Abstract
Background and Aims
It has been documented that African Americans have been significantly affected by COVID-19 infection due to systemic societal factors, which may lead to increases in comorbid medical history and subsequently vulnerability to having higher viral loads as measured by the cycle threshold/number (CT/CN) values by reverse transcriptase polymerase chain reaction (RT-PCR). Differences in CT/CN values by ethnicity and comorbid medical history could play an important role in public health research, particularly in elucidating the reasons for differential public health outcomes by ethnicity, as viral loads are known to correlate with disease severity. However, there is a gap in the literature regarding CT/CN values by ethnicity and comorbid medical history. Therefore, this study seeks to address this literature gap and its important implication for public health research.
Methods
A retrospective review of all SARS-CoV-2 RT-PCR tests collected at the regional Veterans Administration Medical Center (VAMC) serving the Philadelphia area from March 17, 2020, to May 20, 2020, was performed to collect demographic information such as race, gender, and age. In addition, comorbid medical conditions, clinical course, and CT/CN values were obtained for the positive cases.
Results
There was a total of 1524 patients tested for SARS-CoV-2. A total of 713/1524 patients (46.8%) were African American. A total of 187/1524 patients (12%) had tested positive for SARS-CoV-2 from which 139/187 (74%) were African American. African American patients required more intensive unit care. Both African Americans and other ethnicities had similar rates of comorbid medical conditions. On comparison of the ethnic groups, there were lower viral loads in African Americans on admission, though the difference was not statistically significant.
Conclusion
African American Veterans tested positive at higher rates and require more ICU care, despite similar rates of comorbid illness and viral loads.
Keywords: Population demographics, SARS-CoV-2, Access to health care, Molecular pathology, COVID-19, Quality control, Quality assurance, Clinical pathology
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the disease known as coronavirus disease 2019 (COVID-19) [1]. Since its origins in Wuhan China in or around December 2019, SARS-CoV-2 has spread worldwide to become an international pandemic affecting nearly all countries around the globe [1]. The United States of America has been severely affected by the pandemic; within the American population, African Americans are overrepresented in COVID-19 disease cases [2]. This disparity negatively affecting African Americans is likely due to systemic societal factors [3–10]. Even before the pandemic, it had been well documented in the literature that African Americans have reduced access to care, lower levels of health insurance coverage, and generally receive lower quality care compared to other ethnicities [1–5]. These societal factors for African American population may lead to negative outcomes [5–15]. The presence of health disparity or inequality may also increase the likelihood of more comorbid medical conditions (such as cardiac, pulmonary, diabetes, and smoking history) due to lower quality preventive care. The increase in the number of comorbid medical conditions may be hypothesized to lead to poorer health outcomes or higher viral loads when challenged by SARS-CoV-2 infection. Higher viral loads, as inversely measured by the cycle threshold/cycle number (CT/CN) by reverse transcriptase polymerase chain reaction (RT-PCR) such that lower CT/CN values correspond to higher viral loads, are a known negative prognostic marker [16]. However, despite the known differences in outcome between ethnic groups, the differences in the known prognostic marker of CT/CN values or viral load between ethnic groups remains to be fully investigated in the literature. Studying the correlation between ethnicity and comorbid medical conditions with CT/CN values on admission could play an important role in public health research into racial disparity in outcomes, especially for the Veteran population early in the course of the pandemic.
