Abstract
Aims
Heart failure (HF) is a common disease with increasing prevalence and poor prognosis. The vasopressin (VP) marker copeptin predicts development of diabetes mellitus, diabetic heart disease, coronary artery disease, and premature mortality. Copeptin is elevated in HF patients and predicts a worse outcome. This study aims to investigate whether copeptin can predict HF development.
Methods
Copeptin was analysed in 5297 individuals (69.6% men) without prevalent HF from the Malmö Preventive Project, a population‐based prospective cohort. Cox proportional hazards models were used to analyse risk of incident HF by copeptin levels after adjusting for conventional cardiovascular risk factors.
Results
During a median follow‐up time of 11.1 years, 350 subjects (6.6%) were diagnosed with HF. Of these events, 99 were classified as myocardial infarction (MI) related HF and 251 as non‐MI‐related HF. Individuals in the top quartile of copeptin had, after multivariate adjustment for conventional risk factors (age, sex, systolic blood pressure, diabetes mellitus, body mass index, antihypertensive therapy, smoking, low‐density lipoprotein cholesterol, and high‐density lipoprotein cholesterol), a significantly increased risk of developing HF by 1.63 [confidence interval (CI) 1.20–2.21] for HF compared with the reference quartile 1. After adjustment for conventional risk factors, the hazard ratio (HR) per standard deviation increase of log‐transformed copeptin for any HF was 1.30 (95% CI 1.17–1.46), whereas it was 1.39 (CI 1.13–1.71) for MI‐related HF and 1.26 (CI 1.11–1.44) for non‐MI‐related HF. The associations remained after additional adjustment for estimated glomerular filtration rate [HR 1.24 (95% CI: 1.10–1.40)] and for pro atrial natriuretic peptide on top of conventional risk factors [HR 1.14 (95% CI: 1.02–1.28)].
Conclusions
Elevated copeptin predicts development of HF in older adults. Copeptin is a risk marker of VP‐driven HF susceptibility and a candidate to guide prevention efforts of HF targeting the VP system.
Keywords: Heart failure, Vasopressin, Copeptin, Vasopressin antagonists
Introduction
Heart failure (HF) is one of the leading causes of morbidity and mortality worldwide, affecting at least 26 million people globally with annual mortality rates in the end‐stage group of over 50%. 1 , 2 Independently from primary aetiology, neurohormonal activation plays a central role in the progressive development of HF. The renin–angiotensin–aldosterone system (RAAS), the sympathetic nervous system, and the vasopressin (VP) system are all upregulated in HF, contributing to retention of sodium and water, vasoconstriction, and increased heart rate. These effects are driving myocardial dysfunction, hypertrophy, and fibrotic transformation, which are all known as key features of HF. 3 , 4 The positive effects from intervention on the natriuretic peptide (NP) system by neprilysin inhibitors, and lately also from treatment with sodium‐glucose co‐transporter 2 inhibitors, put light on the fact that multiple mechanisms are involved in HF development. 5 , 6
Vasopressin is released from the posterior pituitary lobe as a response to increased plasma osmolality or by a significant fall in systemic blood pressure. 7 The hormone is mainly known for its importance in the regulation of blood osmolality together with its vasopressor and haemostatic effects. However, VP has several other physiological effects through three receptors: the V1AR, V1BR, and the V2R. Stimulation of the renal V2R increases water permeability in the renal tubules leading to increased water reabsorption and, consequently, lowering of blood osmolality. V1ARs and V1BRs have been found in several target organs and tissues such as the pancreas, pituitary gland, vascular smooth muscle cells, platelets, and myocardium. 1 , 7 , 8 , 9
A widely accepted method of measuring VP is to analyse the C‐terminal part of the VP precursor peptide called copeptin. Plasma copeptin is shown to correspond well to VP levels in plasma. Increased VP measured as copeptin has in previous population‐based studies been associated with diabetes development, hypertension, cardiovascular disease, and premature death. 10 , 11 , 12 , 13 Interestingly, elevated copeptin is also shown to be present in chronic HF, to correlate with HF symptoms and may be used as a prognostic marker in HF patients. 1 , 14 , 15 , 16 Even though VP, measured as copeptin, is suggested to be involved in the development of HF, 1 , 7 a predictive value of copeptin in HF development has not so far been possible to establish. To address this uncertainty, our study aims to investigate the possible predictive role of copeptin in HF development.
Methods
The study population was recruited from the Malmö Preventive Project, a Swedish single‐centre population‐based prospective cohort previously described in detail. 17 , 18 Malmö Preventive Project started in 1974 with the aim to invite and examine a large strata of the adult population living in the city of Malmö, Sweden, in order to find high‐risk individuals for preventive intervention on cardiovascular risk factors, alcohol abuse, impaired glucose tolerance, and breast cancer. Between 1974 and 1992, a total of 21 911 male and 8676 female participants attended the screening programme, with an overall attendance rate of 71.2%. The absolute majority of the participants was born in Sweden and was presumed to be of Caucasian ethnicity. 17 Subjects were invited to participate in a comprehensive risk factor screening, including a physical examination, a panel of laboratory tests, a glucose intolerance test, and a mammography for women. Additionally, every participant filled in a self‐administered questionnaire.
