Abstract
Aims
This study aimed to assess short‐term outcomes among emergency department (ED) patients with acute heart failure (AHF) by preserved (≥50%) vs. reduced (<50%) ejection fraction (EF).
Methods and results
We conducted a retrospective, multicentre study of adult ED patients with AHF from 2017 to 2018 in an integrated healthcare system with 21 hospitals. Among patients with known EF, our primary outcome was 30 day all‐cause mortality, comparing patients with heart failure with preserved EF (HFpEF) and heart failure with reduced EF (HFrEF), adjusted for known risk factors. We ran separate multivariate regression models to compare 30 day mortality between HFpEF and HFrEF patients stratified by ED disposition (admit, observe, and discharge). Our secondary outcomes were adjusted 30 day all‐cause return hospital admission and rates of non‐fatal serious adverse events, including new intra‐aorta balloon pump, endotracheal intubation, renal failure requiring dialysis, myocardial infarction, or coronary revascularization. We conducted a sensitivity analysis among patients with EF ≤ 40% and compared our primary and secondary outcomes among patients with EF ≤ 40% with those with EF ≥ 50%. Among the 26 050 total ED encounters for AHF, 15 275 (58.6%) had known EF and 62.4% had HFpEF. The mean age was 76, 49.6% were women, and 60.5% were white. We found that 62.4% of patients were admitted, 18.3% were observed, and 19.3% were discharged from the ED. The 30 day all‐cause mortality rate was lowest among discharged patients (3.9%), intermediate among observed patients (5.9%), and highest among admitted patients (13.9%). Overall, the adjusted 30 day mortality rate was significantly higher among HFpEF patients compared with HFrEF patients (10.2% vs. 8.4%, P = 0.0004). HFpEF patients had higher mortality regardless of ED disposition, although the difference was only significant among admitted patients. The adjusted 30 day return hospital admission rates were not significantly different between HFpEF and HFrEF patients (17.9% vs. 17.8%, P = 0.89). The adjusted 30 day non‐fatal serious adverse event rates were significantly higher among HFrEF patients compared with HFpEF patients (13.7% vs. 11.1%, P < 0.0001), driven by myocardial infarction and coronary revascularization. We found that 3692 patients had EF ≤ 40%. Patients with EF ≥ 50% had significantly higher adjusted 30 day mortality rates compared with those with EF ≤ 40% (10.2% vs. 8.4%, P < 0.05).
Conclusion
In a contemporary population, almost three quarters of ED patients with AHF and known EF have HFpEF. These patients have higher 30 day adjusted mortality compared with those with HFrEF. Further studies might evaluate the underlying factors associated with this difference and target interventions to improve outcomes.
Keywords: Preserved vs. reduced ejection fraction, Emergency department, Outcomes
Introduction
There are 1 million annual US emergency department (ED) visits for acute heart failure (AHF), and 80–85% of these patients are admitted to the hospital. 1 The cost of heart failure (HF) care remains high, and age‐adjusted mortality is increasing. 2 , 3 While a major focus of research and policy has been on improving outcomes and readmission rates 4 , 5 , 6 , 7 among hospitalized HF patients, the specific role of ED management on outcomes, especially in those not hospitalized, has been less well studied.
In addition, several studies have assessed outcomes among hospitalized and community‐dwelling patients comparing those with HF with preserved ejection fraction (HFpEF) with those with HF with reduced ejection fraction (HFrEF). 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 Several have shown better outcomes after hospitalization among patients with HFpEF, 12 , 13 , 15 whereas others have shown similar outcomes. 11 , 16 The short‐term outcomes of patients with HFrEF and HFpEF patients after an ED visit for HF, including patients discharged from the ED, managed under observation status, and admitted to the hospital, are less clear.
In this study of a contemporary population, we sought to characterize clinical characteristics and key outcomes at 30 days after an ED visit for HF in patients with HFpEF compared with those with HFrEF.
Methods
Study setting and population
Kaiser Permanente Northern California (KPNC) is a large integrated healthcare delivery system that provides comprehensive medical care for more than 4 million members with ~1.2 million ED visits per year. KPNC members include ~33% of the population in areas served and are representative of the demographic and socio‐economic diversity of the surrounding and statewide population. 20 , 21
We conducted a retrospective cohort study among KPNC health plan members aged ≥18 years who had an ED visit for AHF between 1 January 2017 and 31 December 2018. Because of known limitations and inaccuracies of relying solely on International Classification of Diseases, Tenth Revision (ICD‐10) coding 22 , 23 , 24 to identify patients with AHF, we developed a novel process to improve case identification. We included patients who had a documented ejection fraction (EF) from an echocardiogram report in the prior 2 years to their index visit (and used the most recent EF) and who had one of the following: (i) chief complaint of shortness of breath; (ii) B‐type natriuretic peptide (BNP) value >499; or (iii) an ICD code for HF (see Supporting Information, Appendix S1 ). Patients were excluded if they had (i) a BNP < 100 and a body mass index <30 and no qualifying ICD‐10 code; (ii) left against medical advice; (iii) left the ED without being seen by a physician; or (iv) no active health plan membership in the month of the ED visit or in 30 days following the ED visit because of the difficulty of capturing outcomes in these patients.
To enrich the study cohort with patients meeting standardized clinical criteria for AHF, we developed a natural language processing algorithm to improve accurate capture of eligible patients. We developed clinically derived lists of alternate phraseologies for each Framingham HF criterion. 25 We then processed the ED providers' notes to search for these identifiers and examined word and phrase variation and order, inflexion, and negation. Two study co‐authors (D. R. S. and D. G. M.) manually reviewed 425 charts to allow for iterative adjustment of search criteria to optimize accurate capture of the Framingham criteria. Please see Supporting Information, Appendix S1 for further explanation of this process. The final algorithm was applied to eligible patient encounters to develop the final study cohort.