Methods
A retrospective review of positive SARS-CoV-2 RT-PCR tests from only Veteran patients performed at the regional Veterans Administration Medical Center (VAMC) patient care areas serving the Philadelphia and surrounding areas from March 17, 2020, to May 20, 2020, was performed. Age, gender, ethnicity, comorbid medical conditions, clinical course (hospitalization, intensive care unit admission, intubation, and death) information, and CT/CN values were obtained in this retrospective review of the medical record (see Table 1). CT/CN values would be obtainable only for positive test results for SARS-CoV-2, and was resulted during the study period from the Abbott RealTime SARS-CoV-2 assay on the Abbott m2000 (Des Plaines, IL) and the Xpert Xpress SARS-CoV-2 assay on the Cepheid GeneXpert Infinity (Sunnyvale, CA). At the beginning of the study period prior to the implementation of both of these in-house SARS-CoV-2 testing platforms, specimens were sent out to reference laboratories for SARS-CoV-2 testing; for these patients, all information was obtainable except for the CT/CN value from the reference laboratory. The Xpert Xpress SARS-CoV-2 assay would give two CT/CN values (one for envelope or E and the other for nucleocapsid or N2 nucleic acid targets) per positive for which the lower CT/CN value (other than 0 or not detected) was recorded and considered for this study. The Abbott RealTime SARS-CoV-2 assay would only give one CT/CN value per positive test, which was recorded and considered for this study. Covering the same time period, a VistA FileMan (Veteran Affairs, Washington DC) report captured the basic demographics of all patients tested for SARS-CoV-2 RT-PCR. The FileMan program is a database management system for the Veterans Health Information systems and Technology Architecture user (VistA) environment that was used in this study to extract the demographic information for all of the patients. The ethnic composition of the tested Veteran population was compared with the published demographic information of the Philadelphia area to (see Table 1) [17, 18].
Table 1.
(A) Demographics of study populations, both those who tested positive and the total population for the ethnic distribution of veterans being tested. Only those tested in patient care areas serving veterans specifically were included in the total population. (B) The average and standard deviations for the CT/CN values for all tested patients including African American patients and others
| A | ||||
| Demographics | Census data | |||
| Age (years of age) | Numbers in veterans that tested positive (percentage of veterans that tested positive) | Numbers in control population | Age | Percentages |
| <40 | 12 (6.4%) | 247 (16.2%) | Under 65 years of age | 86% |
| 40–49 | 10 (5.3%) | 201 (13.2%) | 65 years and over | 14% |
| 50–59 | 37 (19.8%) | 318 (20.9%) | Gender | Percentages |
| 60–69 | 57 (30.5%) | 403 (26.4%) | Male | 47.30% |
| 70–79 | 47 (25.1%) | 268 (17.6%) | Female | 52.70% |
| >80 | 24 (12.8%) | 87 (5.7%) | Race | Percentages |
| Total | 187 | 1524 | African American | 43.60% |
| Gender | Numbers in population that tested positive | Numbers in control population | Caucasian American | 44.80% |
| Male | 178 (95.2%) | 1071 (70.3%) | Asian | 7.80% |
| Female | 9 (4.8%) | 453 (29.7%) | Other | 3.90% |
| Total | 187 | 1524 | ||
| Race | Numbers in population that tested positive | Numbers in control population | ||
| African American | 139 (74.3%) | 713 (46.8%) | ||
| Caucasian American | 39 (20.9%) | 339 (22.2%) | ||
| Asian | 3 (1.6%) | 6 (0.4%) | ||
| Other | 1 (0.5%) | 9 (0.6%) | ||
| Unknown or declined to state | 5 (2.7%) | 457 (30.0%) | ||