Between 2002 and 2006, all subjects who were alive and still residing in Malmö were invited for a re‐examination in which 18 240 individuals participated (63% men, participation rate 72%). The participants were at this point between 53 and 81 years old. Cardiovascular risk factors were reassessed, and plasma was frozen to −80°C for later analyses. At the re‐examination, the participants underwent a medical history, physical examination, and laboratory assessment. Blood pressure was measured using an oscillometric device twice after 10 min of rest in a supine position. Diabetes mellitus was defined as fasting plasma glucose ≥7.0 mmol/L, a self‐reported physician diagnosis of diabetes, or use of antidiabetic medication. Furthermore, prevalent diabetes cases were captured by using six different national and regional diabetes registers, as described in detail previously. 12 Cigarette smoking was assessed by a self‐administered questionnaire, with current cigarette smoking defined as any use within the past year. Measurements of fasting serum total cholesterol, high‐density lipoprotein cholesterol (HDL‐C), triglycerides and creatinine were made according to standard procedures at the Department of Clinical Chemistry, Skane University Hospital Malmö. Low‐density lipoprotein cholesterol (LDL‐C) was calculated according to Friedewald's formula. Copeptin and mid‐regional pro atrial natriuretic peptide (MRproANP) were successfully analysed from stored fasted EDTA plasma, in n = 5410 and n = 5415 individuals, respectively, by using a commercially available immunoassay (B.R.A.H.M.S AG, Hennigsdorf, Germany). The plasma samples selected for analysis of copeptin and MRproANP were randomly chosen with the only exclusion criterion being prior participation in the other large population‐based prospective cohort study from Malmö, called the Malmö Diet and Cancer study. 19 The decision to not analyse in all 18 240 individuals was a result of restricted resources. After excluding 23 participants with incomplete data and 90 participants with HF at baseline, 5297 participants without prevalent HF had copeptin at baseline analysed (Figure 1 ).
Figure 1.
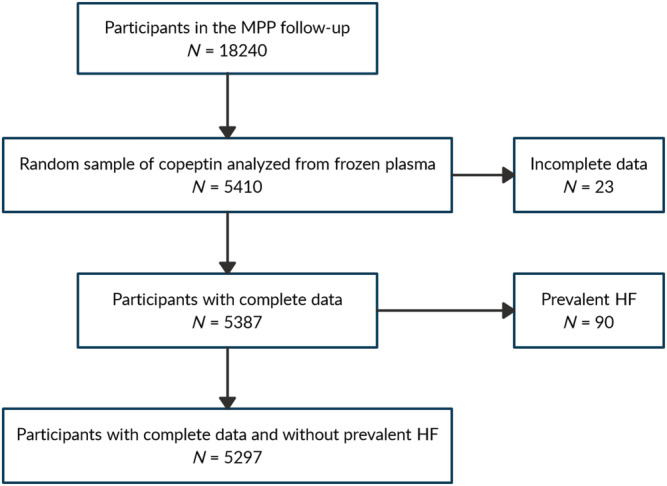
Flow chart of inclusion procedure. HF, heart failure; MPP, Malmö Preventive Project.
Ascertainment of endpoints
Subjects were followed for incident HF and all‐cause mortality until 31 December 2016. Events were identified by linking a 10‐digit personal identification number of each Swedish citizen with three registers: the Swedish National Inpatient Register, the Swedish Hospital‐based outpatient care register, and the Cause‐of‐death Register. The registers have been previously described and validated for classification of outcomes. 20 HF was defined as the diagnosis of HF according to the International Classification of Diseases, ninth revision (ICD‐9) code 148 or International Classification of Diseases, tenth revision (ICD‐10) code I11.1 and I50. Only the primary diagnosis of HF according to the registers was used because previous studies have shown a higher validity (95%) for the diagnosis of HF if using only the primary diagnosis. 21
To identify individuals with HF related to myocardial ischemia, incident HF diagnoses were divided into the two categories myocardial infarction (MI)‐related HF and non‐MI‐related HF. MI‐related HF was defined as a diagnosis of HF on the same day or after a non‐fatal acute MI. Thus, to be classified as MI‐related HF, the MI should have occurred before baseline or during the follow‐up, but not after the diagnosis of HF. Non‐MI‐related HF was defined as a diagnosis of HF without a previous diagnosis of MI, or a HF diagnosis captured at least 1 day before the diagnosis of an MI. Thus, individuals with HF classified as non‐MI related did not have a previous MI diagnose at any time before the diagnosis of HF, including before baseline measurements. MI was defined based on ICD‐9 code 410 or the ICD‐10 code I21.
The research was conducted in accordance with the Declaration of Helsinki, and the study protocols were approved by the regional Ethics Committee of Lund University, Dnr. 85/2004. All the participants provided written informed consent.