Study protocol
We collected patient demographic information including age, gender, race/ethnicity from electronic health record databases, and neighbourhood socio‐economic status at census block group level using the 2010 US Census data. We ascertained information on coexisting illnesses based on diagnoses or procedures using ICD‐10 and Common Procedural Technology codes. For each patient, we obtained an internally derived and validated co‐morbidity risk score (Comorbidity Point Score, COPS2). 26 Laboratory results and ED‐level characteristics were ascertained from administrative databases. HFrEF was defined as EF < 50%, and HFpEF was defined as EF ≥ 50% based on an echocardiogram report within the prior 2 years.
We described ED visit disposition, including discharge, admission to observation status, and full hospital admission. Observed patients were defined as those officially admitted to an observation unit, those placed under observation status, or those managed in the ED for >16 h prior to discharge. During the study period, 16 centres had observation units, although workflows, patient inclusion/exclusion criteria, and care providers (ED physicians vs. hospitalists) varied between sites.
Key outcome measures
The primary outcome measure at 30 days from the index ED visit was all‐cause mortality. Secondary outcome measures were 30 day all‐cause return hospital admission and rates of non‐fatal serious adverse events (SAEs). Return hospital admissions were second episodes of acute care, regardless of disposition after the index ED visit. Non‐fatal SAEs consisted of intra‐aorta balloon pump, endotracheal intubation, renal failure requiring dialysis, myocardial infarction (MI), or coronary revascularization. 27 To avoid misclassifying elevated troponin levels due to HF as an MI, patients had to have either (i) an ICD‐10 code for ST‐elevation MI or (ii) ICD‐10 code for acute coronary syndrome and two troponins greater than the upper 99th percentile reference value within 24 h, with a 20% difference between the lowest and highest values. 28 , 29 See Supporting Information, Appendix S1 for ICD‐10 codes.
Data analysis
We compared baseline characteristics by EF using non‐parametric tests (Kruskal–Wallis test) of continuous variables and χ 2 tests for categorical variables. We performed a Kaplan–Meier analysis to estimate 30 day survival comparing HFrEF and HFpEF patients. To compare the outcomes between patients with HFpEF and those with HFrEF, we used multivariate logistic regression for the one primary and two secondary outcomes adjusted for all patient‐level characteristics in Table 1 (other than outpatient follow‐up at 7 days). For easier interpretation, we reported adjusted rates of the primary and secondary outcome measures by HF classification (generated from the multivariate model). We also ran separate multivariate regression models to compare 30 day mortality between patients with HFpEF and those with HFrEF stratified by ED disposition. We conducted sensitivity analyses to estimate the adjusted 30 day all‐cause mortality, all‐cause return hospital admission, and non‐fatal SAE rates among patients with an EF ≤ 40% compared with those with an EF ≥ 50% and among patients who were alive for the entire 30 days since their index ED visit with an EF < 50% compared with those with an EF ≥ 50%. All analyses were conducted using Stata 14 (StataCorp, College Station, TX).
Table 1.
Patient characteristics among patients visiting an emergency department for acute heart failure, stratified by ejection fraction type: HFrEF (ejection fraction <50%) and HFpEF (ejection fraction ≥50%)
| Characteristic | HFrEF | HFpEF | P value |
|---|---|---|---|
| N | 4843 | 10 432 | |
| Age, median (IQR) | 74.0 (64.0–83.0) | 77.0 (68.0–85.0) | <0.001 |
| Women (%) | 36.3 | 55.7 | <0.001 |
| Race—white/European (%) | 58.7 | 61.4 | 0.0017 |
| Neighbourhood SES, low (%) | 24.3 | 21.8 | 0.0006 |
| Median healthcare utilization in past 365 days, median (IQR) | |||
| ED visits | 1.0 (0.0–3.0) | 1.0 (0.0–3.0) | <0.001 |
| Hospital admissions | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | <0.001 |
| Outpatient primary care visits | 5.0 (2.0–10.0) | 6.0 (3.0–11.0) | <0.001 |
| Cardiovascular history (%) | |||
| Coronary heart disease | 47.1 | 33.9 | <0.001 |