| Total | 187 | 1524 | ||
| B | ||||
| Average CT/CN value | Standard deviation | |||
| African Americans | 19.07 | 9.14 | ||
| Caucasian Americans | 17.61 | 8.44 | ||
| Others | 12.79 | 7.63 | ||
| Student’s T test (African Americans vs. everyone else) = 0.08 | ||||
| Student’s T test (African Americans vs. Caucasian Americans) = 0.41 | ||||
| Average CT/CN value | Standard deviation | |||
| Males | 18.07 | 8.71 | ||
| Females | 22.82 | 11.83 | ||
| Student’s T test (males vs. females) = 0.30 | ||||
| Average CT/CN value | Standard deviation | |||
| <40 years of age | 12.27 | 5.25 | ||
| 40–49 years of age | 17.73 | 8.63 | ||
| 50–59 years of age | 18.1 | 8.53 | ||
| 60–69 years of age | 18.94 | 8.91 | ||
| 70–79 years of age | 18.94 | 9.49 | ||
| >80 years of age | 19.16 | 10.09 | ||
| Student’s T test (<40 years of age vs. >50 years of age) = 0.003 | ||||
Results
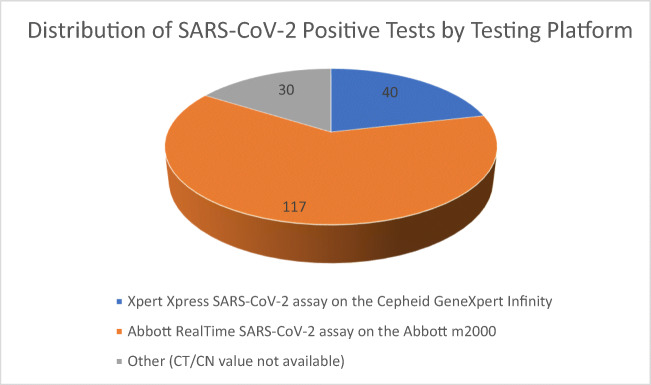
There was a total population of 1524 patients that were tested for SARS-CoV-2, of which 713 out of 1524 or 46.8% were African American (see Table 1). Out of these 1524 patients, there were a total of 187 Veteran patients (12%) that tested positive for SARS-CoV-2, of which 139 out of 187 or 74% were African American (see Table 1). African Americans were overrepresented in proportion of those testing positive in a statistically significant manner (p value by chi squared test <0.05) [19]. The total percentage of the tested population that was African American was similar to the population percentage of African Americans in the surrounding metropolitan area (43.60%) by the local census [17, 18]. Nine patients had a race identification that was something other than African American or Caucasian American, thus leaving a remaining quantity of 178 patients that were African Americans or Caucasian Americans (see Table 2). Two of these 178 patients were treated outside of Veteran Affairs without available information on intensive care unit treatment in the available medical record, and 1 of these 2 was also without information on intubation in the available medical record. African American ethnicity was statistically associated (p value <0.05) with intensive care (ICU) needs compared with Caucasian Americans, odds ratio 3.67 (see Table 2) [20]. There were similar rates of comorbid conditions between African Americans and other ethnicities in the categories of cardiovascular disease other than just hypertension, pulmonary disease, chronic renal disease, hypertension, and diabetes (see Table 2). The rates of the specific presence of certain comorbid medical conditions were similar between the different ethnic groups. Additionally, CT/CN values appeared to be significantly lower in those less than 40 years of age compared to everyone else greater than 50 years of age (p value <0.05, see Table 1). Out of the 187 veteran patients who tested positive for SARS-CoV-2, CT/CN values were available for 157 patients (30 patients had their specimen sent out to the reference laboratory for which a CT/CN value would not be available, see Fig. 1). Forty out of the 187 patients had their SARS-CoV-2 testing performed by the Xpert Xpress SARS-CoV-2 assay on the Cepheid GeneXpert Infinity platform, and 117 out of the 187 patients had their SARS-CoV-2 testing performed by the Abbott RealTime SARS-CoV-2 assay on the Abbott m2000 platform (see Fig. 1). Although the average viral loads (lower cyclic thresholds/numbers) showed lower viral loads in African Americans, a measure of statistical uncertainty (specifically the p value = 0.08 for African Americans vs. everyone else) demonstrated that the difference in values was not statistically different.
Table 2.