Statistics
Copeptin and MRproANP were skewed to the right and were transformed using the natural logarithm. Both the standard deviation (SD) of log‐transformed copeptin and sex‐specific quartiles of copeptin were used, depending on the model, and related to HF risk, using multivariate adjusted Cox proportional hazards models and Kaplan Meier plots. The proportional hazards assumption was tested by including time‐dependent covariates in the Cox regression analysis.
To investigate whether the association between copeptin and HF differed depending on diabetes status, a multiplicative interaction term was calculated between prevalent diabetes and copeptin on incident HF, which was introduced in addition to the main effects and all other covariates in the regression analyses. The analyses were adjusted for conventional cardiovascular risk factors [age, sex, systolic blood pressure, diabetes mellitus, body mass index (BMI), antihypertensive therapy, smoking, LDL‐C, and HDL‐C]. The majority of these risk factors has previously also been shown to be associated with copeptin. 10
The correlation coefficient between copeptin an MRproANP was assessed by using Pearson correlation. Multicollinearity was investigated by using a variance inflation factor.
SPSS statistical software (version 25.0; SPSS, Chicago, Illinois, USA) was used for all calculations, and a two‐sided P value of <0.05 was considered significant.
Results
Baseline characteristics of the population are shown in Table 1 . The median follow‐up time was 11.1 years (8.9–12.1, 25th–75th percentile) with a total follow‐up time of 53 116 person‐years. In total, 350 incident HF events occurred during follow‐up. Of these, 99 HF events were classified as MI related and 251 as non‐MI related.
Table 1.
Baseline characteristics (n = 5297)
| Characteristic | Never HF (n = 4947) | Incident HF (n = 350) | Subgroups of incident HF | |
|---|---|---|---|---|
| MI‐related HF (n = 99) | Non‐MI‐related HF (n = 251) | |||
| Age | 69.1 ± 6.2 | 72.5 ± 5.9 | 71.8 ± 5.1 | 72.8 ± 6.2 |
| Male, n (%) | 3410 (68.9) | 278 (79.4) | 85 (85.9) | 193 (76.9) |
| Smokers, n (%) | 967 (19.5) | 75 (21.4) | 28 (28.3) | 47 (18.7) |
| Systolic blood pressure (mmHg) | 145.8 ± 20.5 | 148.4 ± 21.5 | 146.8 ± 21.8 | 149.1 ± 21.5 |
| Diastolic blood pressure (mmHg) | 84.0 ± 10.70 | 83.2 ± 10.99 | 81.2 ± 11.87 | 84.0 ± 10.55 |
| Diabetes, n (%) | 714 (14.4) | 83 (23.7) | 27 (27.3) | 56 (22.3) |
| LDL‐C a (mmol/L) | 3.63 ± 0.99 | 3.37 ± 0.98 | 3.12 ± 0.99 | 3.47 ± 0.95 |
| HDL‐C b (mmol/L) | 1.38 ± 0.40 | 1.35 ± 0.42 | 1.31 ± 0.38 | 1.36 ± 0.43 |
| BMI c (kg/m2) | 27.1 ± 4.1 | 28.1 ± 4.9 | 27.9 ± 4.4 | 28.2 ± 5.0 |
| Creatinine (μmol/L) | 96.1 (27.3) | 106.3 (29.9) | 109.5 (32.1) | 105.1 (29.0) |
| Copeptin d (pmol/L) | 6.99 (4.24–11.53) | 9.28 (5.36–16.53) | 8.57 (5.67–17.41) | 9.35 (5.10–15.99) |
| Antihypertensive treatment, n (%) | 1866 (37.7) | 216 (61.7) | 76 (76.8) | 140 (55.8) |
| Lipid‐lowering treatment, n (%) | 927 (18.7) | 122 (34.9) | 51 (51.5) | 71 (28.3) |
All values are given as mean (standard deviation) if nothing else specified.
HF, heart failure; MI, myocardial infarction.
Low‐density lipoprotein cholesterol.
High‐density lipoprotein cholesterol.
Body mass index.
Median (25th percentile; 75th percentile).
Cumulative incident rates of HF in different quartiles of baseline copeptin levels are illustrated in Kaplan–Meier curves (Figures 2 , 3 , 4 ), with the highest incident rates of HF in the highest quartile of copeptin for all categories (any HF, MI‐related HF, and non‐MI‐related HF). The difference between curves was tested with log‐rank test with P values of <0.001 for all.
Figure 2.