| Hypertension | 79.7 | 86.4 | <0.001 |
| Myocardial infarction | 29.9 | 19.0 | <0.001 |
| Angina | 11.7 | 9.6 | <0.001 |
| CABG/PCI | 55.3 | 52.7 | 0.0029 |
| Pacemaker | 1.6 | 0.9 | 0.0002 |
| Atrial fibrillation/flutter | 43.7 | 46.9 | 0.0003 |
| HFrEF < 50% | 100.0 | 0.0 | <0.001 |
| Medical history (%) | |||
| ESRD | 8.0 | 10.7 | <0.001 |
| Chronic kidney disease | 48.1 | 51.6 | <0.001 |
| COPD | 15.5 | 19.4 | <0.001 |
| Peripheral vascular disease | 42.5 | 50.4 | <0.001 |
| Active cancer | 9.2 | 11.7 | <0.001 |
| Diabetes | 47.7 | 48.9 | 0.19 |
| Dementia | 3.8 | 4.6 | <0.001 |
| Oxygen dependent a | 0.8 | 1.6 | <0.001 |
| Medications (in past 30 days) (%) | |||
| ACEI or ARB | 54.3 | 45.4 | <0.001 |
| Beta‐blocker | 69.7 | 65.6 | <0.001 |
| Calcium channel blocker | 18.3 | 38.9 | <0.001 |
| Nitrate | 20.0 | 13.0 | <0.001 |
| Arrived by EMS (%) | 30.3 | 33.8 | <0.001 |
| ED triage vital signs, median (IQR) | |||
| Systolic blood pressure (mmHg) | 134.0 (117.0–153.0) | 142.0 (125.0–161.0) | <0.001 |
| Diastolic blood pressure (mmHg) | 79.0 (66.0–93.0) | 74.0 (63.0–88.0) | <0.001 |
| Respiratory rate (per minute) | 20.0 (18.0–24.0) | 20.0 (18.0–24.0) | <0.001 |
| Heart rate (per minute) | 91.0 (76.0–110.0) | 86.0 (72.0–104.0) | <0.001 |
| Oxygen saturation (%) | 97.0 (94.0–98.0) | 95.0 (92.0–98.0) | <0.001 |
| Change in weight over baseline b (%) | |||
| >5 lb above baseline | 19.1 | 23.4 | <0.001 |
| >10 lb above baseline | 10.1 | 13.1 | <0.001 |
| ED laboratory data, median (IQR) | |||
| BNP (pg/mL) | 942 (520–1702) | 480 (239–870) | <0.001 |
| Troponin (pg/mL) | 0.04 (0.03–0.10) | 0.03 (0.02–0.06) | <0.001 |
| Sodium (mmol/L) | 138 (135–140) | 138 (135–140) | 0.0028 |
| BUN (mg/dL) | 25.0 (18.0–37.0) | 24.0 (17.0–37.0) | <0.001 |
| Creatinine (mg/dL) | 1.21 (0.94–1.73) | 1.15 (0.85–1.71) | <0.001 |
| Haemoglobin (g/dL) | 12.3 (10.6–13.7) | 11.4 (9.9–12.9) | <0.001 |
| Chest X‐ray findings (%) | |||
| Cardiomegaly | 48.2 | 42.6 | <0.001 |
| Congestion | 64.7 | 66.2 | 0.079 |
| Pleural effusion | 48.5 | 48.0 | 0.56 |
| Acute illness severity scores, median (IQR) | |||
| COPS2 c score | 67 (35–106) | 75 (43–113) | <0.001 |
| Non‐invasive ventilation used in ED (%) | 13.0 | 13.7 | 0.25 |
| ED disposition (%) | |||
| Discharged | 17.0 | 20.4 | <0.001 |
| Observed | 17.4 | 18.6 | 0.077 |
| Admitted | 65.6 | 61.0 | <0.001 |
| Follow‐up outpatient visit (clinic or telephone) within 7 days of ED visit (%) d | 58.8 | 54.6 | <0.001 |
ACEI, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, B‐type natriuretic peptide; BUN, blood urea nitrogen; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; ED, emergency department; EMS, emergency medical services; ESRD, end‐stage renal disease; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IQR, inter‐quartile range; PCI, percutaneous coronary intervention; SES, socio‐economic status.
There are a small number of patients who died in the ED, so the sum of discharged, observed, and admitted patients appears smaller than the total study population. Overall, rates of missingness of variables were <5%, except for BNP (10.8% missing) and troponin (11.6% missing).
Oxygen dependent defined as requiring O2 to maintain saturation >92%.
Baseline weight was calculated as the average of all weights from all visits over the previous 365 days.
COPS2—internally derived and validated co‐morbidity risk score (Comorbidity Point Score). 26
We did not adjust for 7 day outpatient follow‐up in the analysis of outcomes.
Results
Between 1 January 2017 and 31 December 2018, there were 26 050 total ED encounters for HF based on our study inclusion protocol, and of these, 15 275 (58.6%) had known EF. See Figure 1 for cohort selection. Combining structured data (chief complaint, ICD‐10 code, and BNP values) with the natural language processing algorithm to identify Framingham diagnostic criteria resulted in sensitivity of 87%, specificity of 90%, and positive predictive value of 89% for true AHF when compared with physician adjudication as the gold standard.
Figure 1.
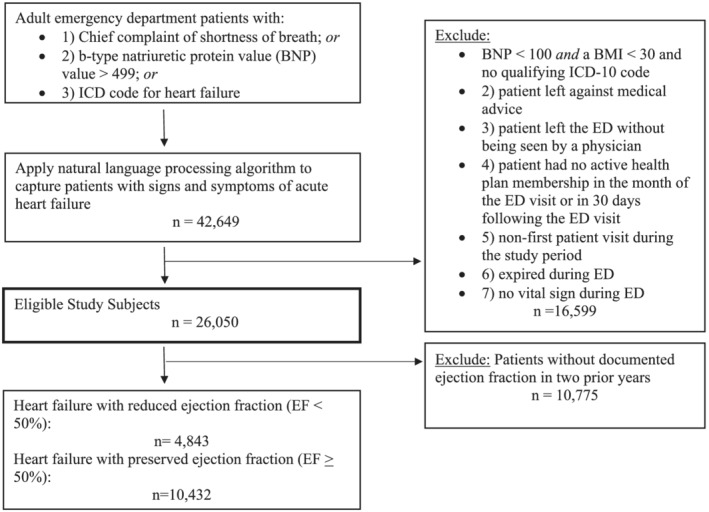
Cohort assembly of 15 275 adult patients treated in the emergency department (ED) for acute heart failure, between 1 January 2017 and 31 December 2018. BMI, body mass index; EF, ejection fraction; ICD, International Classification of Diseases.