Statistical calculations of outcomes with ethnicity. The only outcome with statistical significance for the sample size was admission to the Intensive Care Unit, for which African American patients were more likely to be admitted to the intensive care unit. There were not statistically significant differences in the presence of comorbid medical conditions despite the significant increase in risk of intensive care unit admission. ICU Intensive Care Unit, CKD chronic kidney disease, CV cardiovascular disease other than HTN, HTN hypertension, CI confidence interval, and NS not statistically significant. Statistics calculated as per published guidelines19–20, 34
| Race and outcomes | |||||||||||
| Hospitalized or required medical monitoring | Death | Intubation required | Admitted to ICU | Past history of CKD | |||||||
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | ||
| Race | African American | 70 (39.3%) | 69 (38.8%) | 21 (11.8%) | 118 (66.3%) | 18 (10.2%) | 120 (67.8%) | 48 (27.4%) | 89 (50.3%) | 44 (24.7%) | 95 (53.4%) |
| Caucasian American | 21 (11.8%) | 18 (10.1%) | 1 (0.01%) | 38 (21.3%) | 0 | 39 (22.0%) | 5 (2.3%) | 34 (20%) | 6 (3.4%) | 33 (18.5%) | |
| Odds for African Americans | 1.01 | 0.18 | 0.15 | 0.54 | 0.46 | ||||||
| Odds for Caucasian Americans | 1.17 | 0.03 | 0.00 | 0.15 | 0.18 | ||||||
| Odds ratio | 0.87 | 6.76 | Not calculable | 3.67 | 2.55 | ||||||
| Upper limit of odds ratio (95% CI) | 1.77 | 51.97 | Not calculable | 9.99 | 6.52 | ||||||
| Lower limit of odds ratio (95% CI) | 0.43 | 0.88 | Not calculable | 1.35 | 0.99 | ||||||
| p value | NS | NS | NS | 0.01 | NS | ||||||
| Past history of CV disease | Past history of HTN | Past history of pulmonary disease | Past history of diabetes | ||||||||
| Yes | No | Yes | No | Yes | No | Yes | No | ||||
| Race | African American | 51 (28.7%) | 88 (49.4%) | 108 (60.7%) | 31 (17.4%) | 28 (15.7%) | 111 (62.4%) | 59 (33.1%) | 80 (44.9%) | ||
| Caucasian American | 21 (11.8%) | 18 (10.1%) | 27 (15.1%) | 12 (6.7%) | 11 (6.2%) | 28 (15.7%) | 14 (7.9%) | 25 (14.0%) | |||
| Odds for African Americans | 0.58 | 3.48 | 0.25 | 0.74 | |||||||
| Odds for Caucasian Americans | 1.17 | 2.25 | 0.39 | 0.56 | |||||||
| Odds ratio | 0.50 | 1.55 | 0.65 | 1.32 | |||||||
| Upper limit of odds ratio (95% CI) | 1.02 | 3.41 | 2.27 | 2.75 | |||||||
| Lower limit of odds ratio (95% CI) | 0.24 | 0.70 | 0.29 | 0.63 | |||||||
| p value | NS | NS | NS | NS | |||||||
Fig. 1.
Proportion of positive SARS-CoV-2 tests by platform utilized. CT/CN values were obtained by all positive tests on the Abbott and Cepheid platforms. CT/CN, cycle threshold/cycle number
In addition to an overall comparison of CT/CN of the entire cohort, the CT/CN by ethnic group, gender, and age was looked at with both individual test assays separated (see Table 3). Out of the 40 positive tests performed on the Xpert Xpress SARS-CoV-2 assay on the Cepheid GeneXpert Infinity platform, 31 were from African American patients and 9 on Caucasian American patients. Average CT/CN values was 24.4 for Caucasian American patients and 28.1 for African American patients; although the average viral load from these CT/CN values is lower for African Americans, the measure of statistical uncertainty (p value of 0.26 by the T test) was not statistically different (see Table 3). Of the 117 positive tests performed on the Abbott RealTime SARS-CoV-2 assay on the Abbott m2000 platform, 84 were African American, 25 were Caucasian American, and other/mixed racial backgrounds constituted 8 patient tests (see Table 3). The average CT/CN values on the Abbott platform for the Caucasian American patients (14.4) were similar to the average for the African American patients (15.7). Among the 30 patient specimens sent to a reference laboratory from which a CT/CN value was unavailable, 24 were African American, 5 Caucasian American, and 1 is of unknown or declined to state race.
Table 3.
