |
| Borderline myocarditis | 0.25 | 0.07–0.84 | 0.025 |
CI, confidence interval; LAV, left atrial volume; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction.
Table 6.
Multiple logistic regression for prediction of left ventricular ejection fraction improvement to >35%
| Parameter | Odds ratio | 95% CI | P‐value |
|---|---|---|---|
| Initial LVEF (backward selection) | 1.15 | 1.02–1.28 | 0.018 |
| Initial LVEF (forward selection) | 1.15 | 1.02–1.28 | 0.018 |
CI, confidence interval; LVEF, left ventricular ejection fraction.
Follow‐up after wearable cardioverter defibrillator use
In 32 (54%) patients, 12 months of follow‐up clinical data were available. At that time, an additional seven patients (12%) had recovered LVEF > 35% after ICD implantation.
Two patients had episodes of VT (terminated with anti‐tachycardia pacing) after 447 and 553 days after ICD implantation, and one patient showed an episode of ventricular fibrillation (VF) with appropriate shock delivery 13 days after ICD implantation.
The first patient with an episode of VT after ICD implantation was a 38‐year‐old man with giant cell myocarditis. LVEF was still severely reduced after WCD wearing period but gradually increased after ICD implantation, reaching 64% at last follow‐up.
The second patient with an episode of VT after ICD implantation was a 47‐year‐old man who initially presented with severely reduced LVEF of 25%. EMB revealed Parvovirus B19 associated lymphocytic borderline myocarditis with active transcription of viral genome and high viral load (1882 copies/μg myocardial DNA). LVEF had not improved by the end of WCD wearing time after 5 months but slowly increased to 40% during follow‐up.
The patient who received a shock due to an episode of VF after cardiac resynchronization therapy‐defibrillator implantation was a 55‐year‐old woman, who presented with severely impaired LVEF (29%). Histology of the EMB was consistent with lymphocytic borderline myocarditis with detection of low levels of Parvovirus B19 DNA (65 copies/μg myocardial DNA) and active transcription of viral genome in the molecular biological analysis. LVEF was 25% at last follow‐up after 17 months.
Clinical records of patients who have not undergone ICD implantation after WCD wearing time showed that four additional patients had documented episodes of sustained ventricular tachyarrhythmias, leading to subsequent ICD implantation. Three patients suffered from episodes of VT, and one patient had VF. Emergent treatment of these patients and device implantation were performed at other clinics and added to the patients' clinical records at the following presentation at out clinic. In total, seven patients (11.9%) suffered from sustained ventricular arrhythmias after WCD termination, of whom four (6.8%) were not protected by an ICD by the time of occurrence.
Rehospitalization rates during WCD wearing time and follow‐up were similar, both averaging 0.9 rehospitalizations per patient‐year. Patients with a history of atrial fibrillation were more likely to be readmitted to the hospital after WCD wearing period [OR 1.51; 95% CI (1.11–2.05); P = 0.010]. Furthermore, histologically confirmed giant cell myocarditis was associated with more frequent hospital readmission after the WCD wearing period (P = 0.011). There were no fatal outcomes during the course of this study.
Patients with giant cell myocarditis
There were four patients (6%) with giant cell myocarditis on immunosuppressive treatment. Three patients had a severely to moderately decreased LVEF at baseline (22%, 25%, and 40%, respectively). All of them underwent ICD implantation, and only one of them showed long‐term improvement of LVEF during follow‐up. One patient developed VT, as described in detail earlier.