Of the 15 275 patients with EF data, 10 432 (68.3%) had HFpEF. The mean age was 76, 49.6% were women, and 60.5% were White (Table 1 ). We found that 62.4% of patients were admitted, 18.3% were observed, and 19.3% were discharged from the ED. Among patients with HFpEF, 61.0%, 18.6%, and 20.4% were admitted, observed, and discharged, and among patients with HFrEF, 65.5%, 17.4%, and 17.0% were admitted, observed, and discharged. Among patients discharged from the ED, including those directly discharged and those discharged after a brief observation period, 64.1% had an outpatient follow‐up visit within 7 days. The 30 day all‐cause mortality rate among all patients (regardless of EF) was lowest among discharged patients (3.9%), intermediate among observed patients (5.9%), and highest among admitted patients (13.9%). Discharged and observed patients had comparable 30 day all‐cause return hospital admission rates (14.1% and 13.5%, respectively), while admitted patients had higher rates (18.1%).
Patients with HFpEF were more likely female, older, white, and less likely to come from a low socio‐economic status neighbourhood. They had higher rates of hypertension, atrial fibrillation, kidney disease, chronic obstructive pulmonary disease, peripheral vascular disease, cancer, and dementia but had lower rates of coronary heart disease. They were more likely to arrive by ambulance to the ED, were more frequently above their baseline weight, and had lower BNP (481 vs. 941) and troponin (0.03 vs. 0.05) levels compared with HFrEF patients. Compared with HFrEF patients, HFpEF patients had lower use of beta‐blockers (65.6% vs. 69.7%, P < 0.001) and angiotensin‐converting enzyme inhibitors (45.4% vs. 54.3%, P < 0.001). Data on patients with missing EF are shown in Supporting Information, Appendix S1 ; in general, these patients had fewer co‐morbidities than patients with EF data available and demographic and laboratory characteristics more similar to HFpEF patients.
Figure 2 shows that the adjusted 30 day mortality rate for HFpEF patients was significantly higher compared with HFrEF patients, 10.2% vs. 8.4%, (P = 0.004). The Kaplan–Meier survival curve shows decreased survival of HFpEF patients compared with HFrEF patients over 30 days (Figure 3 , P = 0.054).
Figure 2.
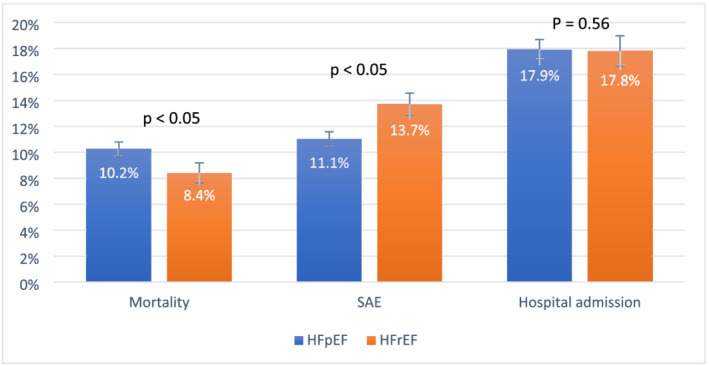
Adjusted 30 day outcome rates comparing patients with heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF): all‐cause mortality, non‐fatal serious adverse events (SAEs), and all‐cause return hospital admission. Non‐fatal SAEs include intra‐aorta balloon pump, endotracheal intubation, renal failure requiring dialysis, myocardial infarction, or coronary revascularization. HFrEF was defined as ejection fraction <50%, and HFpEF was defined as ejection fraction ≥50%. The figure displays outcome rates with 95% confidence intervals. P values represent probability of null hypothesis (no difference in adjusted event rates) comparing patients with EF ≥ 50% with those with EF < 50% for each outcome.
Figure 3.
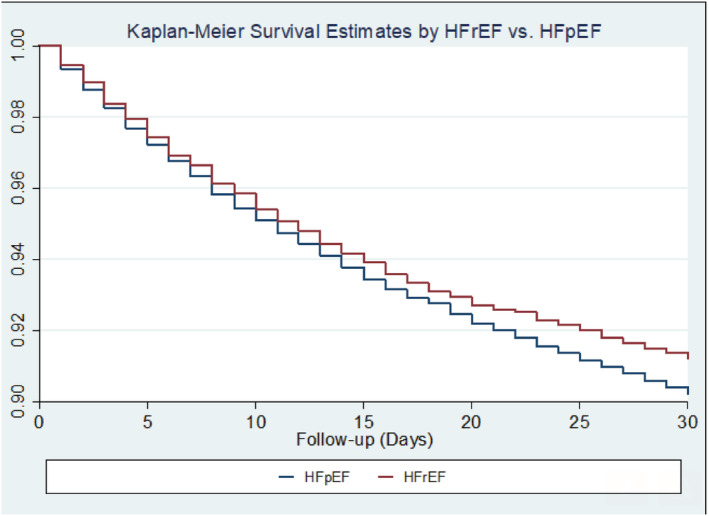
Kaplan–Meier survival curve comparing 30 day all‐cause mortality among patients with an ejection fraction ≥50% [heart failure with preserved ejection fraction (HFpEF)] and those with an ejection fraction <50% [heart failure with reduced ejection fraction (HFrEF)]. P = 0.054 from the log‐rank test for equality of survival distributions.
The adjusted rate of 30 day all‐cause return hospital admission was not significantly different between HFpEF and HFrEF patients, 17.9% vs. 17.8%, respectively, P = 0.89. The adjusted rates of 30 day non‐fatal SAEs were significantly lower among HFpEF patients compared with HFrEF patients, 11.1% vs. 13.7%, P < 0.001 (Figure 2 ). Table 2 displays the unadjusted individual rates of each outcome in the composite non‐fatal SAE outcome among HFrEF and HFpEF patients and suggests that MI and coronary revascularization contributed to higher SAE rates among patients with HFrEF.