Demographic summary by testing platform. (A) Xpert Xpress SARS-CoV-2 assay on the Cepheid GeneXpert Infinity platform, (B) Abbott RealTime SARS-CoV-2 assay on the Abbott m2000 platform, and (C) patients whose specimens were sent to a reference laboratory whereby a CT/CN value would not be available
| A. Cepheid | Total number (%) | Average CT/CN value |
| African Americans | 31 (77.5%) | 28.1 |
| Caucasian Americans | 9 (22.5%) | 24.37 |
| Student’s T test (African Americans vs. Caucasian Americans) = p value of 0.26 | ||
| Total number (%) | Average CT/CN value | |
| Males | 38 (95%) | 26.86 |
| Females | 2 (5%) | 35 |
| Student’s T test (males vs. females) = p value of 0.56 | ||
| Total number (%) | Average CT/CN value | |
| <40 years of age | 1 (2.5%) | 19 |
| 40–49 years of age | 2 (5%) | 21.2 |
| 50–59 years of age | 7 (17.5%) | 28.53 |
| 60–69 years of age | 14 (35%) | 27.64 |
| 70–79 years of age | 8 (20%) | 28.9 |
| >80 years of age | 8 (20%) | 27.25 |
| B. Abbott | Total number (%) | Average CT/CN value |
| African Americans | 84 (71.8%) | 15.73 |
| Caucasian Americans | 25 (21.4%) | 14.44 |
| Other | 8 (6.8%) | 12.79 |
| Student’s T test (African Americans vs. Caucasian Americans) = p value of 0.37 | ||
| Total number (%) | Average CT/CN value | |
| Males | 111 (94.9%) | 15.07 |
| Females | 6 (5.1%) | 18.77 |
| Student’s T test (males vs. females) = p value of 0.35 | ||
| Total number (%) | Average CT/CN value | |
| <40 years of age | 9 (7.7%) | 11.52 |
| 40–49 years of age | 6 (5.1%) | 16.59 |
| 50–59 years of age | 25 (21.4%) | 15.45 |
| 60–69 years of age | 36 (30.8%) | 15.56 |
| 70–79 years of age | 26 (22.2%) | 15.88 |
| >80 years of age | 15 (12.8%) | 14.84 |
| C. Test sent to reference lab | Total number (%) | |
| African Americans | 24 (80%) | |
| Caucasian Americans | 5 (1.7%) | |
| Unknown/Declined to state | 1 (3.3%) | |
| Total number (%) | ||
| Males | 29 (96.7%) | |
| Females | 1 (3.3%) | |
| Total number (%) | ||
| <40 years of age | 2 (6.7%) | |
| 40–49 years of age | 2 (6.7%) | |
| 50–59 years of age | 5 (16.7%) | |
| 60–69 years of age | 7 (23.3%) | |
| 70–79 years of age | 13 (43.3%) | |
| >80 years of age | 1 (3.3%) | |
Discussion
Race and ethnicity are risk markers for worse health outcomes [3–15, 17, 21, 22]. It is known that race and ethnicity is a marker for patients who are more likely to be disadvantaged by society in regard to socioeconomic status, reduced access to quality health care, and even increased likelihood of being an essential worker who could be exposed to the virus [6, 14, 21, 22]. These adverse conditions could lead to increased susceptibility to infection, with a potential biological mechanism being weakened cell-mediated immunity due to adverse conditions [7]. However, it does not appear that race by itself is the factor leading to worse outcomes, but rather that race is a marker of socioeconomic disadvantage [6, 9, 14, 21, 22]. It is then the adverse socioeconomic disadvantage or societal discrimination that is leading to the worse outcomes both in general health, ICU admissions, and in COVID-19 outcomes. Indeed, a study by Kabarriti et al demonstrated that providing complete access to medical services equally in a comprehensive health care environment would significantly ameliorate the observed racial tendency toward worse outcomes for African Americans [9]. The fact that this amelioration occurs constitutes further evidence that race is simply a marker of another socioeconomic factor that is the actual reason for the observed correlation with worse outcomes. This amelioration with comprehensive care, which would be provided by the regional VAMC, provides a reason why the other outcomes (i.e. hospitalization, death, intubation, and comorbid medical conditions) were not statistically worse in African Americans in this study. Given the likelihood that race is a marker for a socioeconomic factor, a potential future direction for additional research in the future would be to evaluate the CT/CN value differences based on a measure of socioeconomic status. Additionally, the observation that comprehensive care can ameliorate the observed differences in outcomes based on race suggests the need for interventions to address the contextual conditions facing vulnerable subgroups (i.e., through improved education, reduction of poverty, and/or other ongoing stressors) in order to ensure the equitable complete access that would reduce or eliminate the disparity in health outcomes. Interestingly, there appeared to be a statistically significant difference in CT/CN values in those <40 years and those >50 years of age. This suggests that those who are younger but still come to medical attention for a positive test tend to have higher viral loads.