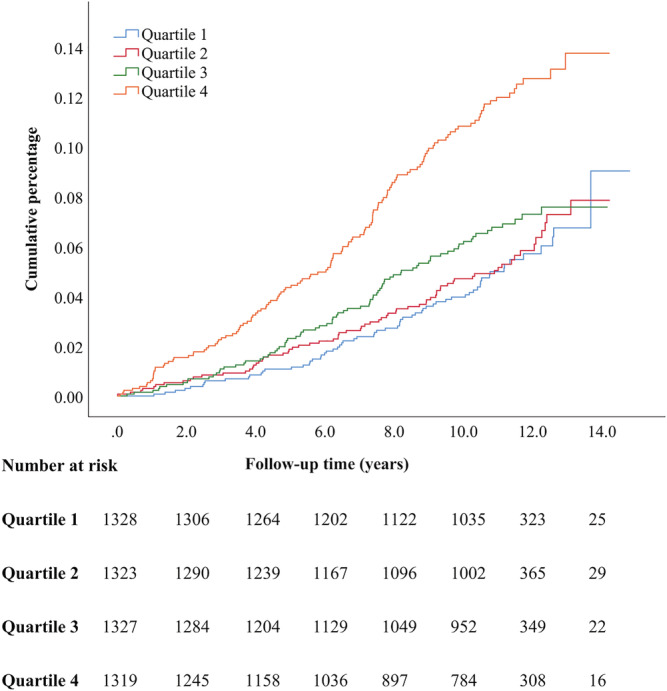
Kaplan–Meier event rates for heart failure according to quartiles of baseline copeptin levels.
Figure 3.
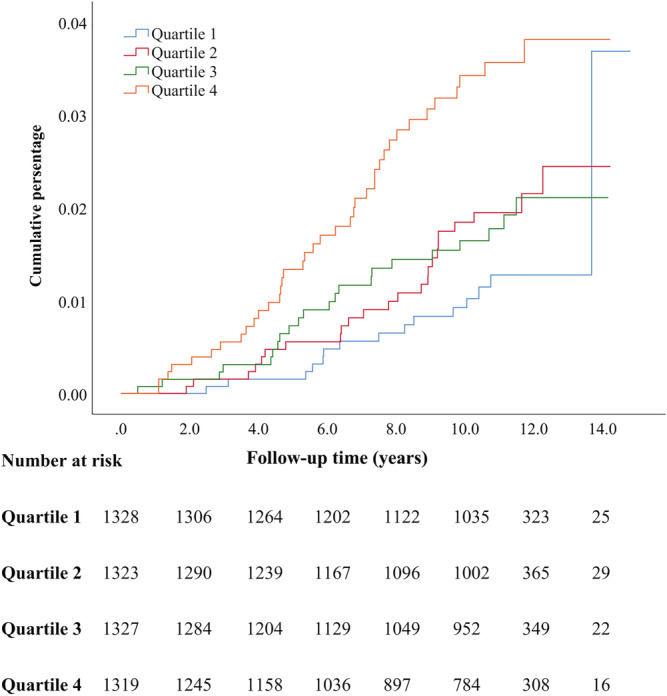
Kaplan–Meier event rates for heart failure classified as myocardial infarction related heart failure according to quartiles of baseline copeptin levels.
Figure 4.
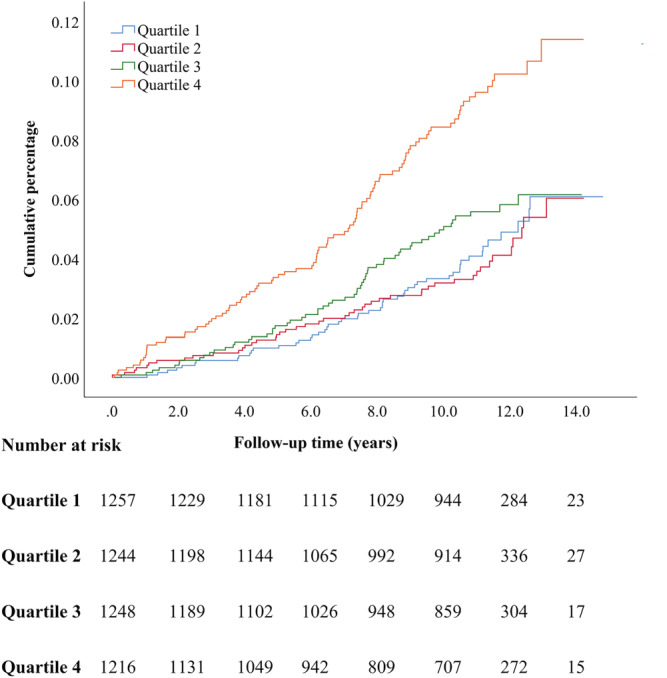
Kaplan–Meier event rates for heart failure classified as non‐myocardial infarction related heart failure according to quartiles of baseline copeptin levels.
Individuals free from HF at baseline and belonging to the top quartile of copeptin had, after multivariate adjustment for conventional risk factors, a significantly increased risk of developing HF by 63% for any HF, by 100% for MI‐related HF and by 47% for non‐MI‐related HF, compared with the reference quartile 1 (Table 2 ).
Table 2.