Discussion
There is currently a major unmet clinical need to identify and protect patients with myocarditis who are at highest risk for developing MACE including SCD. While attempts have been made using various clinical diagnostic tools such as pattern recognition of ECGs 6 , 22 , 23 and CMR, 4 , 24 , 25 as well as identification of risk markers in peripheral blood, 26 , 27 , 28 , 29 individual risk prediction is still not sufficiently accurate with up to 30% of cases with myocarditis found amongst autopsy studies in young adults. 1 , 2 , 3
It has been recognized that the risk for arrhythmia is particularly high during the acute phase of myocarditis, 30 , 31 particularly in the context of severely reduced LVEF (<35%). 32 , 33 The majority of patients with myocarditis have a good long‐term outcome with improvement of left ventricular function. 30 , 34 , 35 Therefore, it is recommended by the ESC position statement that ICD implantation will be deferred during the acute episode. 8 , 9
Even though it has become common practice to at least provide patients with complex arrhythmias (such as frequent ectopy or VT), with a WCD for a minimum of 3 months, literature contains limited data on the outcomes of this practice and whether WCD changes clinical management. The current recommendations of WCD use in patients with myocarditis as stated by the ESC position paper in 2013 8 originated from a case report from Prochnau et al. 36 Since then, myocarditis has evolved to become the second most common reason for WCD prescription in Europe. 37
No additional studies investigated the benefit of a WCD in this particular group of patients to the best of our knowledge. The only randomized controlled WCD trial to date enrolled exclusively patients in early stages after myocardial infarction and focused solely on the mortality due to arrhythmic events. Therefore, its applicability to other cardiomyopathies such as myocarditis is limited. 14 Most WCD studies contained only a small subgroup of patients with myocarditis ranging from a single 38 , 39 , 40 or up to nine patients. 19 , 41 , 42 Klein et al. identified 35 patients with a diagnosis of myocarditis that was based on clinical presentation without the use of CMR or EMB. 43 Subgroup analyses had been performed in patients with myocarditis, and WCD was based on data extracted from the ZOLL database (https://lifevestnetwork.zoll.com). 17 , 44
To evaluate the benefit of a WCD after a diagnosis of myocarditis, in a well‐characterized cohort of patients based on histology, we performed a retrospective observational single centre study in patients who were provided with a WCD between 2009 and 2017; they were followed for up to 83 months. All patients were rated to be at high risk for SCD and thus fitted with a WCD. Although the focus of WCD lies in SCD prevention, we additionally used the data gathered by the WCD for non‐invasive arrhythmia detection. Although, for the sole reason of arrhythmia detection, an implantable loop recorder may be a less invasive option, the WCD could be beneficial in patients at risk for ventricular arrhythmias, as it simultaneously has the ability to provide treatment if indicated.
Our study included 59 patients with myocarditis, two of which had a total of four arrhythmic events, corresponding to a rate of 28 episodes of sustained VT per 100 patient‐years.
Previous WCD studies including patients with myocarditis described events of sustained VT as the main reason for shock delivery in these patients. 17 , 19 , 43 Although no WCD shocks were delivered in our cohort, episodes of sustained VT were the most common type of recorded ventricular arrhythmia, which is in line with previous findings.
Patients who suffered an episode of VT during WCD wearing time had common findings on histology of EMBs. In both biopsy specimens, an increase in CD3 positive lymphocytes along with elevated levels of HLA‐1 and CD54/ICAM‐1 was noticeable. Perforin positive T cells were absent, and there were no signs of necrosis. In both patients, Parvovirus B19 DNA was detected. However, Parvovirus B19 is commonly found in tissues of healthy individuals, and its pathogenicity is currently not well established.
In addition to similarities on histology, both patients had severely impaired LVEF and previously known episodes of supraventricular tachycardia.
Three patients had recorded events of ventricular arrhythmias after WCD use and ICD implantation. One of them was diagnosed with giant cell myocarditis, which is known to implicate a higher risk for arrhythmic events and SCD. 45
Patients with giant cell myocarditis were on average 38 years old, which is considerably younger than the rest of the study population and had a less favourable overall outcome, as it has frequently been outlined in literature. 45 , 46 , 47
Notably, all patients with episodes of ventricular arrhythmia during WCD wearing time and follow‐up, except for one patient with giant cell myocarditis, shared the diagnosis of borderline myocarditis. This emphasizes the assumption that the risk of SCD is unrelated to high levels of myocardial inflammation. 48 , 49
Parvovirus B19 was detectable in 71% of patients and therefore by far the most common virus in our cohort. All patients in our cohort, who developed episodes of ventricular arrhythmia during follow‐up, were positive for Parvovirus on histology. Research shows high prevalence of Parvovirus genome in patients with impairment of left ventricular function, 50 but also healthy individuals, 51 , 52 rendering the significance of this finding questionable.