It is known that the Veteran population is a different population from the rest of the general population [23–25]. These differences occur to such an extent that population-based medical risk factor findings in the general population do not always apply completely to the different Veteran population [23–25]. The Veteran population treated at a VAMC has access to an integrated health care system that is unlike the separate and non-integrated multitude of private health care settings. Particularly, the VAMC is an equal access provider of medical care regardless of socioeconomic status, ethnicity, or the ability to pay. In addition, the Veteran population has been documented to generally have more comorbidities and susceptibilities to disease compared to the general population; Veteran patients at VAMCs are indeed sicker on average [23–25]. Population-based studies oftentimes need to be conducted in not only the general population but also the Veteran population in order to ensure applicability of the population findings. This study involved the Veteran population exclusively, and thus provides useful population-based data by ethnicity, outcomes, and CT/CN values for this population. However, given the sparsity of published literature on CT/CN values for the general population by ethnicity, further study of a non-veteran or general population would be useful to answer the question of whether measured CT/CN values (a surrogate for SARS-CoV-2 viral load) differ by ethnicity in the general population.
Given the documentation about the differences between the Veteran and general population, prior studies of Veterans receiving care by the Department of Veteran Affairs have documented African American Veterans experiencing an excess burden of SARS-CoV-2 infection that is not due exclusively to more comorbid medical conditions [26–28]. Similarly, in this study, African American patients were statistically significantly more likely to test positive and more likely to have severe disease requiring admission to an ICU in this study, despite having comparable comorbid medical conditions to other Veteran patients. Interestingly, though not statistically significant in the entire data set between both testing platforms, it was noted that Veterans who were not African American had on average lower CT/CN values and thus higher viral loads on testing. It is known that CT/CN values or viral loads differ based on when the patient presents during their disease course [29–33]. For patients who become symptomatic, CT/CN values are generally lowest in the first week of symptom onset (corresponding to higher viral load) particularly in the first few days and then increase with the passage of time as the viral load diminishes [29–33]. Therefore, the ability of the patient to seek prompt medical care may impact the CT/CN value measured on presentation; a significant delay to presentation may lead to higher measured CT/CN values. A delay in seeking prompt care in serious medical situations can reasonably lead to worse outcomes. Even though lower CT/CN values are associated with increased mortality and intubation risk, the non-statistically significant inclination toward higher CT/CN values in the African American patients did not lead to better outcomes as African American patients were more likely to require ICU care (Table 2).
Conclusion
While African Americans tended to test positive at higher rates and require more ICU care, viral load and presence of specific comorbid medical conditions were not significantly different in African American Veterans. This again demonstrates the increased risk of COVID-19 disease in African Americans even despite similar comorbid medical conditions and viral load or CT/CN values.
Declarations
Ethics Approval
This retrospective study was reviewed and approved by the IRB of CMCVAMC.