Table 2.
Unadjusted rates of each individual outcome of the non‐fatal serious adverse outcome composite among patients with HFrEF (ejection fraction <50%) and HFpEF (ejection fraction ≥50%)
| Clinical outcome | EF phenotype | 30 day outcome rate |
|---|---|---|
| Intra‐arterial balloon pump | HFpEF | 0.05% |
| HFrEF | 0.02% | |
| Endotracheal intubation | HFpEF | 1.11% |
| HFrEF | 1.24% | |
| Renal failure requiring dialysis | HFpEF | 1.60% |
| HFrEF | 1.59% | |
| Myocardial infarction (MI) or coronary revascularization | HFpEF | 7.35% |
| HFrEF | 15.28% |
EF, ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
There were 3692 patients with EF ≤ 40% (76% of all patients with an EF < 50%). The results from the sensitivity analysis comparing outcomes between patients with EF ≤ 40% and those with EF ≥ 50% were comparable with the findings in the main analysis (see Supporting Information, Appendix S1 ). The 30 day all‐cause adjusted mortality rate among patients with EF ≤ 40% was 8.4%, compared with 10.2% among patients with EF ≥ 50%.
The results on the adjusted 30 day mortality between patients with HFrEF and HFpEF stratified by ED disposition are shown in Figure 4 . HFpEF patients had higher mortality regardless of ED disposition, although the difference was only significant among admitted patients.
Figure 4.
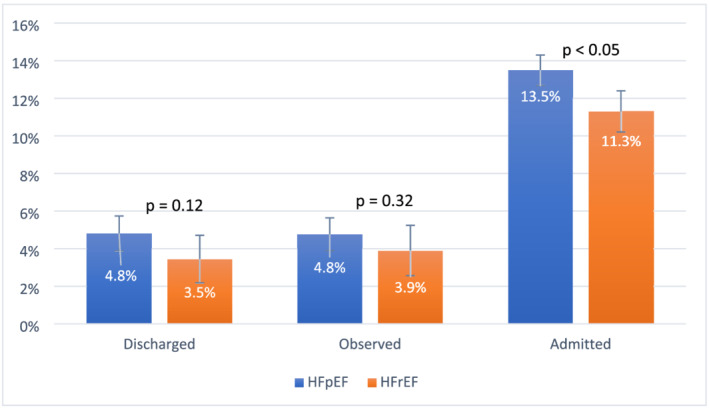
Adjusted 30 day all‐cause mortality (with 95% confidence intervals) by emergency department disposition (direct discharge, observation then discharge, and hospital admission) after the index emergency department visit comparing patients with heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF). HFrEF was defined as ejection fraction <50%, and HFpEF was defined as ejection fraction ≥50%. Observed patients were defined as those officially admitted to an observation unit, those placed under observation status, or those managed in the emergency department for >16 h prior to discharge.
The sensitivity analysis on 30 day return hospital admission and 30 day non‐fatal SAEs among patients alive within 30 days of their index ED visit were comparable with our findings in the main analysis (n = 13 804 patients, 90.4% of full study cohort). The adjusted rates of 30 day all‐cause return hospital admission among those alive at 30 days were not significantly different between HFpEF and HFrEF patients, 16.6% vs. 16.3%, P = 0.92, and the adjusted rates of 30 day non‐fatal SAEs were significantly lower among HFpEF patients compared with HFrEF patients, 9.2% vs. 11.1%, respectively, P < 0.0001.
Discussion
We describe a large, diverse ED population with AHF in a multicentre integrated delivery system. Nearly 40% of patients were discharged, either directly from the ED or after a brief observation stay, a rate substantially higher than national averages. 1 By focusing on outcomes of all ED patients with AHF, we provide a unique perspective on the clinical trajectory of patients with HF symptoms triggering emergent evaluation. Nearly 70% of patients with known EF had HFpEF in our study, highlighting the increasing prevalence of HFpEF patients compared with HFrEF patients. After adjusting for multiple patient‐level characteristics, the 30 day mortality rates were significantly higher among patients with HFpEF compared with those with HFrEF. In contrast, adjusted 30 day return hospital admission rates were not significantly different, and adjusted rates of SAEs were significantly lower among HFpEF patients compared with HFrEF patients. Mortality rates were consistently higher among HFpEF patients regardless of ED disposition but only reached statistical significance among admitted patients.
There remains an urgent need for data on patient outcomes with HFpEF as compared with HFrEF in contemporary populations including the ED population. In comparison with prior studies that found similar 10 , 15 or lower 11 , 12 , 14 mortality among patients with HFpEF compared with those with HFrEF, we found higher adjusted mortality among HFpEF patients. Prior studies assessing outcomes comparing HFrEF patients with HFpEF patients have included study cohorts from the 1990s to 2011. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 To the best of our knowledge, our study cohort from 2017 to 2018 represents the most contemporary assessment of outcomes by EF across the continuum of ED dispositions. Given that HFpEF patients have more non‐cardiovascular deaths compared with HFrEF patients, we suspect that this novel finding of higher all‐cause mortality among HFpEF patients is due to inclusion of a contemporary and aging population with high rates of co‐morbid illness. 30 A recent study found that, due to the ageing population, there has been an 8.5% increase in the number of deaths from heart disease and a 38% rise in deaths specifically from HF. 3 Our study findings confirm this concerning trend and suggest that the higher mortality and growing burden of HFpEF patients are likely driving this trend.