Hazard ratios (confidence interval) of incident HF per copeptin quartiles
| Variable | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|
| Incident HF (N = 5297 a ) | 1 (Ref) | 1.02 (0.72–1.43) | 1.13 (0.81–1.57) | 1.63 (1.20–2.21) |
| Events/person‐years: 350/53 116 | 65/13 856 | 70/13 682 | 81/13 329 | 134/12 249 |
| Myocardial infarction related HF (N = 5297 a ) | 1 (Ref) | 1.52 (0.80–290) | 1.44 (0.74–2.78) | 2.00 (1.09–3.68) |
| Events/person‐years: 99/53 116 | 15/13 856 | 24/13 682 | 22/13 329 | 38/12 249 |
| Non‐myocardial infarction related HF (N = 4965 a ) | 1 (Ref) | 0.85 (0.57–1.28) | 1.05 (0.72–1.53) | 1.47 (1.04–2.10) |
| Events/person‐years: 251/48 668 | 50/12 836 | 46/12 576 | 59/12 162 | 96/11 094 |
Adjusted for sex, age, prevalent diabetes mellitus, systolic blood pressure, antihypertensive treatment, body mass index, low‐density lipoprotein, high‐density lipoprotein, and smoking status.
HF, heart failure.
Total number of individuals. Difference in total number of cases between subgroups is related to how classification of HF was made and how the groups were analysed statistically.
When assessing the risk of HF per SD increase in log‐transformed copeptin, the association was significant after adjustment for conventional risk factors (age, sex, systolic blood pressure, diabetes mellitus, BMI, antihypertensive therapy, smoking, LDL‐C, and HDL‐C). The hazard ratio (HR) per SD increase in log‐transformed copeptin for incident HF was 1.30 (95% CI: 1.17–1.46). The associations remained after additional adjustment for estimated glomerular filtration rate (eGFR) [HR 1.24 (95% CI: 1.10–1.40)]. Furthermore, the association between copeptin and HF remained significant after adjustment for log‐transformed MRproANP on top of conventional risk factors [HR 1.14 (95% CI: 1.02–1.28)]. Finally, the risk of HF per SD increase in log‐transformed copeptin was significant in both men [HR 1.28 (95% CI 1.13–1.46)] and women [HR 1.40 (95% CI 1.11–1.77)] after adjustment for conventional risk factors.
When analysing the association between copeptin and the two HF subgroups, the risk of non‐MI‐related HF per SD increase in log‐transformed copeptin was significant after adjustment for conventional risk factors [HR 1.26 (95% CI 1.11–1.44)], remained significant when additionally adjusted for eGFR [HR 1.20 (95% CI 1.04–1.38)] but became non‐significant when adjusted for MRproANP on top of conventional risk factors [HR 1.12 (95% CI 0.98–1.28)]. For MI‐related HF, the association was significant after adjustment for conventional risk factors [HR 1.39 (95% CI 1.13–1.71)], remained significant after adjustment for eGFR 2 [HR 1.30 (95% CI 1.04–1.63)] but became non‐significant after adjustment for MRproANP on top of conventional risk factors [HR 1.19 (95% CI 0.97–1.47)].
There was no significant interaction between diabetes status and copeptin on the risk of HF [HR 1.22 (95% CI: 0.85–1.74), for any HF; HR 1.05 (95% CI: 0.55–2.01), for MI‐related HF; and HR 1.34 (95% CI: 0.87–2.01), for non‐MI related HF].
The Pearson correlation coefficient between copeptin and MRproANP was 0.218 with a P value of <0.001. The analyses were tested negatively for multicollinearity (all variance inflation factors values obtained were between 1.0 and 1.3).
Discussion
Copeptin as a prognostic and predictive marker
Our key finding is that elevated copeptin is significantly associated with later development of HF independently of several established risk factors in an older middle‐aged population. A predictive and prognostic role of copeptin on HF risk has previously been described after MI. 23 , 24 We previously found a predictive role of copeptin on HF, coronary artery disease (CAD) and premature death in the diabetic subpart (n = 895 diabetes cases) of a middle‐aged Swedish population, 13 whereas there was no such tendency among non‐diabetic cases (n = 4187). The participants had a younger age (mean age 58 years) and a more favourable cardiovascular risk profile than in our cohort, and one may hypothesize that the biological and vascular age of the current population (mean age of approximately 70 years) is similar to the vascular age of younger subjects with diabetes. When the same cohort was investigated without substratification on diabetes status, a predictive value of copeptin on HF risk was found but did not remain significant after multivariate adjustment including N‐terminal pro‐B‐type natriuretic peptide (NTproBNP) and C‐reactive protein (CRP). 25
In the present study, the associations remained significant after adjustment for MRproANP, but unfortunately, neither NTproBNP nor CRP was analysed in our cohort, which prevented us from adjusting for these biomarkers in our multivariate analyses. However, copeptin has been previously shown to correlate only modestly (r = 0.18) with NTproBNP in a male cohort with a similar age span as ours, 26 whereas in the current study, the correlation between copeptin and MRproANP could be considered as moderate (r = 0.218). The release of NPs in HF is an antagonizing reaction to the increased filling pressures activated by increased cardiomyocyte stretching. An activation of the NP system in parallel to that of the VP system, reflected by a rise in both NPs and copeptin concentrations, thus seems like a physiologically accurate response. Whether the VP system is activated independently or not from the activation of the NP system should therefore not affect the possible pathophysiological role of the VP system in the development of HF. Interestingly, it was previously shown that copeptin had a better predictive ability of mortality in HF after MI than NTproBNP. 24 One of the proposed reasons for this was that NTproBNP is known to have a high variability over time and that thus copeptin could be a more stable marker.