In summary, our study has demonstrated that WCD was able to identify ventricular arrhythmias in two patients (3% of the cohort), which would not have been captured otherwise. Prospective trials will be valuable to further investigate the weight of our findings.
Conclusions
We are presenting the results of a retrospective observational single centre study, in which we evaluated the value of a WCD after the diagnosis of myocarditis. WCD use led to the detection of sustained ventricular arrhythmia in two patients (3%) of our cohort. Both patients were diagnosed with only minor changes on EMB consistent with borderline myocarditis, emphasizing the challenge of predicting patients at risk for SCD and the importance of protecting those patients with a WCD. Based on the data presented, we believe the WCD to be a valuable tool that can combine arrhythmia detection and protection from SCD in patients with myocarditis deemed to be at high risk for sustained ventricular arrhythmia, especially in the context of myocarditis representing one of the leading causes of SCD based on autopsy reports. 1 , 2 , 3 Furthermore, our results support the current ESC position statement to defer ICD placement after myocarditis, as a considerable amount of patients will recover left ventricular function.
Limitations
A limitation of this study is its sample size and the associated low occurrence of ventricular arrhythmias. However, even in this small cohort of patients with myocarditis, clinical management changed due to the use of a WCD, emphasizing its clinical value. Compared with prior research, this is the first study in which the focus was on WCD use in patients with myocarditis who also had comprehensive diagnostic workups including EMB in the majority of cases and long‐term follow‐up with a maximum of 83 months.
Conflict of interest
V.T. received travel grants from Biotronik, Biosense Webster, and St. Jude Medical, lecture fees from Biotronik and ZOLL, and a sponsored EP and Devices Fellowship from Biotronik. P.N. received travel grants from Biotronik, Medtronic, St. Jude Medical, and Biosense Webster, lecture fees from ZOLL, and a sponsored EP and Device Fellowship from Boston Scientific. B.H. is an inventor on a patent that uses RNA for the diagnosis of myocarditis. M.H. received travel grants from Novartis and Bayer, lecture fees from Biotronik, and consulting fees from Biosense Webster.
Funding
Open Access funding was enabled and organized by Projekt DEAL.
Supporting information
Figure S1. Correlation of age with daily use of WCD, There was a small positive correlation between a patients age and daily WCD wearing hours, suggesting a trend to more diligent use of WCD with increasing age (R = 0.193; P = 0.144).
Figure S2. Correlation of age with cumulative WCD wearing time, There was a small negative correlation between a patients age and the duration of WCD prescription, indicating longer WCD prescriptions for younger patients, although not statistically significant. (R = −0.063; P = 0.636)
Figure S3. Improvement of left ventricular ejection fraction (LVEF), Changes in LVEF from the time of diagnosis to the end of wearable cardioverter‐defibrillator (WCD) wearing time. Patients with improvement of LVEF are marked in green. Red color highlights patients with decreasing LVEF. Patients without change of LVEF are indicated in black. The purple line illustrates the mean change in LVEF for all patients.
Acknowledgement
We would like to thank Rachel B. Krause, MAT for editorial assistance.
Tscholl, V. , Wielander, D. , Kelch, F. , Stroux, A. , Attanasio, P. , Tschöpe, C. , Landmesser, U. , Roser, M. , Huemer, M. , Heidecker, B. , and Nagel, P. (2021) Benefit of a wearable cardioverter defibrillator for detection and therapy of arrhythmias in patients with myocarditis. ESC Heart Failure, 8: 2428–2437. 10.1002/ehf2.13353.
Bettina Heidecker and Patrick Nagel contributed equally to this manuscript and share senior authorship.