Conflict of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hsu LY, Chia PY, Lim JF. The novel coronavirus (SARS-CoV-2) pandemic. Ann Acad Med Singap. 2020;49(105):105–107. doi: 10.47102/annals-acadmedsg.202051. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. (2020, November). Coronavirus disease 2019 (COVID-19): CDC COVID data tracker, maps, charts, and data provided by the CDC. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
- 3.Egede LE. Race, ethnicity, culture, and disparities in health care. J Gen Intern Med. 2006;21(6):667–669. doi: 10.1111/j.1525-1497.2006.0512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulatao, R.A., & Anderson, N.B., (Eds.). (2004). Understanding racial and ethnic differences in health in late life: a research agenda. National Research Council (US) Panel on Race, Ethnicity, and Health in Later Life. https://www.ncbi.nlm.nih.gov/books/NBK24692/?report=classic doi: 10.17226/11036 [PubMed]
- 5.Mayberry, R.M., Mili, F., & Ofili, E. (2000). Racial and ethnic differences in access to medical care. Medical Care Research and Review, 57(1_suppl), 108-145. [DOI] [PubMed]
- 6.Kim S, Bostwick W. Social vulnerability and racial inequality in COVID-19 deaths in Chicago. Health Educ Behav. 2020;47(4):509–513. doi: 10.1177/1090198120929677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopel J, Perisetti A, Roghani A, Aziz M, Gajendran M, Goyal H. Racial and gender-based differences in COVID-19. Front Public Health. 2020;8:418. doi: 10.3389/fpubh.2020.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes L Jr, Enwere M, Williams J, Ogundele B, Chavan P, Piccoli T, et al. Black-white risk differentials in COVID-19 (SARS-COV2) transmission, mortality and case fatality in the United States: translational epidemiologic perspective and challenges. Int J Environ Res Public Health. 2020;17(12):4322. 10.3390/ijerph17124322. [DOI] [PMC free article] [PubMed]
- 9.Kabarriti R, Brodin NP, Maron MI, Guha C, Kalnicki S, Garg MK, et al. Association of race and ethnicity with comorbidities and survival among patients with COVID-19 at an urban medical center in New York. JAMA Netw Open. 2020;3(9):e2019795–5. [DOI] [PMC free article] [PubMed]
- 10.Kaufman, H. W., Niles, J. K., & Nash, D. B. (2020). Disparities in SARS-CoV-2 positivity rates: associations with race and ethnicity. Population Health Management. [DOI] [PMC free article] [PubMed]
- 11.Vahidy FS, Nicolas JC, Meeks JR, Khan O, Pan A, Jones SL, et al. Racial and ethnic disparities in SARS-CoV-2 pandemic: analysis of a COVID-19 observational registry for a diverse US metropolitan population. BMJ Open. 2020;10(8):e039849. 10.1136/bmjopen-2020-039849. [DOI] [PMC free article] [PubMed]
- 12.Pan D, Sze S, Minhas JS, Bangash MN, Pareek N, Divall P, et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine. 2020;23:100404. 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed]
- 13.Niedzwiedz CL, O’Donnell CA, Jani BD, Demou E, Ho FK, Celis-Morales C, et al. Ethnic and socioeconomic differences in SARS-CoV-2 infection: prospective cohort study using UK Biobank. BMC Med. 2020;18(1):160. 10.1186/s12916-020-01640-8. [DOI] [PMC free article] [PubMed]
- 14.Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2020;72(4):703–6. 10.1093/cid/ciaa815. [DOI] [PMC free article] [PubMed]
- 15.Petersen JM, Dalal S, Jhala D. Ethnic differences in infection with SARS-CoV-2; a Veteran Affairs Medical Center (VAMC) experience. Am J Clin Pathol. 2020;154(Suppl 1):S143–S144. doi: 10.1093/ajcp/aqaa161.314. [DOI] [Google Scholar]
- 16.Magleby, R., Westblade, L. F., Trzebucki, A., Simon, M. S., Rajan, M., Park, J., Goyal, P., Safford, M. M., & Satlin, M. J. (2020). Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, ciaa851. Advance online publication. 10.1093/cid/ciaa851. [DOI] [PMC free article] [PubMed]
- 17.The United States Census Bureau. (2020, November). United States Census for Philadelphia County, Pennsylvania.https://www.census.gov/quickfacts/philadelphiacountypennsylvania.