The observed mortality differences between HFrEF and HFpEF patients may also be due to more comprehensive adjustment than earlier studies. 10 , 11 , 12 , 13 , 14 , 15 , 16 We adjusted for multiple patient characteristics, including demographic information, co‐morbidities, recent healthcare utilization, outpatient medications, ED arrival mode, and acute physiology (ED vital signs, laboratory and chest X‐ray findings, and non‐invasive ventilation use), to give a more direct assessment of outcome differences.
Our findings of higher mortality among HFpEF patients compared with HFrEF patients highlight a pressing need for innovation to develop effective strategies to manage the growing population of patients with HFpEF. While effective, guideline‐directed therapies exist for treating HFrEF patients, 31 no therapies thus far show measurable benefit in HFpEF patients. Current treatment for HFpEF targets symptom relief, quality of life, and reduction of cardiac decompensations by managing co‐morbidities and fluid status. A systematic review of HF disease‐modifying programmes among community‐dwelling adults with HFpEF found that some programmes may improve mortality, hospitalization rates, self‐care, and quality of life in patients. 32 A recent study of patients with AHF discharged from the ED found significant differences in 30 day global rank and health status among patients who received a self‐care intervention, regardless of EF. 33 This is a novel and encouraging finding of improved short‐term outcomes among discharged patients; further analysis of strategies to improve outcomes and quality of life should consider EF phenotype to optimize post‐ED transitions of care in this population.
While international guidelines define HFrEF as an EF ≤ 40% and HFpEF as an EF ≥ 50%, 34 , 35 patients with mid‐range EF (41–49%) share some characteristics with those with EF > 50% and with those 40% or less, and these mid‐range EF patients have intermediate clinical outcomes. 36 In addition, there is less clarity on the management of patients with a borderline or mid‐range EF (41–49%), and whether and how the treatments that have been shown to be effective for HFrEF patients should be applied to these mid‐range EF patients. 37 , 38 , 39 Indeed, Pitt et al. used a cut‐off of ≥45% (rather than ≤40% or ≥50%) for a clinical trial of patients with HFpEF. 38 Because we were primarily interested in comparing outcomes between patients with HFpEF with those without HFpEF, we chose an EF cut‐off of 50% for our primary analysis. Regardless, we observed the similar difference in adjusted 30 day mortality rates (8.4%) whether comparing HFpEF patients with those with an EF < 50% or only patients with an EF ≤ 40%. Rates of 30 day return hospital admissions and non‐fatal SAEs were also consistent in the primary and sensitivity analyses. These findings further highlight the emerging challenge of increasing prevalence and significant mortality risk of patients with HFpEF.
Our study reaffirms co‐morbidity and demographic findings among patients with HFpEF compared with those with HFrEF reported in earlier studies. 7 , 8 , 9 , 10 , 11 , 17 Epidemiological studies have indicated that the proportion of HFpEF patients is increasing, with the proportion in the community estimated at 40–84% 11 , 18 , 40 and, among hospitalized patients, 31–55%. 10 In our sample of ED HF patients, nearly 70% had HFpEF, highlighting an emerging challenge for ED providers. The growing prevalence of HFpEF patients observed in our study likely highlights the increasing prevalence of risk factors for HFpEF, in particular older age, hypertension, metabolic syndrome, renal dysfunction, and obesity.
Most prior studies have assessed longitudinal outcomes in a community population, 7 , 8 , 9 , 15 or short‐term or long‐term outcomes after an AHF hospital admission. 10 , 11 , 12 , 13 , 14 , 16 Evaluating the ED population and including those discharged is unique and provides a more inclusive perspective on all patients who seek emergent care for AHF symptoms, including patients discharged from the ED directly or after a brief observation period. The ED discharge rate, either directly from the ED or after a brief observation period, in our population was significantly higher than in other settings. 1 While the exact reasons for this are not clear, it likely relates to the higher access to follow‐up outpatient care in our setting. We found that 30 day all‐cause mortality rates were 1–2% higher among HFpEF patients compared with HFrEF patients regardless of ED disposition, although the difference was only significant among admitted patients, possibly due to smaller sample sizes among discharged and observed patients.
We found similar rates of 30 day all‐cause return hospital admission comparing HFpEF and HFrEF patients among all ED patients. Earlier studies found similar or higher hospital readmission rates comparing HFpEF patients with HFrEF patients after a hospital admission. 13 , 14 While 30 day mortality rates were higher among HFpEF patients, rates of adjusted 30 day non‐fatal SAEs were significantly lower compared with HFrEF patients. This paradox was due to the higher rates of MI and coronary revascularization among HFrEF patients and highlights the existence of interventions, which likely have a greater potential benefit to patients with HFrEF.
Limitations
Despite our novel findings, our study has several limitations. The retrospective nature of the study design might lead to a higher degree of missing data. Approximately 40% of patients were excluded because they did not have EF data available. However, the shared electronic medical record system among KPNC members across care settings supports availability of patient echocardiogram history for most ED visits. Patients missing these data may not have received a recent (within 2 years) echocardiogram, be new health plan members (prior echocardiograms may be from an outside system), or have a new diagnosis of HF and have not yet had an echocardiogram. This study may not generalize well to these groups. Characteristics of patients without EF data available are found in Supporting Information, Appendix S1 , and overall, these patients more closely resemble patients with HFpEF (more frequently women and older, less frequently having a history of coronary heart disease, and with median troponin and BNP values similar to patients with HFpEF). In addition, we were not able to collect hospital length of stay or cause of death of patients in our study, although others have found that non‐cardiac death is significantly more common among HFpEF patients. 41
Lastly, because this study was conducted within an insured population with readily available access to early follow‐up and lower admission rates than nationwide reports, 1 our findings may not generalize to the uninsured or to other types of practice settings. We previously found that close outpatient follow‐up decreased patients' odds of a short‐term adverse event, 42 suggesting that our results may not generalize to settings with less reliable outpatient follow‐up.