References
- 1. Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, Pearse LA, Virmani R. Sudden death in young adults: a 25‐year review of autopsies in military recruits. Ann Intern Med 2004; 141: 829–834. [DOI] [PubMed] [Google Scholar]
- 2. Kuriachan VP, Sumner GL, Mitchell LB. Sudden cardiac death. Curr Probl Cardiol 2015; 40: 133–200. [DOI] [PubMed] [Google Scholar]
- 3. Neuspiel DR, Kuller LH. Sudden and unexpected natural death in childhood and adolescence. JAMA 1985; 254: 1321–1325. [PubMed] [Google Scholar]
- 4. Berg J, Kottwitz J, Baltensperger N, Kissel CK, Lovrinovic M, Mehra T, Scherff F, Schmied C, Templin C, Lüscher TF, Heidecker B, Manka R. Cardiac magnetic resonance imaging in myocarditis reveals persistent disease activity despite normalization of cardiac enzymes and inflammatory parameters at 3‐month follow‐up. Circ Heart Fail 2017; 10. [DOI] [PubMed] [Google Scholar]
- 5. Grani C, Eichhorn C, Biere L, Murthy VL, Agarwal V, Kaneko K, Cuddy S, Aghayev A, Steigner M, Blankstein R, Jerosch‐Herold M, Kwong RY. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol 2017; 70: 1964–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ukena C, Mahfoud F, Kindermann I, Kandolf R, Kindermann M, Bohm M. Prognostic electrocardiographic parameters in patients with suspected myocarditis. Eur J Heart Fail 2011; 13: 398–405. [DOI] [PubMed] [Google Scholar]
- 7. Sharma PS, Bordachar P, Ellenbogen KA. Indications and use of the wearable cardiac defibrillator. Eur Heart J 2016: ehw353. [DOI] [PubMed] [Google Scholar]
- 8. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss H‐P, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM, European Society of Cardiology Working Group on Myocardial and Pericardial Diseases . Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013; 34: 2636–2648 2648a‐2648d. [DOI] [PubMed] [Google Scholar]
- 9. Priori SG, Blomström‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez‐Madrid A, Nikolaou N, Norekvål TM, Spaulding C, van Veldhuisen D, ESC Scientific Document Group . 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015; 36: 2793–2867. [DOI] [PubMed] [Google Scholar]
- 10. Kutyifa V, Moss AJ, Klein H, Biton Y, McNitt S, MacKecknie B, Zareba W, Goldenberg I. Use of the wearable cardioverter defibrillator in high‐risk cardiac patients: data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT‐II Registry). Circulation 2015; 132: 1613–1619. [DOI] [PubMed] [Google Scholar]
- 11. Zishiri ET, Williams S, Cronin EM, Blackstone EH, Ellis SG, Roselli EE, Smedira NG, Gillinov AM, Glad JA, Tchou PJ, Szymkiewicz SJ, Chung MK. Early risk of mortality after coronary artery revascularization in patients with left ventricular dysfunction and potential role of the wearable cardioverter defibrillator. Circ Arrhythm Electrophysiol 2013; 6: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Epstein AE, Abraham WT, Bianco NR, Kern KB, Mirro M, Rao SV, Rhee EK, Solomon SD, Szymkiewicz SJ. Wearable cardioverter‐defibrillator use in patients perceived to be at high risk early post‐myocardial infarction. J Am Coll Cardiol 2013; 62: 2000–2007. [DOI] [PubMed] [Google Scholar]
- 13. Feldman AM, Klein H, Tchou P, Murali S, Hall WJ, Mancini D, Boehmer J, Harvey M, Heilman MS, Szymkiewicz SJ, Moss AJ, WEARIT investigators and coordinators , BIROAD investigators and coordinators . Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: results of the WEARIT/BIROAD. Pacing Clin Electrophysiol 2004; 27: 4–9. [DOI] [PubMed] [Google Scholar]