Previous studies have shown a clear predictive role of copeptin in diabetes and diabetic heart disease. 12 , 13 A substantial number of patients had prevalent diabetes at start of follow‐up in our cohort (mean of 15.4%). However, the copeptin‐associated risk of HF development was independent of diabetes status in our current study and, as illustrated by the absence of interaction between copeptin and diabetes, diabetes status did not significantly modify the copeptin‐HF association. Impaired kidney function commonly coexists with HF and is also associated with elevated copeptin levels. 27 However, the association between copeptin and HF remained significant after additional adjustment for eGFR.
In a previous study, including only male participants, an association was found between copeptin and incident HF after adjustment for some conventional risk factors including age, smoking, kidney function, and blood pressure, whereas the evidence weakened (P = 0.09) after additional adjustment for eGFR, forced expiratory volume in 1 s, albumin, and CRP. 26 However, the multivariate model was not adjusted for lipids, BMI, or prevalent diabetes. In concordance to our study, the authors suggested that the association between copeptin and HF was driven by individuals with present CVD. The sample size (n = 3560) and age span (mean age 69 years) were comparable with our study. Because the study was performed in a cohort with only men, we investigated if the link between elevated copeptin and HF risk differed between gender in our cohort and found that the increased risk of HF per quartile increase in copeptin was significant in both men and women separately after adjustment for conventional risk factors.
Possible mechanisms underlying a link between copeptin and heart failure
It is not yet known whether copeptin is causally related to HF, although previous data proposes several potential mechanisms. 7 , 28 VP has been suggested to contribute to pathological myocardial remodelling through V1AR‐mediated effects on the cardiomyocytes. 3 , 7 The same receptor is responsible for vasoconstriction in smooth muscle cells. 8 Furthermore, VP mediates water reabsorption in the kidney, which may lead to fluid retention. VP is involved in haemostasis through stimulation of von Willebrand factor, factor VIII, and tissue plasminogen activator produced by endothelial cells. 9 It is also associated with several cardiovascular risk factors such as altered glucose and lipid metabolism, hypertension, and kidney function decline. 10 , 12 , 29
The association between high copeptin concentration and HF appeared slightly more pronounced among individuals classified as having incident MI‐related HF than among individuals classified as having incident non‐MI‐related HF. The classification of MI‐related HF was created to identify subjects with a high likelihood of HF on a coronary atherosclerotic/ischemic basis. We considered this group particularly interesting because previous studies have shown that elevated copeptin is linked to several cardiovascular risk factors, coronary artery disease (CAD) and cardiovascular mortality, 10 , 11 as well as HF development and prognosis after MI (24). In the current study, we suggest a possible impact of CAD on the association between copeptin and HF development, even though our results are only hypothesis‐generating and require further validation. The subgroup of non‐MI‐related HF is probably heterogeneous with regards to HF aetiology, but it cannot be excluded that HF on a coronary atherosclerotic/ischemic basis, but without known MI, constitute a considerable part. Whether the association between copeptin and HF within this group is driven by CAD‐related susceptibility, or if copeptin is a predictor of HF independently of CAD, remains to be clarified.
Antagonism of the VP system has been previously investigated in HF. V2R antagonists are approved for treatment of HF‐related hyponatremia by the Food and Drug Administration. However, V2R antagonism (Tolvaptan) has not shown improved mortality or reduced number of hospitalizations among HF patients. Because blockade of the V2R is associated with a rise in circulating VP, it can be speculated that the treatment is paralleled with enhanced activation of the V1AR. Through its effects on the cardiomyocytes and blood vessels, V1AR stimulation could induce structural changes and myocardial hypertrophy 3 , 7 counteracting the positive effects of the V2R antagonist. Intravenous dual V2R/V1AR antagonism (Conivaptan) has therefore been studied in HF with positive results on diuresis and hemodynamics. 30 Because of the interactions with P450 isoenzyme, the oral version has been withdrawn from further development. 31 In rat models, pharmacological blockade of the VP system with the V2/V1AR inhibitor BAY1753011 has been studied with positive results, both on diuretic effect, obtained without the RAAS activation commonly seen with diuretics, as well as on profibrotic markers on the cardiomyocytes. 32 There are, to the authors' knowledge, so far, no long‐term follow‐up studies investigating the effects of V2/V1AR blockade.
Strengths and limitations
The register‐based prospective design of our study comes with several advantages. First, we have been able to study a relatively large cohort of middle‐aged men and women with access to detailed information about their cardiovascular risk factors and general health. This makes the study results reliable and generalizable. As well, the follow‐up time of in median 11 years made it possible to detect a substantial number of HF cases.
The study does, however, also have several limitations. First, the diagnoses of HF were, as described in the method section, retrieved by using nation‐wide registers collecting diagnoses from outpatient care (i.e. hospitals) and from specialized inpatient care providers. HF diagnoses made in primary health care, as well as non‐diagnosed patients, were however not included in the study. This probably led to an underestimation of the HF incidence which, in turn, would be expected to bias our results toward the null. If a diagnosis of HF in an early stage was missed at the time of inclusion, there is a risk that a high copeptin value was rather a marker of HF in an early stage than a predictive value. If so, the predictive role of copeptin on incident HF in a population without known/diagnosed HF may still be valid.