Conclusions
In a large and contemporary cohort of AHF patients presenting to an ED, we found that nearly 70% with known EF had HFpEF. In contrast to earlier studies, after adjusting for multiple patient‐level characteristics, we found higher rates of 30 day all‐cause mortality among patients with HFpEF compared with those with HFrEF. Further studies might evaluate the underlying factors associated with this difference and target interventions to improve outcomes.
Conflict of interest
There was no outside or industry funding, support, or honoraria involved. The authors have no conflicts of interest.
Funding
This project was supported by The Permanente Medical Group Delivery Science Research Program Oakland, CA.
Supporting information
Appendix S1. Supporting information.
Acknowledgements
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript. The study was approved by the Kaiser Permanente Northern California Institutional Review Board.
Sax, D. R. , Rana, J. S. , Mark, D. G. , Huang, J. , Collins, S. P. , Liu, D. , Storrow, A. B. , Reed, M. E. , and KP CREST Network (2021) Outcomes among acute heart failure emergency department patients by preserved vs. reduced ejection fraction. ESC Heart Failure, 8: 2889–2898. 10.1002/ehf2.13364.
References
- 1. Storrow AB, Jenkins CA, Self WH, Alexander PT, Barrett TW, Han JH, McNaughton CD, Heavrin BS, Gheorghiade M, Collins SP. The burden of acute heart failure on U.S. emergency departments. J Am Coll Cardiol 2014; 2: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire D, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014; 129: e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sidney S, Go AS, Jaffe MG, Solomon MD, Ambrosy AP, Rana JS. Association between aging of the US population and heart disease mortality from 2011 to 2017. JAMA Cardiol 2019; 4: 1280–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khera R, Wang Y, Bernheim SM, Lin Z, Krumholz HM. Post‐discharge acute care and outcomes following readmission reduction initiatives: national retrospective cohort study of Medicare beneficiaries in the United States. BMJ 2020; 368: l6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta A, Allen LA, Bhatt DL, Cox M, DeVore AD, Heidenreich PA, Hernandez AF, Peterson ED, Matsouaka RA, Yancy CW, Fonarow GC. Association of the hospital readmissions reduction program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol 2018; 3: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mizuno M, Kajimoto K, Sato N, Yumino D, Minami Y, Murai K, Munakata R, Asai K, Keida T, Sakata Y, Hagiwawra N, Takano T, ATTEND Investigators . Clinical profile, management, and mortality in very‐elderly patients hospitalized with acute decompensated heart failure: an analysis from the ATTEND registry. European J Int Med 2016; 27: 80–85. [DOI] [PubMed] [Google Scholar]
- 7. Fonarow GC, Abraham WT, Albert NM, Gattis WA, Gheorghiade M, Greenberg B, O'Connor CM, Yancy CW, Young J. Organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE‐HF): rationale and design. Am Heart J 2004; 148: 43–51. [DOI] [PubMed] [Google Scholar]
- 8. Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population‐based cohort. J Am Coll Cardiol 1999; 33: 1948–1955. [DOI] [PubMed] [Google Scholar]
- 9. Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL, CHS Research Group . Importance of heart failure with preserved systolic function in patients ≥65 years of age. Am J Cardiol 2001; 87: 413–419. [DOI] [PubMed] [Google Scholar]
- 10. Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function: the Cardiovascular Health Study. Ann Intern Med 2002; 137: 631–639. [DOI] [PubMed] [Google Scholar]
- 11. Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA 2006; 296: 2209–2216. [DOI] [PubMed] [Google Scholar]
- 12. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006; 355: 251–259. [DOI] [PubMed] [Google Scholar]
- 13. Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population‐based study. N Engl J Med 2006; 355: 260–269. [DOI] [PubMed] [Google Scholar]
- 14. Loop MS, Van Dyke MK, Chen L, Brown TM, Durant RW, Safford MM, Levitan EB. Comparison of length of stay, 30‐day mortality, and 30‐day readmission rates in Medicare patients with heart failure with reduced versus preserved ejection fraction. Am J Cardiol 2016; 118: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng RK, Cox M, Neely ML, Heidenreich PA, Bhatt DL, Eapen ZJ, Hernandez AF, Butler J, Yancy CW, Fonarow GC. Outcomes in patients with heart failure with preserved, borderline, and reduced ejection fraction in the Medicare population. Am Heart J 2014; 168: 721–730. [DOI] [PubMed] [Google Scholar]
- 16. Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, Killian JM, Roger VL. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000–2010. JAMA Intern Med 2015; 175: 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soloman SD, Anavekar N, Skali H, McMurra JV, Swedberg K, Yusuf S, Granger CB, Michelson EL, Wang D, Pocock S, Pfeffer MA, Candasartan in Heart Failure Reduction in Mortality (CHARM) Investigators . Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005; 112: 3738–3744. [DOI] [PubMed] [Google Scholar]
- 18. Vaduganathan M, Michel A, Hall K, Mulligan C, Nodari S, Shah SJ, Senni M, Triggiani M, Butler J, Gheorghiade M. Spectrum of epidemiological and clinical findings in patients with heart failure with preserved ejection fraction stratified by study design: a systematic review. Eur J Heart Fail 2016; 18: 54–65. [DOI] [PubMed] [Google Scholar]