- 14. Olgin JE, Pletcher MJ, Vittinghoff E, Wranicz J, Malik R, Morin DP, Zweibel S, Buxton AE, Elayi CS, Chung EH, Rashba E, Borggrefe M, Hue TF, Maguire C, Lin F, Simon JA, Hulley S, Lee BK, VEST Investigators . Wearable cardioverter–defibrillator after myocardial infarction. N Engl J Med 2018; 379: 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duncker D, Haghikia A, Konig T, Hohmann S, Gutleben K‐J, Westenfeld R, Oswald H, Klein H, Bauersachs J, Hilfiker‐Kleiner D, Veltmann C. Risk for ventricular fibrillation in peripartum cardiomyopathy with severely reduced left ventricular function‐value of the wearable cardioverter/defibrillator. Eur J Heart Fail 2014; 16: 1331–1336. [DOI] [PubMed] [Google Scholar]
- 16. Klein HU, Goldenberg I, Moss AJ. Risk stratification for implantable cardioverter defibrillator therapy: the role of the wearable cardioverter‐defibrillator. Eur Heart J 2013; 34: 2230–2242. [DOI] [PubMed] [Google Scholar]
- 17. Wassnig NK, Gunther M, Quick S, Pfluecke C, Rottstädt F, Szymkiewicz SJ, Ringquist S, Strasser RH, Speiser U. Experience with the wearable cardioverter‐defibrillator in patients at high risk for sudden cardiac death. Circulation 2016; 134: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Duncker D, Konig T, Hohmann S, Bauersachs J, Veltmann C. Ventricular arrhythmias in patients with newly diagnosed nonischemic cardiomyopathy: insights from the PROLONG study. Clin Cardiol 2017; 40: 586–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quast A, van Dijk VF, Wilde AAM, Knops RE, Boersma LVA. Outpatient treatment with the wearable cardioverter defibrillator: clinical experience in two Dutch centres. Netherlands Heart Journal: Monthly Journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation 2017; 25: 312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Association WM . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 21. Van Linthout S, Elsanhoury A, Klein O, Sosnowski M, Miteva K, Lassner D, Abou‐El‐Enein M, Pieske B, Kühl U, Tschöpe C. Telbivudine in chronic lymphocytic myocarditis and human parvovirus B19 transcriptional activity. ESC Heart Fail 2018; 5: 818–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morgera T, di Lenarda A, Dreas L, Pinamonti B, Humar F, Bussani R, Silvestri F, Chersevani D, Camerini F. Electrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changes. Am Heart J 1992; 124: 455–467. [DOI] [PubMed] [Google Scholar]
- 23. Bozkurt B, Colvin M, Cook J, Cooper LT, Deswal A, Fonarow GC, Francis GS, Lenihan D, Lewis EF, McNamara D, Pahl E, Vasan RS, Ramasubbu K, Rasmusson K, Towbin JA, Yancy C, American Heart Association Committee on Heart Failure and Transplantation of the Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; and Council on Quality of Care and Outcomes Research . Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation 2016; 134: e579–e646. [DOI] [PubMed] [Google Scholar]
- 24. Friedrich MG, Sechtem U, Schulz‐Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel‐Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P, International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis . Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol 2009; 53: 1475–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kuruvilla S, Adenaw N, Katwal AB, Lipinski MJ, Kramer CM, Salerno M. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta‐analysis. Circ Cardiovasc Imaging 2014; 7: 250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coronado MJ, Bruno KA, Blauwet LA, Tschöpe C, Cunningham MW, Pankuweit S, van Linthout S, Jeon E‐S, McNamara DM, Krejčí J, Bienertová‐Vašků J, Douglass EJ, Abston ED, Bucek A, Frisancho JA, Greenaway MS, Hill AR, Schultheiss H‐P, Cooper LT Jr, Fairweather DL. Elevated sera sST2 is associated with heart failure in men ≤50 years old with myocarditis. J Am Heart Assoc 2019; 8: e008968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kottwitz J, Bruno KA, Berg J, Salomon GR, Fairweather DL, Elhassan M, Baltensperger N, Kissel CK, Lovrinovic M, Baltensweiler A, Schmied C, Templin C, Lima JAC, Landmesser U, Lüscher TF, Manka R, Heidecker B. Myoglobin for detection of high‐risk patients with acute myocarditis. J Cardiovasc Transl Res 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lauer B, Niederau C, Kühl U, Schannwell M, Pauschinger M, Strauer BE, Schultheiss HP. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol 1997; 30: 1354–1359. [DOI] [PubMed] [Google Scholar]