The use of primary diagnosis of HF from Swedish national registers has been previously validated. However, there was no accessible data on the type or severity of HF in this cohort. For example, we were not able to classify HF with regard to reduced, mid‐range, or preserved ejection fraction. This restricted us from performing analyses depending on different HF‐classifications.
Finally, copeptin was only measured at baseline, which prevented us from studying dynamic aspects of copeptin concentrations in HF development. This would have been particularly interesting because a second measurement of copeptin has been shown to add prognostic value in HF after MI. 24
Clinical implications
Identifying high‐risk individuals for HF is of great value for clinicians in the choice of primary prevention and follow‐up. In routine practice, physicians consider classical HF risk factors (CAD, hypertension, diabetes, and smoking) along with physical examination in the assessment of HF risk. HF risk scores, mainly based on similar parameters, have also been developed. 22 Biomarkers as risk indicators are relatively simple to use and to implement in risk scores. If future studies can show a sharpened predictive capacity of HF risk scores by adding copeptin, it has an interesting potential to be useful in clinical practice.
Conclusions
Copeptin predicts development of HF in older adults, independently of diabetes diagnosis or conventional cardiovascular risk factors. We suggest that copeptin has the potential to be used as a risk marker for incident HF and propose that an overactive VP system warrants further attention in HF development.
Conflict of interest
None declared.
Funding
This study was supported by ALF funds; Ernhold Lundström Foundation; Albert Påhlsson Foundation; the Swedish Heart‐Lung Foundation (grant 20200126); Region Skåne and Skåne University Hospital, Lund University Infrastructure grant “Malmö population‐based cohorts” (grant 2019/2046).
Schill, F. , Timpka, S. , Nilsson, P. M. , Melander, O. , and Enhörning, S. (2021) Copeptin as a predictive marker of incident heart failure. ESC Heart Failure, 8: 3180–3188. 10.1002/ehf2.13439.
References
- 1. Balling L, Gustafsson F. Copeptin in heart failure. Adv Clin Chem 2016; 73: 29–64. [DOI] [PubMed] [Google Scholar]
- 2. Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev 2017; 3: 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chatterjee K. Neurohormonal activation in congestive heart failure and the role of vasopressin. Am J Cardiol 2005; 95: 8B–13B. [DOI] [PubMed] [Google Scholar]
- 4. Hartupee J, Mann DL. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol 2017; 14: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- 6. Jackson AM, Dewan P, Anand IS, Bělohlávek J, Bengtsson O, Boer RAM, Boulton DW, Chopra VK, DeMets DL, Docherty KF, Dukát A, Greasley PJ, Howlett JG, Inzucchi SE, Katova T, Køber L, Kosiborod MN, Langkilde AM, Lindholm D, Ljungman CEA, Martinez FA, O'Meara E, Sabatine MS, Sjöstrand M, Solomon SD, Tereshchenko S, Verma S, Jhund PS, McMurray JJV. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA‐HF. Circulation 2020; 142: 1040–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schweiger TA, Zdanowicz MM. Vasopressin‐receptor antagonists in heart failure. Am J Health Syst Pharm 2008; 65: 807–817. [DOI] [PubMed] [Google Scholar]
- 8. Morgenthaler NG. Copeptin: a biomarker of cardiovascular and renal function. Congest Heart Fail 2010; 16: S37–S44. [DOI] [PubMed] [Google Scholar]
- 9. Mavani GP, DeVita MV, Michelis MF. A review of the nonpressor and nonantidiuretic actions of the hormone vasopressin. Front Med 2015; 2: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Enhorning S, Struck J, Wirfalt E, Hedblad B, Morgenthaler NG, Melander O. Plasma copeptin, a unifying factor behind the metabolic syndrome. J Clin Endocrinol Metab 2011; 96: E1065–E1072. [DOI] [PubMed] [Google Scholar]
- 11. Tasevska I, Enhorning S, Persson M, Nilsson PM, Melander O. Copeptin predicts coronary artery disease cardiovascular and total mortality. Heart 2016; 102: 127–132. [DOI] [PubMed] [Google Scholar]
- 12. Enhorning S, Wang TJ, Nilsson PM, Almgren P, Hedblad B, Berglund G, Struck J, Morgenthaler NG, Bergmann A, Lindholm E, Groop L, Lyssenko V, Orho‐Melander M, Newton‐Cheh C, Melander O. Plasma copeptin and the risk of diabetes mellitus. Circulation 2010; 121: 2102–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Enhorning S, Hedblad B, Nilsson PM, Engstrom G, Melander O. Copeptin is an independent predictor of diabetic heart disease and death. Am Heart J 2015; 169: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wasilewski MA, Myers VD, Recchia FA, Feldman AM, Tilley DG. Arginine vasopressin receptor signaling and functional outcomes in heart failure. Cell Signal 2016; 28: 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin‐Toretsky E, Yusuf S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the Studies of Left Ventricular Dysfunction (SOLVD). Circulation 1990; 82: 1724–1729. [DOI] [PubMed] [Google Scholar]