- 19. Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res 2019; 124: 1598–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census‐based methodology. Am J Public Health 1992; 82: 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gordon N, Lin T. The Kaiser Permanente Northern California Adult Member Health Survey. Perm J 2016; 20: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shivade C, Raghavan P, Fosler‐Lussier E, Embi PJ, Elhadad N, Johnson SB, Lai AM. A review of approaches to identifying patient phenotype cohorts using electronic health records. J Am Med Inform Assoc 2014; 21: 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sczynski JS, Andrade SE, Harrold LR, Tijia J, Cutrona SL, Dodd KS, Goldberg RJ, Gurwitz JH. A systemic review of validated methods for identifying heart failure using administrative data. Pharacoepidemiol Drug Saf 2012; 21: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee DS, Donavan L, Austin PC, Gong Y, Liu PP, Rouleau JL, Tu JV. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care 2005; 43: 182–188. [DOI] [PubMed] [Google Scholar]
- 25. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med 1971; 285: 1441–1446. [DOI] [PubMed] [Google Scholar]
- 26. Escobar GJ, Ragins A, Scheirer P, Liu V, Robles J, Kipnis P. Nonelective rehospitalizations and post‐discharge mortality: predictive models suitable for use in real time. Med Care 2015; 53: 916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Collins SP, Jenkins CA, Harrell FE Jr, Dr L, Miller KF, Lindsell CJ, Naftilan CJ, McPherson JA, Maron DJ, Sawyer DB, Weintraub NL, Fermann GJ, Roll SK, Sperling M, Storrow AB. Identification of emergency department patients with acute heart failure at low risk for 30‐day adverse events: the STRATIFY decision tool. JACC Heart Fail 2015; 3: 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meigher S, Thode HC, Peacock WF, Bock JL, Gruberg L, Singer AJ. Causes of elevated cardiac troponins in the emergency department and their associated mortality. Acad Emerg Med 2016; 23: 1267–1273. [DOI] [PubMed] [Google Scholar]
- 29. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, The Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018; 72: 2231–2264. [DOI] [PubMed] [Google Scholar]
- 30. Zile MR, Gaasch WH, Anand IS, Haass M, Little WC, Miller AB, Lopez‐Sendon J, Teerlink JR, White M, McMurray J, Komajda M, McKelvie R, Ptaszynska A, Hetzel SJ, Massie BM, Carson PE, I‐Preserve Investigators . Mode of death in patients with heart failure and a preserved ejection fraction: results from the Ibresartan in Heart Failure with Preserved Ejection Fraction Study (I‐Preserve) trial. Circulation 2010; 121: 1393–1405. [DOI] [PubMed] [Google Scholar]
- 31. Saltzberg MT. 2016. Update to heart failure clinical practice guidelines. https://www.heart.org/idc/groups/heart‐public/@wcm/@mwa/documents/downloadable/ucm_489089.pdf. Accessed November 1, 2020.
- 32. Kalogirou F, Forsyth F, Kyriakou M, Mantle R, Deaton C. Heart failure disease management: a systematic review of effectiveness in heart failure with preserved ejection fraction. ESC Heart Fail 2020; 7: 194–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Collins SP, Liu D, Jenkins CA, Storrow AB, Levy PD, Pang PS, Chang AM, Char D, Diercks DJ, Fermann GJ, Han JH. Effect of self‐care intervention on 90‐day outcomes in patients with acute heart failure discharged from the emergency department: a randomized clinical trial. JAMA Cardioli 2020. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ponikowski P, Voors AA, Anker SD, BuenoH CJG, Coats AJ, Falk V, Gonzalez‐Juanetey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Rushitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 35. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
- 36. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo‐Leiro MG, Harjola VP, Parissis J, Laroche C, Francesco Piepoli M, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitzka F, Maggioni AP, Filippatos G. Epidemiology and one‐year outcomes in patients with chronic heart failure and preserved, mid‐range, and reduced ejection fraction: an analysis of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017; 19: 1574–1585. [DOI] [PubMed] [Google Scholar]
- 37. Tomasoni D, Adamo M, Lombardi CM, Metra M. Highlights in heart failure. ESC Heart Fail 2019; 6: 1105–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai A, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis FL, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM, TOPCAT Investigators . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 39. Abraham WT, Psotka MA, Fiuzat M, Filippatos G, Lindenfeld J, Mehran R, Ambardekar AV, Carson PE, Jacob R, Januzzi JL, Konstam MA, Krucoff MW, Lewis EF, Piccini JP, Solomon SD, Stockbridge N, Teerlink JR, Unger EF, Zeitler EP, Anker SD, O'Connor CM. Standardized definitions for evaluation of heart failure therapies: scientific expert panel from the Heart Failure Collaboratory and Academic Research Consortium. Eur J Heart Fail 2020; 22: 2175–2186. [DOI] [PubMed] [Google Scholar]
- 40. Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003; 289: 194–202. [DOI] [PubMed] [Google Scholar]
- 41. Vergaro G, Chionzoli N, Innocenti L, Taddei C, Giannoni A, Valleggi A, Borreli C, Senni M, Passino C, Emdin M. Noncardiac versus cardiac mortality in heart failure in preserved, midrange, and reduced ejection fraction. JAHA 2019; 8: 3013441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sax DR, Mark DG, Hsia RY, Tan TC, Tabada GH, Go AS. Short‐term outcomes and factors associated with adverse events among adults discharged from the emergency department after treatment for acute heart failure. Circ Heart Fail 2017; 10: e004144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information.