- 29. Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997; 95: 163–168. [PubMed] [Google Scholar]
- 30. Aoyama N, Izumi T, Hiramori K, Isobe M, Kawana M, Hiroe M, Hishida H, Kitaura Y, Imaizumi T, Japanese Investigators of Fulminant Myocarditis. National survey of fulminant myocarditis in Japan: therapeutic guidelines and long‐term prognosis of using percutaneous cardiopulmonary support for fulminant myocarditis (special report from a scientific committee). Circulation Journal: Official Journal of the Japanese Circulation Society 2002; 66: 133–144. [DOI] [PubMed] [Google Scholar]
- 31. Kohno K, Aoyama N, Shimohama T, Yoshida M, Machida Y, Fukuda N, Aizaki T, Suzuki K, Kurosawa T, Izumi T. Resuscitation from fulminant myocarditis associated with refractory ventricular fibrillation. Jpn Circ J 2000; 64: 139–143. [DOI] [PubMed] [Google Scholar]
- 32. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med 2005; 352: 225–237. [DOI] [PubMed] [Google Scholar]
- 33. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346: 877–883. [DOI] [PubMed] [Google Scholar]
- 34. Krejci J, Hude P, Ozabalova E, Mlejnek D, Zampachova V, Svobodova I, Stepanova R, Spinarova L. Improvement of left ventricular systolic function in inflammatory cardiomyopathy: what plays a role? Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2016; 160: 524–532. [DOI] [PubMed] [Google Scholar]
- 35. D'Ambrosio A, Patti G, Manzoli A, Sinagra G, di Lenarda A, Silvestri F, di Sciascio G. The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review. Heart 2001; 85: 499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prochnau D, Surber R, Kuehnert H, Heinke M, Klein HU, Figulla HR. Successful use of a wearable cardioverter‐defibrillator in myocarditis with normal ejection fraction. Clinical Research in Cardiology: Official Journal of the German Cardiac Society 2010; 99: 129–131. [DOI] [PubMed] [Google Scholar]
- 37. Lenarczyk R, Potpara TS, Haugaa KH, Hernandez‐Madrid A, Sciaraffia E, Dagres N. The use of wearable cardioverter‐defibrillators in Europe: results of the European Heart Rhythm Association survey. Europace 2016; 18: 146–150. [DOI] [PubMed] [Google Scholar]
- 38. Bhaskaran A, Bartlett M, Kovoor P, Davis LM. The wearable cardioverter defibrillator: an early single centre Australian experience. Some pitfalls and caveats for use. Heart Lung Circ 2016; 25: 155–159. [DOI] [PubMed] [Google Scholar]
- 39. Feldman AM, Klein H, Tchou P, Murali S, Hall WJ, Mancini D, Boehmer J, Harvey M, Heilman MS, Szymkiewicz SJ, Moss AJ, WEARIT investigators and coordinators , BIROAD investigators and coordinators . Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death: results of the WEARIT/BIROAD. Pacing and Clinical Electrophysiology: PACE 2004; 27: 4–9. [DOI] [PubMed] [Google Scholar]
- 40. Leyton‐Mange JS, Hucker WJ, Mihatov N, Reynolds M, Albert C, Lubitz SA, Milan DJ. Experience with wearable cardioverter‐defibrillators at 2 academic medical centers. JACC Clin Electrophysiol 2018; 4: 231–239. [DOI] [PubMed] [Google Scholar]
- 41. Duncker D, Konig T, Hohmann S, Bauersachs J, Veltmann C. Avoiding untimely implantable cardioverter/defibrillator implantation by intensified heart failure therapy optimization supported by the wearable cardioverter/defibrillator—the PROLONG study. J Am Heart Assoc 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Erath JW, Vamos M, Sirat AS, Hohnloser SH. The wearable cardioverter‐defibrillator in a real‐world clinical setting: experience in 102 consecutive patients. Clinical Research in Cardiology: Official Journal of the German Cardiac Society 2017; 106: 300–306. [DOI] [PubMed] [Google Scholar]