- 16. Goldsmith SR, Francis GS, Cowley AW, Barry Levine T, Cohn JN. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J Am Coll Cardiol 1983; 1: 1385–1390. [DOI] [PubMed] [Google Scholar]
- 17. Berglund G, Nilsson P, Eriksson KF, Nilsson JA, Hedblad B, Kristenson H, Lindgärde F. Long‐term outcome of the Malmo preventive project: mortality and cardiovascular morbidity. J Intern Med 2000; 247: 19–29. [DOI] [PubMed] [Google Scholar]
- 18. Leosdottir M, Willenheimer R, Persson M, Nilsson PM. The association between glucometabolic disturbances, traditional cardiovascular risk factors and self‐rated health by age and gender: a cross‐sectional analysis within the Malmo Preventive Project. Cardiovasc Diabetol 2011; 10: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berglund G, Elmstahl S, Janzon L, Larsson SA. The Malmo diet and cancer study. Design and feasibility. J Intern Med 1993; 233: 45–51. [DOI] [PubMed] [Google Scholar]
- 20. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail 2005; 7: 787–791. [DOI] [PubMed] [Google Scholar]
- 22. Kalogeropoulos A, Psaty BM, Vasan RS, Georgiopoulou V, Smith AL, Smith NL, Smith NL, Kritchevsky SB, Wilson SWF, Newman AB, Harris TB, Butler J. Validation of the health ABC heart failure model for incident heart failure risk prediction. Circ Heart Fail 2010; 3: 495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kelly D, Squire IB, Khan SQ, Quinn P, Struck J, Morgenthaler NG, Davies JE, Ng LL. C‐terminal provasopressin (copeptin) is associated with left ventricular dysfunction, remodeling, and clinical heart failure in survivors of myocardial infarction. J Card Fail 2008; 14: 739–745. [DOI] [PubMed] [Google Scholar]
- 24. Voors AA, von Haehling S, Anker SD, Hillege HL, Struck J, Hartmann O, Bergmann A, Squire I, van Veldhuisen DJ, Dickstein K, for the OPTIMAAL Investigators . C‐terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: results from the OPTIMAAL study. Eur Heart J 2009; 30: 1187–1194. [DOI] [PubMed] [Google Scholar]
- 25. Smith JG, Newton‐Cheh C, Almgren P, Struck J, Morgenthaler NG, Bergmann A, Platonov PG, Hedblad B, Engström G, Wang TJ, Melander O. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol 2010; 56: 1712–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wannamethee SG, Welsh P, Whincup PH, Lennon L, Papacosta O, Sattar N. N‐terminal pro brain natriuretic peptide but not copeptin improves prediction of heart failure over other routine clinical risk parameters in older men with and without cardiovascular disease: population‐based study. Eur J Heart Fail 2014; 16: 25–32. [DOI] [PubMed] [Google Scholar]
- 27. Tasevska I, Enhorning S, Christensson A, Persson M, Nilsson PM, Melander O. Increased levels of copeptin, a surrogate marker of arginine vasopressin, are associated with an increased risk of chronic kidney disease in a general population. Am J Nephrol 2016; 44: 22–28. [DOI] [PubMed] [Google Scholar]
- 28. Balling L, Thomsen JH, Wolsk E, Hassager C, Boesgaard S, Goldsmith SR, Gustafsson F. Hemodynamic effects of short‐term infusion of a vasopressin V1A/V2 receptor antagonist conivaptan in patients withchronic heart failure during submaximal exercise. Am Heart J 2018; 203: 101–104. [DOI] [PubMed] [Google Scholar]
- 29. Enhorning S, Tasevska I, Roussel R, Bouby N, Persson M, Burri P, Bankir L, Melander O. Effects of hydration on plasma copeptin, glycemia and gluco‐regulatory hormones: a water intervention in humans. Eur J Nutr 2019; 58: 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Udelson JE, Smith WB, Hendrix GH. Acute hemodynamic effects of conivaptan, a dual V1A and V2 vasopressin receptor antagonist, in patients with advanced heart failure. ACC Curr J Rev 2002; 11: 56. [DOI] [PubMed] [Google Scholar]
- 31. Ghali JK, Tam SW. The critical link of hypervolemia and hyponatremia in heart failure and the potential role of arginine vasopressin antagonists. J Card Fail 2010; 16: 419–431. [DOI] [PubMed] [Google Scholar]
- 32. Kolkhof P, Pook E, Pavkovic M, Kretschmer A, Buchmuller A, Tinel H, Delbeck M, Mondritzki T, Wasnaire P, Dinh W, Truebel H, Hüser J, Schmeck C. Vascular protection and decongestion without renin‐angiotensin‐aldosterone system stimulation mediated by a novel dual‐acting vasopressin V1a/V2 receptor antagonist. J Cardiovasc Pharmacol 2019; 74: 44–52. [DOI] [PubMed] [Google Scholar]