- 18.Petersen JM, Dalal S, Jhala D. Improved across the board access to SARS-CoV-2 laboratory testing in an integrated medical system; the Veteran Affairs Medical Center (VAMC) experience. Am J Clin Pathol. 2020;154(Suppl 1):S144–S145. doi: 10.1093/ajcp/aqaa161.316. [DOI] [Google Scholar]
- 19.Swinscow, T. (Eds.). (1997). Statistics at Square One 9th edition, The BMJ. BMJ, https://www.bmj.com/about-bmj/resources-readers/publications/statistics-square-one
- 20.Tenny, S., & Hoffman, M.R. (2020) Odds ratio, StatPearls, https://www.ncbi.nlm.nih.gov/books/NBK431098/ [PubMed]
- 21.Centers for Disease Control and Prevention. (2020, October). Coronavirus disease 2019 (COVID-19): COVID-19 hospitalization and death by race/ethnicity.https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html.
- 22.Centers for Disease Control and Prevention. (2020, October). Coronavirus disease 2019 (COVID-19): health equity considerations and racial and ethnic minority groups.https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/race-ethnicity.html.
- 23.Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker?: a comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252–3257. doi: 10.1001/archinte.160.21.3252. [DOI] [PubMed] [Google Scholar]
- 24.Eibner C, Krull H, Brown KM, Cefalu M, Mulcahy AW, Pollard M, et al. Current and projected characteristics and unique health care needs of the patient population served by the Department of Veterans Affairs. Rand health quarterly. 2016;5(4):13. [PMC free article] [PubMed]
- 25.Morgan, R. O., Teal, C. R., Reddy, S. G., Ford, M. E., & Ashton, C. M. (2005). Measurement in Veterans Affairs Health Services Research: veterans as a special population. Health services research, 40(5p2), 1573-1583. [DOI] [PMC free article] [PubMed]
- 26.Rentsch CT, Kidwai-Khan F, Tate JP, Park LS, King JT Jr, Skanderson M, et al. Patterns of COVID-19 testing and mortality by race and ethnicity among United States veterans: a nationwide cohort study. PLoS Med. 2020;17(9):e1003379. 10.1371/journal.pmed.1003379. [DOI] [PMC free article] [PubMed]
- 27.Ioannou GN, Locke E, Green P, Berry K, O’Hare AM, Shah JA, Crothers K, Eastment MKC, Dominitz JA, Fan VS. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3(9):e2022310. doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferguson JM, Magid HSA, Purnell AL, et al. Differences in COVID-19 testing and test positivity among veterans, United States, 2020. Public Health Rep. 2021;136(4):483–492. doi: 10.1177/00333549211009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He X, Lau E, Wu P, Deng X, Wang J, Hao X, Lau YC, Wong JY, Guan Y, Tan X, Mo X, Chen Y, Liao B, Chen W, Hu F, Zhang Q, Zhong M, Wu Y, Zhao L, Zhang F, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 30.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–9. 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed]
- 31.Singanayagam, A., Patel, M., Charlett, A., Lopez Bernal, J., Saliba, V., Ellis, J., Ladhani, S., Zambon, M., & Gopal, R. (2020). Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin, 25(32), 2001483. 10.2807/1560-7917.ES.2020.25.32.2001483, 25. [DOI] [PMC free article] [PubMed]
- 32.Salvatore, P. P., Dawson, P., Wadhwa, A., Rabold, E. M., Buono, S., Dietrich, E. A., Reses, H. E., Vuong, J., Pawloski, L., Dasu, T., Bhattacharyya, S., Pevzner, E., Hall, A. J., Tate, J. E., & Kirking, H. L. (2020). Epidemiological correlates of PCR cycle threshold values in the detection of SARS-CoV-2. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. Clin Infect Dis 2021;72(11):e761–767. [DOI] [PMC free article] [PubMed]
- 33.Kissler, S. M., Fauver, J. R., Mack, C., Tai, C., Shiue, K. Y., Kalinich, C. C., Jednak S., Ott I.M., Vogels C.B.F., Wohlgemuth J., Weisberger J., Difiori J., Anderson D.J., Mancell J., Ho D.D., Grubaugh N.D., & Grad, Y. H. (2020). Viral dynamics of SARS-CoV-2 infection and the predictive value of repeat testing. medRxiv.