- 43. Klein HU, Meltendorf U, Reek S, Smid J, Kuss S, Cygankiewicz I, Jons C, Szymkiewicz S, Buhtz F, Wollbrueck A, Zareba W, Moss AJ. Bridging a temporary high risk of sudden arrhythmic death. Experience with the wearable cardioverter defibrillator (WCD). Pacing and Clinical Electrophysiology: PACE 2010; 33: 353–367. [DOI] [PubMed] [Google Scholar]
- 44. Odeneg T, Ebner C, Mortl D, Keller H, Dirninger A, Stix G, Föger B, Grimm G, Steinwender C, Gebetsberger F, Stühlinger M, Mastnak B, Haider C, Manninger M, Scherr D. Indications for and outcome in patients with the wearable cardioverter‐defibrillator in a nurse‐based training programme: results of the Austrian WCD Registry. Eur J Cardiovasc Nurs 2019; 18: 75–83. [DOI] [PubMed] [Google Scholar]
- 45. Cooper LT Jr, Berry GJ, Shabetai R. Idiopathic giant‐cell myocarditis—natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N Engl J Med 1997; 336: 1860–1866. [DOI] [PubMed] [Google Scholar]
- 46. Peretto G, Sala S, Rizzo S, de Luca G, Campochiaro C, Sartorelli S, Benedetti G, Palmisano A, Esposito A, Tresoldi M, Thiene G, Basso C, Della Bella P. Arrhythmias in myocarditis: state of the art. Heart Rhythm 2019; 16: 793–801. [DOI] [PubMed] [Google Scholar]
- 47. Cooper LT Jr. Ventricular arrhythmias and sudden cardiac death in lymphocytic myocarditis. J Am Coll Cardiol 2020; 75: 1058–1060. [DOI] [PubMed] [Google Scholar]
- 48. Weber MA, Ashworth MT, Risdon RA, Malone M, Burch M, Sebire NJ. Clinicopathological features of paediatric deaths due to myocarditis: an autopsy series. Arch Dis Child 2008; 93: 594–598. [DOI] [PubMed] [Google Scholar]
- 49. Angelini A, Crosato M, Boffa GM, Calabrese F, Calzolari V, Chioin R, Daliento L, Thiene G. Active versus borderline myocarditis: clinicopathological correlates and prognostic implications. Heart 2002; 87: 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tschöpe C, Bock CT, Kasner M, Noutsias M, Westermann D, Schwimmbeck P‐L, Pauschinger M, Poller W‐C, Kühl U, Kandolf R, Schultheiss H‐P. High prevalence of cardiac parvovirus B19 infection in patients with isolated left ventricular diastolic dysfunction. Circulation 2005; 111: 879–886. [DOI] [PubMed] [Google Scholar]
- 51. Schenk T, Enders M, Pollak S, Hahn R, Huzly D. High prevalence of human parvovirus B19 DNA in myocardial autopsy samples from subjects without myocarditis or dilative cardiomyopathy. J Clin Microbiol 2009; 47: 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Halle M, Binzenhöfer L, Mahrholdt H, Johannes Schindler M, Esefeld K, Tschöpe C. Myocarditis in athletes: a clinical perspective. Eur J Prev Cardiol 2020: 2047487320909670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Correlation of age with daily use of WCD, There was a small positive correlation between a patients age and daily WCD wearing hours, suggesting a trend to more diligent use of WCD with increasing age (R = 0.193; P = 0.144).
Figure S2. Correlation of age with cumulative WCD wearing time, There was a small negative correlation between a patients age and the duration of WCD prescription, indicating longer WCD prescriptions for younger patients, although not statistically significant. (R = −0.063; P = 0.636)
Figure S3. Improvement of left ventricular ejection fraction (LVEF), Changes in LVEF from the time of diagnosis to the end of wearable cardioverter‐defibrillator (WCD) wearing time. Patients with improvement of LVEF are marked in green. Red color highlights patients with decreasing LVEF. Patients without change of LVEF are indicated in black. The purple line illustrates the mean change in LVEF for all patients.


